Abstract
Transfer RNA is one of the most richly modified biological molecules. Biosynthetic pathways that introduce these modifications are underexplored, largely because their absence does not lead to obvious phenotypes under normal growth conditions. Queuosine (Q) is a hypermodified base found in the wobble positions of tRNA Asp, Asn, His, and Tyr from bacteria to mankind. Using liquid chromatography MS methods, we have screened 1,755 single gene knockouts of Escherichia coli and have identified the key final step in the biosynthesis of Q. The protein is homologous to B12-dependent iron-sulfur proteins involved in halorespiration. The recombinant Bacillus subtilis epoxyqueuosine (oQ) reductase catalyzes the conversion of oQ to Q in a synthetic substrate, as well as undermodified RNA isolated from an oQ reductase knockout strain. The activity requires inclusion of a reductant and a redox mediator. Finally, exogenously supplied cobalamin stimulates the activity. This work provides the framework for studies of the biosynthesis of other modified RNA components, where lack of accessible phenotype or obvious gene clustering has impeded discovery. Moreover, discovery of the elusive oQ reductase protein completes the biosynthetic pathway of Q.
Keywords: biochemistry, queuosine, reductive dehalogenation
Nearly 100 modifications have been identified in RNA, many of which are found in tRNA and are common to eukaryotes, bacteria, and archaea (1). Most of these modifications are likely not essential under normal laboratory conditions, making discovery of biosynthetic pathways by phenotypic methods impossible. Queuosine (Q), a hypermodified RNA base containing a 7-deazapurine core, is among the more complex RNA modifications described to date. Q replaces the guanine in the wobble positions of the subset of tRNA molecules with a 5′-GUN-3′ sequence in their anticodon loops (His, Asp, Asn, and Tyr). Conservation of Q in RNA of organisms in nearly all kingdoms of life (2) suggests that the modification may be of cardinal importance. A physiological role for Q has eluded discovery partly because of gaps in understanding of the biosynthetic pathway. De novo biosynthesis of Q occurs in bacteria whereas eukaryotes acquire the free base, queuine, from dietary sources (3–5). The bacterial pathway for biosynthesis of Q has been elucidated up to the penultimate intermediate, epoxyqueuosine (oQ) (6, 7). However, the enzyme that catalyzes the final step in the pathway, conversion of oQ to Q, had yet to be identified. Because no selectable phenotypes have been demonstrated for the modification, discovery of the final step in the pathway required a different approach that melded modern analytical methods and emerging tools in bacterial genetics. This methodology has led to successful identification of the final step and can be generalized to other RNA modifications where lack of phenotype has hindered discovery of the biosynthetic pathway.
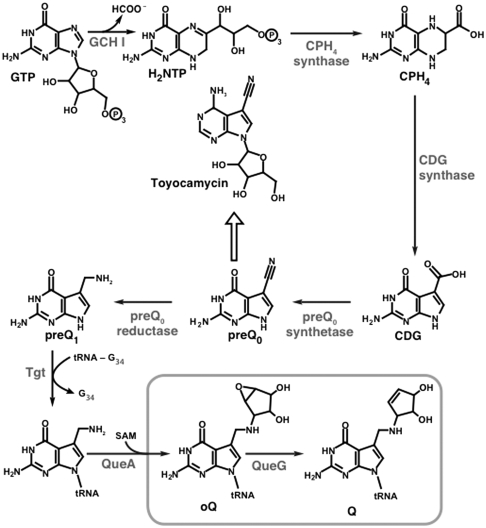
Structural parallels between the 7-deazapurine core of queuosine and 7-deazapurine-containing antibiotic toyocamycin were noted long before the various elements of the biosynthetic pathway leading to either compound was reconstructed in vitro (7–9). This in vitro reconstitution of the 7-deazapurine core was aided by the discovery of the toyocamycin biosynthetic gene cluster in Streptomyces rimosus, which contains three genes that had been previously shown to be required for the biosynthesis of queuosine in Bacillus subtilis (10–13). The cluster also contained a GTP cyclohydrolase I (GCH I) homolog, which hinted at GTP as being the primary metabolic precursor. GCH I catalyzes the conversion of GTP to 7,8-dihydroneopterin triphosphate (H2NTP) in the first step in the biosynthesis of both folic acid and biopterin. GCH I was subsequently shown to be required for the biosynthesis of Q in Escherichia coli (14). In vitro reconstitution was accomplished by the successive actions of GCH I, 6-carboxy-5,6,7,8-tetrahydropterin (CPH4) synthase, 7-carboxy-7-deazaguanine (CDG) synthase, and preQ0 synthetase, demonstrating that four enzymatic transformations are required to convert GTP to preQ0, a previously identified intermediate in the biosynthesis of queuosine (see Fig. 1). At this point, the biosynthetic pathway toward toyocamycin diverges from that of queuosine with preQ0 being elaborated to toyocamycin in a series of steps that are analogous to those in purine biosynthesis/salvage. By contrast, the biosynthetic pathway to queuosine entails the NADPH-dependent conversion of preQ0 to 7-amino-7-deazaguanine (preQ1) by preQ0 reductase (15, 16). PreQ1 base is then exchanged for guanine in the anticodon loop of tRNA by tRNA:guanine transglycosylase (Tgt) (6, 17, 18) and elaborated to oQ by addition of a cyclopentanediol epoxide derived from S-adenosyl-l-methionine by oQ synthase (QueA) (7). The penultimate intermediate to queuosine, oQ, was isolated and characterized 23 y ago (19) and B12 was subsequently implicated in its conversion to Q (20). The identity of the enzyme that catalyzes the transformation and in vitro reconstitution of oQ reductase activity are reported in this manuscript.
Fig. 1.
Biosynthetic pathway for production of queuosine with the oQ reductase step elucidated in this manuscript boxed. The block arrow denotes a series of steps analogous to purine salvage/repair, which convert the 7-cyano-7-deazaguanine base to toyocamycin.
Results and Discussion
Screening of the Keio Collection.
The availability of a series of isogenic variants of E. coli K-12, the Keio collection (21), in which each of the approximate 4,000 nonessential genes are individually disrupted by a kanamycin resistance cassette, provided a valuable opportunity to mount a search for the elusive oQ reductase. We identified 1,755 knockout strains carrying deletions in genes of unknown function, annotated as “y” genes in the National Center for Biotechnology Information genome database (see Dataset S1 for a complete list of deletion strains examined in this study), as potential candidates for genes that encode oQ reductase. Each deletion strain was grown in Lennox broth, RNA was isolated from each culture, digested, and dephosphorylated (22–24). Nucleosides were resolved by reverse phase HPLC and detected by tandem UV-visible spectrophotometry and mass spectrometry. Under these conditions, oQ and Q, which are present in miniscule amounts, are readily detected and identified.
Representative UV-visible and extracted ion chromatograms obtained from wild-type E. coli as processed and described above are shown in Fig. 2A. Queuosine and epoxyqueuosine typically elute at approximately 30–32 min in chromatograms. Extracted ion chromatograms monitoring oQ (m/z = 426) and Q (m/z = 410) show that under normal growth conditions both forms are present in RNA (Fig. 2B). However, the intensity of the peak for oQ is typically 0–30% of that of Q. RNA isolated from any strain carrying deletions up to and including Tgt would be predicted to be completely devoid of Q and oQ (6). Indeed, deletion of CPH4 synthase (b2765), CDG synthase (b2777), preQ0 synthethase (b0444), preQ0 reductase (b2794), and Tgt (b0406) lead to disappearance of both Q and oQ from RNA. GCH I is an essential gene for E. coli because it also catalyzes the first step in biosynthesis of folic acid (25, 26), and is therefore not included in the Keio collection. Interestingly, although oQ or Q are absent in extracted ion chromatograms of RNA from the QueA (b0405) deletion strain, the precursor to oQ, preQ1 nucleoside (m/z = 312), is clearly present (see Fig. 1 for structure).
Fig. 2.
Analysis of RNA from Keio collection deletion strains for presence and absence of Q and oQ. A shows a representative UV-visible trace of the RNA samples, highlighting the region where oQ and Q elute. Wild-type E. coli contain very little oQ when grown in LB. B shows extracted ion chromatograms of wild-type and deletion strains corresponding to each step in the pathway. Deletion of any gene up to and including Tgt leads to complete absence of oQ (m/z = 426) or Q (m/z = 410). Deletion of queA leads to accumulation of preQ1 nucleoside (m/z = 312), as would be expected (see Fig. 1). Deletion of queG leads to complete absence of Q and accumulation of oQ. A seven-point data smoothing algorithm was applied to all of the extracted ion chromatograms.
Liquid chromatography (LC) MS analysis of y genes in the Keio collection revealed a single gene, yjeS (b4166), which is required for conversion of oQ to Q. RNA from the ΔyjeS strain clearly lacks Q and accumulates oQ, as would be expected if YjeS catalyzes conversion of oQ to Q in vivo. We observed the identical phenotype with the deletion of tonB (see Fig. S1), which is a periplasmic protein that is required for import of cobalamin and Fe (27)—both of which are cofactors for oQ reductase (see below). The YjeS protein will hereafter be referred to as QueG or oQ reductase.
Bioinformatic Analysis of oQ Reductase.
QueG is annotated to be a Fe-S containing protein involved in electron transport. A BLAST (28, 29) search with the QueG sequence, however, reveals similarity to a class of corrinoid Fe-S containing proteins from anaerobic halorespiring bacteria. These proteins are found in organisms that grow in biological niches that are heavily contaminated by halogenated organics in which halogenated compounds serve as terminal electron acceptors (see ref. 30 for a review). Recent genome sequencing of a perchloroethene dechlorinating bacterium, Dehalococcoides ethenogenes, has revealed 17 homologs in this organism alone, which are presumably specialized for breakdown of different halogenated compounds (31). Fig. S2 shows an alignment of several bacterial QueG homologs and the biochemically studied (32) tetrachloroethene reductase from Dehalobacter restrictus highlighting conservation in QueG of the eight Cys residues that comprise the two 4Fe-4S clusters in this and other reductive dehalogenase proteins studied to date. Sequence similarities between QueG and reductive dehalogenases are localized to the C-terminal half of the dehalogenases. The N termini of dehalogenase proteins often contain a region involved in membrane association, which is presumably to proximate the protein with the electron transport chain. A cobalamin cofactor is found in all reductive dehalogenase proteins studied to date (30, 32–36), which is intriguing given the long-standing observation from Kersten and coworkers suggesting the involvement of cobalamin in the conversion of oQ to Q (20) and the requirement for presence of TonB demonstrated in this manuscript. Intriguingly, we also find conserved D109xH111 (E. coli numbering) motif in the reductase, which we hypothesize, by analogy to cobalamin-dependent methionine synthase (37), may be involved in the base-off binding of the cobalamin cofactor. Such a motif is also found in a subset of reductive dehalogenase proteins (38). Although the motif is not universally conserved in all reductive dehalogenase proteins, spectroscopic evidence suggests base-off binding of the cofactor to several dehalogenases (32, 35).
In Vitro Reconstitution of oQ Reductase Activity.
Because of solubility issues relating to the expression of the E. coli YjeS protein, we chose to isolate the B. subtilis protein which is annotated as YhbA (BSU08910) and shares 30% sequence identity with the E. coli protein; all of the key sequence features of the E. coli homolog are conserved in the B. subtilis protein. Heterologous expression of His6-YhbA in E. coli in the presence of Fe and cobalamin at 18 °C led to accumulation of significant quantities of soluble protein. As with the anaerobic reductive dehalogenases, we expect oQ reductase to be oxygen sensitive. Hence the protein was purified and characterized under strictly anaerobic conditions (95–97% N2/3–5% H2). Fractions from the Ni-affinity column were orange/brown. Attempts at desalting the protein to remove imidazole and NaCl led to loss of the chromophore. Inclusion of glycerol (20% vol/vol), however, did improve retention. Because the protein was judged to be > 85% pure (based on SDS-PAGE) as it eluted from the column, we opted to utilize it without any additional purification steps.
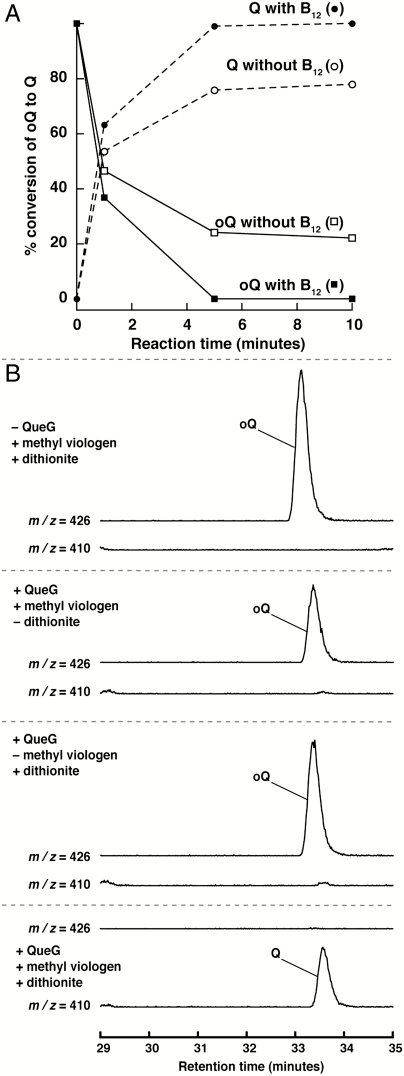
We utilized a synthetic substrate to establish QueG activity in vitro. Both Tgt and QueA have been shown to catalyze their respective transformations on a synthetic 17-mer oligoribonucleotide stem loop substrate corresponding to nucleotides 28–44 of the E. coli tyrT tRNA (39). To prepare the substrate for the analysis, preQ1 was generated enzymatically from synthetic preQ0 (40), exchanged into the wobble position of the stem loop with Tgt, and modified to oQ with QueA. Control experiments in which the substrate was digested to the corresponding nucleosides and analyzed by LC-MS show that > 85% of the substrate is modified to oQ nucleoside (m/z = 426), as judged by comparison of the oQ peak to that of guanosine. The initial assays with the protein were carried out with all of the components that we reasoned might be required for activity. The purified protein catalyzes the conversion of oQ in the synthetic substrate to Q under anaerobic conditions in a time-dependent manner (Fig. 3A), as monitored by disappearance of oQ and appearance of Q. The reaction is complete in approximately 5 min. As shown in Fig. 3B, no turnover is observed when either the enzyme, dithionite, or methyl viologen are removed. Intriguingly, the reactions are stimulated in the presence of cobalamin (see Fig. 3A). The stimulation is not large (approximately 1.3-fold) but it is reproducibly observed, suggesting that approximately 30% of the active protein contains Fe-S but no cobalamin. The requirement for dithionite and methyl viologen, however, was established by showing complete absence of Q in the synthetic stem loop substrate even after a longer incubation period (15 h) and in the presence of large amounts of enzyme (20-fold higher); the extracted ion chromatograms from these studies are identical to controls shown in Fig. 3B. We note that the requirement for a reductant and a mediator, such as dithionite and methyl viologen, have also been demonstrated for all reductive dehalogenase proteins studied to date (30). The cellular reductant has never been identified.
Fig. 3.
Activity assays with oQ reductase. Incubation of oQ reductase in the presence of methyl viologen, dithionite, and B12 leads to time-dependent disappearance of the oQ and appearance of Q (A). In the time course, the areas of the extracted ion chromatograms at m/z = 426 or 410, corresponding to oQ or Q, respectively, were measured and plotted. The intensity of the peaks at each time point were referenced to the intensities of the unreacted substrate (100% oQ) or full conversion (100% Q). B12 stimulates activity by approximately 30%. Control experiments show that methyl viologen and dithionite are absolutely required as shown in extracted ion chromatograms in B. A seven-point data smoothing algorithm was applied to all of the extracted ion chromatograms.
To further confirm the oQ reductase activity in the context of a representative biological sample, the RNA purified from a ΔqueG knockout of E. coli, which contains only oQ (see Fig. 2B), was treated with purified oQ reductase protein. The nucleosides obtained after digestion and dephosphorylation of the RNA were analyzed by LC-MS. As with the synthetic substrate, QueG catalyzes the conversion of oQ (presumably associated with Asp, Asn, His, and Tyr tRNA) to Q (see Fig. S3). These results confirm the role of oQ reductase and bacterial homologs in conversion of oQ to Q in vivo.
The role of B12 in the reaction catalyzed by oQ reductase remains to be established. However, one may envision two potential roles for the cofactor, as for example proposed for reductive dehalogenation of perchloroethylene and trichloroethylene (41). First, cobalamin may be intimately involved in the chemical transformation either as a covalent or direct electron transfer catalyst (41). For example, cobalamin in the +1 oxidation state may attack the C-O bond of the epoxide leading to ring opening. Heterolytic cleavage of the bond followed by elimination of water would lead to formation of Q and oxidation (by two electrons) of the cobalamin to the cob(III)alamin state. In this scenario, the Fe-S clusters serve to deliver reducing electrons to the cobalamin cofactor to regenerate the nucleophilic +1 state (see Fig. S4). A second mechanistic paradigm would utilize the cobalamin cofactor as a conduit through which electrons are transferred to the Fe-S cluster(s), where conversion of oQ to Q takes place by an unknown mechanism. Studies to differentiate between these and other mechanistic possibilities could be undertaken with recombinant oQ reductase using site-directed mutagenesis experiments in which the Fe-S cluster(s) or the cobalamin binding determinants are removed. Moreover, given the protocols that permit isolation and analysis of cellular RNA for oQ and Q, it should be possible to obtain RNA from cells grown under various conditions, to probe the role of the cofactors in vivo with chromosomal copies of the oQ reductase gene where Fe-S or cobalamin binding determinants have been deleted. Moreover, examination of deletion strains may also provide insights into the in vivo reductant for the oQ reductase reaction.
The TonB requirement for the modification may be important physiologically. In addition to B12 and iron, TonB has been implicated in the import of colicins into E. coli (42). Colicins are bacterial toxins, which upon uptake into a susceptible host, exert their lethal effects. ColE5, for example, is a ribonuclease that specifically cleaves on the 3′ side of Q-containing tRNAs in E. coli (43). Many colicins share the same BtuB–TonB system that is used for import of iron and B12. Although a role for TonB in mediating ColE5 uptake has not been established, at present one cannot rule out a link between TonB and import of ColE5, B12, and iron.
Summary.
These studies complete the biosynthetic pathway for queuosine and pave the way for probing the physiological role of queuosine, which, based on its ubiquity, we suspect serves a central role in biology. Moreover, the methodology used in this study to identify a B12-dependent protein involved in the biosynthesis of queuosine is relatively simple and generally applicable to studies aimed at identification of proteins involved in other RNA modification pathways where physiological function of the modification has remained unknown.
Methods
Growth of E. coli Strains and Isolation of Total RNA.
Wild-type and E. coli deletion strains from the Keio collection were inoculated into 50 mL Lenox Broth (LB) containing 34 μg/mL kanamycin sulfate, grown for 16 h at 37 °C, and harvested by centrifugation. Total cellular RNA was extracted using a protocol based on Kingston et al. (24). Each E. coli cell pellet was resuspended in 0.6 mL of a denaturation solution containing 4 M guanidine thiocyanate, 25 mM sodium citrate (pH 7.0), 0.5% N-laurylsarcosine, and 0.1 M β-mercaptoethanol. The following were added to the resuspended cells in the order listed: 0.1 mL of 2 M sodium acetate (pH 4.0), 1 mL phenol, and 200 μL of bromochloropropane. Each was mixed in by vortexing upon addition. The mixture was placed on ice for 15 min and centrifuged at 15,000 × g for 15 min to separate aqueous and organic phases. The aqueous layer was removed, combined with 1 vol of isopropanol, chilled at -20 °C for 30 min, and centrifuged for 10 min at 21,000 × g to pellet the precipitated RNA. The RNA pellet was dissolved in 50 μL of RNase free water and further purified as follows using RNeasy RNA purification kit (Qiagen) according to manufacturer’s protocol.
Digestion and Dephosphorylation of Isolated RNA.
Total cellular RNA purified as described above was hydrolyzed to its constitutive nucleotides and dephosphorylated using a procedure that yields samples that are suitable for LC-MS analysis in positive ion mode (22). Each RNA sample (50 μL in water) obtained as described above was combined with 5 μL of 0.1 M ammonium acetate (pH 5.3) and 1 μL of nuclease P1 (1.4 U/μL) (Sigma) and incubated for 6 h at 45 °C. Next, 5 μL of 1 M ammonium bicarbonate and 3 μL of phosphodiesterase I (2 U/μL) (Sigma) were added and the mixture was incubated at 37 °C for 2 h. The reaction was subsequently combined with 0.1 mL of a solution containing 10 mM Tris•HCl (pH 7.5) and 10 mM MgCl2 and 3 μL of bacterial alkaline phosphatase (150 U/μL) (Invitrogen) and incubated at 37 °C for 2 h. The total reaction was filtered through a YM-10 centrifugal filter (Pall Life Sciences) at 12,000 × g for 10 min to remove protein and the flow-through containing the nucleosides was analyzed as described below.
LC-MS Analysis of RNA Hydrolysates.
The presence or absence of oQ and queuosine from cellular RNA was determined by LC-MS of the dephosphorylated RNA nucleotides. The procedure described here is based on analytical methods reported by Pomerantz and McCloskey (23) for detection and identification of modified bases in RNA. An aliquot (30 μL) of RNA nucleosides prepared as described above was injected onto a 4.6 × 250-mm Eclipse XDB-C18 column (Agilent) which had been preequilibrated in 19.3% (wt/vol) ammonium acetate adjusted to pH 6.0 with glacial acetic acid (solution A) and run at 0.3 mL/ min. Nucleosides were eluted from the column with a complex gradient from solution A and a solution composed of 40% acetonitrile and 60% H2O (solution B). The separation program was as follows: 0–3 min, 0% solution B; 3.0–4.4 min 0–0.2% solution B; 4.4–5.8 min, 0.2–0.8% solution B; 5.8–7.2 min, 0.8–1.8% solution B; 7.2–8.6 min, 1.8–3.2% solution B; 8.6–10 min, 3.2–5% solution B; 10–25 min, 5–25% solution B; 25–40 min, 25–50% solution B; 40–49 min, 50–75% solution B; 49–52 min, 75% solution B; 52–60 min, 75–100% solution B. The elution was monitored by UV-visible and MS detection. UV-visible spectra (220–500 nm) were recorded on a ThermoFinnigan Surveyor photodiode array detector. MS was carried out with an in-line electrospray (ES) ionization-equipped LCQ ThermoFinnigan Deca XP mass spectrometer, which was operated in the positive ion mode. The m/z range of 90–500 atomic mass unit was scanned and the ES was set at a 7 V ionization energy and a 200 °C ion source temperature.
Cloning of B. subtilis QueG.
The open reading frame encoding B. subtilis QueG (yhbA) was amplified from B. subtilis chromosomal DNA by PCR using the following primers: 5′- CATATGAACGTTTATCAGCTCAAAGAAGAATTAATTGAATACGCG -3′ (forward) and 5′- CTCGAGTCATCAGGACAGGCCTTGTTTAGTCATGCCTGAAG -3′ (reverse) at an annealing temperature of 53 °C. The resulting PCR product was subcloned into pGEM-T Easy vector (Promega) prior to being excised by NdeI and XhoI and being cloned into similarly digested pET28a (Novagen) to yield pZM002 for expression of His6-tagged QueG.
Expression of B. subtilis oQ Reductase.
E. coli HMS-174 (DE3) containing pZM002 (for expression of QueG) and pBD1282 (from Dennis Dean, Virginia Tech, Blacksburg, VA, through Squire Booker, Pennsylvania State University, University Park, PA), which contains the isc operon of Azotobacter vinelandii, were grown in 6 L of LB containing 34 μg/mL kanamycin and 100 μg/mL ampicillin at 37 °C to an OD600 of approximately 0.3, at which point solid arabinose was added to a final concentration of 0.05% (wt/vol) to induce transcription of the genes in pBD1282. The cells were grown further to an OD600 of approximately 0.5, at which point ferric chloride (final concentration of 50 μM) was added and expression of QueG was induced by addition of IPTG (0.1 mM). Hydroxocobalamin (acetate salt, 10 μM final concentration) was added as well. Cells were grown for 8 h at 18 °C, harvested by centrifugation (5,000 × g), frozen in N2, and stored at -80 °C.
Purification of E. coli QueG.
Purification of QueG was carried out in a Coy anaerobic chamber (approximately 95–97% N2, 3–5% H2). Cells (approximately 3 g) were suspended in buffer containing 20 mM potassium phosphate (pH 7.2), 0.5 M NaCl, 5 mM imidazole, 20% glycerol, and 1 mM PMSF and sonified using a Branson digital sonifier (45% amplitude). The lysate was centrifuged for 20 min at 14,500 × g and 4 °C to remove cellular debris. The cleared lysate was loaded onto a 1 mL HisTrapHP column (GE Healthcare), which had been charged with NiSO4 and equilibrated with a solution containing 20 mM potassium phosphate (pH 7.2), 0.5 M NaCl, 5 mM imidazole, and 20% glycerol (buffer A). The column was rinsed with 15 mL of buffer A. The column was washed with 2 mL of buffer A containing 0.1 M imidazole to remove nonspecifically bound proteins. Epoxyqueuosine reductase was eluted with 6 mL of buffer A containing 300 mM imidazole. Fractions containing the desired protein were identified using SDS-PAGE, and stored at -80 °C in small aliquots. Protein concentration was determined by the binchoninic acid method using bovine serum albumin (Thermo Scientific) as a standard.
Assay for Conversion of oQ to Q by E. coli QueG.
The conversion of oQ to queuoseine by QueG was carried out using either a 17-mer oQ-containing RNA stem loop, prepared as described in SI Text, or total RNA from the ΔqueG strain of E. coli. The assays contained 12 mM Hepes•NaOH (pH 7.2), 1.5 mM hydoxocobalamin (acetate salt), 1.5 mM methyl viologen, 20 mM sodium dithionite, 4 μL of either the synthetically modified 17-mer stem D loop (35 μM) containing epoxyqueuosine or purified total RNA (3.2 mg/mL) from the ΔqueG strain of E. coli, and 6 μM QueG protein. The final solutions also contained 1.3 mM potassium phosphate (pH 7.2), 33.3 mM sodium chloride, 20 mM imidizole (Sigma), and 1% (vol/vol) glycerol carried over from the QueG stock solution. The reactions were initiated by addition of QueG and quenched by addition of 0.25 mL QIAzol reagent (which contains phenol). RNA was purified from the reaction mixtures using the protocol described above for the cleanup of cellular RNA with the RNAeasy kit, digested, dephosphorylated, and analyzed by LC-MS as described.
Supplementary Material
Acknowledgments.
V.B. is grateful for National Institutes of Health (NIH) (GM72623) and Burroughs Wellcome Fund for funding various aspects of the work. R.M.M. acknowledges support from Biological Chemistry Training grant from NIH (T32 GM008804). Z.D.M. is supported by Integrative Graduate Education and Research Traineeship Program in Comparative Genomics (National Science Foundation 0654435).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018636108/-/DCSupplemental.
References
- 1.Rozenski J, Crain PF, McCloskey JA. The RNA modification database: 1999 update. Nucleic Acids Res. 1999;27:196–197. doi: 10.1093/nar/27.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katze JR, Basile B, McCloskey JA. Queuine, a modified base incorporated posttranscriptionally into eukaryotic transfer RNA: Wide distribution in nature. Science. 1982;216:55–56. doi: 10.1126/science.7063869. [DOI] [PubMed] [Google Scholar]
- 3.Gaur R, Bjork GR, Tuck S, Varshney U. Diet-dependent depletion of queuosine in tRNAs in Caenorhabditis elegans does not lead to a developmental block. J Biosci. 2007;32:747–754. doi: 10.1007/s12038-007-0074-4. [DOI] [PubMed] [Google Scholar]
- 4.Gunduz U, Katze JR. Queuine salvage in mammalian cells. Evidence that queuine is generated from queuosine 5′-phosphate. J Biol Chem. 1984;259:1110–1113. [PubMed] [Google Scholar]
- 5.Reyniers JP, Pleasants JR, Wostmann BS, Katze JR, Farkas WR. Administration of exogenous queuine is essential for the biosynthesis of queuosine-containing transfer RNAs in the mouse. J Biol Chem. 1981;256:11591–11594. [PubMed] [Google Scholar]
- 6.Okada N, Nishimura S. Isolation and characterization of a guanine insertion enzyme, a specific tRNA transglycosylase, from Escherichia coli. J Biol Chem. 1979;254:3061–3066. [PubMed] [Google Scholar]
- 7.Slany RK, Bösl M, Crain PF, Kersten H. A new function of S-adenosylmethionine: The ribosyl moiety of AdoMet is the precursor of the cyclopentanediol moiety of the tRNA wobble base queuine. Biochemistry. 1993;32:7811–7817. doi: 10.1021/bi00081a028. [DOI] [PubMed] [Google Scholar]
- 8.Niguchi S, et al. Isolation of Q nucleoside precursor present in tRNA of an E. coli mutant and its characterization as 7-(cyano)-7-deazaguanosine. Nucleic Acids Res. 1978;5:4215–4223. doi: 10.1093/nar/5.11.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kasai H, et al. Structure of the modified nucleoside Q isolated from Escherichia coli transfer ribonucleic acid. 7-(4,5-cis-dihydroxy-1-cyclopenten-3-ylaminomethyl)-7-deazaguanosine. Biochemistry. 1975;14:4198–4208. doi: 10.1021/bi00690a008. [DOI] [PubMed] [Google Scholar]
- 10.Reader JS, Metzgar D, Schimmel P, de Crecy-Lagard V. Identification of four genes necessary for biosynthesis of the modified nucleoside queuosine. J Biol Chem. 2004;279:6280–6285. doi: 10.1074/jbc.M310858200. [DOI] [PubMed] [Google Scholar]
- 11.McCarty RM, Bandarian V. Deciphering deazapurine biosynthesis: Pathway for pyrrolopyrimidine nucleosides toyocamycin and sangivamycin. Chem Biol. 2008;15:790–798. doi: 10.1016/j.chembiol.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCarty RM, Somogyi A, Bandarian V. Escherichia coli QueD is a 6-carboxy-5,6,7,8-tetrahydropterin synthase. Biochemistry. 2009;48:2301–2303. doi: 10.1021/bi9001437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCarty RM, Somogyi A, Lin G, Jacobsen NE, Bandarian V. The deazapurine biosynthetic pathway revealed: In vitro enzymatic synthesis of PreQ(0) from guanosine 5′-triphosphate in four steps. Biochemistry. 2009;48:3847–3852. doi: 10.1021/bi900400e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phillips G, et al. Biosynthesis of 7-deazaguanosine-modified tRNA nucleosides: A new role for GTP cyclohydrolase I. J Bacteriol. 2008;190:7876–7884. doi: 10.1128/JB.00874-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee BW, Van Lanen SG, Iwata-Reuyl D. Mechanistic studies of Bacillus subtilis QueF, the nitrile oxidoreductase involved in queuosine biosynthesis. Biochemistry. 2007;46:12844–12854. doi: 10.1021/bi701265r. [DOI] [PubMed] [Google Scholar]
- 16.Van Lanen SG, et al. From cyclohydrolase to oxidoreductase: Discovery of nitrile reductase activity in a common fold. Proc Natl Acad Sci USA. 2005;102:4264–4269. doi: 10.1073/pnas.0408056102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okada N, Harada F, Nishimura S. Specific replacement of Q base in the anticodon of tRNA by guanine catalyzed by a cell-free extract of rabbit reticulocytes. Nucleic Acids Res. 1976;3:2593–2603. doi: 10.1093/nar/3.10.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okada N, et al. Structure determination of a nucleoside Q precursor isolated from E. coli tRNA: 7(-aminomethyl)-7-deazaguanosine. Nucleic Acids Res. 1978;5:2289–2296. doi: 10.1093/nar/5.7.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phillipson DW, et al. Isolation and structure elucidation of an epoxide derivative of the hypermodified nucleoside queuosine from Escherichia coli transfer RNA. J Biol Chem. 1987;262:3462–3471. [PubMed] [Google Scholar]
- 20.Frey B, McClosky JA, Kersten W, Kersten H. New function of vitamin B12: Cobamide-dependent reduction of epoxyqueuosine to queuosine in tRNAs of Escherichia coli and Salmonella typhimurium. J Bacteriol. 1988;170:2078–2082. doi: 10.1128/jb.170.5.2078-2082.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baba T, et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol Syst Biol. 2006;2:1–11. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crain PF. Preparation and enzymatic hydrolysis of DNA and RNA for mass spectrometry. Methods Enzymol. 1990;193:782–790. doi: 10.1016/0076-6879(90)93450-y. [DOI] [PubMed] [Google Scholar]
- 23.Pomerantz SC, McCloskey JA. Analysis of RNA hydrolyzates by liquid chromatography-mass spectrometry. Methods Enzymol. 1990;193:796–824. doi: 10.1016/0076-6879(90)93452-q. [DOI] [PubMed] [Google Scholar]
- 24.Kingston RE, Chomczynski P, Sacchi N, editors. Guanidine Methods for Total RNA Preparation. Vol 36. New York: Wiley; 1996. pp. 4.2.1–4.2.9. [DOI] [PubMed] [Google Scholar]
- 25.Burg AW, Brown GM. The biosynthesis of folic acid. VI. Enzymatic conversion of carbon atom 8 of guanosine triphosphate to formic acid. Biochim Biophys Acta. 1966;117:275–278. doi: 10.1016/0304-4165(66)90180-2. [DOI] [PubMed] [Google Scholar]
- 26.Burg AW, Brown GM. The biosynthesis of folic acid. 8. Purification and properties of the enzyme that catalyzes the production of formate from carbon atom 8 of guanosine triphosphate. J Biol Chem. 1968;243:2349–2358. [PubMed] [Google Scholar]
- 27.Kadner RJ. Vitamin B12 transport in Escherichia coli: Energy coupling between membranes. Mol Microbiol. 1990;4:2027–2033. doi: 10.1111/j.1365-2958.1990.tb00562.x. [DOI] [PubMed] [Google Scholar]
- 28.Altschul SF, et al. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marchler-Bauer A, et al. CDD: A conserved domain database for protein classification. Nucleic Acids Res. 2005;33:D192–196. doi: 10.1093/nar/gki069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wohlfarth G, Diekert G. Reductive dehalogenases. In: Banerjee R, editor. Chemistry and Biochemistry of B12. New York: Wiley; 1999. pp. 871–893. [Google Scholar]
- 31.Seshadri R, et al. Genome sequence of the PCE-dechlorinating bacterium Dehalococcoides ethenogenes. Science. 2005;307:105–108. doi: 10.1126/science.1102226. [DOI] [PubMed] [Google Scholar]
- 32.Maillard J, et al. Characterization of the corrinoid iron-sulfur protein tetrachloroethene reductive dehalogenase of Dehalobacter restrictus. Appl Environ Microbiol. 2003;69:4628–4638. doi: 10.1128/AEM.69.8.4628-4638.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schumacher W, Holliger C, Zehnder AJ, Hagen WR. Redox chemistry of cobalamin and iron-sulfur cofactors in the tetrachloroethene reductase of Dehalobacter restrictus. FEBS Lett. 1997;409:421–425. doi: 10.1016/s0014-5793(97)00520-6. [DOI] [PubMed] [Google Scholar]
- 34.Neumann A, Wohlfarth G, Diekert G. Purification and characterization of tetrachloroethene reductive dehalogenase from Dehalospirillum multivorans. J Biol Chem. 1996;271:16515–16519. doi: 10.1074/jbc.271.28.16515. [DOI] [PubMed] [Google Scholar]
- 35.van de Pas BA, et al. Purification and molecular characterization of ortho-chlorophenol reductive dehalogenase, a key enzyme of halorespiration in Desulfitobacterium dehalogenans. J Biol Chem. 1999;274:20287–20292. doi: 10.1074/jbc.274.29.20287. [DOI] [PubMed] [Google Scholar]
- 36.Krasotkina J, Walters T, Maruya KA, Ragsdale SW. Characterization of the B12- and iron-sulfur-containing reductive dehalogenase from Desulfitobacterium chlororespirans. J Biol Chem. 2001;276:40991–40997. doi: 10.1074/jbc.M106217200. [DOI] [PubMed] [Google Scholar]
- 37.Drennan CL, Huang S, Drummond JT, Matthews RG, Ludwig ML. How a protein binds B12: A 3.0 Å X-ray structure of B12-binding domains of methionine synthase. Science. 1994;266:1669–1674. doi: 10.1126/science.7992050. [DOI] [PubMed] [Google Scholar]
- 38.Holscher T, et al. Multiple nonidentical reductive-dehalogenase-homologous genes are common in Dehalococcoides. Appl Environ Microbiol. 2004;70:5290–5297. doi: 10.1128/AEM.70.9.5290-5297.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Lanen SG, et al. tRNA modification by S-adenosylmethionine∶tRNA ribosyltransferase-isomerase. Assay development and characterization of the recombinant enzyme. J Biol Chem. 2003;278:10491–10499. doi: 10.1074/jbc.M207727200. [DOI] [PubMed] [Google Scholar]
- 40.Quaranta D, McCarty R, Bandarian V, Rensing C. The copper-inducible cin operon encodes an unusual methionine-rich azurin-like protein and a preQ0 reductase in Pseudomonas putida KT2440. J Bacteriol. 2007;189:5361–5371. doi: 10.1128/JB.00377-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pratt DA, van der Donk WA. On the role of alkylcobalamins in the vitamin B12-catalyzed reductive dehalogenation of perchloroethylene and trichloroethylene. Chem Commun. 2006:558–560. doi: 10.1039/b513624e. [DOI] [PubMed] [Google Scholar]
- 42.Cascales E, et al. Colicin biology. Microbiol Mol Biol Rev. 2007;71:158–229. doi: 10.1128/MMBR.00036-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ogawa T, et al. A cytotoxic ribonuclease targetting specific transfer RNA anticodons. Science. 1999;283:2097–2100. doi: 10.1126/science.283.5410.2097. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.