This study identifies a mechanism for neutrophil-enhanced early biofilm development on contact lens surfaces and indicates a potential new strategy in the prevention of pathogenic biofilm formation during contact lens wear.
Abstract
Purpose.
To evaluate the capacity of neutrophils to enhance biofilm formation on contact lenses by an infectious Pseudomonas aeruginosa (PA) corneal isolate. Agents that target F-actin and DNA were tested as a therapeutic strategy for disrupting biofilms formed in the setting of neutrophils in vitro and for limiting the infectious bioburden in vivo.
Methods.
Biofilm formation by infectious PA strain 6294 was assessed in the presence of neutrophils on a static biofilm plate and on unworn etafilcon A soft contact lenses. A d-isomer of poly(aspartic acid) was used alone and with DNase to reduce biofilm formation on test contact lenses. The gentamicin survival assay was used to determine the effectiveness of the test compound in reducing subsequent intracellular bacterial load in the corneal epithelium in a contact lens infection model in the rabbit.
Results.
In a static reactor and on hydrogel lenses, PA biofilm density was enhanced 30-fold at 24 hours in the presence of neutrophils (P < 0.0001). The combination of DNase and anionic poly(aspartic acid) reduced the PA biofilms formed in the presence of activated neutrophils by 79.2% on hydrogel contact lenses (P < 0.001). An identical treatment resulted in a 41% reduction in internalized PA in the rabbit corneal epithelium after 24 hours (P = 0.03).
Conclusions.
These results demonstrate that PA can exploit the presence of neutrophils to form biofilm on contact lenses within a short time. Incorporation of F-actin and DNA represent a mechanism for neutrophil-induced biofilm enhancement and are targets for available agents to disrupt pathogenic biofilms formed on contact lenses and as a treatment for established corneal infections.
Microbial keratitis (MK) is the most severe and visually devastating complication associated with contact lens wear. Pseudomonas aeruginosa (PA), an opportunistic Gram-negative bacteria, has been identified as the primary pathogen in all reported series of contact lens–related MK for the past two decades.1–4 PA-mediated MK is characterized clinically by the presence of a light-blocking infiltrate and overlying epithelial defect. Destruction of corneal cells, which leads to scarring and vision loss, is the result of intense neutrophil-mediated inflammation.5 Epidemiologic studies in the late 1980s estimated the annualized incidence of MK with conventional hydrogel lens wear to be 4.1 per 10,000 per year for daily wear and 20.9 per 10,000 per year with extended wear.1,6 Chronic hypoxia from wearing these lenses has been thought to contribute to inflammation and infection, as contact lens–mediated reduction in oxygen is associated with significant alterations in the corneal epithelium, including an inhibition of epithelial renewal mechanisms and a corresponding increase in PA adherence to exfoliated corneal epithelia cells.7–10 Despite the much-anticipated reduction in MK after the wide acceptance of hyper–oxygen-transmissible silicone hydrogel lenses, epidemiologic studies continue to report incidence rates of 25.4 per 10,000 per year.11,12
Biofilms are heterogeneous bacterial populations encased in an extracellular matrix that enables the organism to survive in a harsh environment and confers protection against traditional antimicrobial agents.13 In contact lens wear, biofilms have been shown to form on the posterior surface of contact lenses in vivo and on lens storage cases and have been associated with culture-proven microbial keratitis.14,15Adequate compliance with cleaning regimens has not been shown to reduce biofilm formation.16 Likewise, chemically preserved multipurpose lens care solutions (MPS), which constitute more than 90% of the lens care market, have limited effectiveness against biofilm formation on lens surfaces and are unable to penetrate the heavier, more mature biofilms that can accumulate in lens storage cases.17–19 The use of MPS is further limited by potential toxic effects on surface corneal epithelial cells, which have been associated with alterations in surface epithelial permeability and increases in PA binding in clinical studies20,21 (Paugh JR, et al. IOVS 2009;50:ARVO E-Abstract 6358), as well as ZO-1-mediated tight junction breakdown, downregulation of epithelial mucin production, and corresponding loss of cell viability in monolayer culture models22–25 (Imayasu M, et al. IOVS 2010;51:ARVO E-Abstract 1525; Gorbet M, et al. IOVS 2010;50:ARVO E-Abstract 3415).
In human disease, chronic infection by PA results from biofilm formation. Conditions complicated by PA biofilms include pulmonary cystic fibrosis (CF), severe burns, wounds of the skin, and contact lens–associated MK. In each case, exuberant neutrophil recruitment and impaired clearance of the dead and dying cells is followed by PA infection. We have reported that the presence of neutrophils dramatically enhances early biofilm formation in vitro by the laboratory strain PAO126 and by clinical isolates from the CF airway.27 PA utilizes filaments of neutrophil DNA and F-actin as an initial scaffolding to accelerate biofilm development.26 Negatively charged F-actin and DNA are electrostatically linked to positively charged histones and cations.28,29 Polyanionic peptides have the capacity to disassociate DNA and F-actin from histones and to disaggregate F-actin bundles via electrostatic competition.28,29 Recently, we reported that by targeting F-actin and DNA with anionic poly(aspartic acid) and DNase, we were able to effectively disrupt PA biofilms that form in the presence of neutrophils.26,27
In this study, the presence of human neutrophils dramatically enhanced the formation of biofilms caused by an infectious corneal isolate of PA, in the initial stages of their development on conventional hydrogel contact lenses. This effect was seen on both conventional biofilm plates and etafilcon A contact lenses. Biofilms formed in the presence of human neutrophils were disrupted in vitro by a synthetic isomer of polyaspartic acid combined with DNase. The identical mixture of polyaspartic acid with DNase also reduced the burden of infection in a clinical rabbit model of contact lens–related PA infection and was not associated with ocular irritation. Together, these results advance our understanding of how PA overcomes the innate immune defense of the eye and offer a potential therapeutic strategy based on this mechanism.
Materials and Methods
Bacterial Strains, Media, and Culture Conditions
All PA strains were derived from stock plates of Luria broth (LB) agar, grown overnight in RPMI 1640, supplemented with l-glutamine and 2% heat-inactivated (human) platelet-poor plasma (HIPPP), at 37°C with moderate shaking (225 rpm) and stored as frozen stocks, to achieve a consistent growth phase. The bacteria were then grown overnight in RPMI+2% HIPPP at 37°C, with final concentrations adjusted in a spectrophotometer (DU 640; Beckman, Fullerton, CA) at 650 nm to an OD of 0.30 (5 × 108 CFU mL−1). PA strain 6294 was an ocular isolate that was stably conjugated with GFP and was the gift of Suzanne M. Fleiszig (University of California, Berkeley).
Neutrophil Isolation
Neutrophils for all assays were isolated from healthy volunteers by the plasma Percoll method, as described elsewhere.30 The blood sampling procedure was approved by the National Jewish Health Institutional Review Board.
Crystal Violet Biofilm Assay
Human neutrophils were resuspended at a concentration of 16.6 × 106 cells mL−1 in RPMI+2% HIPPP in a round-bottom, 96-well plate (Nunc, Roskilde, Denmark). The cellular control was a plate identical with the test plate, save for the absence of neutrophils. Bacteria were tested at a 1:0.1 ratio, with a final concentration of 16.6 × 105 CFU mL−1. A screening assembly (Transferable Solid Phase [TSP] Screening System, cat. no.445497, Nunc), composed of a noncoated polystyrene 96-well plate lid with pegs that extended into each well, was placed on a 96-well plate of the same material. This assembly was incubated for 24 hours with rocking (three to four oscillations per minute) at 37°C. At 24 hours, the peg lids with adherent biofilms were removed and rinsed for 2 minutes in sterile normal saline to remove nonadherent bacteria and cellular debris. The pegs were treated for 20 minutes with a combination of DNase (8.25 μg mL−1) and 5 μM poly(d-aspartic acid) or poly(l-aspartic acid). After treatment, the peg lid was air dried for 5 minutes and fixed for 10 minutes in 100% ethanol. The ethanol was evaporated for 5 minutes at room temperature and stained in 1% crystal violet (CV) for 15 minutes. Excess CV was removed by two rinses in normal saline. Decolorization took place in a 96-well, flat-bottom plate (Nunc) with 200 μL 100% methanol/well for 45 minutes. Readings were obtained in a plate reader at 550 nm (μQuant, with KCjunior ver. 1.403 software; BioTek Instruments, Winooski, Vermont). Values in the control samples were averaged before comparison of each test well.
Reagents
The peptide used was poly(d-aspartic acid) synthesized at the Peptide and Protein Chemistry Core Laboratory (University of Colorado Denver), initially diluted in PCR grade dH2O to 1 M and then serially diluted in normal saline. DNase (Dornase alpha; Genentech, South San Francisco, CA) was added at a final concentration of 8.25 or 33 μg mL−1.
Formation of PA Biofilms on Contact Lenses
The contact lenses were etafilcon A hydrogel lenses (Vistakon Division of Johnson & Johnson Vision Care, Jacksonville, FL) that were rinsed in saline and divided in half before the assay. The half lenses were placed in wells of a cell culture plate (Costar 3524; Corning, Corning, NY) containing 500 μL RPMI and 2% HIPPP. Human neutrophils were resuspended at a concentration of 16.6 × 106/mL and activated by the addition of 25 nM (or 60 ng/mL) phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich, St. Louis, MO). After 1 minute of stimulation, the neutrophils were pelleted by centrifugation and the supernatant was removed and replaced with an equal volume of fresh medium. A volume of 500 μL of neutrophil suspension was added to each lens-containing well, for a final well volume of 1 mL. Activation was microscopically verified after 4 hours of incubation at 37°C, and 33.3 μL PA was added to the well with a concentration of 5 × 107 CFU/mL. Control lenses were combined with PA in the absence of neutrophils. Lenses and bacteria were incubated at 37°C for 24 to 48 hours to allow biofilm growth.
Microscopic Evaluation of Biofilm Formation
PA 6294 was allowed to form a biofilm on the contact lenses in the presence and absence of neutrophils. After 24 hours, the lenses were air dried and fixed for 30 seconds in methanol. The lenses were stained with Wright's Giemsa (Diff-Quik solution II; Fisher Scientific, Pittsburgh, PA) for 30 seconds and drained. They were counterstained (Diff-Quik solution I) for 30 seconds, rinsed in tap water to remove excess stain, and rapidly dehydrated in absolute alcohol. The bacteria were imaged with light microscopy at 40× magnification.
Quantization of Bacteria Adhering to Contact Lenses
In addition to assaying biofilm density by CV staining, we used two methods to quantify the viable PA adhering to the contact lenses. Viable bacteria were quantified by their determining their metabolic activity with alamar blue reduction27 and verifying the result by the standard colony-counting method. At the time of biofilm analysis, the lenses were washed twice in saline, then individually placed in 500 μL of RPMI for homogenization (Tissue Tearor 985370-395 homogenizer; Biospec Products, Bartlesville, OK). After homogenization, the samples were serially diluted in RPMI and plated on LB agar for overnight growth and colony counts. In parallel, the diluted homogenate (200 μL) was used to inoculate alamar blue development medium (1 mL total well volume, composed of 450 μL RPMI 1640, 250 μL 2× LB broth, and 100 μL of alamarBlue [Invitrogen, Carlsbad, CA] in a culture plate [Costar 3524; Corning]. The plates were read (FLX 800 plate reader; BioTek Instruments) every half hour at 37°C with shaking. Bacterial counts were derived from plotting the V50 of sample wells calculated by a Boltzmann sigmoidal nonlinear regression against the V50 of standard curves generated by defined concentrations of bacteria that were plotted in a linear regression against log CFU.
In Vivo Analysis of the Burden of Infection in the Corneal Epithelium
Twelve New Zealand White rabbits (3.5–3.5 kg body weight) were used. All animals were treated according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Before lens fitting, all rabbits underwent a partial nictitating membranectomy while under anesthesia, to facilitate lens retention, as previously described,31 and were allowed to recover for 1 week before the lens-wearing test was initiated. To establish an in vivo model of infection, we fitted one eye of each rabbit by using fluorescein with a polymethylmethacrylate (PMMA) lens (diameter, 14.0 mm; base curves, 7.60–8.20 mm). After 24 hours of lens wear, the lens was removed, 50 μL of a 1 × 109 CFU PA/mL suspension was applied to the posterior concave surface, and the lens was reinserted for an additional 24 hours of wear (n = 5). Bacterial adherence to the lens surface was verified by laser scanning confocal microscopy. To test whether targeting DNA and F-actin polymers during lens wear would reduce the infection load, we fitted both eyes with PMMA lenses, as just described, for 24 hours (n = 7), the lenses were removed, and PA was added as described. In one eye, the test compound was instilled into the post lens tear film four times over a 24-hour period, by gently lifting the lens edge under light anesthesia with an intramuscular injection of 50 mg/mL ketamine (Fort Dodge Animal Health, Fort Dodge, IA) and 5 mg/kg xylazine (AnaSed; Lloyd Inc. Veterinary Products, Shenandoah, IA). The test compound, 33 μg/mL DNase and 40 μM d-Asp30, was instilled with a preservative-free artificial tear as the vehicle (Refresh Tears; Allergan, Irvine, CA). The preservative-free artificial tear was instilled into the contralateral eye as the control. After lens wear, the animals were euthanatized by intravenous injection of 120 mg/kg pentobarbital sodium (Sleepaway; Fort Dodge Animal Health), and whole globes were enucleated for subsequent analysis.
Draize Ocular Irritation Score
Ocular toxicity with the test compound was evaluated in three rabbits. The rabbits were treated in one eye four times a day with the test compound in the vehicle and in the contralateral eye with only the vehicle, and both were scored on the standard Draize scale.32
Gentamicin Survival Assay
To quantify residual intracellular invasion of PA into corneal epithelial cells, we performed a standard gentamicin survival assay (GSA), as previously reported.33 Each globe was removed, placed in a 12-well plate with 1 mL gentamicin (200 μg/mL) in minimum essential medium (MEM) for 2 hours at 37°C, and subsequently washed three times with MEM. The corneal epithelium was removed by scraping with a surgical blade, and the resultant tissue was incubated in 5 mg/mL saponin-MEM for 30 minutes at room temperature. Viable bacteria were quantified after serial dilution and plating on Mueller Hinton II agar plates. CFUs were counted after overnight incubation (20 hours) at 37°C.
Statistical Analysis
The t-test (Student's or one-tailed, where appropriate) was used to determine the significance of paired data. In analysis of lenses from the rabbit model (see Fig. 4), data were nonparametric, and so paired comparisons were performed with the Wilcoxon signed rank test. One-way and Kruskal-Wallis ANOVA were performed on data where multiple testing resulted in data sets that required additional post test analysis, as well as on unpaired t-tests. Dunn's multiple-comparison post test, Bonferroni's multiple-comparison test, and Dunnett's test, where multiple data sets are compared to a single control set, were used as noted, with significance at P < 0.05 (all analyses: Prism 4; GraphPad Software, Inc., San Diego, CA, or Excel; Microsoft, Redmond, WA).
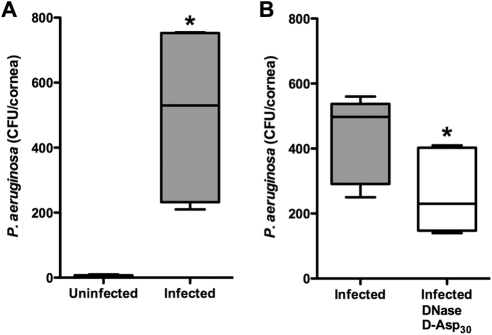
Figure 4.
Targeting F-actin and DNA polymers reduced the burden of infection induced in the corneal epithelium by PMMA lens wear. (A) Establishing PA internalization in vivo. One eye of each rabbit was fit with a PMMA lens. Both eyes were inoculated with 1 × 109 CFU/mL PA 6294. Only the combination of the lens with PA resulted in internalization in the epithelium (CFU/cornea). n = 5 animals per group; *P = 0.03, Wilcoxon signed rank test. (B) Both eyes were fit with PMMA lenses for 48 hours. The lenses were inoculated with PA after 24 hours of wear. One eye of each rabbit was treated four times over a 24-hour period with a combination of DNase and d-Asp30. There was a significant reduction in internalized PA in the corneal epithelium compared with that in the vehicle-treated contralateral control eye. n = 6 animals per group; *P = 0.03, Wilcoxon signed rank test.
Results
Human Neutrophils Induced Enhanced Biofilm Formation by PA
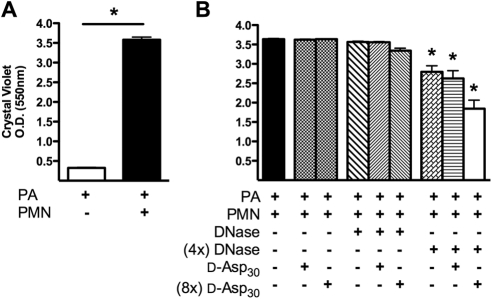
An infectious ocular isolate of PA strain 6294 was combined with activated human neutrophils in a static reactor to model early biofilm development. The presence of human neutrophils with PA at a multiplicity of infection (MOI) of 0.1 resulted in a significant increase in peg-associated biofilm density after 24 hours, when compared with PA biofilms allowed to form in the absence of neutrophils (P < 0.0001; Fig. 1A). For 20 minutes, a synthesized 30-mer d-isomer of poly(aspartic acid) at two concentrations (5 or 40 μM) was introduced, alone or combined with two concentrations of DNase (33 or 8.25 μg/mL), to a PA biofilm formed in the presence of neutrophils (Fig. 1B). Under these conditions, significant reductions in biofilm density occurred in response to the combination of DNase and d-Asp30. This effect was concentration dependent, with the greatest disruption of biofilms achieved with the highest concentration of d-Asp30 combined with DNase (P < 0.01).
Figure 1.
Human neutrophils enhance the capacity of the ocular strain PA 6294 to form biofilms on plates. (A) PA 6294, incubated in astatic biofilm plate reactor in the presence of neutrophils (24 hours), formed a much more dense biofilm than PA grown under identical conditions in the absence of neutrophils (*P < 0.0001 by unpaired t-test). (B) PA biofilms grown on plates in the presence of neutrophils were disrupted by a combination of DNase and poly(d-aspartic) acid in a concentration-dependent manner. PA 6294 was incubated in a static reactor for 48 hours in the presence of human neutrophils. The biofilms were then treated for 20 minutes at 37°C with two concentrations of DNase (1× and 4×), with and without d-Asp30 at two concentrations (1× and 8×), and compared to a saline control. Under these conditions, the highest concentration of DNase resulted in a small but significant reduction in biofilm density. However, the combination of the highest concentration of DNase with the highest concentration of d-Asp30 resulted in the greatest reduction in biofilm density. The data represent results from eight separate experiments, with six replicate wells examined per trial. One-way ANOVA, followed by Dunnett's multiple-comparison test (*P < 0.01).
Neutrophil-Induced Enhancement of PA Biofilm Formation on Contact Lenses
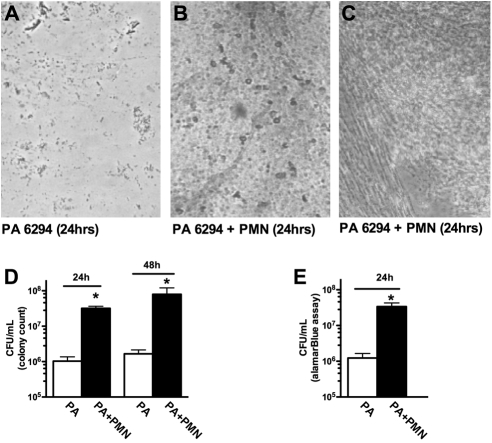
The capacity of activated human neutrophils to enhance biofilm formation by PA 6294 on contact lenses was tested by incubating etafilcon A contact lenses in cultures of PA 6294, in the presence and absence of human neutrophils. After 24 hours, light microscopy showed sparse adherence of PA to the lenses (Fig. 2A). When co-incubated with neutrophils, a greater density of PA was apparent on the lenses (Figs. 2B, 2C). When quantified by standard colony counts, the presence of neutrophils increased the quantity of PA adhering to the lenses by 30.8-fold after 24 hours and by 47.9-fold at 48 hours (Fig. 2D). When bacterial density was analyzed by metabolic activity after 24 hours, an equivalent neutrophil-induced increase in density was confirmed (Fig. 2E).
Figure 2.
Neutrophil-induced enhancement of PA biofilm formation on contact lenses. (A) The ocular isolate PA 6294 was incubated alone or in the presence of human neutrophils. Microscopic examination (40×) of the contact lens demonstrated sparse adherent PA 6294, visible as single bacteria or small clusters. (B, C) PA 6294 incubated in the presence of human neutrophils for 24 hours. Microscopic examination (40×) of the contact lens–associated biofilms displayed a more organized biofilm architecture when compared with PA grown in the absence of neutrophils. (D) Quantification of viable lens-associated PA demonstrated that lenses co-incubated in the presence of activated neutrophils contained a greater amount of PA at both 24 and 48 hours. Quantification performed by direct plating of homogenized lenses, followed by serial dilution and colony counting. *P < 0.001, by Kruskal-Wallis ANOVA with Dunn's multiple-comparison post test. (E) Bacteria associated with contact lenses after 24 hours' incubation was also quantified by determining metabolic activity with alamar blue reduction. With this method, the extent of neutrophil-induced PA biofilm enhancement was found to be equivalent to that determined by colony counts, with a 27-fold increase. (A–C) Images are representative of typical regions from three separate experiments. (D, E) A composite of four separate experiments (*P < 0.0001 by unpaired Student's t-test).
Agents that Target F-actin and DNA Polymers Disrupt Lens-Associated PA Biofilm Formation in the Presence of Neutrophils
PA 6294 biofilms were allowed to form in the presence of human neutrophils for 24 hours on etafilcon A contact lenses and then were treated with either DNase (8.25 μg/mL) or RNase (8.25 μg/mL; Figs. 3A, 3B). DNase was effective in reducing the bacteria associated with the contact lens, but no effect was detected in response to the RNase control. Biofilm density was determined by colony counts (Fig. 3A) and metabolic activity via an alamar blue assay (Invitrogen; Fig. 3B). In subsequent trials, exposure of the biofilm to d-Asp30 also resulted in disruption of the biofilm; however, the addition of DNase, even at low concentrations, enhanced the capacity of d-Asp30 to disrupt the biofilm (Fig. 3C; P < 0.001). Together, the combination of d-Asp30 and DNase resulted in a 79.2% decrease in the CFUs associated with the contact lens after treatment.
Figure 3.
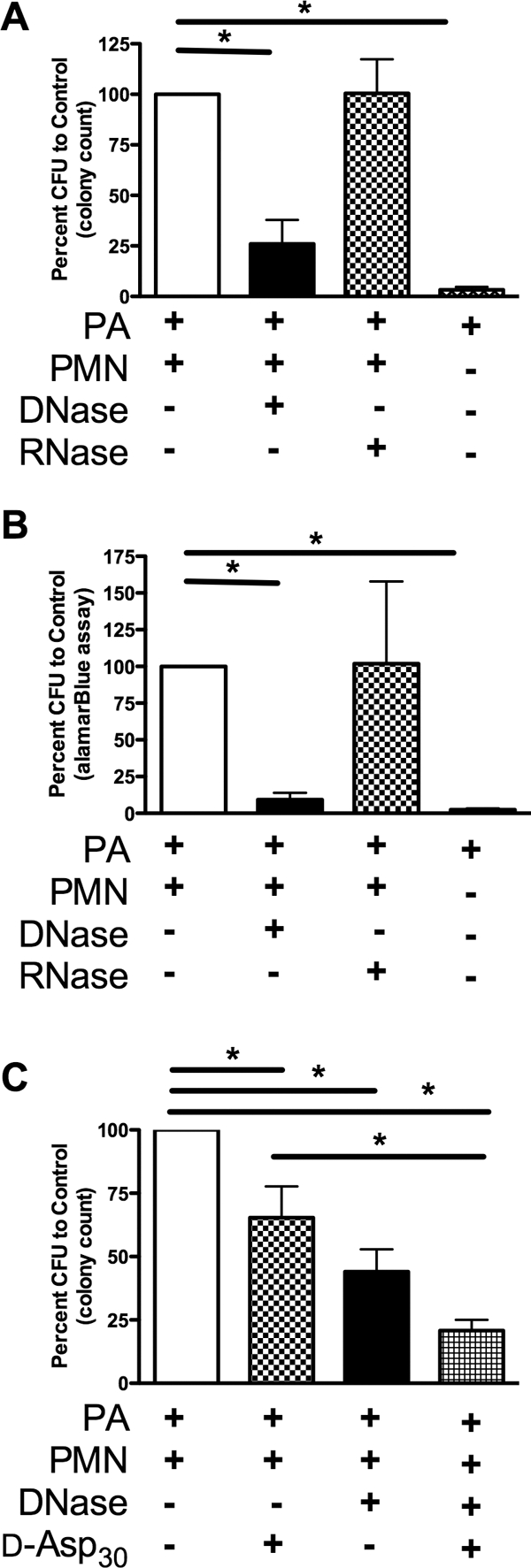
Agents targeting F-actin and DNA disrupted lens-associated PA biofilms formed in the presence of neutrophils. (A) The ocular isolate PA 6294 was incubated on contact lenses, with or without neutrophils, for 24 hours. The lenses were treated with equal concentrations of DNase or RNase for 20 minutes at 37°C. Quantification of the adherent PA was determined by colony counts after serial dilution and plating. (B) In parallel experiments, the quantity of adherent PA was determined by alamar blue reduction. (C) PA 6294, incubated on contact lenses in the presence of neutrophils for 24 hours, was treated with DNase and d-Asp30, alone and in combination, for 20 minutes at 37°C. (A, B) n = 4; (C) n = 6. (*P < 0.01 by one-way ANOVA, followed by Dunnett's multiple-comparison post test).
Targeting F-actin and DNA Polymers Reduces the Burden of Infection Induced in the Corneal Epithelium by PMMA Lens Wear
The utility of the combination of DNase and d-Asp30 in reducing the formation of lens-associated PA biofilm was tested in vivo in a standard rabbit contact lens model of infection. A GSA demonstrated that 48 hours of PMMA lens wear in the presence of bacteria resulted in significant PA internalization in the corneal epithelium, whereas the cornea challenged with PA alone failed to demonstrate any intracellular invasion (Fig. 4A). Pretesting using the Draize scoring model demonstrated no evidence of toxicity after 72 hours of repeated doses (not shown). Treatment with DNase and d-Asp30 four times over the 24-hour PA challenge resulted in a 41% reduction in the lens-associated PA burden (Fig. 4B).
Discussion
The results of this study demonstrate that in eyes inflamed in the setting of contact lens wear, the robust neutrophil response to the cornea has the potential to significantly enhance biofilm formation on hydrogel contact lens surfaces. Specifically, PA strain 6294, an infectious corneal isolate that has been found to be internalized in corneal epithelial cells via lipid raft–mediated endocytosis,33 had little capacity for forming biofilms in vitro in a static reactor or on hydrogel lenses. However, when the model was expanded to include activated neutrophils, bacterial biofilms were rapidly generated (Figs. 1, 2). The presence of neutrophils in conjunction with PA significantly enhanced biofilm formation on the etafilcon A lenses, resulting in an approximately 30-fold increase in the first 24 hours of exposure and a further increase to nearly 50-fold at 48 hours. It has been well established in a variety of models and experimental designs5,34–40 that significant neutrophil accumulation occurs in the corneal epithelium during extended contact lens wear with concurrent bacterial challenge. Collectively, these data support the conclusion that neutrophil accumulation and death have the potential to accelerate infection by PA biofilms in contact lens–related MK.
The pathogenesis of contact lens–related MK caused by PA has been clearly linked to loss of the clearance effect provided by eyelid blinking, stagnation of the posterior lens tear film, and accelerated neutrophil recruitment.5,41 In a rat contact lens model of infection, PA biofilms were shown to preferentially form on the posterior surface of a hydrogel lens.15 The absence of PA from the anterior lens surface is most likely due in part to continuous tear flow across the lens during blinking and to mechanical friction from the eyelid, which would facilitate removal of bacteria. Stagnation of the posterior tear volume under a soft lens, however, may compromise the integrity of epithelial tight junctions, as well as the expression of defensins, surfactant D, mucins, and cytokines.41–44 Overnight lens wear alters cytokine levels and neutrophil infiltration in tears of healthy subjects,45 and this effect is dramatically increased in the pathogenesis of contact lens–induced acute red eye (CLARE) and contact lens–induced peripheral ulcer (CLPU).46 There is also a clear association between prolonged neutrophil infiltration of the cornea and corneal damage5,34–40; however, an inadequate neutrophil response has been linked to more severe PA infection.40 Associated with prolonged neutrophil influx is the death of the cell, whose lifespan in inflamed tissue is estimated to be only a few hours.47 This environmental niche of intense inflammation in the setting of impaired clearance of neutrophil debris closely resembles the conditions associated with severe skin burns or the CF airway,48,49 which are highly predisposed toward PA biofilm infection.
Our results demonstrated that PA successfully exploits the ineffective inflammatory response associated with MK by adhering to polymers of F-actin and DNA that are released from dying neutrophils. Increased adherence of bacteria to the lens has been identified as an initiating factor for MK and other significant lens-related adverse events, including CLARE, CLPU, and infiltrative keratitis.50–53 Negatively charged strands of DNA, when present with F-actin, are linked via positively charged histones and cations.28 Previously, early biofilm formation by the strain PAO1 has been shown to be increased by the adherence of the bacteria to or the delayed detachment from filaments of neutrophil-derived DNA and F-actin.26,27 Polyanionic peptides have the capacity to disassociate DNA histone, as well as F-actin histone complexes and to disaggregate F-actin bundles.28,29 This F-actin and DNA framework represents an attractive potential target for therapeutic intervention,27 as a DNA-based biofilm that has incorporated F-actin could be disrupted by anionic polymers via electrostatic competition.
In the present study, the combination of DNase and anionic polyaspartic acid was used to target PA biofilms on hydrogel lenses in vitro. Previously, DNase has been shown to disrupt PA biofilms,26,27,54 supporting the concept that DNA is also an essential component of the scaffolding or extracellular matrix thought to be involved in biofilm integrity. Inhaled DNase is an established therapy for the reduction of the viscosity of mucus in CF lung disease, and the use of this medication is associated with a decrease in the burden of infection or frequency of pulmonary exacerbations.55,56 Improvement in early biofilm disruption through incorporation of an anionic amino acid in conjunction with DNase may arise from the capacity of poly(Asp) to sequester histones as well as disassociate F-actin along with associated bacteria and saccharides, allowing exposure of a greater number of sites to DNase for cleavage in the actin-DNA polymer.27–29 Previously, we found that neutrophil proteases released in an uncoordinated manner by dying leukocytes degrade long chains of poly(l-aspartic acid), and co-incubation with high concentrations of protease inhibitors is necessary to achieve biofilm disruption with this agent.27 In the present study, a poly(d-aspartic acid) that is typically less susceptible to protease degradation was synthesized, thus eliminating the need for exogenous protease inhibitors.
In contrast to in vitro models, tear film absorption on lens surfaces in vivo has been evaluated as a factor in mediating PA lens adherence.57 In addition, antimicrobial components of the tear film act in concert to limit PA adherence and facilitate effective removal, necessitating the use of an animal model to prospectively evaluate the clinical effectiveness of this compound. Using a modified rabbit model of contact lens–associated PA internalization in vivo, we confirmed that after 48 hours of PMMA lens wear, bacterial internalization in the corneal epithelium was significantly increased compared with that in the non–lens-wearing eye. Although lens wear was not maintained until disease onset, evidenced by the presence of corneal opacity, the successful reduction in internalized PA in the epithelium represents initial success in limiting the cascade of events that ultimately contributes to infection. Since differences in the rabbit precorneal tear film effectively limit any assessment of corneal toxicity by fluorescein staining, preliminary safety of the compound was assessed by using the Draize test for ocular irritation. In an important finding, the results of repeated administration of the DNase and poly(aspartic acid) compound over 72 hours failed to induce any visible level of toxicity, with a response equivalent to that seen after instillation of a preservative-free artificial tear in the contralateral eye.
In summary, these data highlight the novel finding that neutrophil-mediated biofilm formation on contact lens surfaces can be disrupted by targeting neutrophil-derived polymers. The treatment reduced contact lens–related bioburden of infection in vitro, in the absence of a tear film and associated inflammatory response, and in an in vivo animal model. The data further indicate the potential for a new strategy in the prevention of pathogenic biofilm formation during contact lens wear. The novel therapeutic approach to disrupting or inhibiting PA biofilm by targeting neutrophil F-actin and DNA represents a new avenue in maximizing contact lens safety by contributing to the overall goal, which is to reduce infectious bacterial bioburden at the ocular surface and ultimately to ameliorate the number of inflammatory and infectious adverse events currently associated with lens wear.
Acknowledgments
The authors thank Edward Williams, OD, (Denver, CO) for his kind gift of the contact lenses that were used in the in vitro assessment of biofilm formation.
Footnotes
Supported by Grants R01HL 090991 and HL34303 from the National Heart, Lung, and Blood Institute (JAN); a Rebecca Runyon Bryan Chair in Cystic Fibrosis (JAN); a Colorado Bioscience Discovery Evaluation Grant (JAN); The Max and Yetta Karasik Foundation (JAN); National Institutes of Health Grant R01EY018219 (DMR) and Core Grant EY020799; OneSight Research Foundation, Dallas, Texas (DMR); and a Career Development Award (DMR) and an unrestricted grant from Research to Prevent Blindness, Inc., New York, New York.
Disclosure: D.M. Robertson, None; Q.M. Parks, P; R.L. Young, None; J. Kret, None; K.R. Poch, None; K.C. Malcolm, None; D.P. Nichols, None; M. Nichols, None; M. Zhu, None; H.D. Cavanagh, None; J.A. Nick, P
References
- 1. Schein OD, Glynn RJ, Poggio EC, et al. The relative risk of ulcerative keratitis among users of daily-wear and extended-wear soft contact lenses: a case-control study. Microbial Keratitis Study Group. N Engl J Med. 1989;321:773–778 [DOI] [PubMed] [Google Scholar]
- 2. Pachigolla G, Blomquist P, Cavanagh HD. Microbial keratitis pathogens and antibiotic susceptibilities: a 5-year review of cases at an urban county hospital in North Texas. Eye Contact Lens. 2007;33:45–49 [DOI] [PubMed] [Google Scholar]
- 3. Ormerod LD, Smith RE. Contact lens-associated microbial keratitis. Arch Ophthalmol. 1986;104:79–83 [DOI] [PubMed] [Google Scholar]
- 4. Mondino BJ, Weissman BA, Farb MD, et al. Corneal ulcers associated with daily-wear and extended-wear contact lenses. Am J Ophthalmol. 1986;102:58–65 [DOI] [PubMed] [Google Scholar]
- 5. Willcox MD. Pseudomonas aeruginosa infection and inflammation during contact lens wear: a review. Optom Vis Sci. 2007;84:273–278 [DOI] [PubMed] [Google Scholar]
- 6. Poggio EC, Glynn RJ, Schein OD, et al. The incidence of ulcerative keratitis among users of daily-wear and extended-wear soft contact lenses. N Engl J Med. 1989;321:779–783 [DOI] [PubMed] [Google Scholar]
- 7. Ladage PM, Yamamoto K, Ren DH, et al. Effects of rigid and soft contact lens daily wear on corneal epithelium, tear lactate dehydrogenase, and bacterial binding to exfoliated epithelial cells. Ophthalmology. 2001;108:1279–1288 [DOI] [PubMed] [Google Scholar]
- 8. Cavanagh HD, Ladage PM, Li SL, et al. Effects of daily and overnight wear of a novel hyper oxygen-transmissible soft contact lens on bacterial binding and corneal epithelium. Ophthalmology. 2002;109:1957–1969 [DOI] [PubMed] [Google Scholar]
- 9. Ladage PM, Yamamoto K, Li L, et al. Corneal epithelial homeostasis following daily and overnight lens wear. Contact Lens Anterior Eye. 2002;25:11–21 [DOI] [PubMed] [Google Scholar]
- 10. Ren DH, Yamamoto K, Ladage PM, et al. Adaptive effects of 30-night wear of hyper-O2 transmissible contact lenses on bacterial binding and corneal epithelium. Ophthalmology. 2002;109:27–39 [DOI] [PubMed] [Google Scholar]
- 11. Dart JK, Radford CF, Minassian D, et al. Risk factors for microbial keratitis with contemporary contact lenses: a case-control study. Ophthalmology. 2008;115:1647–1654 [DOI] [PubMed] [Google Scholar]
- 12. Stapleton F, Keay L, Edwards K, et al. The incidence of contact lens related microbial keratitis in Australia. Ophthalmology. 2008;115:1655–1662 [DOI] [PubMed] [Google Scholar]
- 13. Zegans ME, Becker HI, Budzik J, et al. The role of bacterial biofilms in ocular infections. DNA Cell Biol. 2002;21:415–420 [DOI] [PubMed] [Google Scholar]
- 14. McLaughlin-Borlace L, Stapleton F, Matheson M, et al. Bacterial biofilm on contact lenses and lens storage cases in wearers with microbial keratitis. J Appl Microbiol. 1998;84:827–838 [DOI] [PubMed] [Google Scholar]
- 15. Tam C, Mun JJ, Evans DJ, et al. The impact of inoculation parameters on the pathogenesis of contact lens-related infectious keratitis. Invest Ophthalmol Vis Sci. 2010;51:3100–3106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stapleton F, Dart JK, Seal DV, Matheson M. Epidemiology of Pseudomonas aeruginosa keratitis in contact lens wearers. Epidemiol Infect. 1995;114:395–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morgan PB, Efron NA. A decade of contact lens prescribing trends in the United Kingdom. Contact Lens Anterior Eye. 2006;29:59–68 [DOI] [PubMed] [Google Scholar]
- 18. Szczotka-Flynn LB, Imamura Y, Chandra J, et al. Increased resistance of contact lens-related bacterial biofilms to antimicrobial activity of soft contact lens care solutions. Cornea. 2009;28:918–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wilson LA, Sawant AD, Ahearn DG. Comparative efficacies of soft contact lens disinfectant solutions against microbial films in lens cases. Arch Ophthalmol. 1991;109:1155–1157 [DOI] [PubMed] [Google Scholar]
- 20. Robertson DM, Petroll WM, Cavanagh HD. The effect of nonpreserved care solutions on 12 months of daily and extended silicone hydrogel contact lens wear. Invest Ophthalmol Vis Sci. 2008;49:7–15 [DOI] [PubMed] [Google Scholar]
- 21. Li SL, Ladage PM, Yamamoto T, et al. Effects of contact lens care solutions on surface exfoliation and bacterial binding to corneal epithelial cells. Eye Contact Lens. 2003;29:27–30 [DOI] [PubMed] [Google Scholar]
- 22. Chuang EY, Li DQ, Bian F, et al. Effects of contact lens multipurpose solutions on human corneal epithelial survival and barrier function. Eye Contact Lens. 2008;34:281–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Imayasu M, Shiraishi A, Ohashi Y, et al. Effects of multipurpose solutions on corneal epithelial tight junctions. Eye Contact Lens. 2008;34:50–55 [DOI] [PubMed] [Google Scholar]
- 24. Lim MJ, Hurst RK, Konynenbelt BJ, et al. Cytotoxicity testing of multipurpose contact lens solutions using monolayer and stratified cultures of human corneal epithelial cells. Eye Contact Lens. 2009;35:287–296 [DOI] [PubMed] [Google Scholar]
- 25. Forte R, Cennamo G, Del Prete S, et al. Scanning electron microscopy of corneal epithelium in soft contact lens wearers. Cornea. 2010;29:732–736 [DOI] [PubMed] [Google Scholar]
- 26. Walker TS, Tomlin KL, Worthen GS, et al. Enhanced Pseudomonas aeruginosa biofilm development mediated by human neutrophils. Infect Immun. 2005;73:3693–3701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Parks QM, Young RL, Poch KR, et al. Neutrophil enhancement of Pseudomonas aeruginosa biofilm development: human F-actin and DNA as targets for therapy. J Med Microbiol. 2009;58:492–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tang JX, Wen Q, Bennett A, et al. Anionic poly(amino acid)s dissolve f-actin and DNA bundles, enhance DNase activity, and reduce the viscosity of cystic fibrosis sputum. Am J Physiol Lung Cell Mol Physiol. 2005;289:L599–L605 [DOI] [PubMed] [Google Scholar]
- 29. Tang JX, Janmey PA. The polyelectrolyte nature of f-actin and the mechanism of actin bundle formation. J Biol Chem. 1996;271:8556–8563 [DOI] [PubMed] [Google Scholar]
- 30. Haslett C, Guthrie LA, Kopaniak MM, et al. Modulation of neutrophil function by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am J Pathol. 1985;119:101–110 [PMC free article] [PubMed] [Google Scholar]
- 31. Ladage PM, Yamamoto K, Ren DH, et al. Proliferation rate of rabbit corneal epithelium during overnight rigid contact lens wear. Invest Ophthalmol Vis Sci. 2001;42:2804–2812 [PubMed] [Google Scholar]
- 32. Wilhelmus KR. The Draize eye test. Surv Ophthalmol. 2001;45:493–515 [DOI] [PubMed] [Google Scholar]
- 33. Yamamoto N, Yamamoto N, Petroll WM, et al. Internalization of Pseudomonas aeruginosa is mediated by lipid rafts in contact lens-wearing rabbit and cultured human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2005;46:1348–1355 [DOI] [PubMed] [Google Scholar]
- 34. Kernacki KA, Barrett RP, McClellan SA, et al. Aging and PMN response to P. aeruginosa infection. Invest Ophthalmol Vis Sci. 2000;41:3019–3025 [PubMed] [Google Scholar]
- 35. Kumar A, Hazlett LD, Yu FS. Flagellin suppreses the inflammatory response and enhances bacterial clearance in a murin model of Pseudomonas aeruginosa keratitis. Infect Immun. 2008;76:89–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Szliter EA, Barrett RP, Gabriel MM, Zhang Y, Hazlett LD. Pseudomonas aeruginosa-induced inflammation in the rat extended-wear contact lens model. Eye Contact Lens. 2006;32:12–18 [DOI] [PubMed] [Google Scholar]
- 37. Hazlett LD. Role of innate and adaptive immunity in the pathogenesis of keratitis. Ocul Immunol Inflamm. 2005;13:133–138 [DOI] [PubMed] [Google Scholar]
- 38. Chusid MJ, Nelson DB, Meyer LA. The role of the polymorphonuclear leukocyte in the induction of corneal edema. Invest Ophthalmol Vis Sci. 1986;27:1466–1469 [PubMed] [Google Scholar]
- 39. Thakur A, Xue M, Stapleton F, et al. Balance of pro- and anti-inflammatory cytokines correlates with outcome of acute experimental Pseudomonas aeruginosa keratitis. Infect Immun. 2002;70:2187–2197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zaidi TS, Zaidi T, Pier GB. Role of neutrophils, myd88-mediated neutrophil recruitment, and complement in antibody-mediated defense against Pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci. 2010;51:2085–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fleiszig SMJ, Evans DJ. The pathogenesis of bacterial keratitis: studies with Pseudomonas aeruginosa. Clin Exp Optom. 2002;85:271–278 [DOI] [PubMed] [Google Scholar]
- 42. McDermott AM. The role of antimicrobial peptides at the ocular surface. Ophthalmic Res. 2009;41:60–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ni M, Evans DJ, Hawgood S, et al. Surfactant protein D is present in human tear fluid and the cornea and inhibits epithelial cell invasion by Pseudomonas aeruginosa. Infect Immun. 2005;73:2147–2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lin MC, Polse KA. Hypoxia, overnight wear, and tear stagnation effects on the cornea epithelium: data and proposed model. Eye Contact Lens. 2007;33:378–381 [DOI] [PubMed] [Google Scholar]
- 45. Thakur A, Wilcox MDP. Contact lens wear alters the production of certain inflammatory mediators in tears. Exp Eye Res. 2000;70:255–259 [DOI] [PubMed] [Google Scholar]
- 46. Thakur A, Wilcox MDP. Cytokine and lipid inflammatory mediator profile of human tears during contact lens associated inflammatory diseases. Exp Eye Res. 1998;67:9–19 [DOI] [PubMed] [Google Scholar]
- 47. Hofman P. Molecular regulation of neutrophil apoptosis and potential targets for therapeutic strategy against the inflammatory process. Curr Drug Targets Inflamm Allergy. 2004;3:1–9 [DOI] [PubMed] [Google Scholar]
- 48. Lethem MI, James SL, Marriott C, Burke JF. The origin of DNA associated with mucus glycoprotenis in cystic fibrosis sputum. Eur Respir J. 1990;3:19–23 [PubMed] [Google Scholar]
- 49. Sheils CA, Kas J, Travassos W, et al. Actin filaments mediate DNA fiber formation in chronic inflammatory airway disease. Am J Pathol. 1996;148:919–927 [PMC free article] [PubMed] [Google Scholar]
- 50. Holden BA, La Hood D, Grant T, et al. Gram-negative bacteria can induce contact lens related acute red eye (CLARE) responses. CLAO J. 1996;22:47–52 [PubMed] [Google Scholar]
- 51. Szczotka-Flynn LB, Lass JH, Sethi A, et al. Risk factors for corneal infiltrative events during continuous wear of silicone hydrogel contact lenses. Invest Ophthalmol Vis Sci. 2010;51:5421–5430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sankaridurg PR, Sharma S, Willcox M, et al. Colonization of hydrogel lenses with Streptococcus pneumoniae: risk of development of corneal infiltrates. Cornea. 1999;18:289–295 [DOI] [PubMed] [Google Scholar]
- 53. Jalbert I, Willcox MD, Sweeney DF. Isolation of Staphylococcus aureus from a contact lens at the time of a contact lens-induced peripheral ulcer: case report. Cornea. 2000;19:116–120 [DOI] [PubMed] [Google Scholar]
- 54. Whitchurch CB, Tolker-Nielsen T, Ragas PC, et al. Extracellular DNA required for bacterial biofilm formation. Science. 2002;295:1487. [DOI] [PubMed] [Google Scholar]
- 55. Robinson PJ. Dornase alfa in early cystic fibrosis lung disease. Pediatr Pulmonol. 2002;34:237–241 [DOI] [PubMed] [Google Scholar]
- 56. Frederiksen B, Pressler T, Hansen A, et al. Effect of aerosolized rhDNase (pulmozyme (r)) on pulmonary colonization in patients with cystic fibrosis. Acta Paediatr. 2006;95:1070–1074 [DOI] [PubMed] [Google Scholar]
- 57. Bruinsma GM, van der Mei HC, Busscher HJ. Bacterial adhesion to surface hydrophilic and hydrophobic contact lenses. Biomaterials. 2001;22:3217–24 [DOI] [PubMed] [Google Scholar]