Abstract
The West Antarctic Peninsula (WAP) and adjacent Scotia Sea support abundant wildlife populations, many of which were nearly extirpated by humans. This region is also among the fastest-warming areas on the planet, with 5–6 °C increases in mean winter air temperatures and associated decreases in winter sea-ice cover. These biological and physical perturbations have affected the ecosystem profoundly. One hypothesis guiding ecological interpretations of changes in top predator populations in this region, the “sea-ice hypothesis,” proposes that reductions in winter sea ice have led directly to declines in “ice-loving” species by decreasing their winter habitat, while populations of “ice-avoiding” species have increased. However, 30 y of field studies and recent surveys of penguins throughout the WAP and Scotia Sea demonstrate this mechanism is not controlling penguin populations; populations of both ice-loving Adélie and ice-avoiding chinstrap penguins have declined significantly. We argue in favor of an alternative, more robust hypothesis that attributes both increases and decreases in penguin populations to changes in the abundance of their main prey, Antarctic krill. Unlike many other predators in this region, Adélie and chinstrap penguins were never directly harvested by man; thus, their population trajectories track the impacts of biological and environmental changes in this ecosystem. Linking trends in penguin abundance with trends in krill biomass explains why populations of Adélie and chinstrap penguins increased after competitors (fur seals, baleen whales, and some fishes) were nearly extirpated in the 19th to mid-20th centuries and currently are decreasing in response to climate change.
Sea ice plays a critical role in structuring ecosystem dynamics throughout the West Antarctic Peninsula (WAP) and Scotia Sea, and variations in sea-ice extent are hypothesized to affect penguin populations directly. As seasonal sea-ice extent and duration declines in this region, the Adélie penguin (Pygoscelis adeliae), which favors pack-ice habitat in winter, should decline in population size, whereas the closely related chinstrap penguin (Pygoscelis antarctica), which forages in ice-free water during winter, should increase (1–5). The foundation for this hypothesis is based on a short (7-y) series of simultaneously observed decreases in nesting populations of Adélie penguins and increases in chinstrap penguins following winters with low sea ice in the South Shetland Islands during the 1970s and 1980s (1). However there now is overwhelming evidence that, in contrast to expectations, both Adélie and chinstrap penguin populations are declining throughout the WAP and broader Scotia Sea region.
Results and Discussion
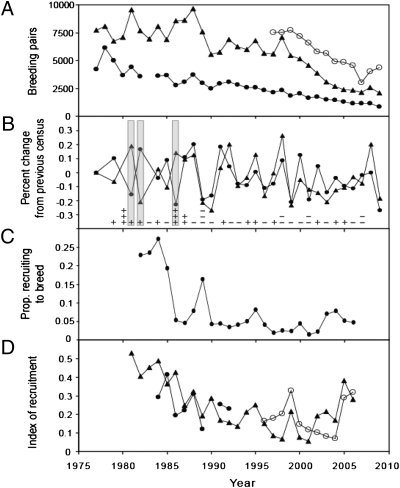
Adélie and chinstrap penguin populations have declined more than 50% during the last 30 y at study colonies in the South Shetland Islands (Fig. 1A). Moreover, since 1987, interannual changes in Adélie and chinstrap breeding populations have been positively correlated (Pearson's r = 0.7; P < 0.001; n = 21; Fig. 1B). These findings are in contrast with the negative correlation (Pearson's r = −0.8; P < 0.05; n = 7; Fig. 1B) reported at these same colonies between 1977 and 1986 (6) and from which the sea-ice hypothesis was originally inferred. The contrasting patterns of population change observed before and after 1986 are explained by recruitment trends. During the first decade of our studies, 40–60% of the penguins banded as fledglings recruited back to natal colonies, and first-time breeders constituted 20–25% of the breeding population annually (Fig. 1 C and D). Subsequently, survival to first breeding dropped precipitously in the 1980s, and the recruitment rates of both species have declined (7). Less than 10% of Adélie penguins banded as chicks survive to breed (Fig. 1C), and recruitment rates remain well below historical maximums for both species (Fig. 1D). Analyses of cohorts produced during the first decade of our study revealed a large effect of winter sea ice on juvenile recruitment. Strong cohorts of Adélie penguins recruited to their natal colonies following cold winters with extensive sea ice, whereas strong cohorts of chinstrap penguins followed warm, ice-free winters. When juvenile penguin survival rates were higher, from the late 1970s to the mid-1980s, variability in winter sea-ice extent coincided with the strong, negatively correlated changes in the breeding populations of these two species (1, 6). Young prebreeding (2- to 4-y-old) penguins do not recruit to the breeding colony until favorable conditions arise; then, when conditions are favorable, young from several cohorts recruit at high rates in the same year (7). Because of recent declines in juvenile survival and subsequent recruitment, breeding populations of Adélie and chinstrap penguins no longer are dominated by influxes of large numbers of 2- to 4-y-old birds, and therefore the contrasting pattern of recruitment no longer provides the dominant mechanism driving annual abundance estimates of the two species (Fig. 1B) (7).
Fig. 1.
Indices of Adélie and chinstrap population responses. Closed circles (●) indicate chinstrap penguins at Admiralty Bay, King George Island. Open circles (○) indicate chinstrap penguins at Cape Shirreff, Livingston Island. Closed triangles (▲) indicate Adélie penguins at Admiralty Bay. (A) Number of breeding pairs of Adélie and chinstrap penguins at all colonies. (B) Percent change in breeding population size of Adélie and chinstrap penguins at Admiralty Bay. Gray bars highlight the years in which the percent changes in Adélie and chinstrap breeding populations exceed 10% in opposite directions. Annual ice conditions are indicated as SDs from the long-term mean; + or − is between 0 and 1 SD; + + or − − is between 1 and 2 SD; and + + + or − − − is between 2 and 3 SD. No ice data are available for the winters of 2008 and 2009. (C) Recruitment of first-time breeders into the Adélie population at Admiralty Bay. (D) Index of recruitment to the natal colony for Adélie and chinstrap penguins (7). Chinstrap recruitment before 1984 at Admiralty Bay is excluded because of inconsistent resighting effort.
Population declines at our two study sites in the South Shetland Islands are not an anomaly; Adélie and chinstrap penguin populations have declined throughout the Scotia Sea (Table 1). Both species have declined during the past 30 y in the South Orkney Islands (8) and at colonies in the Antarctic Peninsula region (9). In the South Sandwich Islands, long considered the center of the chinstrap penguin's distribution, both Adélie and chinstrap penguin populations have declined by ∼75% (10). Although variability in sea ice remains a principal physical driver on the ecosystem in the WAP and Scotia Sea, we suggest that sea ice no longer drives trends in penguin populations through direct, physical effects on habitat. Rather, sea ice is one of several factors that mediate prey availability to penguins. Antarctic krill (Euphausia superba) is the dominant prey of nearly all vertebrates in this region, including Adélie and chinstrap penguins (6, 11–17). Large-scale changes in krill biomass best explain why populations of Adélie and chinstrap penguins increased as a result of competitive release following the harvesting of the whales and seals (the krill-surplus hypothesis) (18, 19) and why more recently they have decreased as a result of climate change and the recovery of pinnipeds and baleen whale populations (7–10).
Table 1.
Average annual percent (and absolute) changes in the abundances of Adélie and chinstrap penguins from breeding colonies in the Western Antarctic Peninsula and Scotia Sea region
| Area/Region | Adélie | Chinstrap |
| Admiralty Bay | −2.3 (−13,787) | −2.5 (−4,255) |
| South Sandwich Islands | −3.9 (−20,000) | −4.4 (−3,715,000) |
| South Orkney Islands | −4.5 (−482) | −1.9 (−18,066) |
| South Shetland Islands* | −2.0 (−7,247) | −2.7 (−21,809) |
| Western Antarctic Peninsula | −2.5 (−20,439) | −0.17 (−35)† |
| Overall | −2.9 (−61,955) | −4.3 (−3,759,165) |
Averages are weighted by absolute changes in abundance and are limited to colonies with a minimum of 10 y between the first and last counts.
*Not including data from Admiralty Bay.
†Not including data from the Palmer Long-Term Ecological Research Program.
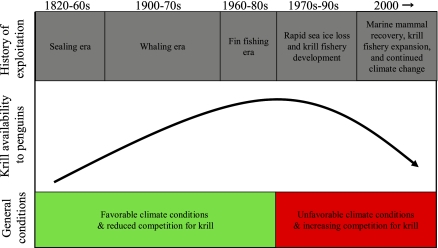
Laws (18) estimated that 150 million tons of krill were available to support other krill predators, such as penguins, after humans depleted the world's whale populations during the historic whaling era. Historical data on penguin populations and trends are few and largely anecdotal. However, the data that do exist are intriguing and support this hypothesis; Adélie and chinstrap penguin populations increased up to fivefold at breeding colonies in the Scotia Sea region from the 1930s to the 1970s (19–22). The large populations of Adélie and chinstrap penguin were not sustained for long, however, and now are declining precipitously (7–10, 23, 24). Concomitantly, increasing temperatures and reductions in sea ice have altered the physical environment necessary to sustain large krill populations (25–27). We hypothesize that the amount of krill available to penguins has declined because of increased competition for krill from recovering whale and fur seal populations (28–32) and from bottom-up, climate-driven changes that have altered this ecosystem significantly during the last 2–3 decades (4, 7, 8, 33–36; Fig. 2).
Fig. 2.
Schematic diagram of ecosystem perturbations in the Scotia Sea. From 1820–1860 Antarctic fur seal populations were extirpated rapidly after the discovery of South Shetland Islands (28–30). From 1900–1970 commercial whaling resulted in the near extirpation of all large baleen whales (31, 32). From 1960–1980 fishing for ice fishes and Notothenioids resulted in severe population declines in the Scotia Sea; populations remain well below historical levels (44). The serial depletion of krill predators was mirrored by large increases in Adélie and chinstrap penguin populations throughout the Scotia Sea region (6–9, 19–22, 42, 45, 46). From 1970–1990 climate-change effects were becoming evident in this highly altered ecosystem, with marked declines in sea ice, episodic recruitment in krill populations, and declining krill density. A pelagic trawl fishery for krill developed at this time. In the 2000s once-depleted marine mammal populations have recovered or are recovering; the krill fishery is expanding; rapid, well-documented climate change is progressing; and Adélie and chinstrap penguin populations are declining.
Recent analyses of Adélie penguin diets using fossil eggshell material from extinct colonies throughout the Scotia Sea region revealed an abrupt shift in their diets within the last 200 y (37). Only recently, contemporary with the removal of baleen whales and krill-eating seals, have Adélie penguins relied on krill as a major dietary item. Although this dietary change suggests that penguins could buffer the impact of further declines in krill biomass by returning to a diet dominated by fishes, we are unsure whether this reversal can or will occur and whether a fish-based diet would support large penguin populations. Diet data collected during the last 30 y have shown that krill continue to dominate the diet of Adélie and chinstrap penguins (7) despite an estimated 38–81% reduction in krill biomass during that period (35). Although we have found evidence of fishes (e.g., tissue, scales, and otoliths) in the diets of Adélie and chinstrap penguins breeding at our study sites in the South Shetland Islands (Admiralty Bay and Cape Shirreff) from about 25–30% of all samples annually, there has been no upward trend over the 30-y period. Furthermore, fish biomass in the diet averaged only 1–2% by mass of stomach contents with a maximum of 5% per year (14, 38).
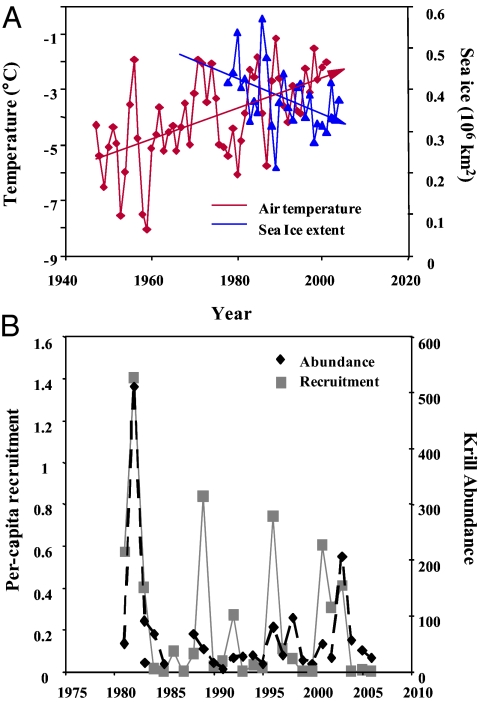
Interannual variability in the abundance and biomass of krill in the WAP and Scotia Sea is substantial and can be attributed to the aperiodic nature of recruitment since the late 1980s. Sea-ice extent and duration have been correlated with the reproductive success of krill, and in years following winters with expansive ice area and temporal duration, krill reproductive success increases (34, 36). Mean annual sea-ice extent in the WAP and Scotia Sea is inversely related to mean annual air temperature (Fig. 3A), and the rapid warming experienced in the WAP and Scotia Sea (25, 26) is correlated with regional declines in sea-ice extent and duration that affect krill productivity. Long-term, climate-driven declines in krill abundance are evident in this region. Krill density has declined by as much as 80% from the mid 1970s to the present, and this decline is associated with reductions in sea ice (35). Results from annual net surveys of krill populations around the South Shetland Islands suggest that the size of recruiting cohorts has declined, but the average time between recruitment events (4–5 y) has remained fairly consistent (Fig. 3B) (36). The decline in recruitment strength is an important factor determining the amount and mean size of krill available for predators and may be especially important for fledging penguins. Fledging masses of both chinstrap and Adélie penguins have declined (7), and therefore fledglings have smaller buffers against low krill availability when they depart from breeding colonies to start feeding independently. The decline in the reproductive capacity of the krill population, associated with the overall decline in sea ice, suggests that food resources for penguins and other predators may continue to decline in the near future.
Fig. 3.
Large-scale changes in krill populations and physical conditions in the Scotia Sea. (A) Mean annual (January through December) air temperature (°C) and sea-ice extent (>15% ice concentration) around the Antarctic Peninsula. Air temperature and sea-ice extent are significantly correlated (r ≥ 0.7; P < 0.05) over the 30-y time series. (B) Per-capita krill recruitment and krill abundance for the Elephant Island region of the South Shetland Islands derived from annual net-tow surveys of the region (36).
The krill fishery is expanding and recorded its largest catch in more than a decade during the 2009–2010 fishing season (December 1, 2009, through November 30, 2010) (39). Krill catches from around the WAP, South Shetland, South Orkney, and South Sandwich Islands during the past 10 fishing seasons have increased from 50,804 tons in 2002–2003, to 202,346 tons in 2009–1010 [catches reported through September 24, 2010 (39, 40)]. In addition, the Marine Stewardship Council's recent certification of one company's krill fishing as being sustainable* and the introduction of new products (e.g., Omega-3 krill oil, a popular dietary supplement) suggest that the fishery may be poised to expand further in the near future (41).
There now is overwhelming evidence to confirm significant declines in both Adélie and chinstrap penguin populations throughout the WAP and Scotia Sea and therefore to discount the hypothesis that future changes in Adélie and chinstrap populations will be directly related to sea-ice extent and inversely related to each other (1–5). There is a contrasting report of an increase in population size for a small colony (200-400 pairs) of chinstrap penguins near Palmer Station in the WAP; however, data from this colony were unavailable, and figures in two papers summarizing these reported increases are not in agreement (3, 33). Thus, we excluded this colony from our analysis. Other chinstrap populations in this region have declined less than in any other areas inhabited by this species (Table 1), but the small sizes of all colonies in the WAP and the magnitude of declines elsewhere in the Scotia Sea suggest these colonies, near the southern limit of the chinstrap distribution, are not representative of processes occurring throughout the core of the chinstrap penguin's range (Table 1).
Conclusion
If the warming trend in the WAP and Scotia Sea continues (25, 26), winter sea ice will be absent from much of this region in the near future, krill abundance may be reduced further, krill recruitment events will remain episodic (34–36), and Adélie and chinstrap penguin populations probably will continue to decline. These conditions are particularly critical for chinstrap penguins, because this species breeds almost exclusively in the WAP and Scotia Sea, where they have sustained declines in excess of 50% throughout their breeding range. Unlike Adélie penguins, which may be buffered by large, stable populations in the Ross Sea and Indian Ocean sector of Antarctica, chinstrap penguins have no southern breeding refuges. Given the magnitude of their global population decline, the predictions of further warming in this region (27), and the links between climate change and reductions in krill biomass (34), the primary food of the chinstrap penguin, we suggest that chinstrap penguin populations should be monitored carefully and their status reviewed by organizations such as the International Union for the Conservation of Nature. Long thought to be ecological winners in the climate-warming scenario (1–5), the chinstrap penguin instead may be among the most vulnerable species affected by a warming climate.
Materials and Methods
Penguin colonies included in the population changes presented in Table 1 are listed here. Sources for the data used to construct Table 1 are indicated in parentheses. Adélie colonies from the WAP are at Berthelot Islands (9), Booth Island (9), Detaille Island (9), Fish Islands (9), Palmer Station (3), Petermann Island (9), and Yalour Islands (9). Adélie colonies from the South Orkney Islands are at Shingle Cove (9), Signy Island (8), and Watson Point (4). Adélie colonies from the South Shetland Islands (excluding Admiralty Bay) are at Penguin Island (23) and Stranger Point (42). Adélie colonies from Admiralty Bay are at Point Thomas (6, 7) and Copacabana (6, 7). Specific Adélie colonies from the South Sandwich archipelago (10) are not identified. Chinstrap colonies from the WAP are at Eckener Point (9), Georges Point (9), Useful Island (9), Waterboat Point (9), and Booth Island (9). Chinstrap colonies from the South Orkney Islands are at Cape Robertson (42), Pirie Peninsula (42), Port Martin (23), Signy Island (8), South Coast (43), and Watson Peninsula (43). Chinstrap colonies from the South Shetland Islands (excluding Admiralty Bay) are at Cape Shirreff (7), Cecilia Island (9), Entrance Bay (9), Hannah Point (9), Penguin Island (23), President Head (9), and Vapour Col (43). Chinstrap colonies from Admiralty Bay are at Chabrier Rocks (24), Demay (6, 7), Uchatka (6, 7), and Patelnia (6, 7). Specific colonies from the South Sandwich archipelago (10) are not identified.
Acknowledgments
This research was conducted as part of the US Antarctic Marine Living Resources Program of the National Oceanic and Atmospheric Administration (NOAA). It was supported by the NOAA and by several grants from the National Science Foundation's Office of Polar Programs. Further support and assistance was provided by the Lenfest Ocean Program of the Pew Charitable Trusts and the Oceanites Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Data are held in the US Antarctic Marine Living Resources Program database and have also been linked to a publicly available, federal website that is managed by the US AMLR Program (http://swfsc.noaa.gov/textblock.aspx?Division=AERD&id=3156).
*Moody International (2010) Fisheries Management Certificate of Registration issued to Aker Biomarine, Oslo, Norway, and stating that the certificate holder “has been assessed and conforms with the requirements of the MSC Principles and Criteria for Sustainable Fishing.” Available at http://www.msc.org/track-a-fishery/certified/southern-ocean/aker-biomarine-antarctic-krill/assessment-downloads (accessed March 18, 2011).
References
- 1.Fraser WF, Trivelpiece WZ, Ainley DG, Trivelpiece SG. Increases in Antarctic penguin populations: Reduced competition with whales or a loss of sea-ice due to environmental warming? Polar Biol. 1992;11:525–531. [Google Scholar]
- 2.Smith RC, et al. Marine ecosystem sensitivity to climate change. Bioscience. 1999;49:393–404. [Google Scholar]
- 3.Ducklow HW, et al. Marine pelagic ecosystems: The west Antarctic Peninsula. Philos Trans R Soc Lond B Biol Sci. 2007;362:67–94. doi: 10.1098/rstb.2006.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McClintock J, Ducklow HW, Fraser W. Ecological responses to climate change on the Antarctic Peninsula. Am Sci. 2008;96:302–310. [Google Scholar]
- 5.Montes-Hugo M, et al. Recent changes in phytoplankton communities associated with rapid regional climate change along the western Antarctic Peninsula. Science. 2009;323:1470–1473. doi: 10.1126/science.1164533. [DOI] [PubMed] [Google Scholar]
- 6.Trivelpiece WZ, Trivelpiece SG, Geupel GR, Kjelmyr J, Volkman NJ. In: Antarctic Ecosystems – Ecological Change and Conservation. Kerry K, Hempel G, editors. Berlin: Springer; 1990. pp. 191–202. [Google Scholar]
- 7.Hinke JT, Salwicka K, Trivelpiece SG, Watters GM, Trivelpiece WZ. Divergent responses of Pygoscelis penguins reveal a common environmental driver. Oecologia. 2007;153:845–855. doi: 10.1007/s00442-007-0781-4. [DOI] [PubMed] [Google Scholar]
- 8.Forcada J, Trathan PN, Reid K, Murphy J, Croxall JP. Contrasting population changes in sympatric penguin species in association with climate warming. Glob Change Biol. 2006;12:411–423. [Google Scholar]
- 9.Lynch HJ, Naveen R, Fagan WF. Censuses of penguin, blue-eyed shag (Phalacrocorax atriceps) and southern giant petrel (Macronectes giganteus) populations on the Antarctic Peninsula. Mar Ornithol. 2008;36:83–97. [Google Scholar]
- 10.Poncet J. Report to Commissioner South Georgia and South Sandwich Islands, Seabird Species Account. Falkland Islands: Government Printing Office; 1997. [Google Scholar]
- 11.Volkman NJ, Pressler P, Trivelpiece W. Diets of Pygoscelid penguins at King George Island, Antarctica. Condor. 1980;82:373–378. [Google Scholar]
- 12.Jablonski B. The diet of penguins on King George Island, South Shetland Islands. Acta Zool Cracov. 1985;29:117–186. [Google Scholar]
- 13.Lishman GS. The food and feeding ecology of Adélie penguins (Pygoscelis adeliae) and chinstrap penguins (Pygoscelis antarctica) at Signy Island, South Orkney Islands. J Zool. 1985;205:245–263. [Google Scholar]
- 14.Trivelpiece WZ, Trivelpiece SG, Volkman NJ. Ecological segregation of Adélie, gentoo, and chinstrap penguins at King George Island, Antarctica. Ecology. 1987;68:351–361. [Google Scholar]
- 15.Jensen JK, Boveng PL, Bengston JL. Foraging modes of chinstrap penguins: Contrasts between day and night. Mar Ecol Prog Ser. 1998;165:161–172. [Google Scholar]
- 16.Lynnes AS, Reid K, Croxall JP. Diet and reproductive success of Adélie and chinstrap penguins: Linking responses of predators to prey population dynamics. Polar Biol. 2004;27:544–554. [Google Scholar]
- 17.Rombola E, Marschoff E, Coria N. Interannual study of chinstrap penguin's diet and reproductive success at Laurie Island, South Orkney Islands, Antarctica. Polar Biol. 2006;29:502–509. [Google Scholar]
- 18.Laws R. Seals and whales of the Southern Ocean. Philos Trans R Soc Lond. 1977;279:81–96. [Google Scholar]
- 19.Sladen WJL. In: Biologie Antarctique. Carrick R, Holdgate MW, Prevost J, editors. Paris: Hermann; 1964. pp. 359–365. [Google Scholar]
- 20.Conroy JWH. In: The Biology of Penguins. Stonehouse B, editor. Baltimore: University Park; 1975. pp. 321–336. [Google Scholar]
- 21.Croxall JP, Kirkwood ED. The Breeding Distribution of Penguins on the Antarctic Peninsula and Islands of the Scotia Sea. Cambridge, UK: British Antarctic Survey; 1979. [Google Scholar]
- 22.Croxall JP, Prince PA, Hunter I, McInnes SJ, Copestake PG. In: Status and Conservation of the Worlds Seabirds. Croxall JP, Evans PGH, Schreiber RW, editors. Cambridge, UK: International Council for Bird Preservation; 1984. pp. 637–666. [Google Scholar]
- 23.Sander M, Balbao M, Costa TC, Dos Santos ES, Petry MV. Decline of the breeding population of Pygoscelis antarctica and Pygoscelis adeliae on Penguin Island, South Shetland, Antarctica. Polar Biol. 2007a;30:651–654. [Google Scholar]
- 24.Sander M, Balbao M, Costa TC, Dos Santos ES, Petry MV. Recent decrease in chinstrap penguin (Pygoscelis antarctica) populations at two of Admiralty Bay's islets on King George Island, South Shetland Islands, Antarctica. Polar Biol. 2007b;30:659–661. [Google Scholar]
- 25.Vaughan DG, et al. Recent rapid regional climate warming on the Antarctic Peninsula. Clim Change. 2003;60:243–274. [Google Scholar]
- 26.Meredith MP, King JC. Rapid climate change in the ocean west of the Antarctic Peninsula during the second half of the 20th century. Geophys Res Lett. 2004;32:L-19604. [Google Scholar]
- 27.Christensen JH, et al. In: Climate Change 2007: The Physical Basis. Contributions of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Solomon S, et al., editors. Cambridge, UK: Cambridge Univ. Press; 2007. pp. 847–940. [Google Scholar]
- 28.O'Gorman FA. Fur seals breeding in the Falkland Islands Dependencies. Nature. 1961;192:914–916. [Google Scholar]
- 29.Boyd IL. Pup production and distribution of breeding Antarctic fur seals (Arctocephalus gazelle) at South Georgia. Antarct Sci. 1993;5:17–24. [Google Scholar]
- 30.Hucke-Gaete R, Osman LP, Moreno CA, Torres D. Examining natural population growth from near extinction: The case of the Antarctic fur seal at the South Shetlands, Antarctica. Polar Biol. 2004;27:304–312. [Google Scholar]
- 31.Clapham PJ, Baker CS. In: Encyclopedia of Marine Mammals. Perrin WF, Wursig B, Thewissen JGM, editors. New York: Academic; 2002. pp. 1328–1332. [Google Scholar]
- 32.Reilly S, et al. Biomass and energy transfer to baleen whales in the South Atlantic sector of the Southern Ocean. Deep Sea Res Part II Top Stud Oceanogr. 2004;51:1397–1409. [Google Scholar]
- 33.Schofield O, et al. How do polar marine ecosystems respond to rapid climate change? Science. 2010;328:1520–1523. doi: 10.1126/science.1185779. [DOI] [PubMed] [Google Scholar]
- 34.Loeb V, et al. Effects of sea-ice extent and krill or salp dominance on the Antarctic food web. Nature. 1997;387:897–900. [Google Scholar]
- 35.Atkinson AA, Siegel V, Pakhomov E, Rothery P. Long-term decline in krill stock and increase in salps within the Southern Ocean. Nature. 2004;432:100–103. doi: 10.1038/nature02996. [DOI] [PubMed] [Google Scholar]
- 36.Reiss CS, Cossio AM, Loeb V, Demer DA. Variations in the biomass of Antarctic krill (Euphausia superba) around the South Shetland Islands, 1996-2006. ICES J Mar Sci. 2008;65:497–508. [Google Scholar]
- 37.Emslie SD, Patterson WP. Abrupt recent shift in δ 13C and δ 15N values in Adélie penguin eggshell in Antarctica. Proc Natl Acad Sci USA. 2007;104:11666–11669. doi: 10.1073/pnas.0608477104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller AK, Kappes MA, Trivelpiece SG, Trivelpiece WZ. Foraging niche separation of breeding gentoo and chinstrap penguins, South Shetland Islands, Antarctica. Condor. 2010;112:684–695. [Google Scholar]
- 39.Scientific Committee for the Conservation of Antarctic Marine Living Resources . Report of the Twenty-Ninth Meeting of the Scientific Committee, Hobart, Australia, October 25–29, 2010. Scientific Committee for the Conservation of Antarctic Marine Living Resources, Hobart, Australia; 2010. [Google Scholar]
- 40.Commission for the Conservation of Antarctic Marine Living Resources Statistical Bulletin. 2010;Vol 22 [Google Scholar]
- 41.Nicol S, Foster J, Kawaguchi S. The fishery for Antarctic krill – recent developments. Fish Fish. 2011 10.1111/j.1467-2979.2011.00406.x. [Google Scholar]
- 42.Woehler EJ, Croxall JP. The status and trends of Antarctic and sub-Antarctic seabirds. Mar Ornithol. 1997;25:43–66. [Google Scholar]
- 43.Barbosa A, Moreno J, Potti J, Merino S. Breeding group size, nest position and breeding success in the chinstrap penguin. Polar Biol. 1997;18:410–414. [Google Scholar]
- 44.Kock KH, Jones CD. Fish stocks in the southern Scotia Arc region: A review and prospects for future research. Rev Fish Sci. 2005;13:75–108. [Google Scholar]
- 45.Croxall JP, Trathan PN, Murphy EJ. Environmental change and Antarctic seabird populations. Science. 2002;297:1510–1514. doi: 10.1126/science.1071987. [DOI] [PubMed] [Google Scholar]
- 46.Trathan PN, Croxall JP, Murphy EJ. Dynamics of Antarctic penguin populations in relation to inter-annual variability in sea-ice distribution. Polar Biol. 1996;16:321–330. [Google Scholar]