Abstract
The thymus lacks self-renewing hematopoietic cells, and thymopoiesis fails rapidly when the migration of progenitor cells to the thymus ceases. Hence, the process of thymus homing is an essential step for T-cell development and cellular immunity. Despite decades of research, the molecular details of thymus homing have not been elucidated fully. Here, we show that chemotaxis is the key mechanism regulating thymus homing in the mouse embryo. We determined the number of early thymic progenitors in the thymic rudiments of mice deficient for one, two, or three of the chemokine receptor genes, chemokine (C-C motif) receptor 9 (Ccr9), chemokine (C-C motif) receptor 7 (Ccr7), and chemokine (C-X-C motif) receptor 4 (Cxcr4). In the absence of all three chemokine receptors, thymus homing was reduced about 100-fold both before and after vascularization of the thymic rudiment. In the absence of only two of these three chemokine receptor genes, thymus homing was much less affected (only two- to 10-fold), indicating that the chemotactic regulation of thymus homing is remarkably robust. Our results reveal the redundant roles of Ccr9, Ccr7, and Cxcr4 for thymic homing and provide a framework to examine the regulation of progenitor homing in the postnatal thymus.
T-cell development occurs in the thymus. Beginning at midgestation (1), lymphocyte progenitors migrate to the thymus anlage (2, 3), and there they develop into mature T cells that emigrate to the periphery. The colonization of the thymic rudiment is the essential starting point of thymopoiesis; without this colonization, intrathymic T-cell development does not occur. More than 30 y ago, chemotactic mechanisms were proposed to underlie the thymus homing process (4, 5). However, so far, no single factor has been shown to regulate this process, leading to the suggestion that the combinatorial activity of many factors is required (6). The nature of these factors has not yet been defined.
From the earliest stages of thymus development [embryonic day (E) 11.5–12.5] onwards, chemokines such as chemokine (C-C motif) ligand 21 (Ccl21), chemokine (C-C motif) ligand 25 (Ccl25), and chemokine (C-X-C motif) ligand 12 (Cxcl12) are expressed in the thymic anlage (7–10). Cxcl12 also is expressed in the tissue surrounding the thymus, the perithymic mesenchyme (7, 10); in addition, Ccl21 is expressed in the primordium of the parathyroid (7–10). At E12.5, the receptors for Ccl21, Ccl25, and Cxcl12 chemokines (Ccr7, Ccr9, and Cxcr4, respectively) are expressed in CD45+ cells isolated from the perithymic mesenchyme (10).
Several studies support the notion that chemokines and their receptors play a role in the thymus homing process. Ccr9-deficient embryos display a reduced thymocyte number at early, but not later, stages of thymus development (8, 11, 12). Paucity of lymph node T cells (plt)/plt embryos, which lack functional chemokine (C-C motif) ligand 19 (Ccl19) and Ccl21 genes, and Ccr7-deficient embryos exhibit an early impairment of thymus colonization but have attained normal thymic cellularity by E14.5 (9). Compound Ccr9 and Ccr7 deficiency leads to an even stronger defect; at E11.5, thymic primordia of Ccr9−/−;Ccr7−/− embryos are poorly colonized by hematopoietic cells. Nonetheless, these effects are transient, because thymocyte numbers are normal at birth (8). These results were interpreted as indicating that Ccr9/Ccl25 and Ccr7/Ccl21 are essential only for the prevascular stage of thymus colonization. Interestingly, two recent reports demonstrate that adult mice lacking both Ccr7 and Ccr9 display severe reductions in the number of early thymic progenitors and suggest that compensatory expansion of intrathymic populations could explain, at least in part, normal thymic cellularity (13, 14). Although these studies ascribe important roles to Ccr9 and Ccr7 in thymus colonization, these receptors do not appear to be essential, suggesting that other molecules might be involved, for instance Cxcl12 and its receptor Cxcr4. In initial analyses of Cxcr4- and Cxcl12-deficient mice, no defect in T-cell development was observed (15–17), although it was found subsequently that thymocyte numbers were reduced at later stages of embryonic development (18, 19), and this reduction recently was attributed to the contribution of the Cxcr4/Cxcl12 signaling pathway to efficient β selection (19, 20). In medaka (Oryzias latipes) and zebrafish (Danio rerio) embryos, simultaneous interference with ccl25a and cxcl12 specifically and completely blocked thymopoiesis, suggesting that cxcr4/cxcl12 and ccr9/ccl25 cooperate in guiding progenitors to settle in the thymus of fish embryos (21).
In this study, we therefore examined the relative contributions of Cxcr4, Ccr9, and Ccr7 chemokine receptors in the homing of T-cell progenitors to the mouse embryonic thymic rudiment.
Results
Combined Absence of Ccr9 and Cxcr4 Chemokine Receptor Genes Does Not Abolish Thymopoiesis.
In lower vertebrates, the cooperative activities of Ccr9/Ccl25 and Cxcr4/Cxcl12 chemokine receptor/chemokine pairs regulate thymus homing (21). To examine whether the same is true for thymus homing in mammals, we generated mice deficient for both Ccr9 and Cxcr4. Because it is not possible to perform live imaging of intact mouse embryos, we used the number of thymocytes in the thymic rudiments at E14.5 and 17.5 as a surrogate parameter of thymus homing. E17.5 was the latest time point that could be examined reliably because of embryonic lethality of Cxcr4-deficient mice (16, 17, 22). Unexpectedly, thymopoiesis was not completely abolished in Ccr9;Cxcr4 double-deficient embryos. As shown in Fig. 1, Ccr9 deficiency alone led to only a slight reduction in the number of thymocytes at E14.5 and E17.5, as described previously (8, 11); the effect of Cxcr4 deficiency on thymocyte numbers was more pronounced, particularly at E17.5. Although thymocyte numbers were reduced more substantially in Ccr9;Cxcr4 double-deficient embryos (by about 3.4-fold at E14.5 and 9.3-fold at E17.5) than in embryos deficient for either chemokine receptor gene alone, significant numbers of thymocytes were still detectable at both stages examined (Fig. 1). These results differ from those observed in fish (21) and indicate that the regulation of thymus homing in mice is more complex than in lower vertebrates.
Fig. 1.
Combined lack of Ccr9 and Cxcr4 chemokine receptors does not abolish intrathymic T-cell development. The number of thymocytes is shown for embryos of the indicated genotypes at E14.5 and E17.5. The number of analyzed embryos is indicated (n).
Chemotaxis Regulates the Distribution of Hematopoietic Precursors in the Proximity of the Common Parathyroid/Thymus Anlage.
Previous work in mice has suggested a role for the Ccr7 chemokine receptor in thymus homing (8, 9, 13, 14). Therefore, we proceeded to examine the additional role of Ccr7 in the context of single and combined deficiencies of Ccr9 and Cxcr4. To this end, we examined the distribution of CD45+ hematopoietic cells in and around the thymic anlage by immunohistochemistry. At E12.5, the epithelial anlagen of the thymus and parathyroid have budded off the pharynx but have not yet fully separated into individual organ primordia (23). Hence, this time point affords a comprehensive analysis of the distribution of hematopoietic progenitors in and around these organs. Our breeding scheme generated the expected 27 genotypes in nearly normal Mendelian ratios up to E17.5. For the sake of simplicity, only the results for heterozygous and nullizygous genotypes (n = 8) are reported here; hence, mice heterozygous at all three loci provide the baseline reference in the experiments described below. At E12.5, all thymocytes express the cell-surface marker CD45 (24, 25); thus, these cells most likely include the thymic immigrants and their immediate progeny. The epithelium of organ primordia is positive for cytokeratin 8; therefore, sagittal sections were immunostained for cytokeratin 8 and CD45 to reveal the precise positions of hematopoietic cells (Fig. 2). In control embryos (Ccr9+/−;Ccr4+/−;Ccr7+/−), CD45+ cells were located inside and around the thymic primordium but also around the parathyroid anlage (Fig. 2A). In Ccr9-deficient embryos (Ccr9−/−;Cxcr4+/−;Ccr7+/−) reduced numbers of cells were found within the thymus but not around the parathyroid (Fig. 2B); in contrast, the distribution of cells in Cxcr4-deficient embryos (Ccr9+/−;Cxcr4−/−;Ccr7+/−) appeared similar to that in controls (Fig. 2C). This distribution is in keeping with the results shown in Fig. 1 and with previous observations (18). In embryos deficient for both Ccr9 and Cxcr4 (Ccr9−/−;Cxcr4−/−;Ccr7+/−), CD45+ cells were found mainly around the parathyroid anlage (Fig. 2D). These findings are compatible with the notion that the high levels of Ccl21 expression in the parathyroid contribute to the attraction of progenitors to the perithymic area (7, 9). Accordingly, the distribution of CD45+ cells in Ccr9+/−;Cxcr4+/−;Ccr7−/−embryos is characterized by the conspicuous absence of hematopoietic cells near the parathyroid and reduced numbers within and around the thymus (Fig. 2E), a reciprocal distribution compared with Ccr9−/−;Cxcr4−/−;Ccr7+/− embryos (Fig. 2D). In Ccr9−/−;Cxcr4+/−;Ccr7−/− and Ccr9+/−;Ccr4−/−;Ccr7−/−embryos, the thymus still contained CD45+ cells, albeit in reduced numbers (Fig. 2 F and G), showing that Ccr9 and Cxcr4 on their own are sufficient to sustain the homing process. Notably, triple-deficient embryos (Ccr9−/−;Cxcr4−/−;Ccr7−/−) lacked CD45+ cells in and around the primordia of the thymus and the parathyroid. Collectively, these results suggest that chemotaxis regulates the distribution of progenitor cells in and around the thymus.
Fig. 2.
Spatial distribution of CD45+ cells in the pharyngeal region of E12.5 embryos. Cryosections were stained for cytokeratin 8 (green fluorescence) and CD45 (red fluorescence). The boundaries between the anlagen of the parathyroid (pointing to the upper left corner) and the thymus (lower right) are indicated by the dashed lines. The genotypes of embryos are indicated above A–H. The panels are representative of two to four thymic lobes. (Scale bar, 50 μm.)
Thymopoiesis in Ccr9;Cxcr4;Ccr7 Triple-Deficient Mice Is Severely Impaired.
These results suggest that all three chemokine receptors contribute to thymus homing, albeit in different ways. To assess the effect of triple deficiency on thymopoiesis quantitatively, we determined the number and phenotype of thymocytes in the thymic rudiments of E14.5 mice by flow cytometry. In heterozygous control mice, about 20,000 cells were recovered, whereas in triple-deficient mice, only about 400 cells were obtained (about 2% of control values; Fig. 3A). At this time point, thymopoiesis already has begun, and the differentiation of thymocytes has progressed from the DN1 (CD45+CD44+CD25−) to the DN4 stage (CD45+CD44−CD25−). The DN1 population contains early thymic progenitors (ETPs), defined by the presence of c-kit (CD117) and the absence of lineage markers, including CD25 (26). When we determined the number of ETPs [lineage marker-negative (Lin−)CD117+CD25−] (Fig. 3B), about 2,400 ± 800 cells were found in the heterozygous controls (Fig. 3C). By contrast, fewer than 10 such cells could be found in the triple-deficient thymic rudiments (Fig. 3C). The thymocyte numbers in single- and double-deficient mice again suggest that the relative contributions of the three chemokine receptors differ (Fig. 3A). Loss of Cxcr4 function reduced thymocyte numbers by about 30%; loss of Ccr7 function had a slightly more pronounced effect (a reduction of about 40%). The strongest effect was seen with Ccr9 deficiency, which reduced thymocyte numbers by 85%; the same level of reduction was seen in combined deficiency of Ccr7 and Cxcr4. Combined deficiency of Ccr9 and Cxcr4 reduced thymocyte numbers to about 10% of control values. The strongest effect of double deficiency was observed with Ccr7 and Ccr9 mutants (about 5% of control values). From these results, we infer that Ccr9 is the most important factor in regulating thymus homing at this stage. The results for ETPs were qualitatively similar, although the effects tended to be more pronounced (Fig. 3C). When immature thymocytes were defined by a different cell surface marker combination [Lin−CD117+/Paired immunoglobulin receptor (PIR+)] (27), similar findings were made (Fig. S1). Collectively, our results indicate that at E14.5, the thymic rudiment in triple-deficient mice lacks T-cell progenitors.
Fig. 3.
T-cell progenitors in the thymus of E14.5 embryos deficient for chemokine receptors. (A) Total number of thymocytes in embryos of the indicated genotypes. (B) Representative flow cytometric profile of a Ccr9+/−;Ccr7+/−;Cxcr4+/− embryo to illustrate the identification of ETPs. Numbers represent percentages of total thymocytes. (C) Number of ETPs in embryos of the indicated genotypes. Note that the scale is expanded in the lower part of the y axis; the inset is a magnification to show the absolute number of ETPs for the three color-coded genotypes. Average values are indicated; each data point represents one embryo, and the total number of embryos per genotype is indicated (n).
Triple-Deficient Mice Possess the Same Number of T-Cell Progenitors in the Fetal Liver.
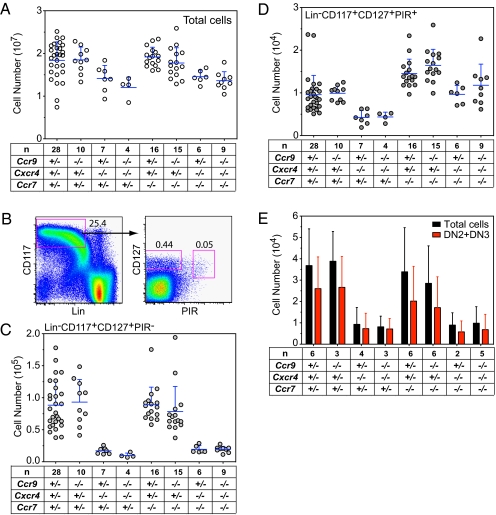
Instead of being the result of impaired homing, the lack of T-cell progenitors in the thymic anlage of mutant mice might have been caused by a failure in their production in the fetal liver. To examine this possibility, we determined the numbers of various progenitor populations in this tissue. Cxcr4 deficiency always causes a 30% reduction in the numbers of total fetal liver cells (Fig. 4A). The Lin−CD117+/ stem cell antigen 1 (Sca-1+) population, enriched in multipotent progenitors (28), was affected only marginally, as were the mostly lymphoid-primed Lin−CD117+ Sca-1+/FMS-related tyrosine kinase 3 (Flt3)hi multipotent progenitors (29) (Fig. S2 A–C). The Lin−CD117+CD127+ population in fetal liver (Fig. 4B) is enriched in progenitors with B and T/natural killer (NK) cell potential, respectively (30), which can be assigned to PIR− and PIR+ subpopulations: the Lin−CD117+CD127+PIR− subset contains B-cell progenitors, and the Lin−CD117+CD127+PIR+ subset contains exclusively T/NK/dentritic cell progenitors (27). In keeping with these and previous findings (15–17, 31, 32), Lin−CD117+CD127+PIR− cells were decreased drastically in all Cxcr4-deficient embryos, but the absence of the other chemokine receptor genes had no significant influence (Fig. 4C). By contrast, the effect of Cxcr4 deficiency on Lin−CD117+CD127+PIR+ cells (henceforth designated “PIR+ cells”) was much less pronounced (Fig. 4D). Although Ccr9 deficiency alone did not appear to have any effect on PIR+ cells, lack of Ccr7 increased their number ∼1.5-fold; the number of PIR+ cells was normalized in embryos double-deficient for Ccr7 and Cxcr4, irrespective of the Ccr9 genotype (Fig. 4D). To examine the capacity of mutant PIR+ cells to generate T cells in vitro, they were cultured on OP9-DL1 stromal cells (33). Although deficiency of Cxcr4 caused a reduction in cell numbers after 8 d of in vitro growth, this inhibition was comparable for PIR+ cells of Ccr9+/−;Cxcr4−/−;Ccr7+/− and Ccr9−/−;Cxcr4−/−;Ccr7−/− embryos. Importantly, however, no qualitative defect in T lineage differentiation, as measured by the number of DN2/3 cells, was detectable (Fig. 4E). The same is true when Lin−CD117+Sca-1+ cells were subjected to in vitro differentiation (Fig. S2D). Collectively, these data suggest that T-cell progenitors are generated in the fetal liver of Ccr9−/−;Cxcr4−/−;Ccr7−/−embryos in nearly normal numbers and that they are capable of differentiating normally into T cells in vitro.
Fig. 4.
Early progenitor cells in the fetal liver of E14.5 embryos. (A) Total number of cells for embryos of the indicated genotypes. (B) Representative flow cytometric profile of a Ccr9+/−;Ccr7+/−;Cxcr4+/− embryo to illustrate the identification of Lin−CD117+CD127+PIR− and Lin−CD117+CD127+PIR+ populations. Numbers refer to percentages of total cells. (C) Number of Lin−CD117+CD127+PIR− cells in the fetal liver of embryos with the indicated genotypes. (D) Number of Lin−CD117+CD127+PIR+ cells. (E) Total number of cells and number of DN2 + DN3 cells obtained after in vitro culture of 100 Lin−CD117+CD127+PIR+ cells for 8 d on OP9-DL1 stroma. The total number of embryos analyzed per genotype is indicated (n).
Thymus Homing in Triple-Deficient Mice Does Not Resume After Vascularization.
These experiments addressed thymopoiesis at the prevascularized stage of thymus development. It has been argued, however, that vascularization affects the homing process (8). Hence, it was important to determine whether the observed effects on thymopoiesis persist beyond E14.5. To this end, we examined the state of thymopoiesis at E17.5, well beyond the onset of vascularization (Fig. 5 A–E). The total number of thymocytes in triple-deficient mice (about 3,000 cells) was lower by more than two orders of magnitude than in controls (about 1,000,000 cells) and by at least one order of magnitude than in other mutant embryos (Fig. 5F). The distribution of CD45+ cells found within the epithelium (Fig. 5E) appears to be essentially random with respect to cortical and medullary areas (Fig. S3). The number of ETPs in the thymic lobes mirrored this situation; in triple-deficient embryos, only about 50 ETPs per thymus were found, whereas about 5,000 cells of this phenotype were observed in controls (Fig. 5 G and H). The same is true when early progenitors are defined as Lin−CD117+PIR+ cells (Fig. S4). Hence, even after vascularization, the number of ETPs remains at extremely low levels in triple-deficient thymi, suggesting that the combined functions of three chemokine receptors account for >99% of homing activity during the entire embryonic period.
Fig. 5.
T-cell progenitors in the thymus of E17.5 embryos deficient for chemokine receptors. (A–E) Distribution of CD45+ cells in the thymic rudiment of E17.5 embryos. The genotype is indicated at the top of each panel. Cryosections were stained for cytokeratin 8 (epithelial marker, green fluorescence), CD45 (marker of hematopoietic cells, red fluorescence), and CD31 (endothelial marker, blue fluorescence). The panels are representative of two to six thymic lobes. (Scale bar, 100 μm.) (F) Number of total thymocytes in embryos of the indicated genotypes. (G) Representative flow cytometric profile of a Ccr9+/−;Ccr7+/−;Cxcr4+/− embryo to illustrate the identification ETPs. Numbers refer to percentages of total thymocytes. (H) Number of ETPs in embryos of the indicated genotypes. Note that the scale is expanded in the lower part of the y axis. The total number of embryos per genotype is indicated (n).
To exclude the possibility that the absence of all three chemokine receptors affected the general physiology of thymocytes in a way that interfered with their normal intrathymic development, E17.5 embryos were subjected to further analyses. The presence of more mature DN thymocyte populations (Lin−CD117+CD25+ and Lin−CD117-/loCD25+) in mutant thymi indicated that the triple-deficient cells can develop further (Fig. S5A). When thymic lobes were removed from the embryos and allowed to develop further in fetal thymus organ cultures for 8 d, CD4/CD8 double-positive thymocytes developed (Fig. S5B). The same result was obtained when the thymic lobes were transplanted under the kidney capsule of syngeneic wild-type mice and analyzed 12 d later (Fig. S5C). Moreover, the colonization of triple-deficient thymic lobes by wild-type host cells was not impaired (Fig. S5D). Collectively, these results demonstrate that, although the mutant cells are severely impaired in their ability to colonize the thymus, they nonetheless are capable of progressing through all stages of T-cell development.
Discussion
Here we show that chemotaxis is the key mechanism regulating thymus homing in mouse embryos before and after vascularization of the thymus. This chemotactic regulation is remarkably robust because of the redundant activities of the Ccr9, Ccr7, and Cxcr4 chemokine receptors.
Our results indicate that the presumptive T-cell progenitor populations, each identified by a characteristic combination of cell surface markers, are present in normal numbers in the fetal liver of triple-deficient embryos. Because they neither accumulate in nor disappear from the fetal liver, it seems unlikely that they are functionally incapacitated. Indeed, when assayed in vitro, these cells are capable of differentiating into T cells. Although we have not been able to isolate T-cell progenitors directly from fetal blood, it appears unlikely that their ability to enter the circulation and to survive in this environment is affected by the lack of chemokine receptors, because neither Ccr7 nor Ccr9 has been implicated in these processes (8). Cxcr4 is known to regulate, among other processes, the adhesion of hematopoietic cells to the stromal microenvironment (32). Hence, progenitors arriving in the thymus might be nonspecifically lost because of their diminished retention. However, we note that such cells are found in large numbers in the thymus of Cxcr4;Ccr7 and Cxcr4;Ccr9 double-deficient embryos. Therefore, we believe that the lack of thymocytes in triple-deficient embryos is a direct result of impaired responses to chemokine gradients emanating from the thymic microenvironment. Indeed, intrathymic development of the few progenitors arriving in the thymus of triple-deficient embryos into mature T cells is not impaired.
Previous studies on thymus homing have focused on the roles of Ccr9 and Ccr7, whereas the potential function of Cxcr4 in this process has been largely ignored. We were prompted to analyze the contribution of Cxcr4 to thymus homing in mice following our demonstration that thymus homing in fish could be abolished by simultaneously interfering with ligands for Ccr9 and Cxcr4 (21). Our present analysis supports the notion that chemotactic regulation of thymus homing is a conserved feature of vertebrate thymus function and that this process has evolved to become more redundant and robust in mammals. Several factors contribute to this enhanced redundancy. First, in contrast to the situation in fish, Cxcl12 is expressed not only by the capsular mesenchyme but also by the thymic epithelium. Second, thymus homing is helped, at least initially, by the close proximity of the thymus to the parathyroid, which expresses copious amounts of the chemokine Ccl21, the ligand for the Ccr7 chemokine receptor. Ccl21 appears to attract, albeit inefficiently, Ccr9−/−;Cxcr4−/−-deficient progenitors to the vicinity of the thymic rudiment, where some might come under the influence of “thymic” Ccl21.
Our data suggest the following sequence of events of thymus homing during embryogenesis. At early stages, prothymocytes arrive in the pharyngeal region via the blood stream, where they respond to chemokines emanating from the rudiments of the thymus and the parathyroid. Although they are heterogeneous with respect to chemokine receptor expression (10), they nonetheless represent a developmentally homogeneous population (34). Because Ccl25 and Cxcl12 (both of thymus origin) are considerably more effective in attracting progenitor cells than is Ccl21 (predominantly of parathyroid origin) (7–10), progenitor cells collect around and in the thymic rudiment rather than near the parathyroid. Hence, progenitor accumulation in the vicinity of the thymus of early embryos is most effective when all three chemokine receptors are functionally competent. Once thymus and parathyroid have become two separate organs and have located to different regions of the embryo, the importance of parathyroid-derived Ccl21 for thymus homing diminishes; however, this chemokine continues to be expressed by the thymic epithelium and contributes to thymus homing. As a result of these redundancies, homing to the thymus is remarkably robust. Our results do not exclude the possibility that other molecules apart from chemokines and their receptors are involved in the homing process. However, their direct involvement during the embryonic phase is likely to be marginal or indirect and then possibly dependent on intracellular signals emanating from chemokine receptors (35).
Materials and Methods
Animals.
Mouse strains deficient for Ccr9, Ccr7, and Cxcr4 have been described (12, 17, 36). Ccr7-deficient mice with the C57BL/6 genetic background were obtained from Jackson Laboratories, and Ccr7-deficient mice with the BALB/c background were obtained from M. Lipp (Max Delbrück Center for Molecular Medicine, Berlin). Cxcr4-deficient mice were from Jackson Laboratories. Mice were kept and bred under specific pathogen-free conditions at the animal facility of the Max Planck Institute of Immunobiology and Epigenetics in Freiburg, Germany. Genotyping was performed as described in SI Materials and Methods. Embryos were obtained from timed pregnancies; the day of the vaginal plug was counted as day 0.5 of pregnancy.
Immunohistochemistry.
E12.5 and E17.5 embryos were embedded in optimal cutting temperature (OCT) medium and snap frozen. E12.5 sagittal and E17.5 transversal sections (7-μm thickness) were fixed in acetone/methanol (3:1; vol/vol).The following antibodies and reagents were used for staining: anti-cytokeratin 8 [Troma-1; a gift from R. Kemler (Max Planck Institute of Immunobiology and Epigenetics, Freiburg, Germany)], allophycocyanin-conjugated anti-CD45 (30-F11; eBioscience), biotinylated anti-CD31 (MEC 13.3; BD Bioscience), Alexa Fluor 488 goat anti-rat IgG (Invitrogen), and Cy3-conjugated streptavidin (Jackson ImmunoResearch). Fluorochrome-labeled antibodies were detected using a Zeiss AxioImager.Z1 microscope with ApoTome slider and Axiovision 4.8 software.
Flow Cytometric Analysis and Cell Sorting.
Embryonic thymi were minced carefully with two thin forceps to prepare single-cell suspensions. Thymocyte numbers were determined using CountBright absolute counting beads (Invitrogen). Analytical cytometry was performed using an LSRII, and cell sorting was done using a FACSAria (BD Biosciences).
OP9-DL1 Culture.
E14.5 fetal liver progenitors (Lin−CD117+CD127+PIR+ at 100 cells per well or Lin−CD117+Sca-1+ at 200 cells per well) were sorted directly onto an OP9-DL1 layer in 96-well plates. OP9-DL1 cells (kindly provided by J. C. Zuniga-Pflücker, University of Toronto, Toronto) were plated at 2 × 103 cells per well in a 96-well plate 48 h before the addition of progenitor cells. Progenitors were cultured in the presence of recombinant murine IL-7, Flt3L, and stem-cell factor (SCF) (5 ng/mL each; PeproTech) (37). Cells were analyzed by flow cytometry after 8 d in culture.
Fetal Thymus Organ Culture and Thymus Transplantations.
Fetal thymus organ culture and thymus transplantations were carried out as described previously (37, 38).
Statistical Analysis.
Average values and SDs are shown.
Supplementary Material
Acknowledgments
We thank Drs. Conrad Bleul and Martin Lipp for mice, and J. C. Zuniga-Pflücker for OP9-DL1 cells. We are grateful to J. B. Swann for advice and critical reading of the manuscript. Financial support for these studies was provided by the Max Planck Society and the Deutsche Forschungsgemeinschaft. L. C. is a member of the International Max Planck Research School for Molecular and Cellular Biology, a joint international PhD program of the Max Planck Institute of Immunobiology and Epigenetics and the University of Freiburg, Germany.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1016428108/-/DCSupplemental.
References
- 1.Owen JJ, Ritter MA. Tissue interaction in the development of thymus lymphocytes. J Exp Med. 1969;129:431–442. doi: 10.1084/jem.129.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore MA, Owen JJ. Experimental studies on the development of the thymus. J Exp Med. 1967;126:715–726. doi: 10.1084/jem.126.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ritter MA. Embryonic mouse thymus development: Stem cell entry and differentiation. Immunology. 1978;34:69–75. [PMC free article] [PubMed] [Google Scholar]
- 4.Pyke KW, Bach JF. The in vitro migration of murine fetal liver cells to thymic rudiments. Eur J Immunol. 1979;9:317–323. doi: 10.1002/eji.1830090413. [DOI] [PubMed] [Google Scholar]
- 5.Jotereau FV, Houssaint E, Le Douarin NM. Lymphoid stem cell homing to the early thymic primordium of the avian embryo. Eur J Immunol. 1980;10:620–627. doi: 10.1002/eji.1830100809. [DOI] [PubMed] [Google Scholar]
- 6.Boehm T, Bleul CC. Thymus-homing precursors and the thymic microenvironment. Trends Immunol. 2006;27:477–484. doi: 10.1016/j.it.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Bleul CC, Boehm T. Chemokines define distinct microenvironments in the developing thymus. Eur J Immunol. 2000;30:3371–3379. doi: 10.1002/1521-4141(2000012)30:12<3371::AID-IMMU3371>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 8.Liu C, et al. Coordination between CCR7- and CCR9-mediated chemokine signals in prevascular fetal thymus colonization. Blood. 2006;108:2531–2539. doi: 10.1182/blood-2006-05-024190. [DOI] [PubMed] [Google Scholar]
- 9.Liu C, et al. The role of CCL21 in recruitment of T-precursor cells to fetal thymi. Blood. 2005;105:31–39. doi: 10.1182/blood-2004-04-1369. [DOI] [PubMed] [Google Scholar]
- 10.Jenkinson WE, et al. Chemokine receptor expression defines heterogeneity in the earliest thymic migrants. Eur J Immunol. 2007;37:2090–2096. doi: 10.1002/eji.200737212. [DOI] [PubMed] [Google Scholar]
- 11.Wurbel MA, et al. Mice lacking the CCR9 CC-chemokine receptor show a mild impairment of early T- and B-cell development and a reduction in T-cell receptor gammadelta(+) gut intraepithelial lymphocytes. Blood. 2001;98:2626–2632. doi: 10.1182/blood.v98.9.2626. [DOI] [PubMed] [Google Scholar]
- 12.Benz C, Heinzel K, Bleul CC. Homing of immature thymocytes to the subcapsular microenvironment within the thymus is not an absolute requirement for T cell development. Eur J Immunol. 2004;34:3652–3663. doi: 10.1002/eji.200425248. [DOI] [PubMed] [Google Scholar]
- 13.Krueger A, Willenzon S, Lyszkiewicz M, Kremmer E, Förster R. CC chemokine receptor 7 and 9 double-deficient hematopoietic progenitors are severely impaired in seeding the adult thymus. Blood. 2010;115:1906–1912. doi: 10.1182/blood-2009-07-235721. [DOI] [PubMed] [Google Scholar]
- 14.Zlotoff DA, et al. CCR7 and CCR9 together recruit hematopoietic progenitors to the adult thymus. Blood. 2010;115:1897–1905. doi: 10.1182/blood-2009-08-237784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagasawa T, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 16.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 17.Ma Q, et al. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci USA. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ara T, et al. A role of CXC chemokine ligand 12/stromal cell-derived factor-1/pre-B cell growth stimulating factor and its receptor CXCR4 in fetal and adult T cell development in vivo. J Immunol. 2003;170:4649–4655. doi: 10.4049/jimmunol.170.9.4649. [DOI] [PubMed] [Google Scholar]
- 19.Janas ML, et al. Thymic development beyond beta-selection requires phosphatidylinositol 3-kinase activation by CXCR4. J Exp Med. 2010;207:247–261. doi: 10.1084/jem.20091430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trampont PC, et al. CXCR4 acts as a costimulator during thymic beta-selection. Nat Immunol. 2010;11:162–170. doi: 10.1038/ni.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bajoghli B, et al. Evolution of genetic networks underlying the emergence of thymopoiesis in vertebrates. Cell. 2009;138:186–197. doi: 10.1016/j.cell.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 22.Tachibana K, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 23.Blackburn CC, Manley NR. Developing a new paradigm for thymus organogenesis. Nat Rev Immunol. 2004;4:278–289. doi: 10.1038/nri1331. [DOI] [PubMed] [Google Scholar]
- 24.Hattori N, Kawamoto H, Katsura Y. Isolation of the most immature population of murine fetal thymocytes that includes progenitors capable of generating T, B, and myeloid cells. J Exp Med. 1996;184:1901–1908. doi: 10.1084/jem.184.5.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amagai T, Itoi M, Kondo Y. Limited development capacity of the earliest embryonic murine thymus. Eur J Immunol. 1995;25:757–762. doi: 10.1002/eji.1830250320. [DOI] [PubMed] [Google Scholar]
- 26.Godfrey DI, Zlotnik A. Control points in early T-cell development. Immunol Today. 1993;14:547–553. doi: 10.1016/0167-5699(93)90186-O. [DOI] [PubMed] [Google Scholar]
- 27.Masuda K, et al. Prethymic T-cell development defined by the expression of paired immunoglobulin-like receptors. EMBO J. 2005;24:4052–4060. doi: 10.1038/sj.emboj.7600878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawamoto H, Ohmura K, Katsura Y. Direct evidence for the commitment of hematopoietic stem cells to T, B and myeloid lineages in murine fetal liver. Int Immunol. 1997;9:1011–1019. doi: 10.1093/intimm/9.7.1011. [DOI] [PubMed] [Google Scholar]
- 29.Månsson R, et al. Molecular evidence for hierarchical transcriptional lineage priming in fetal and adult stem cells and multipotent progenitors. Immunity. 2007;26:407–419. doi: 10.1016/j.immuni.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 30.Kawamoto H, Ikawa T, Ohmura K, Fujimoto S, Katsura Y. T cell progenitors emerge earlier than B cell progenitors in the murine fetal liver. Immunity. 2000;12:441–450. doi: 10.1016/s1074-7613(00)80196-x. [DOI] [PubMed] [Google Scholar]
- 31.Egawa T, et al. The earliest stages of B cell development require a chemokine stromal cell-derived factor/pre-B cell growth-stimulating factor. Immunity. 2001;15:323–334. doi: 10.1016/s1074-7613(01)00185-6. [DOI] [PubMed] [Google Scholar]
- 32.Ma Q, Jones D, Springer TA. The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity. 1999;10:463–471. doi: 10.1016/s1074-7613(00)80046-1. [DOI] [PubMed] [Google Scholar]
- 33.Schmitt TM, Zúñiga-Pflücker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 34.Desanti GE, et al. Clonal analysis reveals uniformity in the molecular profile and lineage potential of CCR9+ and CCR9- thymus-settling progenitors. J Immunol. 2011;186 doi: 10.4049/jimmunol.1002686. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lei Y, Liu C, Saito F, Fukui Y, Takahama Y. Role of DOCK2 and DOCK180 in fetal thymus colonization. Eur J Immunol. 2009;39:2695–2702. doi: 10.1002/eji.200939630. [DOI] [PubMed] [Google Scholar]
- 36.Förster R, et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 37.Benz C, Martins VC, Radtke F, Bleul CC. The stream of precursors that colonizes the thymus proceeds selectively through the early T lineage precursor stage of T cell development. J Exp Med. 2008;205:1187–1199. doi: 10.1084/jem.20072168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramsdell FJ, Zuniga-Pflucker JC, Takahama Y. In vitro systems for the study of cell development: fetal thymus organ culture and OP9-DL1 cell coculture. Curr Protoc Immunology. 2006;71:3.18.1–3.18.18. doi: 10.1002/0471142735.im0318s71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.