Altered Drp1 activity after acute IOP elevation may be an important component of a biochemical cascade leading to RGC death in ischemic retina. Thus, the results support further studies to determine whether inhibition of Drp1 by mdivi-1 may provide a novel mechanism to protect RGCs against pressure-related ischemic damage.
Abstract
Purpose.
To determine whether acute intraocular pressure (IOP) elevation alters dynamin-related protein 1 (Drp1) as well as whether a selective inhibitor of Drp1, mdivi-1, can block apoptotic cell death and subsequently increase retinal ganglion cell (RGC) survival in ischemic mouse retina.
Methods.
C57BL/6 mice received injections of mdivi-1 (50 mg/kg) or vehicle, and then transient retinal ischemia was induced by acute IOP elevation. RGC survival was measured after FluoroGold labeling. Drp1 and glial fibrillary acidic protein (GFAP) protein expression and distribution were assessed at 12 hours after ischemia–reperfusion by Western blot and immunohistochemistry. Apoptotic cell death was assessed by TUNEL staining.
Results.
Drp1 and GFAP protein expression was significantly increased in the early neurodegenerative events (within 12 hours) of ischemic mouse retina. Mdivi-1 treatment blocked apoptotic cell death in ischemic retina, and significantly increased RGC survival at 2 weeks after ischemia. In the normal mouse retina, Drp1 is expressed in the ganglion cell layer (GCL) as well as the inner plexiform layer, the inner nuclear layer (INL), and the outer plexiform layer (OPL). In the GCL, Drp1 immunoreactivity was strong in RGCs. While Drp1 protein expression was increased in the GCL of vehicle-treated ischemic retina at 12 hours. Mdivi-1 treatment did not change this increase of Drp1 protein expression but significantly decreased GFAP protein expression.
Conclusions.
These findings suggest that altered Drp1 activity after acute IOP elevation may be an important component of a biochemical cascade leading to RGC death in ischemic retina.
Elevated intraocular pressure (IOP) is an important risk factor for optic nerve damage and retinal ganglion cell (RGC) death in glaucoma.1 However, the precise pathophysiological relationships among elevated IOP, mitochondrial dysfunction, and RGC death are poorly understood. Recently, we found that elevated IOP can trigger abnormal mitochondrial cristae depletion, mitochondrial fission, and OPA1 and cytochrome c release in the optic nerve or retina, and that it can induce apoptotic cell death in RGCs in a mouse model of glaucoma.2,3 Also, acute IOP elevation induces OPA1 and cytochrome c release, as well as apoptotic cell death in RGCs of ischemic rat retina.4 These observations raise the possibility that pressure-induced mitochondrial dysfunction is a part of ischemic damage-related RGC death in glaucomatous retina.
Mitochondria are autonomous and morphologically dynamic organelles that structurally reflect a precise balance of ongoing fission and fusion within a cell.5–8 This balance is regulated by a family of dynamin-related GTPases that exert opposing effects. Drp1, the human orthologue of Dnm1 in mice, regulates mitochondrial fission, while OPA1, the human orthologue of Mgm1p/Msp1p, and the mitofusins are required for mitochondria fusion.5,7
Drp1 assembles from the cytosol onto mitochondria at focal sites of mitochondrial fission.7,9–11 Emerging evidence indicates that Drp1 is concomitantly involved in apoptosis with mitochondrial outer membrane permeabilization (MOMP) and that inhibition of Drp1 prevents partially intrinsic apoptosis.10,12–14 Mitochondrial division inhibitor (mdivi)-1 is a derivative of quinazolinone and a selective inhibitor of mitochondrial fission Drp1. It inhibits GTPase activity by blocking the self-assembly of Drp1 in vitro and causes the rapid, reversible and dose-dependent formation of netlike mitochondria in wild-type cells.10,11
Recent evidence demonstrates that mdivi-1 has a protective effect in an experimental model of renal ischemia–reperfusion-induced acute kidney injury, as well as in an in vitro culture model that induces apoptotic cell death in mammalian COS cells by staurosporine.10,15 Previously, we reported that elevated hydrostatic pressure triggers Drp1 translocation from the cytosol into the mitochondria and induces apoptotic cell death in cultured RGC-5 cells. However, the specific functional role of Drp1 in pressure-induced ischemic retina remains unknown.
Hence, the present study was undertaken to determine whether acute IOP elevation alters mitochondrial fission mediator, Drp1, as well as whether mdivi-1 can block apoptotic cell death and subsequently increase RGC survival in ischemic mouse retina.
Materials and Methods
Transient Retinal Ischemia
All procedures concerning animals were in accordance with the ARVO Statement for the Use of Animals in Ophthalmic Vision Research and under protocols approved by institutional IACUC committees at the University of California-San Diego. C57BL/6 mice, 3 months of age (20–25 g in weight; Harlan Laboratories, Indianapolis, IN) were housed in covered cages, fed with a standard rodent diet ad libitum, and kept on a12-hour light/12-hour dark cycle. For transient retinal ischemia, the mice were anesthetized with a mixture of ketamine (100 mg/kg, Ketaset; Fort Dodge Animal Health, Fort Dodge, IA) and xylazine (9 mg/kg, TranquiVed; Vedeco, Inc., St. Joseph, MO) by intraperitoneal (IP) injection. A cannula was inserted into the anterior chamber that was connected by flexible tubing to a reservoir. By raising the reservoir, we elevated IOP elevated above systolic blood pressure (100–120 mm Hg) for 60 minutes. The animals were allowed to recover for 6 to 24 hours or for 2 weeks.
Pharmacologic Treatment
Two groups of mice were studied after unilateral transient retinal ischemia: a group treated with vehicle (dimethyl sulfoxide [DMSO], IP injection; n = 30 mice/group) and a group treated with mdivi-1 (50 mg/kg in DMSO, IP injection; n = 30 mice/group) given 60 minutes before and 6 hours after ischemia.
Tissue Preparations
The mice were anesthetized with isoflurane followed by an IP injection of a mixture of ketamine and xylazine. Both eyes were enucleated, and then the mice were killed by CO2 inhalation. The retinas were dissected from the choroid and fixed in the 4% paraformaldehyde in phosphate-buffered saline (PBS, pH 7.4) for 2 hours at 4°C. They were dehydrated through graded ethanols and then embedded in polyester wax, as previously described.16 For Western blot analyses, whole retinas were immediately used or frozen in liquid nitrogen and stored at −70°C until use.
TUNEL Staining
Sections were incubated with proteinase K (10 μg/mL, 10 mM Tris [pH 7.4–8.0]) for 10 minutes at 37°C. After rinsing in PBS, the sections were incubated with terminal deoxynucleotidyl transferase plus nucleotide mixture in reaction buffer for 60 minutes at 37°C (In situ Cell Death Detection kit; Roche Applied Science, Indianapolis, IN) as previously described.4 To count TUNEL-positive cells, the areas were divided into three layers by ganglion cell layer (GCL), inner nuclear layer (INL), and outer nuclear layer (ONL). TUNEL-positive cells were counted in eight microscopic fields of 0.2 mm from retinal sections per condition (n = 5 retinas) by two investigators in a masked fashion, and the scores were averaged.
Retrograde Labeling and Counting of RGCs
One week before death, anesthetized mice situated in a stereotactic apparatus received FluoroGold (1 μL/injection of 4%; Fluorochrome, Inc., Englewood, CO) diluted in saline and microinjected bilaterally into the superior colliculi with a mixture of ketamine and xylazine as previously described.16 To count RGCs, each retinal quadrant was divided into three zones by center, middle, and peripheral retina (one sixth, three sixths, and five sixths of the retinal radius). RGC densities were measured in 28 distinct areas of 0.344 mm2 (two areas at center and middle, and three areas at periphery per retinal quadrant) per condition by two investigators in a masked fashion, and the scores were averaged.
Western Blot Analysis
The retinas were homogenized in a glass-Teflon Potter homogenizer in lysis buffer (20 mM HEPES [pH 7.5], 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 0.5% CHAPS, and complete protease inhibitors; Roche Applied Science). Each sample (10 μg) was separated by PAGE and electrotransferred to PVDF membranes. The membranes were blocked with 5% nonfat dry milk and 0.05% in Tween-20 in PBS; incubated with mouse monoclonal anti-Drp1 antibody (1:1000; BD Transduction Laboratories, San Diego, CA), mouse monoclonal anti-GFAP antibody (1:3000; Sigma-Aldrich, St. Louis, MO), or mouse monoclonal anti-actin antibody (Ab-1; 1:5000; Calbiochem, La Jolla, CA); rinsed with 0.05% Tween-20 in PBS; incubated with peroxidase-conjugated goat anti-mouse IgG (1:2000; Bio-Rad, Hercules, CA); and developed using chemiluminescence detection (ECL Plus; GE Health care Bio-Sciences, Piscataway, NJ). The images were analyzed as previously described.17
Immunohistochemical Analysis
Immunohistochemical staining of 7-μm wax sections of full-thickness retina was done by an immunofluorescence method, as previously described.16 Five sections per wax block from each group (n = 4 retinas/group) were used for immunohistochemical analysis. The primary antibodies were rabbit polyclonal anti-Drp1 (1:50; Santa Cruz Biotechnology, Santa Cruz, CA), mouse monoclonal anti-Thy1.1 (1:500; Millipore, Billerica, MA), or mouse monoclonal anti-GFAP (1:200; Sigma-Aldrich). To prevent nonspecific background staining, tissues were incubated with 1% bovine serum albumin/PBS for 1 hour at room temperature and then with the primary antibodies against Drp1, Thy1.1, and GFAP for 16 hours at 4°C. After several washes, the sections were incubated with Fluor 488-conjugated goat anti-rabbit IgG or Fluor 568-conjugated goat anti-mouse IgG (1:200; Invitrogen, Carlsbad, CA) for 4 hours at 4°C and then washed with PBS. The sections were counterstained with Hoechst 33342/PBS (1 μg/mL; Invitrogen). Images were analyzed, as previously described.17
Measurement of Blood Pressure and Body Weight
To determine the effect of mdivi-1 on blood pressure, systolic and diastolic blood pressure were measured by tail-cuff plethysmography in conscious mice prewarmed for 10 minutes in a thermostatically controlled restrainer (XBP1000; Kent Scientific Corp., Torrington, CT). After three training sessions during the week before measurement, blood pressure was assessed before an intraperitoneal injection of mdivi-1 or vehicle, and at one day and 1 week after ischemia–reperfusion. The average of 10 separate recordings was taken to represent systolic and diastolic blood pressure in each time point and per condition. Body weight was measured with a scale before an intraperitoneal injection of mdivi-1 or vehicle and at 1 and 2 weeks after ischemia–reperfusion.
Statistical Analysis
Experiments presented were repeated at least three times. The data are presented as the mean ± SD. Comparison of two or three experimental conditions was evaluated by using the unpaired Student's t-test or one-way analysis of variance (ANOVA) and the Bonferroni t-test. A P < 0.05 was considered to be statistically significant.
Results
Differential Expression of Drp1 and GFAP on Ischemic Retina
As shown in Figure 1, normal retinas of 3-month-old C57BL/6 mice contained Drp1 (84 kDa) and GFAP (50 kDa). Increases in retinal Drp1 and GFAP protein induced by acute IOP elevation were maximal 12 hours later (2.53 ± 0.24- and 2.54 ± 0.18-fold, respectively; P < 0.05, Fig. 1). The relative concentration of each of these proteins was less at 24 hours than at 12 hours after ischemia.
Figure 1.
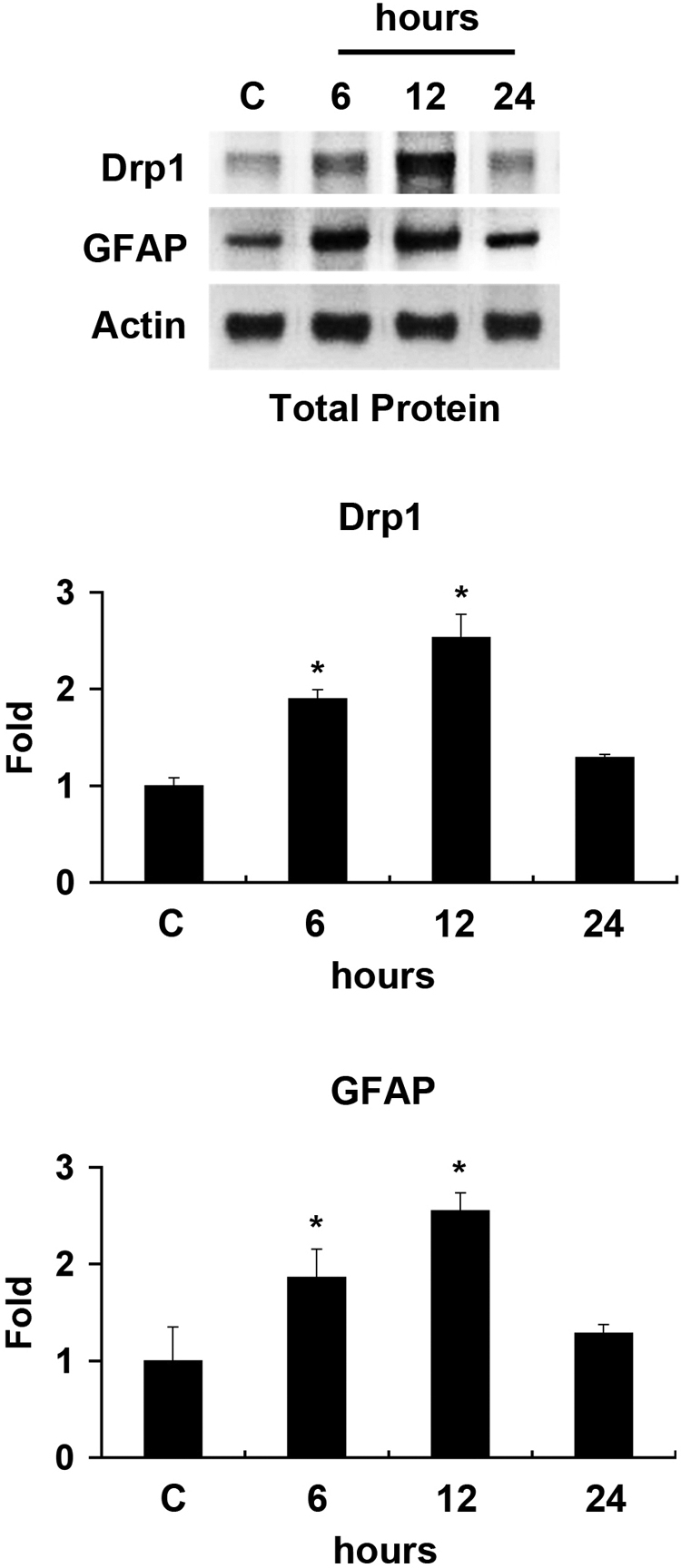
Differential expression of Drp1 and GFAP in ischemic retina. The Drp1 and GFAP protein bands showed a major band at 84 kDa and 50 kDa, respectively, in the retinas of 3-month-old C57BL/6 control mice. Of note, Drp1 and GFAP protein expression peaked at 12 hours after ischemia. Relative intensity of chemiluminescence for bands Drp1 and GFAP protein was normalized using actin as a calibrator. Error bars, SD. *Significant at P < 0.05 compared with 3-month-old C57BL control mice; n = 4 retinas per pool.
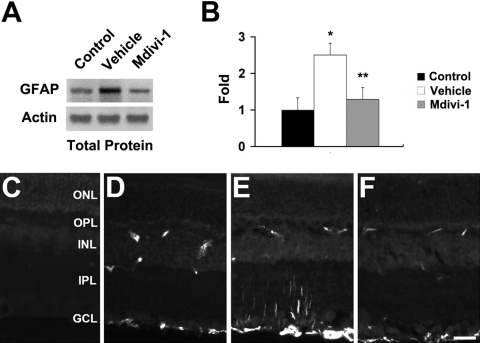
Effect of Mdivi-1 on Apoptotic Cell Death
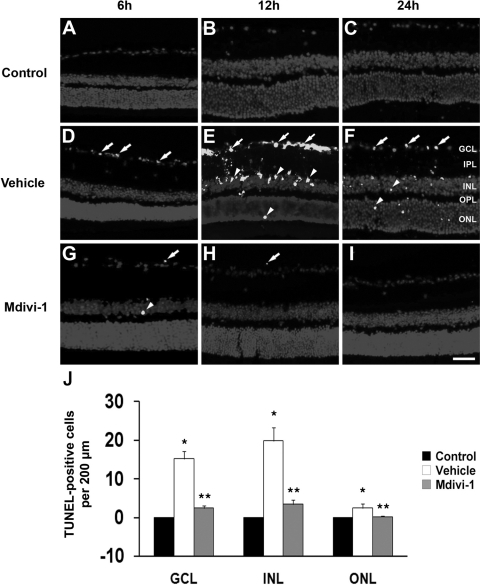
Compared with the retinas of 3-month-old C57BL/6 control mice (Figs. 2A–C), ischemic retina from vehicle-treated eyes showed TUNEL-positive apoptotic cell death in the ganglion cell layer (GCL) and inner nuclear layer (INL) at 6 hours (Fig. 2D) and at 12 hours (Fig. 2E) and in the GCL, INL, and outer nuclear layer (ONL) at 24 hours (Fig. 2F). In contrast, mdivi-1 treatment reduced this apoptotic cell death in all layers of ischemic retina (Figs. 2G–2I). Compared with the retinas of 3-month-old C57BL/6 control mice, vehicle-treated ischemic retina had more apoptotic cells (n = 5 retinas, P < 0.05, Fig. 2J). In contrast, mdivi-1 treatment significantly reduced apoptotic cells by approximately 84% in the GCL, 83% in the INL, and 92% in the ONL compared with vehicle-treated ischemic retina at 12 hours after ischemia (n = 5 retinas, P < 0.05, Figs. 2B, 2E, 2H, 2J).
Figure 2.
Apoptotic cell death in ischemic retina after mdivi-1 treatment. There were no TUNEL-positive cells in the retinas of 3-month-old C57BL/6 control mice (A–C). Vehicle-treated ischemic retinas showed TUNEL-positive apoptotic cell death in the GCL (arrows) and in the INL and ONL (arrowheads, D–F). Of note, mdivi-1 treatment blocked this apoptosis in ischemic retina (G–I). The quantitative analysis of TUNEL-positive cells (J). *Significant at P < 0.05 compared with 3-month-old C57BL control mice; **significant at P < 0.05 compared with vehicle-treated 3-month-old C57BL/6 ischemic mice; n = 5 retinal flat mounts per group. Scale bar, 50 μm.
Effect of Mdivi-1 on RGC Loss
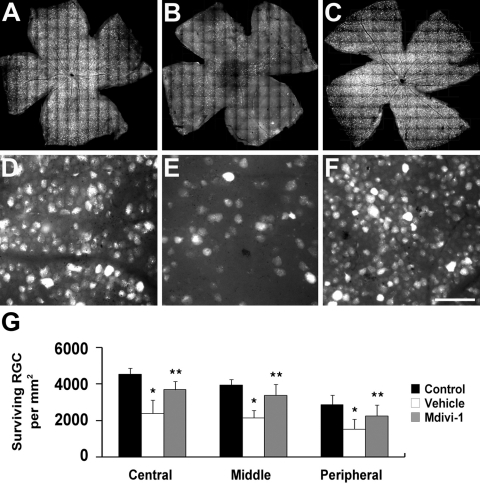
Normal 3-month-old C57BL/6 mouse retina had an average of 4418 RGCs in the central, 3843 RGCs in the middle, and 2911 RGCs in the peripheral areas (n =10 retinas, Figs. 3A, 3D, 3G; Table 1). Compared with the retinas of 3-month-old C57BL/6 control mice, vehicle-treated ischemic retina had greater RGC loss by approximately 46% in the central, 44% in the middle, and 48% in the peripheral areas compared with that in nontreated control mice (n = 10 retinas, P < 0.05, Figs. 3B, 3E, 3G; Table 1). In contrast, mdivi-1 treatment significantly increased RGC survival by approximately 54% in the central, 58% in the middle, and 48% in the peripheral areas compared with vehicle-treated ischemic retina (n = 10 retinas, P < 0.05, Figs. 3C, 3F, 3G; Table 1).
Figure 3.
RGC survival in ischemic retina after mdivi-1 treatment. The retinal flat mounts of 3-month-old C57BL/6 (A, D), vehicle-treated 3-month-old ischemic C57BL/6 mice (B, E), and mdivi-1-treated 3-month-old ischemic C57BL/6 mice (C, F). High magnification showed a significant increase in RGC survival after mdivi-1 treatment compared with vehicle treatment. The quantitative analysis of RGC survival (G). Error bars, SD. *Significant at P < 0.05 compared with 3-month-old C57BL control mice; **significant at P < 0.05 compared with vehicle-treated 3-month-old C57BL/6 ischemic mice; n = 10 retinal flat mounts per group. Scale bar: (D, E, F) 50 μm.
Table 1.
Effect of Mdivi-1 on Central, Middle, and Peripheral RGC Loss at 2 Weeks after Ischemia
| Treatment | Central | Middle | Peripheral |
|---|---|---|---|
| N/A | 4418 ± 532 | 3843 ± 468 | 2911 ± 354 |
| Ischemia/vehicle | 2384 ± 652* | 2145 ± 511* | 1522 ± 478* |
| Ischemia/mdivi-1 | 3690 ± 390† | 3386 ± 452† | 2252 ± 577† |
Data are expressed as the mean RGC density per retina (RGCs/mm2) ± SD.
Comparison of the three groups was made with ANOVA and the Bonferroni t-test.
Significant at P < 0.05 compared with untreated control C57BL/6 mice.
Significant at P < 0.05 compared with vehicle-treated ischemic C57BL/6 mice, n = 5 retinal flat mounts per mouse group.
Effect of Mdivi-1 on Drp1 and GFAP Protein Expression
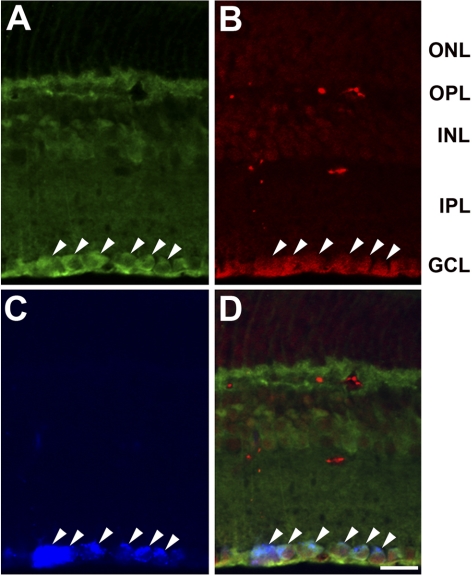
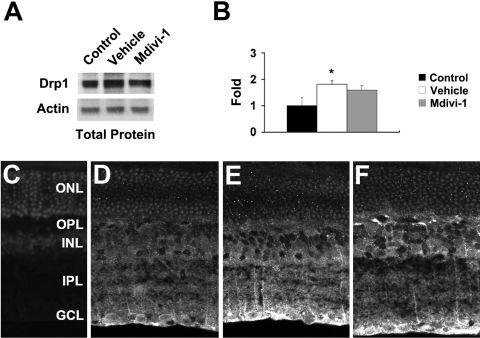
Drp1immunoreactivity was observed in the GCL, inner plexiform layer (IPL), INL, and outer plexiform layer (OPL) in the retina of normal 3-month-old C57BL/6 mice (Fig. 4A). To assess whether RGCs express Drp1, RGCs labeled with FluoroGold were co-immunostained with Drp1 and Thy1.1. As shown in Figure 4, retinal neurons in the GCL were identified that were immunoreactive for both Drp1 and Thy1.1. Moreover, many of these double-labeled GCL neurons also contained FluoroGold, confirming their identification as RGCs. In comparison with the normal retina, vehicle-treated ischemic retina had significantly increased Drp1 protein expression at 12 hours (Figs. 5A, 5B). However, Drp1 protein expression at 12 hours in ischemic retina pretreated with mdivi-1 was not changed (P < 0.05; Figs. 5A, 5B). When the primary antibody was omitted, as a control for Drp1 immunohistochemistry, there was no labeling by the secondary antibody in the retina of normal 3-month-old C57BL/6 control mice (Fig. 5C). Similarly, Drp1 immunoreactivity in GCL neurons of ischemic retina was increased in the absence of mdivi-1 (Figs. 5D, 5E), and this increase was not changed by treatment with mdivi-1 (Fig. 5F).
Figure 4.
Cellular localization of Drp1 in the normal mouse retina determined by Drp1 and Thy1.1 double immunohistochemistry in a GCL that was labeled with FluoroGold. Drp1 immunoreactivity was observed in the GCL, IPL, INL, and OPL in the normal mouse retina (A). Note that Drp1-positive neurons colocalized with Thyl.1, a marker for RGCs (B) and FluorGold (C). Merged image (D). These findings indicate that the RGCs contained Drp1 protein (arrowheads). Scale bar, 20 μm.
Figure 5.
Drp1 expression in ischemic retina after mdivi-1 treatment. Drp1 protein expression was significantly increased in ischemic retina at 12 hours. However, mdivi-1 treatment did not change Drp1 protein expression (A, B). The relative intensity of chemiluminescence for Drp1 protein bands was normalized using actin as a calibrator. Error bars, SD. *Significant at P < 0.05 compared with 3-month-old C57BL control mice; n = 4 retinas per pool. When the primary antibody for Drp1 was omitted, there was no binding of the secondary antibody (C). Compared to normal mouse retina (D), Drp1 immunoreactivity was increased in the GCL of vehicle-treated 3-month-old ischemic C57BL/6 mouse retina (E). However, mdivi-1 did not change Drp1 immunoreactivity in the GCL (F). Scale bar, 20 μm.
Western blot analysis showed that the increase in retinal GFAP induced by ischemia–reperfusion was blocked by pretreatment with mdivi-1 (P < 0.05, Figs. 6A, 6B). When the primary antibody was omitted, as a control for GFAP immunohistochemistry, there was no labeling by the secondary antibody in the retina of normal 3-month-old C57BL/6 control mice (Fig. 6C). Compared with that in normal 3-month-old C57BL/6 control mice (Fig. 6D), GFAP immunoreactivity was increased in Müller cells of the IPL and in astrocytes of the nerve fiber layer (NFL) of vehicle-treated ischemic retina at 12 hours (Fig. 6E). In contrast, mdivi-1 treatment decreased GFAP immunoreactivity in ischemic retina (Fig. 6F). In addition, GFAP immunoreactivity was localized in the blood vessels. This finding likely reflects staining of the processes of Müller cells that were localized around the blood vessels.
Figure 6.
GFAP expression in ischemic retina after mdivi-1 treatment. Mdivi-1 treatment significantly decreased GFAP protein expression in ischemic retina at 12 hours (A, B). Relative intensity of the chemiluminescence band for GFAP protein was normalized using actin as a calibrator. Error bars, SD. *Significant at P < 0.05 compared with 3-month-old C57BL control mice; **significant at P < 0.05 compared with vehicle-treated 3-month-old C57BL/6 ischemic mice; n = 4 retinas per pool. When the primary antibody for GFAP was omitted, there was no binding of the secondary antibody (C). GFAP immunoreactivity in 3-month-old C57BL/6 control mouse retina (D), in vehicle-treated 3-month-old ischemic C57BL/6 mouse retina (E), and in mdivi-1-treated 3-month-old ischemic C57BL/6 mouse retina (F). Scale bar, 20 μm.
Effect of Mdivi-1 on Blood Pressure and Body Weight
In comparison with the vehicle-treated ischemic retina, mdivi-1 treatment did not change mean arterial blood pressure (Table 2) or body weight (Table 3). There were no significant differences in body weight, animal appearance or behavior between vehicle and mdivi-1 treatment.
Table 2.
Effect of Mdivi-1 on Mean Arterial Blood Pressure
| Vehicle | Mdivi-1 | |
|---|---|---|
| Before ischemia | 98.5 ± 6.7 | 99.8 ± 9.5 |
| 1 Day after ischemia | 98.1 ± 5.4 | 99.8 ± 7.3 |
| 1 Week after ischemia | 100.0 ± 7.0 | 99.6 ± 8.3 |
Data are expressed as the mean arterial blood pressure (mm Hg) ± SD. Comparison of the three groups was made with ANOVA and the Bonferroni t-test. Mean arterial blood pressure = diastolic blood pressure + [(systolic blood pressure − diastolic blood pressure)/3].
Table 3.
Effect of Mdivi-1 on Body Weight
| Vehicle | Mdivi-1 | |
|---|---|---|
| Before ischemia | 21.7 ± 0.6 | 21.9 ± 0.4 |
| 1 Week after ischemia | 22.1 ± 0.6 | 22.2 ± 0.5 |
| 2 Weeks after ischemia | 22.6 ± 0.5 | 22.5 ± 0.5 |
Data are expressed as the mean body weight (g) ± SD. Comparison of the three groups was made with ANOVA and the Bonferroni t-test.
Discussion
These results provide the first direct evidence that mdivi-1, a selective inhibitor of Drp1, protects against RGC death after ischemic insult by acute IOP elevation. Interestingly, Drp1 protein expression was increased in the GCL of ischemic retina at 12 hours. However, mdivi-1 treatment did not change the Drp1 protein expression that occurred within 12 hours. Mdivi-1 treatment also significantly reduced activation of astroglia in ischemic retina. These findings suggest that blockade of Drp1 assembly may be a novel mechanism to protect retinal cells, particularly RGCs, against pressure-induced ischemic damage in glaucomatous retina.
Growing evidence suggests that mitochondrial structural and functional dynamics play an important role in cell and animal physiology. Imbalance in the control of mitochondrial fusion and fission dramatically alters overall mitochondrial morphology.7,8 Recent studies suggest that excessive mitochondrial fission can lead to breakdown of the mitochondrial network, loss of mitochondrial DNA, and respiratory defects in mammalian cells.8,18,19 However, the functional relationship between mitochondrial fission protein, Drp1, and RGC death in ischemic retina remains unknown. In the present study, we found that acute IOP elevation significantly increased total Drp1 protein expression in the early neurodegenerative events (within 12 hours) of ischemic mouse retina. Emerging evidence indicates that increased Drp1 expression triggers excessive mitochondrial fission and induces apoptotic cell death.14,20
We observed that inhibiting Drp1 assembly with mdivi-1 can result in increased RGC survival in ischemic retina. Inhibition of Drp1 by overexpression of a dominant-negative mutant of Drp1, Drp K38A, or knockdown of Drp1 blocks mitochondrial fission during apoptosis.20,21 A recent study reported that a general Drp inhibitor, (Dynasore; Tocris Cookson, Inc., Ellisville, MO), inhibits the GTP hydrolysis of dynamin-1, dynamin-2, and Drp1, in noncompetitive manner by binding to the GTPase domain in both assembled and unassembled states.10,22 Intriguingly, Cassidy-Stone et al.10 demonstrated that mdivi-1 selectively inhibits the activity of mitochondrial division of DRPs by binding to an allosteric site that does not exclusively act through the GTPase domain; blocks apoptotic cell death by inhibiting MOMP; and reduces mitochondrial fission and Drp1 self-assembly during apoptosis. Further, midiv-1 attenuates mitochondrial fragmentation and apoptosis in an acute animal model of kidney ischemic injury.15 These observations suggest that mdivi-1 functions by inhibiting Drp1 assembly onto mitochondria or MOMP.
We show here the first demonstration that mdivi-1 treatment significantly increases RGC survival as well as blocks apoptotic cell death in retinas with pressure-induced ischemia. Moreover, our group recently reported that elevated hydrostatic pressure in vitro triggers mitochondrial fission, cellular ATP reduction, OPA1 and cytochrome c release, and apoptotic cell death in differentiated RGC-5 cells.3,17 We also showed in vivo that elevated IOP induces OPA1 and cytochrome c release and apoptotic cell death in ischemic rat retina and glaucomatous DBA/2J mouse retina.4,23 Together, these findings suggest that modulation of mitochondria fission/fusion proteins may be an important factor in ischemic retinal neurodegeneration.
Of note, we observed that Drp1 protein is expressed in RGCs within normal mouse retina. Ischemia increased retinal Drp1 expression in the GCL at 12 hours, and this response was not blocked by mdivi-1. However, mdivi-1 treatment also blocked the increase in retinal GFAP that occurs after ischemia–reperfusion injury. These findings raise the possibility that Drp1-mediated mitochondrial fission may alter the function of retinal glial cells after ischemic damage. Further, it is possible that mdivi-1 facilitates cell survival and protects against pressure-induced ischemic insult by inhibiting Drp1-mediated excessive mitochondrial fission, rather than by altering Drp1 protein expression. These results suggest that inhibiting Drp1 activity may be useful for protecting RGCs against pressure-induced mitochondrial dysfunction in ischemic retina.
Acknowledgments
The authors thank Ohkyung Kwon of the National Center for Microscopy and Imaging Research in University of California San Diego for technical support and advice in Olympus spinning disc confocal microscope.
Footnotes
Supported by National Institutes of Health Grant EY018658 (WKJ) and National Center for Research Resources Grant P41 RR00405 (MHE).
Disclosure: S.W. Park, None; K.-Y. Kim, None; J.D. Lindsey, None; Y. Dai, None; H. Heo, None; D.H. Nguyen, None; M.H. Ellisman, None; R.N. Weinreb, None; W.-K. Ju, None
References
- 1. Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–1720 [DOI] [PubMed] [Google Scholar]
- 2. Ju WK, Kim KY, Lindsey JD, et al. Intraocular pressure elevation induces mitochondrial fission and triggers OPA1 release in glaucomatous optic nerve. Invest Ophthalmol Vis Sci. 2008;49:4903–4911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ju WK, Kim KY, Lindsey JD, et al. Elevated hydrostatic pressure triggers release of OPA1 and cytochrome C, and induces apoptotic cell death in differentiated RGC-5 cells. Mol Vis. 2009;15:120–134 [PMC free article] [PubMed] [Google Scholar]
- 4. Ju WK, Lindsey JD, Angert M, Patel A, Weinreb RN. Glutamate receptor activation triggers OPA1 release and induces apoptotic cell death in ischemic rat retina. Mol Vis. 2008;14:2629–2638 [PMC free article] [PubMed] [Google Scholar]
- 5. Karbowski M, Youle RJ. Dynamics of mitochondrial morphology in healthy cells and during apoptosis. Cell Death Differ. 2003;10:870–880 [DOI] [PubMed] [Google Scholar]
- 6. Okamoto K, Shaw JM. Mitochondrial morphology and dynamics in yeast and multicellular eukaryotes. Annu Rev Genet. 2005;39:503–536 [DOI] [PubMed] [Google Scholar]
- 7. Chen H, Chan DC. Emerging functions of mammalian mitochondrial fusion and fission. Hum Mol Genet. 2005;1:R283–R289 [DOI] [PubMed] [Google Scholar]
- 8. Bossy-Wetzel E, Barsoum MJ, Godzik A, Schwarzenbacher R, Lipton SA. Mitochondrial fission in apoptosis, neurodegeneration and aging. Curr Opin Cell Biol. 2003;15:706–716 [DOI] [PubMed] [Google Scholar]
- 9. Labrousse AM, Zappaterra MD, Rube DA, van der Bliek AM. C. elegans dynamin-related protein DRP-1 controls severing of the mitochondrial outer membrane. Mol Cell. 1999;4:815–826 [DOI] [PubMed] [Google Scholar]
- 10. Cassidy-Stone A, Chipuk JE, Ingerman E, et al. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell. 2008;14:193–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tanaka A, Youle RJ. A chemical inhibitor of DRP1 uncouples mitochondrial fission and apoptosis. Mol Cell. 2008;29:409–410 [DOI] [PubMed] [Google Scholar]
- 12. Frank S, Gaume B, Bergmann-Leitner ES, et al. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell. 2001;1:515–525 [DOI] [PubMed] [Google Scholar]
- 13. Lee YJ, Jeong SY, Karbowski M, Smith CL, Youle RJ. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell. 2004;15:5001–5011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jagasia R, Grote P, Westermann B, Conradt B. DRP-1-mediated mitochondrial fragmentation during EGL-1-induced cell death in C. elegans. Nature. 2005;433:754–760 [DOI] [PubMed] [Google Scholar]
- 15. Brooks C, Wei Q, Cho SG, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest. 2009;119:1275–1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ju WK, Misaka T, Kushnareva Y, et al. OPA1 expression in the normal rat retina and optic nerve. J Comp Neurol. 2005;488:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ju WK, Liu Q, Kim KY, et al. Elevated hydrostatic pressure triggers mitochondrial fission and decreases cellular ATP in differentiated RGC-5 cells. Invest Ophthalmol Vis Sci. 2007;48:2145–2151 [DOI] [PubMed] [Google Scholar]
- 18. Yaffe MP. Dynamic mitochondria. Nat Cell Biol. 1999;1:E149–E150 [DOI] [PubMed] [Google Scholar]
- 19. Youle RJ, Karbowski M. Mitochondrial fission in apoptosis. Nat Rev Mol Cell Biol. 2005;6:657–663 [DOI] [PubMed] [Google Scholar]
- 20. Martinou JC, Youle RJ. Which came first, the cytochrome c release or the mitochondrial fission? Cell Death Differ. 2006;13:1291–1295 [DOI] [PubMed] [Google Scholar]
- 21. Karbowski M, Lee YJ, Gaume B, et al. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J Cell Biol. 2002;159:931–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell. 2006;10:839–850 [DOI] [PubMed] [Google Scholar]
- 23. Ju WK, Kim KY, Angert M, et al. Memantine blocks mitochondrial OPA1 and cytochrome c release and subsequent apoptotic cell death in glaucomatous retina. Invest Ophthalmol Vis Sci. 2009;50:707–716 [DOI] [PMC free article] [PubMed] [Google Scholar]