Abstract
CD4+CD25+ regulatory T cells (Treg) play a crucial role in the regulation of immune responses. Although many mechanisms of Treg suppression in vitro have been described, the mechanisms by which Treg modulate CD8+ T cell differentiation and effector function in vivo are more poorly defined. It has been proposed, in many instances, that modulation of cytokine homeostasis could be an important mechanism by which Treg regulate adaptive immunity; however, direct experimental evidence is sparse. Here we demonstrate that CD4+CD25+ Treg, by critically regulating IL-2 homeostasis, modulate CD8+ T-cell effector differentiation. Expansion and effector differentiation of CD8+ T cells is promoted by autocrine IL-2 but, by competing for IL-2, Treg limit CD8+ effector differentiation. Furthermore, a regulatory loop exists between Treg and CD8+ effector T cells, where IL-2 produced during CD8+ T-cell effector differentiation promotes Treg expansion.
CD4+CD25+Foxp3+ regulatory T cells (Treg) maintain immune homeostasis by limiting T-cell responses to self-, environmental-, and pathogen-associated antigens and by modulating innate immune responsiveness. The critical role of Treg in preventing autoimmunity is highlighted when Treg are developmentally absent or depleted (1–3). In vivo, the modulatory effects of Treg on adaptive immunity are likely mediated through suppression of both CD4+ and CD8+ T cells. However, although Treg suppression of CD4+ T-cell responses has been widely studied both in vitro and in vivo, modulation of CD8+ T-cell responses by Treg is less well understood. Depleting Treg promotes CD8+ T-cell–dependent responses, such as virus and tumor clearance (4, 5). In many of these studies, however, whether Treg depletion affects primarily the afferent phase of CD8+ T cell expansion and effector differentiation or the later efferent phases has been largely undistinguished.
In CD4+ T-cell–dependent autoimmune responses, Treg limit both T cell priming in lymph node (LN) (6) and effector activity at sites of inflammation (7). For CD8+ T cells, it has been elegantly shown in a tumor setting that Treg directly inhibit CD8+ T cell-mediated cytolysis through, for example, TGF-β–dependent inhibition of degranulation (8, 9) without modulating effector differentiation. However, in settings that lead to strong priming, such as vaccination, Treg can restrain CD8+ T cell expansion (10). Such disparate observations could reflect differences between T cell activation occurring under either weak or strong innate immune cell or antigen-presenting cell activation, respectively. It is, however, also possible that Treg function is enhanced by T cells undergoing effector differentiation in response to strongly immunogenic T cell priming, facilitating Treg control under these conditions.
Mechanisms of CD4+ T cell inhibition by Treg in vitro have been well described (reviewed in refs. 11 and 12) but in vivo mechanisms of CD8+ T cell suppression are poorly understood. Consumption of IL-2 is often proposed as a mechanism of Treg suppression. Evidence is strong that CD4+ T-cell effector function is inhibited in vitro by this mechanism (13, 14), but direct experimental evidence for this mechanism in vivo is lacking. Existing evidence is correlative, which most likely reflects the technical challenge of blocking IL-2 activity without disturbing T-cell developmental homeostasis and studies have relied on knockout or immunodeficient mice in which homeostasis of T cells and cytokines is highly perturbed. Recent studies have shown that both Treg and CD8+ T cells are exquisitely sensitive to modulation of IL-2 homoeostasis in vivo (15–17). Emerging evidence implicates alterations in IL-2 homeostasis in development of a range of autoinflammatory responses (18–20). Given the importance of CD8 T-cell responses in tumor clearance and autoimmunity, we investigated the interrelationship of IL-2, Treg, and CD8+ T-cell effector differentiation. We demonstrate, in vivo, regulation of CD8+ effector differentiation by Treg-mediated modulation of IL-2 bioavailability.
Results
Regulatory T Cells Restrain CD8+ T-Cell Expansion and Inhibit Effector Differentiation.
To define the influence of Treg on CD8+ T cells, ovalbumin (OVA)-specific T-cell receptor (TCR) transgenic CD8+ T cells (OT-I) were transferred and recipient mice immunized with or without Treg depletion. Immunization with OVA/QuilA led to rapid expansion of OT-I T cells in the draining (inguinal) LN, which was followed by accumulation of differentiated effectors in spleen (Fig. 1 A and B), indicating migration of differentiated effectors from the site of priming. After transient accumulation in both sites, the number of OT-I T cells waned (Fig. 1 A and B), consistent with normal postexpansion population contraction. Administration of αCD25 effectively depleted Treg throughout the course of the experiments (Fig. S1 A and B) and substantially increased OT-I T cell expansion, prolonged their accumulation in draining LN, and increased their accumulation in the spleen (Fig. 1 A and B). When Treg were depleted, more OT-I T cells were recovered from mice challenged with OVA 21 d after immunization (Fig. 1D), consistent with increased memory cell formation. Thus, after immunization, Treg modulated OT-I T cell expansion and memory formation, as observed previously for other CD8+ T-cell responses (10).
Fig. 1.
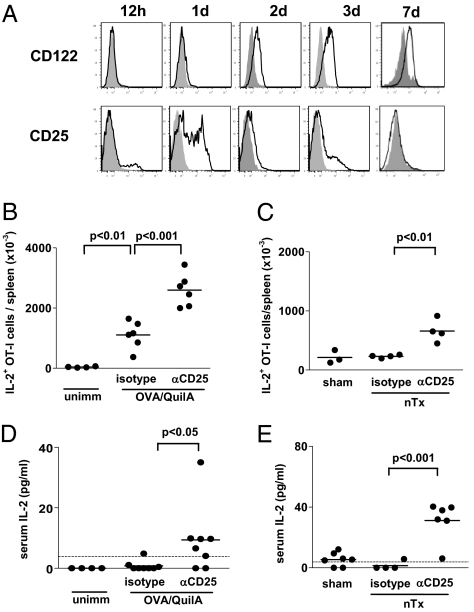
Regulatory T cells restrain CD8+ expansion and effector differentiation. (A–C) CD45.1+ OT-I T cells were transferred to anti-CD25 or isotype-treated C57BL/6 mice immunized with OVA/QuilA at the time of transfer. At the indicated time points, inguinal LN (A) or spleens (B and C) were collected and OT-I number determined by a FACS counting assay and intracellular cytokine staining performed. (D) CD45.1+ OT-I T cells were transferred to anti-CD25 or isotype-treated C57BL/6 mice immunized with OVA/QuilA at the time of transfer and 21 d later. A further 7 d later, spleens were collected and the total number of OT-I cells determined using a FACS counting assay. (E–H) CD45.1+ OT-I T cells were transferred to nTx or sham-Tx 11c.OVA mice treated with either anti-CD25 or isotype-control mAb. At the indicated time points (E and F) or 3 d after transfer (G and H), spleens were collected and OT-I T-cell number determined by a flow-cytometric counting assay and intracellular cytokine staining performed. Data depict mean ± SEM or individual values for 4 to 10 mice for each time point (A–C) pooled from a minimum of two experiments, except inguinal LN 1 d posttransfer, where two mice from a single experiment are represented, and (D–H) four to six mice per group pooled from two to three individual experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
Treg modulate immune responses to self-antigens and, in so doing, prevent autoimmune disease (2, 3). Current paradigms suggest steady-state dendritic cell (DC) tolerise T cells, but loss of Treg converts tolerogenic to immunogenic T cell activation under steady-state conditions (21). Neonatal thymectomy (nTx) is classically used to limit Treg development and promotes autoimmunity (1). Therefore, we used 11c.OVA mice, in which OVA is expressed by DCs (22), to test whether nTx modulated steady-state DC activation of naive CD8+ T cells to confirm Treg control of afferent CD8+ T-cell responses. In the 11c.OVA model, transferred OT-I T cells undergo proliferation and population expansion, followed by deletion and, ultimately, tolerance (22). The number of B220+ B cells or CD11b+ myeloid cells in blood or spleen of 11c.OVA mice was not altered by nTx, whereas Treg, CD8+, and CD4+ T cells were all numerically reduced relative to sham-Tx controls (Fig. S1C). As reported (1), Treg were not completely absent, but additional treatment with anti-CD25 resulted in almost complete ablation of Treg from nTx 11c.OVA mice (Fig. S1C). Neither thymectomy nor anti-CD25–mediated depletion of Treg alone altered the kinetics or extent of OT-I T cell expansion and contraction in 11c.OVA mice (Fig. 1E). Anti-CD25 treatment of nTx 11c.OVA mice substantially increased expansion and delayed contraction of the OT-I T-cell population (Fig. 1E), and substantially increased total IFN-γ−producing OT-I T cells relative to controls (Fig. 1F). Differentiating cytotoxic T lymphocytes (CTL) show hierarchical production of IFN-γ, TNF-α, and IL-2 and polyfunctional CTL are considered higher-quality effectors. Consistent with increased effector differentiation, the proportion of IFN-γ+/TNF-α+, and IFN-γ+/IL-2+ OT-I T cells was much greater when Treg were depleted (Fig. 1 G and H). Taken together, these data demonstrate that, under immunogenic conditions, Treg modulate CD8+ T cell expansion and effector differentiation.
Circulating IL-2 Is Increased During CD8+ T-Cell Activation in the Absence of Treg.
IL-2 is crucial not only for the function and maintenance of Treg but also plays an important role in CD8+ T cell homeostasis, priming, and memory formation. IL-2 production is a hallmark of differentiating CD8+ effector T cells and production is lost as terminal effector status is acquired. As the effect of Treg depletion was most profound during the phase of effector differentiation, we investigated the role of IL-2. We first defined IL-2R expression on OT-I T cells. After immunization, CD122 (IL-2Rβ) was expressed by almost all OT-I T cells within 2 d and then remained stably expressed (Fig. 2A, Upper). CD25 (IL-2Rα), on the other hand, was transiently up-regulated by OT-I T cells 1 d after OVA immunization, then returned to low levels within 2 d (Fig. 2A, Lower). In contrast, CD25 expression by Treg did not change after immunization. Thus, during the effector differentiation phase, CD8+ OT-I T cells primarily express the βγ low-affinity IL-2R. Given that the high affinity IL-2R, comprised of the αβγ chains, has a 100-fold higher affinity for IL-2 and was expressed at higher levels by Treg than OT-I T cells, Treg might effectively compete with OT-I T cells for IL-2. Alternatively, removal of Treg could increase CD8+ T-cell effector differentiation by IL-2–independent mechanisms. To distinguish between these possibilities, we next looked at IL-2 production by differentiating CD8+ T cells and its homeostasis in the presence and absence of Treg. The total number of IL-2–producing OT-I T cells accumulating in spleens of OVA-immunized or nTx 11c.OVA OT-I recipients at the peak of the expansion response was substantially increased by Treg depletion (Fig. 2 B and C), consistent with the increase in polyfunctional OT-I T cells in the absence of Treg (Fig. 1). Given that the number of IL-2–producing OT-I T cells was increased, we next determined whether circulating IL-2 was increased in the absence of Treg. Although normally tightly regulated to almost undetectable levels, IL-2 was detected in the serum of six of eight OVA-immunized Treg-depleted mice (Fig. 2D) and reached high levels in nTx OT-I recipients (Fig. 2E). Thus, IL-2 levels were markedly increased, either from increased differentiation of OT-I T cells or decreased consumption in the absence of Treg.
Fig. 2.
Systemic IL-2 is increased in the absence of Treg. (A) CD45.1+ OT-I T cells were transferred to C57BL/6 mice immunized with OVA/QuilA and at the indicated time points, expression of CD25 and CD122 by OT-I T cells was determined by FACS. (B and D) CD45.1+ OT-I T cells were transferred to C57BL/6 mice treated with anti-CD25 or isotype control mAb and immunized or not with OVA/QuilA at the time of transfer. Seven days later, spleens were collected and the number of IL-2–producing OT-I T cells determined by intracellular cytokine staining and a FACS counting assay (B) or serum collected and IL-2 concentration determined by ELISA (D). (C and E) CD45.1+ OT-I T cells were transferred to anti-CD25 or isotype-treated nTx or sham-Tx 11c.OVA mice. Three days later, spleens were collected and the number of IL-2–producing OT-I T cells determined by intracellular cytokine staining and a FACS counting assay (C) and serum collected, and IL-2 measured by ELISA (E). Limit of IL-2 detection is denoted by the horizontal dashed line. Data are representative of (A) four mice from two individual experiments or (B–E) pooled from at least two separate experiments with each point representing an individual mouse.
Increased Expansion of CD8+ T Cells in the Absence of Treg Is IL-2–Dependent.
To test the possibility that increased IL-2 availability in the absence of Treg promoted expansion and differentiation of OT-I T cells, we compared accumulation of OT-I T cells in spleens of OVA-immunized OT-I recipients or nTx 11c.OVA OT-I recipients depleted of Treg with or without additional IL-2 blockade. To achieve IL-2 blockade, we treated mice with the combination of two αIL-2 mAb (S4B6, JES6-1) that alternately block the high- and low-affinity IL-2R binding sites, respectively, of the IL-2 molecule (15). In the absence of Treg depletion, IL-2 blockade had little if any effect on OT-I T cell accumulation in spleen 7 d after immunization (Fig. 3A). As expected, depletion of Treg promoted OT-I T-cell accumulation in spleens of OVA-immunized recipient mice and this was completely reversed by IL-2 blockade. Similarly, in nTx 11c.OVA mice, IL-2 blockade almost completely abrogated the additional OT-I T cell accumulation in spleen resulting from Treg depletion (Fig. 3B). Because CD25 expression is not strictly limited to Treg and is transiently expressed by activated OT-I T cells (Fig. 2), we employed two further strategies to verify that our results reflected Treg control of CD8+ T cell expansion and effector differentiation, rather than off-target effects of CD25 blockade.
Fig. 3.
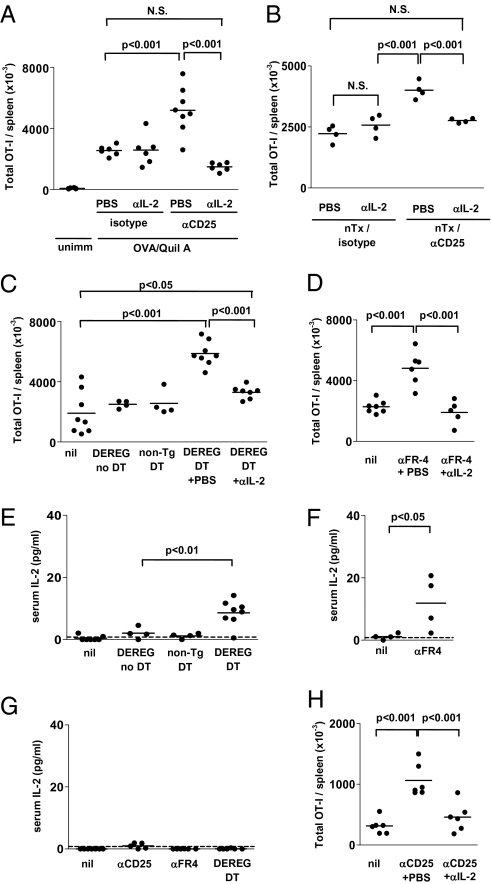
Increased expansion and effector differentiation in the absence of Treg is IL-2–dependent. (A) CD45.1+ OT-I T cells (2 × 106) were transferred to anti-CD25 or isotype-treated C57BL/6 mice immunized or not with OVA/QuilA at the time of transfer and additionally administered anti–IL-2 (JES6.1 plus S4B6) or PBS, as indicated. Seven days later, spleens were collected and the total number of OT-I T cells determined by FACS counting assay. (B) CD45.1+ OT-I T cells were transferred to anti-CD25 or isotype-treated nTx 11c.OVA mice administered either anti–IL-2 or PBS. Three days later, spleens were collected and the total number of OT-I T cells determined by FACS counting assay. (C and E) CD45.1+ OT-I T cells were transferred to DEREG or nontransgenic mice treated or not with DT, anti–IL-2, or PBS and immunized with QuilA at the time of transfer. Seven days later, spleens (C) were collected and the total number of OT-I T cells determined by FACS counting assay and serum IL-2 determined by ELISA (E). (D and F) CD45.1+ OT-I T cells were transferred to C57BL/6 mice treated or not with anti-FR4 and anti–IL-2 and immunized with OVA/QuilA at the time of transfer. Seven days later (D), spleens were collected and the total number of OT-I T cells determined by FACS counting assay and serum IL-2 determined by ELISA (F). (G) C57BL/6 mice were treated or not with anti-CD25 or anti-FR4. DEREG mice were treated with DT and 7 d later serum collected and IL-2 measured by ELISA. (H) CD45.1+ OT-I T cells (2 × 104) were transferred to anti-CD25 or isotype-treated C57BL/6 mice administered either anti–IL-2 or PBS and immunized with OVA/QuilA at the time of transfer. Seven days later, spleens were collected and the total number of OT-I T cells determined by FACS counting assay. Data are pooled from at least two separate experiments and each datapoint portrays an individual mouse.
In DEREG (depletion of regulatory T cells) mice expressing human diphtheria toxin (DT) receptor targeted by the FoxP3 promoter, FoxP3+ Treg are depleted by administration of DT (3). Accumulation of OT-I T cells in DEREG recipient spleens 7 d after OVA/QuilA immunization (Fig. 3C) was significantly increased by administration of DT and to a level similar to that following Treg depletion with anti-CD25 mAb (Fig. 3A). IL-2 blockade significantly reduced the DT-induced additional OT-I T cell accumulation (Fig. 3C). Folate receptor 4 (FR4) is an alternate Treg-specific marker expressed at high levels by CD25hi Treg. Thus, anti-FR4 mAb depletes CD25hi Treg, as does anti-CD25, but not effector T cells (23). Depletion of Treg using anti-FR4 significantly increased the number of OT-I T cells in spleen 7 d postimmunization (Fig. 3D), and this was completely prevented by IL-2 blockade (Fig. 3D). As increased OT-I accumulation after DT treatment of DEREG mice, which depletes both CD25+FoxP3+ and CD25−FoxP3+ Treg, was not completely reversed by IL-2 blockade, it is possible that—and consistent with IL-2 uptake via the high-affinity αβγ receptor—only CD25hi Treg modulate IL-2 availability. In mice depleted of Treg using either DT treatment of DEREG mice or anti-FR4 antibody, serum IL-2 was increased relative to undepleted mice (Fig. 3 E and F), similar to the increase seen after anti-CD25 treatment (Fig. 2 D and E), confirming that IL-2 availability was modulated by the presence of Treg. In contrast, depletion of Treg by anti-CD25, anti-FR4 treatment, or DT treatment of DEREG mice did not lead to significant accumulation of IL-2 in serum in the absence of OT-I transfer and OVA immunization (Fig. 3G).
The conditions tested to this point reflect a CD8+ T-cell response corresponding in magnitude range to a potent antiviral response. Under these conditions it is possible large amounts of IL-2 could be produced, possibly overrepresenting the importance of Treg modulation of IL-2. To determine whether alteration of IL-2 homeostasis by Treg also modulated less-potent responses, where less IL-2 may be produced, we transferred 100-fold fewer OT-I T cells. Even under these conditions, depletion of Treg boosted accumulation of OT-I T cells and this was reversed by blockade of IL-2 (Fig. 3H).
Effector Differentiation of CD8+ T Cells Modulates IL-2–Mediated Treg Homeostasis.
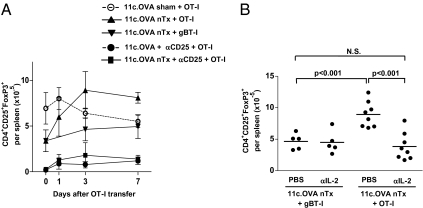
As Treg numbers and function are highly dependent on IL-2 (17), it is possible that IL-2 produced during CD8+ T-cell effector differentiation could feedback on Treg homeostasis. Neonatal thymectomy results in a partial, but stable, Treg deficiency in 11c.OVA mice, which can be further manipulated by depleting (Fig. 4A and Fig. S1) or adoptively transferring Treg, therefore providing an ideal system in which to monitor influences on Treg homeostasis. Adoptive transfer of OT-I T cells to nTx 11c.OVA mice resulted in a rapid and significant increase in the total number of splenic Treg and restored Treg to levels equivalent to that in sham-Tx controls (Fig. 4A, days 0, 1, 3), whereas transfer of irrelevant antigen-specific CD8+ (gBT-I) T cells did not. In sham-Tx 11c.OVA mice, Treg numbers did not change after adoptive transfer of OT-I T cells (Fig. 4A). Treatment with αCD25 maintained Treg at low levels, regardless of whether 11c.OVA recipients were nTx or not (Fig. 4A), indicating a failure of Treg restoration either because of depletion or functional inhibition of high-affinity IL-2 signaling. Therefore, inducing effector differentiation of antigen-specific CD8+ T cells resulted in IL-2 production (Fig. 2B) and led to concurrent expansion of Treg that was preventable by blockade of CD25. To determine whether this truly reflected an IL-2–dependent expansion of Treg, OT-I or irrelevant-specificity CD8+ (gBT-I) T cells were transferred to nTx 11c.OVA mice with or without IL-2 blockade. Treg expanded after transfer of OT-I but not antigen-irrelevant gBT-I T cells, and this was completely prevented by IL-2 blockade (Fig. 4B).
Fig. 4.
Effector differentiation of CD8+ T cells induces IL-2–dependent Treg expansion. (A) CD45.1+ OT-I or CD45.1+ gBT-I T cells were transferred to nTx or sham-Tx 11c.OVA mice treated with anti-CD25 or isotype control mAb. At the indicated time points, spleens were collected and the number of CD4+CD25+FoxP3+ Treg determined by FACS counting assay. (B) CD45.1+ OT-I or CD45.1+ gBT-I T cells were transferred to nTx 11c.OVA mice treated with anti–IL-2 (JES6.1 plus S4B6) or PBS, as indicated. Three days after transfer, spleens were collected and the number of CD4+CD25+FoxP3+ Treg determined by flow cytometry. Data are (A) mean ± SD (n = 4–7 per group) or (B) individual values pooled from two to three experiments.
Discussion
Regulatory T cells are important controllers of adaptive and innate immune responses. In vitro mechanisms of CD4+ T cell inhibition by Treg are well described (11, 12), but suppression mechanisms in vivo, particularly of CD8+ T-cell responses, are poorly defined. Here we provide compelling direct evidence that Treg inhibit CD8+ T cell expansion and effector differentiation in vivo by limiting IL-2 availability.
Release of Treg control of afferent effector differentiation or the efferent function of terminally differentiated CTLs, or possibly both, may underlie promotion of CD8+ T-cell–dependent responses, such as virus and tumor clearance, which occurs after Treg depletion (4, 5). That Treg suppress CD8+ T-cell effector responses by inhibiting CTL degranulation is well established (8, 9). Our results now clearly demonstrate that Treg also strongly modulate CD8 T cell expansion and effector differentiation, and this is supported by recent studies from others (24, 25). IL-2 crucially governs homeostasis of effector and regulatory T cells and both cell types are exquisitely sensitive to modulation of IL-2 availability in vivo (15). The present study shows, when present, Treg effectively modulate the bioavailability of IL-2 as CD8+ T cells expand and differentiate after immunogenic stimuli. Furthermore, where Treg uptake of IL-2 is blocked, here achieved by anti-CD25 treatment or Treg depletion, CD8+ T cells are able to expand without Treg competition and concomitantly effector differentiation is also promoted. That availability of IL-2 could promote CD8+ effector differentiation in the absence of Treg is supported by demonstrations that increased IL-2 signaling in CD8+ T cells, either in the form of IL-2/anti–IL-2 complexes (15) or prolonged IL-2R signaling (26), promotes their responsiveness.
Many mechanisms have been proposed to underlie suppression of T-cell responses in vivo by Treg. However, it is largely unclear which of these mechanisms apply to CD8+ T-cell responses. Historical and more recent evidence indicates that CD8+ T cells are exquisitely sensitive to the effects of IL-2 availability, but whether—and by how much—Treg might control CD8+ responses by modulating IL-2 was previously unclear. In DEREG mice, where DT depletes both FoxP3+CD25+ and FoxP3+CD25− Treg, the observation that blockade of IL-2 incompletely reversed the effect of Treg depletion suggests release from both IL-2–dependent and IL-2–independent suppression. Conversely, when anti-CD25 or anti-FR4 mAb were used, only FoxP3+CD25+ Treg but not Foxp3+CD25− were depleted and enhanced OT-I expansion was virtually all IL-2–dependent. We propose that these data support a model where, by virtue of their high-affinity IL-2R (CD25) expression, FoxP3+CD25+ Treg modulate IL-2 homeostasis, whereas FoxP3+CD25− Treg appear able to exert regulation independent of this.
Further to understanding how Treg inhibit CD8+ T-cell effector differentiation, our findings highlight the complex interplay between effector differentiation and Treg homeostasis mediated by IL-2 signals. We found that IL-2 produced during CD8+ T-cell effector differentiation induced homeostatic expansion of Treg, which can then contribute to a feedback loop regulating afferent CD8+ T-cell responses. These data suggest that the “rheostat” for Treg number is determined by the overall population of effector and naive T cells, and the extent of their activation, with the “set-point” being readily adjusted as IL-2 becomes available from differentiating effector T cells. In our studies, this significantly modulated Treg numbers, most likely through proliferation of preexisting Treg, although this was not directly tested. Such a proposal is supported by studies in which the abundance of IL-2 in vivo has been increased by administration (19, 27), enforced expression (16), or by increasing bioavailability to the αβγ high-affinity receptor (28). Previously, it has been widely observed that induction of antigen-specific immune responses can promote Treg function, contributing to control of antigen-specific responses, but a mechanism for this remains poorly defined. We show that feed-forward, via IL-2, from differentiating effector CD8+ T cells, plays an important role in expanding Treg that can subsequently limit CD8+ effector responses, indicating this mechanism as an important contributor. Therefore, IL-2 produced during effector T cell differentiation is a crucial component of efficient regulation of adaptive immunity. The importance of this mechanism for immune homeostasis is confirmed by studies in which Treg control over pathogenic self-reactive T-cell responses is promoted by nonspecific or pathogen activation of effector T-cell responses (29, 30) or provision of exogenous IL-2 (19, 28, 31). Conversely, when IL-2 availability is limited, through genetic perturbations for instance, autoinflammation ensues (18–20).
In summary, using a unique approach, we provide direct experimental evidence that consumption of IL-2 is a key in vivo mechanism by which Treg control CD8+ T-cell effector differentiation. Altered numbers or dysfunction of Treg will thus impact CD8+ effector responses in tumor and viral immunity as well as autoimmune disease.
Materials and Methods
Mice.
Mice were from the Animal Resources Centre or bred and maintained at the Princess Alexandra Hospital Biological Research Facility (Brisbane, Queensland, Australia). OT-I mice (32) carrying a transgenic TCR for H-2Kb/OVA257–264 were bred with C57BL/6.SJLptprca mice to generate CD45.1+ OT-I cells. The gBT-I mice carry a transgenic TCR for H-2Kb/HSV-1 glycoprotein B498–505 (33) and were a kind gift from Francis Carbone (University of Melbourne, Australia). CD11c.OVA (22) and DEREG (3) mice have been described. All animal experiments were approved by The University of Queensland animal ethics committee.
Thymectomy and Treg Depletion.
Three-day neonatal thymectomy was performed as described (34). Neonates were anesthetized on ice and thymic lobes removed by suction through a sternal incision. Sham-thymectomized mice were treated identically but without thymus removal. Peripheral blood leukocytes were screened at 4 to 6 wk of age and successfully thymectomized mice were selected based on peripheral blood lymphopenia (<9% of normal CD4+ T cells). CD25+ cells were depleted using αCD25 (PC61, 1 mg) 3d before adoptive transfer of T cells and every 3 d thereafter for the course of the experiment. Isotype controls were treated identically with αphytochrome (MAC-4). Anti-FR4 was administered 2 d before OT-I transfer and every 3 d thereafter. For the memory and recall experiments, mice were treated with a single dose of αCD25 (PC61, 1 mg) 3 d before adoptive transfer and immunization. In some studies, DEREG and control mice were treated with DT as described (3).
Antibodies and Flow Cytometry.
Unless stated, mAb for FACS were from Biolegend, BD, or grown, purified, and conjugated in-house. Anti-CD25 (PC61, rat IgG1) and antiphytochrome (Avena sativa) (MAC49, rat IgG1) were purified from hybridoma supernatants in house, anti-FR4 mAb was kindly supplied by Shimon Sakaguchi (Kyoto University, Japan) and anti–IL-2 for in vivo blockade (JES6-1, S4B6) were purchased from BioXcell. Mouse/rat FoxP3 staining set and ELISA mAb were from eBioscience, used in accordance with instructions. Flow-Count Fluorospheres were from Beckman-Coulter Inc. Cells were stained and analyzed as described previously (22). For analysis of Treg depletion by anti-CD25 (PC61), cell suspensions were stained using an alternate anti-CD25 clone (7D4; Cymbus Biotechnology) for FACS analysis.
Cell Preparation, Transfer, and Immunization.
Brachial, axillary, inguinal, and mesenteric LN were collected from CD45.1+ OT-I or gBT-I mice and CD8+ T cells purified (>90% CD8+, <3% CD4+) by negative selection using magnetic beads according to the manufacturers instruction (Miltenyi Biotec) and 2 × 106 cells injected intravenously. In a small number of experiments, OT-I donor mice were treated with αCD25 and bulk LN cells containing 2 × 106 CD8+ (OT-I) T cells transferred. In a small number of experiments OT-I T cells were prepared as described and 2 × 104 transferred intravenously. Mice were immunized subcutaneously at the tail-base with OVA/QuilA [100 μg OVA (Grade V; Sigma), 20 μg QuilA (Soperfos Biosector DK)].
IL-2 Blockade and ELISA.
The mAb S4B6 and JES6-1A12 individually bind high- or low-affinity IL-2R binding sites on the IL-2 molecule (15) and were injected intraperitoneally (100–200 μg daily) in combination to achieve IL-2 blockade in vivo. For ELISA, serum was prepared from blood obtained by cardiac puncture. ELISA plates were coated with αIL-2 (JES6-1A12), washed, blocked, and incubated with serum and standards overnight. Biotinylated αIL-2 detection mAb (JES6-5H4) was followed by avidin-horseradish peroxidize (Sigma) subsequently visualized with TMB (Biolegend) according to standard protocols.
Statistical Analyses.
Student t test was used for comparison of means and one-way ANOVA followed by Newman-Keuls posttest (GraphPad Software) for multiple pairwise comparisons.
Supplementary Material
Acknowledgments
This work was supported by project Grant 401620 from the National Health and Medical Research Council (NHMRC) of Australia (R.J.S.); a University of Queensland Postgraduate Research Award (to A.M.); NHMRC Career Development Award 519768 (to R.J.S.); an Australian Research Council Future Fellowship and support from Arthritis Queensland (R.T.); and an NHMRC Practitioner Fellowship (to G.R.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1103782108/-/DCSupplemental.
References
- 1.Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184:387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 3.Lahl K, et al. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J Exp Med. 2007;204:57–63. doi: 10.1084/jem.20061852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suvas S, Kumaraguru U, Pack CD, Lee S, Rouse BT. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J Exp Med. 2003;198:889–901. doi: 10.1084/jem.20030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onizuka S, et al. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor α) monoclonal antibody. Cancer Res. 1999;59:3128–3133. [PubMed] [Google Scholar]
- 6.Tang Q, et al. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Z, Herman AE, Matos M, Mathis D, Benoist C. Where CD4+CD25+ T reg cells impinge on autoimmune diabetes. J Exp Med. 2005;202:1387–1397. doi: 10.1084/jem.20051409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen ML, et al. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proc Natl Acad Sci USA. 2005;102:419–424. doi: 10.1073/pnas.0408197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mempel TR, et al. Regulatory T cells reversibly suppress cytotoxic T cell function independent of effector differentiation. Immunity. 2006;25:129–141. doi: 10.1016/j.immuni.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Toka FN, Suvas S, Rouse BT. CD4+ CD25+ T cells regulate vaccine-generated primary and memory CD8+ T-cell responses against herpes simplex virus type 1. J Virol. 2004;78:13082–13089. doi: 10.1128/JVI.78.23.13082-13089.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: A jack of all trades, master of regulation. Nat Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pandiyan P, Zheng L, Ishihara S, Reed JC, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8:1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 15.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 16.Brandenburg S, et al. IL-2 induces in vivo suppression by CD4(+)CD25(+)Foxp3(+) regulatory T cells. Eur J Immunol. 2008;38:1643–1653. doi: 10.1002/eji.200737791. [DOI] [PubMed] [Google Scholar]
- 17.Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201:723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamanouchi J, et al. Interleukin-2 gene variation impairs regulatory T cell function and causes autoimmunity. Nat Genet. 2007;39:329–337. doi: 10.1038/ng1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang Q, et al. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28:687–697. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Humrich JY, et al. Homeostatic imbalance of regulatory and effector T cells due to IL-2 deprivation amplifies murine lupus. Proc Natl Acad Sci USA. 2010;107:204–209. doi: 10.1073/pnas.0903158107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schildknecht A, et al. FoxP3+ regulatory T cells essentially contribute to peripheral CD8+ T-cell tolerance induced by steady-state dendritic cells. Proc Natl Acad Sci USA. 2010;107:199–203. doi: 10.1073/pnas.0910620107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steptoe RJ, et al. Cognate CD4+ help elicited by resting dendritic cells does not impair the induction of peripheral tolerance in CD8+ T cells. J Immunol. 2007;178:2094–2103. doi: 10.4049/jimmunol.178.4.2094. [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi T, et al. Control of immune responses by antigen-specific regulatory T cells expressing the folate receptor. Immunity. 2007;27:145–159. doi: 10.1016/j.immuni.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 24.Chappert P, et al. Antigen-specific Treg impair CD8(+) T-cell priming by blocking early T-cell expansion. Eur J Immunol. 2010;40:339–350. doi: 10.1002/eji.200839107. [DOI] [PubMed] [Google Scholar]
- 25.Heit A, et al. Circumvention of regulatory CD4(+) T cell activity during cross-priming strongly enhances T cell-mediated immunity. Eur J Immunol. 2008;38:1585–1597. doi: 10.1002/eji.200737966. [DOI] [PubMed] [Google Scholar]
- 26.Cheng LE, Greenberg PD. Selective delivery of augmented IL-2 receptor signals to responding CD8+ T cells increases the size of the acute antiviral response and of the resulting memory T cell pool. J Immunol. 2002;169:4990–4997. doi: 10.4049/jimmunol.169.9.4990. [DOI] [PubMed] [Google Scholar]
- 27.Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood. 2006;107:2409–2414. doi: 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Webster KE, et al. In vivo expansion of T reg cells with IL-2-mAb complexes: Induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J Exp Med. 2009;206:751–760. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belghith M, et al. TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med. 2003;9:1202–1208. doi: 10.1038/nm924. [DOI] [PubMed] [Google Scholar]
- 30.Filippi CM, Estes EA, Oldham JE, von Herrath MG. Immunoregulatory mechanisms triggered by viral infections protect from type 1 diabetes in mice. J Clin Invest. 2009;119:1515–1523. doi: 10.1172/JCI38503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grinberg-Bleyer Y, et al. IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. J Exp Med. 2010;207:1871–1878. doi: 10.1084/jem.20100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hogquist KA, et al. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 33.Mueller SN, Heath WR, McLain JD, Carbone FR, Jones CM. Characterization of two TCR transgenic mouse lines specific for herpes simplex virus. Immunol Cell Biol. 2002;80:156–163. doi: 10.1046/j.1440-1711.2002.01071.x. [DOI] [PubMed] [Google Scholar]
- 34.Reeves JP, Reeves PA, Chin LT. Survival surgery: removal of the spleen or thymus. In: Coliga JE, Bierer BE, Margulies DH, Shevach EA, Strober W, editors. Current Protocols in Immunology. Vol. 1. Hoboken, NJ: John Wiley and Sons; 1991. Unit 1.10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.