Abstract
Most studies on the ability of insect populations to transmit pathogens consider only constant temperatures and do not account for realistic daily temperature fluctuations that can impact vector–pathogen interactions. Here, we show that diurnal temperature range (DTR) affects two important parameters underlying dengue virus (DENV) transmission by Aedes aegypti. In two independent experiments using different DENV serotypes, mosquitoes were less susceptible to virus infection and died faster under larger DTR around the same mean temperature. Large DTR (20 °C) decreased the probability of midgut infection, but not duration of the virus extrinsic incubation period (EIP), compared with moderate DTR (10 °C) or constant temperature. A thermodynamic model predicted that at mean temperatures <18 °C, DENV transmission increases as DTR increases, whereas at mean temperatures >18 °C, larger DTR reduces DENV transmission. The negative impact of DTR on Ae. aegypti survival indicates that large temperature fluctuations will reduce the probability of vector survival through EIP and expectation of infectious life. Seasonal variation in the amplitude of daily temperature fluctuations helps to explain seasonal forcing of DENV transmission at locations where average temperature does not vary seasonally and mosquito abundance is not associated with dengue incidence. Mosquitoes lived longer and were more likely to become infected under moderate temperature fluctuations, which is typical of the high DENV transmission season than under large temperature fluctuations, which is typical of the low DENV transmission season. Our findings reveal the importance of considering short-term temperature variations when studying DENV transmission dynamics.
Keywords: arbovirus, climate, vectorial capacity
Incidence, seasonal variation, and global distribution of vector-borne diseases are known to be influenced by climate (1). What is controversial is exactly how climatic factors—and thus climate change—impact the intrinsic transmission intensity of most vector-borne pathogens (1–5). Part of the problem derives from the interplay of multiple factors, such as spatial heterogeneity (6) or differing socioeconomic and demographic backgrounds (3, 7) that combine with climate to influence overall transmission dynamics. In addition, our ability to accurately define the impact of climatic factors on the risk of vector-borne disease is limited by poor understanding of the mechanistic link between environmental variables, such as temperature, and the vectorial capacity of insect vector populations (1, 8–12).
Vectorial capacity captures key components of an insect's role in pathogen transmission, which is influenced by many environmental, ecological, behavioral, and molecular factors (13). Mathematically, it can be described by:
 |
where m is vector density per person, a is daily probability of a vector biting a human host, p is daily probability of vector survival, n is duration in days of the pathogen extrinsic incubation period (EIP) in the vector, and b is vector competence (13). Vector competence is defined as the intrinsic ability of a vector to become infected and subsequently transmit a pathogen to a susceptible host (13). In the absence of host recovery, V is equal to the number of new mosquito infectious bites that arise from one infected person introduced into a population of entirely susceptible hosts.
Insects are small-bodied ectotherms, which makes their life cycle duration, survival, and behavior dependent on ambient temperature (14–16). The EIP of many vector-borne pathogens is similarly known to be temperature sensitive (17–22). Vectorial capacity, therefore, is influenced by environmental temperature (1). With a few exceptions (9, 23), the vast majority of studies examining the effects of temperature on vectorial capacity consider set point temperatures representative of average conditions (8). Likewise, mathematical models of the relationships between climatic factors and vector-borne disease incidence most often use averaged monthly temperature and parameter values derived from experimental data based on constant temperatures (1). In nature, however, mosquitoes and their pathogens do not simply experience mean conditions, but are instead subjected to temperatures that fluctuate throughout the day.
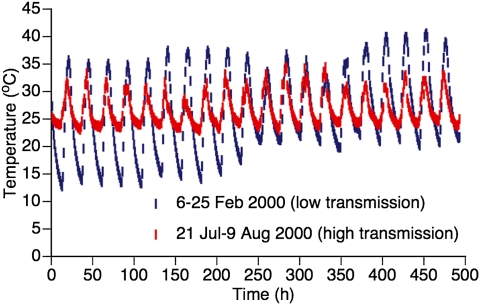
Here, we investigated whether short-term temperature fluctuations could play a role in intraannual fluctuations (i.e., seasonal forcing) of dengue virus (DENV) transmission. Our study was motivated by observations from rural Thai villages near Mae Sot, Tak Province where daily temperature fluctuation varies considerably between low and high seasons of DENV transmission, even though mean air temperature remains relatively constant (Fig. 1). Dengue is endemic throughout Thailand where the four antigenically related, but distinct virus serotypes (DENV-1, -2, -3, and -4) cocirculate (24, 25) and are a leading cause of morbidity (26). These RNA viruses in the genus Flavivirus are transmitted primarily by the mosquito Aedes aegypti, a species well adapted to the domestic, human environment (27). Because immature Ae. aegypti in Thailand develop principally in managed water containers located in and around dwellings, they are not strongly affected by seasonal varition in rain-filled natural development sites (28, 29). Accordingly, standard entomological measures of mosquito abundance (parameter m in Eq. 1) are not positively associated with high and low DENV transmission seasons in Thailand (30, 31), indicating that marked seasonal fluctuations in DENV transmission (24, 25) are likely governed by factors other than simple changes in vector abundance.
Fig. 1.
Seasonal DTR differences associated with the intensity of natural DENV transmission. Representative time series of daily temperature fluctuations measured during the high DENV transmission season (red symbols) and the low DENV transmission season (blue symbols) at Mae Sot, Tak Province, Thailand. The average temperature over the entire period is 26.7 °C for the high transmission season and 26.1 °C for the low transmission season.
We evaluated experimentally the effect of diurnal temperature range (DTR) on the potential for DENV transmission by Ae. aegypti females under three temperature regimes defined by field temperature profiles (Fig. 1). Each treatment had the same average temperature (26 °C), but a different amplitude of daily variation; control (DTR = 0 °C), moderate (DTR = 10 °C), and large (DTR = 20 °C). Moderate and large DTRs were characteristic of high and low DENV transmission seasons, respectively, at Mae Sot. We conducted two experiments, the first with three temperature regimes using a DENV-2 isolate and a second, independent experiment in a different laboratory using a DENV-1 isolate with the constant and large DTR regimes. We examined three components of vectorial capacity over a 32-d period following mosquito exposure to virus-infected blood meals (days postinfection, dpi), namely, vector survival, EIP duration (measured by the time required for viral dissemination from the midgut to other tissues), and vector competence (measured by the prevalence of midgut infection and viral dissemination), which represent parameters p, n, and b, respectively, in Eq. 1. We used thermodynamic models to explore the implications of daily temperature fluctuations for DENV transmission potential.
Results
Vector Competence and EIP.
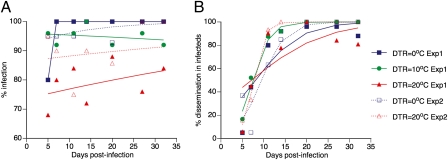
In the first experiment, rates of infection and dissemination by a DENV-2 isolate were measured in a total of 525 Ae. aegypti females (175 in each of the three temperature regimes). Overall, 474 females (90.3%) had a DENV-2 infected midgut. In the second experiment, rates of infection and dissemination by a DENV-1 isolate were measured in 227 females (132 under the constant temperature regime and 95 under the large DTR regime). Overall, 212 females (93.4%) had a DENV-1 infected midgut. Logistic regression analysis of results from both experiments indicated that although the proportion of infected females did not change significantly over the 32-d time course of the experiments, mosquito infection was significantly influenced by the DTR regime (Table 1 and Fig. 2A). Averaged across the entire time course of the first experiment, 97.1, 94.9, and 78.9% of females were DENV-2 infected under DTRs of 0 °C, 10 °C, and 20 °C, respectively. Averaged across the entire time course of the second experiment, 97.0 and 88.4% of females were DENV-1 infected under DTRs of 0 °C and 20 °C, respectively. In the first experiment, separate analyses indicated that the proportion of infected females significantly differed between large DTR and the two other temperature regimes, but not between the moderate DTR and constant temperature regimes. Slight variations in prevalence were observed over time (Fig. 2A), which could be due to stochastic effects (sampling error) and/or temporal dynamics in vector–virus interplay. Nonetheless, increasing DTR reduced the likelihood that a female became infected, a necessity for virus to ultimately be transmitted from salivary glands and a major component of vector competence (parameter b in Eq. 1).
Table 1.
Test statistics of DTR effects on Ae. aegypti experimental vector competence for DENV
| Infection |
Dissemination |
||||
| Source | df | χ2 | P value | χ2 | P value |
| Experiment | 1 | 1.09 | 0.296 | 6.63 | 0.0100 |
| EIP | 6 | 5.46 | 0.486 | 150 | <0.0001 |
| Experiment * EIP | 6 | 11.6 | 0.072 | 24.1 | 0.0005 |
| DTR (within experiment) | 3 | 43.1 | <0.0001 | 4.26 | 0.234 |
| EIP * DTR (within experiment) | 18 | NS | NS | 25.5 | 0.112 |
Logistic analysis of the proportion of females with a midgut infection and the proportion of infected females (excluding uninfected) with a disseminated infection as a function of the experiment, the extrinsic incubation period (EIP) and the diurnal temperature range (DTR). Because the number of temperature regimes is different in each experiment, DTR is nested in the experiment. NS means that the effect was strongly nonsignificant and thus removed from the final statistical model.
Fig. 2.
Effects of EIP and DTR on Ae. aegypti experimental vector competence for DENV. Time course of (A) the percentage of females with a midgut infection and (B) the percentage of infected (excluding uninfected) females with a disseminated infection as a function of DTR regimes in two independent experiments (Exp1 and Exp2). Each data point represents 25 females in Exp1 and 3–28 females (mean 16) in Exp2. Lines are the logistic fits of the data. Overall, DTR significantly influences the percentage of infected females (P < 0.0001) but not the percentage of females with a disseminated infection (P = 0.234).
In the first experiment, 353 (74.5%) female mosquitoes with infected midguts had a disseminated DENV-2 infection, as indicated by detection of virus in leg tissues. In the second experiment, 143 (67.5%) midgut-infected females had a disseminated DENV-1 infection. The prevalence of disseminated infections increased sharply during the first 2 wk following the infectious feed and then reached a plateau (Fig. 2B). These kinetics are consistent with an EIP of 8–14 d usually reported for DENV in Ae. aegypti at 26–30 °C (27). Logistic regression revealed that the proportion of infected females with a disseminated infection over time did not differ between DTR regimes (Table 1). Thus, although the EIP (parameter n in Eq. 1) of many mosquito-borne viruses is sensitive to mean temperature (17–22), it did not seem, in our experiments, to be significantly affected by DTR.
Thermodynamic Models.
The lack of DTR effect on the rate of virus dissemination from the midgut under sinusoidal temperature fluctuations is consistent with results from earlier studies on yellow fever and eastern equine encephalitis viruses, indicating that cyclical temperature regimes have little impact on duration of the EIP (17, 18). This conclusion is supported by the results from our modeling of EIP in which we used a simple temperature-dependent model (Fig. S1) based on standard enzyme kinetics (32). When daily temperature dynamics were described by a simple sine function, no effect of DTR on EIP was observed relative to estimates based on mean temperatures alone (Fig. S2). Because the sine function is symmetrical around the mean and our DENV growth model has a constant increase in rate per degree, increases in growth above the mean are offset by slower growth below the mean. Our empirical and EIP model results, therefore, indicate that DTR does not affect EIP when temperature follows strict sinusoidal variation.
Temperature in the field, however, does not strictly follow a sine function between maximum and minimum temperatures. It is more accurately described using a sinusoidal progression during daytime and a decreasing exponential curve at night (Fig. S3) (33). Using this more realistic temperature function to drive DENV growth, our model indicates that larger DTRs will tend to shorten EIP duration relative to equivalent constant mean temperatures (Fig. S2). In this case, although the DENV growth model is the same, the slight asymmetry in the temperature function between day and night results in shorter EIP under larger DTR. The effect is modest, even at the greatest DTR. A shorter EIP (and hence greater vectorial capacity) is, however, inconsistent with our observation that DENV transmission intensity in the field is lower under larger DTR (Fig. 1). Thus, although such an effect of DTR on EIP may occur in nature, we suggest that it is offset by other factors.
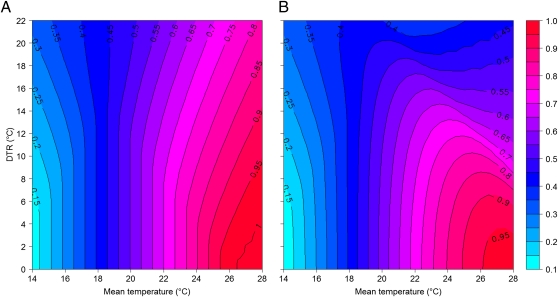
Results from our empirical studies indicated that rather than affecting EIP, a more important effect is that increasing DTR decreased the likelihood of midgut infection (Fig. 2). To investigate this effect over a wider range of conditions, we developed a second thermodynamic model describing the relationship between temperature and vector competence (Fig. S4). This model predicts infection and transmission probabilities using published empirical reports (references in SI Materials and Methods) of the highest proportion of infected and transmitting mosquitoes measured at various constant temperatures for a variety of mosquito–flavivirus systems. Output from this model indicates that DTR can have a marked effect on vector competence through changes in infection probability, with the sign of the effect dependent on mean temperature (Fig. 3A). Specifically, at the coolest mean temperatures, larger DTRs increase infection probability relative to baseline mean temperature. Conversely, at the warmest mean temperatures, larger DTRs reduce infection probability (Fig. 3A). Although we only considered a single mean temperature (26 °C) in our experiments, model predictions correspond well with our experimental data within the range we examined. At a mean of 26 °C, the percentage of infection is predicted to be 99, 88, and 76% under DTRs of 0 °C, 10 °C, and 20 °C, respectively. Given that infection probability appears to reach a maximum once mean temperatures exceed 26 °C (Fig. S4), these results indicate that exposure to lower temperatures during the evening, night, and early morning likely act to reduce virus infection under large DTRs in Thailand and in our experiments. This result applies whether using a simple sine function or a more realistic combination of sine and exponential functions to model daily temperature dynamics (Table S1).
Fig. 3.
Combined theoretical effects of mean temperature and DTR on Ae. aegypti vector competence for DENV. The probability (right-hand bar) that the virus (A) infects the mosquito and (B) is subsequently transmitted across a range of mean temperatures and diurnal temperature ranges according to vector competence models (Fig. S4) In both panels, temperature variation is described by combined sine and exponential functions (a sinusoidal progression during daytime and a decreasing exponential curve during the night).
Extending this approach to epidemiologically important transmission events, we speculate on the basis of data from a range of mosquito–flavivirus systems reported in the literature (references in SI Materials and Methods) that transmission probability (typically measured by analyzing mosquito salivary secretions for presence of virus or by exposing a susceptible animal host to mosquito bites) exhibits a stronger nonlinear response to temperature than infection probability (Fig. S4). Capturing this effect with an appropriate thermodynamic model indicates potentially greater effects of DTR on transmission probability than on infection probability (Fig. 3). In areas with a mean temperature of 18 °C, the model predicts that the effect of DTR on transmission is nonsignificant (Fig. 3B). In areas with means <18 °C, however, the effect of DTR is larger: as DTR increases so does the probability of transmission (Fig. 3B). For instance, whereas transmission probability is estimated to be 0.11 at a constant mean temperature of 14 °C, it shows a 2.7-fold increase with a DTR of 20 °C (Table S1). In this case, it appears that exposure to warmer temperatures for at least part of the day increases transmission probability relative to the cooler mean temperatures. In contrast, at mean temperatures >18 °C, DTR has the reverse effect; i.e., larger DTRs decrease transmission probability (Fig. 3B). Presumably, periodic exposure to cooler temperatures, and/or hot temperatures that exceed the optimum, leads to a lower transmission probability than expected under a constant temperature model. Thus, the predicted transmission probability of 0.95 at a constant temperature of 26 °C is more than halved under a DTR of 20 °C (Table S1). Although we did not measure actual transmission in our experiments, the output from our model suggests that the strongly nonlinear effect of temperature on transmission probability is an additional mechanism by which DTR can affect the seasonal dynamics of DENV transmission observed in Thailand.
Survival Analysis.
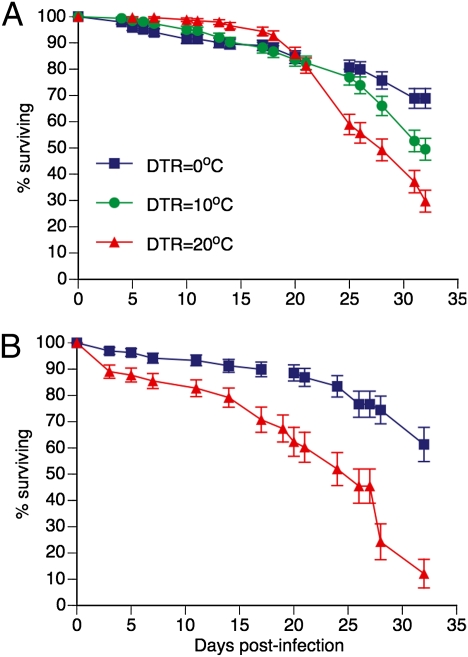
A total of 900 Ae. aegypti females were included in survival analysis in the first experiment with DENV-2, of which 244 died during the course of the experiment and 656 were censored; i.e., were included in survival analysis up to the point when they were removed for virus detection. Although mean survival times were similar across DTR regimes (27.6, 27.9, and 27.3 dpi under DTRs of 0 °C, 10 °C, and 20 °C, respectively), DTR had a statistically significant influence on overall survival rates (log-rank test: χ2 = 22.8, P < 0.0001). This effect appeared to be due to higher mortality rates of mosquitoes held under fluctuating temperatures at the end of the experiment, most markedly under the DTR of 20 °C from 24 dpi onward (Fig. 4A). Survival curves differed significantly both between DTRs of 0 °C and 10 °C (χ2 = 5.03, P = 0.0249) and between DTRs of 20 °C and 10 °C (χ2 = 7.33, P = 0.0068). Only ~30% of females under the DTR of 20 °C survived to the end of the experiment, compared with ~50 and ~70% under a DTR of 10 °C and constant temperature, respectively.
Fig. 4.
Effect of DTR on experimental survival of Ae. aegypti females after oral exposure to DENV. Kaplan–Meier analysis of survival rates following exposure to an infectious blood meal as a function of diurnal temperature regimes. In A, mosquitoes were exposed to a DENV-2 isolate. Survival curves are significantly different according to log-rank tests (overall: P < 0.0001; DTR = 0 °C vs. DTR = 10 °C: P = 0.0249; DTR = 10 °C vs. DTR = 20 °C: P = 0.0068). In B, mosquitoes were exposed to a DENV-1 isolate. Survival curves are significantly different according to a log-rank test (P < 0.0001). Vertical bars indicate SE.
Survival results of the first experiment were confirmed in the second experiment with DENV-1 (Fig. 4B). From a total of 317 Ae. aegypti females, 85 died during the course of the second experiment and 232 were censored. Mean survival times were 28.5 and 22.2 dpi under DTRs of 0 °C and 20 °C, respectively. DTR had a statistically significant influence on survival rates (log-rank test: χ2 = 31.4, P < 0.0001).
Discussion
Our results support the hypothesis that the potential for DENV transmission by Ae. aegypti is influenced by the amplitude and pattern of daily temperature variation. This is due to a negative effect of DTR on two important components of vectorial capacity, vector competence and vector survival (parameters b and p in Eq. 1, respectively). Vectorial capacity is influenced linearly by variations in vector competence and exponentially by differences in survival (34, 35). Although additional research is needed to determine whether our conclusions extend across all four DENV serotypes, our results were consistent in two independent experiments conducted in different laboratories and using different mosquitoes and different DENV serotypes. Thus, our findings did not depend on a particular set of experimental conditions. They help to explain seasonal variation in the intensity of DENV transmission in Thailand where mean temperature does not significantly differ throughout the year and there is no simple relationship between mosquito abundance and dengue incidence (30, 31). The low DENV transmission season occurs during months when the amplitude of DTR is large and mosquito infection and transmission potential are relatively low. Conversely, the high DENV transmission season corresponds to a time of the year when DTR amplitude is comparatively low and mosquito infection and transmission potential are elevated.
Amplitude of daily temperature fluctuations adds to the list of factors that influence Ae. aegypti vector competence for DENV, such as mean temperature (22), infectious virus dose (36), and the specific combination of vector and virus genotypes (37). The underlying mechanism for the negative impact of DTR on vector competence is unclear, but could be due to deleterious effects of low and/or high temperatures on key steps of the progression of virus infection in the mosquito (20, 38). For example, extreme temperatures experienced by mosquitoes under large DTRs may affect the efficacy of a midgut infection barrier to virus propagation, which accounts for a large portion of variation in vector competence in natural mosquito populations (39). Short periods of time spent at high or low temperatures under large DTRs could adversely impact the efficacy of midgut infection by limiting entry into midgut epithelial cells or initial replication in midgut cells (13). This proposition is supported by studies indicating temperature-dependent efficacy of barriers to virus propagation in Culex tarsalis mosquitoes infected with western equine encephalitis virus (20, 40).
We do not know whether the negative effect of DTR on mosquito survival that we detected in our experiments would have been observed if mosquitoes had not been exposed to virus. The potential for this effect merits additional investigation because decreased values of p act to reduce vectorial capacity in an exponential fashion through both the probability that a mosquito will become infectious (i.e., survive through n days of EIP, pn in Eq. 1) and the number days it will survive after EIP is completed [1/−ln(p) in Eq. 1]. Daily vector survival has long been recognized as one of the most influential parameters in transmission of vector-borne pathogens because small changes in p have relatively large effects on vectorial capacity and thus the basic reproductive rate (R0) of a virus (34, 35). Even slight reductions in mosquito survival due to increased DTR, as observed in our experiment, could lead to a noticeable drop in the extent of DENV transmission.
Our study design does not allow us to rule out nonmutually exclusive, alternative explanations. First, temperature profiles obtained in Mae Sot, Thailand were measured with indoor data loggers that may not have captured subtle temperature differences between microhabitats within human dwellings where female Ae. aegypti spend most of their time. Although we expect that it is unlikely that such differences would dramatically reduce the range of temperature fluctuations experienced by infected mosquitoes in the field, further investigation is warranted to determine the importance for DENV transmission of the actual temperatures to which wild female Ae. aegypti are exposed (12). Second, high DENV transmission in Thailand coincides with the rainy season and low transmission is associated with the dry season. In addition to average temperature and the pattern of temperature fluctuations, other climatic factors such as atmospheric pressure or humidity likely differ between dry and rainy seasons and may also contribute to variation in Ae. aegypti vectorial capacity. Additional research is again needed to determine the respective contribution of climatic factors other than temperature. Third, although we examined several components of vectorial capacity, we did not carry out behavioral experiments to determine the effect of temperature fluctuations on mosquito biting rate (parameter a in Eq. 1). Even though there is evidence that the frequency at which Ae. aegypti imbibes blood meals is positively associated with temperature (41), we know of no data indicating that DTR alone can affect blood-feeding frequency (but see ref. 9 for related data from Anopheles mosquitoes).
Our results reveal a potentially significant role, which has not previously been considered, for environmental variability in the dynamics of DENV transmission. Like several earlier studies (10, 12, 42), our research highlights the need for a better mechanistic understanding of the environmental determinants of vector–pathogen interactions. Although different in detail, our general conclusion about the importance of DTR on vectorial capacity for DENV is similar to conclusions from recent theoretical and empirical studies on malaria (8, 9). We show that, as for malaria, intensity of DENV transmission can be influenced by the specific combination of mean and range in temperature fluctuations. Inclusion of the range of short-term, daily temperature variation in analyses of vector–pathogen interactions will provide insights into environmental factors that influence pathogen transmission dynamics and heterogeneity in the risk of mosquito-borne disease.
Materials and Methods
Mosquitoes.
The first experiment was carried out at the Wadsworth Center Arbovirus Laboratory, Slingerlands, NY, using Ae. aegypti nine generations after their collection from Nakhon Ratchasima Province, Thailand during May 2006. The second experiment was carried out at the Institut Pasteur in Paris, France, using Ae. aegypti two generations after their collection from Kamphaeng Phet Province, Thailand during December 2009. In both experiments larvae were reared under constant, standard conditions. Three days before the infectious feed, adults were separated into incubators programmed to follow different temperature regimes with the same average temperature of 26 °C. In fluctuating temperature treatments, temperature followed 24-h sinusoidal cycles.
Experimental Infections.
In the first experiment, mosquitoes were orally challenged with a low-passage DENV-2 strain isolated from a child in 1999 in Kamphaeng Phet, Thailand. In the second experiment, mosquitoes were orally challenged with a low-passage DENV-1 strain isolated in 2009 from a patient in Kamphaeng Phet, Thailand. Virus was grown in Aedes albopictus (C6/36) cells to prepare an artificial infectious blood meal to which 5- to 8-d-old adult females were exposed through a membrane feeding system. After feeding, fully engorged females were transferred into four to five replicate cartons per treatment and returned to their respective incubators. In both experiments, mortality was monitored in each carton by removing and counting dead mosquitoes every 1–3 d.
Vector Competence.
Viral infection and dissemination were monitored for 32 d after the infectious blood meal by detection of infectious DENV in mosquito bodies and legs, respectively. The number of mosquitoes randomly sampled at each time point was 25 per treatment in the first experiment and 3–28 (mean 16) in the second experiment. The proportion of infected bodies and legs was determined by plaque assay on African green monkey kidney (Vero) cells in the first experiment and by immunofluorescent assay on C6/36 cells in the second experiment. The presence of infectious virus in each sample was determined qualitatively; i.e., either positive or negative.
Data Analysis.
The proportion of mosquitoes with infected bodies (midgut infection) and legs (disseminated infection) was analyzed with a nominal logistic regression as a function of EIP, DTR, and their interaction. The experiment was included as a covariate in the analysis. Because the number of temperature regimes was not the same in both experiments, DTR was nested within the experiment. EIP was considered an ordinal variable. The minimal model was obtained by stepwise removal of strongly nonsignificant effects. Survival distributions were analyzed using the Kaplan–Meier method for univariate survival with censored data; i.e., mosquitoes collected for virus detection were included in the analysis. Homogeneity of survival functions between temperature treatments was tested with a log-rank test.
Temperature and EIP Models.
The diurnal fluctuation in air temperature (Ta) was approximated using two commonly applied models (Fig. S3). The first model was a simple sine function fitted between daily maximum and minimum temperatures, as was used in the vector competence assays. The second model was a sinusoidal progression during daytime and a decreasing exponential curve during the night (12:12 h day:night cycle), which better captures the slight asymmetries that can occur during the daily heating and cooling phases that are not captured in a simple sine model. Under both scenarios, Ta was calculated at 30-min intervals for a wide range of mean temperature and DTR combinations. We used an established model based on enzyme kinetics (Fig. S1) to estimate DENV growth rate at 30-min intervals across the diurnal cycle according to the two temperature models described above. EIP completion was defined when cumulative growth rate reached one.
Vector Competence Models.
EIP describes the time required for pathogen incubation in the vector and is usually considered separately from vector competence, which describes intrinsic susceptibility and does not have a temporal dimension. Because the effect of temperature on DENV infection and transmission probabilities is not well documented, data from other mosquito–flavivirus systems were used to model the relationship between vector competence and temperature. We modeled both infection and transmission probabilities using empirical reports of the highest proportion of infected and transmitting vectors measured at various constant temperatures (Fig. S4). Probabilities of DENV infection and transmission were estimated by averaging probabilities calculated at 30-min intervals across the diurnal cycle according to the two temperature models described above, over a 24-h period.
Full methods and associated references are available in the SI Materials and Methods.
Supplementary Material
Acknowledgments
The authors are grateful to Laura C. Harrington and Alongkot Ponlawat for providing mosquito eggs and field temperature data from Thailand. We thank Richard G. Jarman and Alan L. Rothman for providing the DENV isolates, Pam Chin for expert technical support, and Linda M. Styer and Caroline Lego for their help during vector competence assays in the first experiment. Anna-Bella Failloux, Marie Vazeille, Laurence Mousson, Estelle Martin, and Camilo Arias-Goeta provided support during the second experiment. Christopher M. Barker and Romain Gallet helped with fruitful discussions. Two anonymous reviewers provided helpful comments on a previous version of the manuscript. L.L. was supported by Marie Curie Fellowship MOIF-CT-2006-039855 from the European Commission and French Agence Nationale de la Recherche Grant ANR-09-RPDOC-007-01. T.W.S. received support from the Research and Policy for Infectious Disease Dynamics program of the Science and Technology Directorate, Department of Homeland Security, and Fogarty International Center, National Institutes of Health (NIH), Bethesda, MD. This study was supported by National Science Foundation Ecology of Infectious Disease Program Grant EF-0914384 and National Institutes of Health Grant R01 GM-083224.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1101377108/-/DCSupplemental.
References
- 1.Rogers DJ, Randolph SE. Climate change and vector-borne diseases. Adv Parasitol. 2006;62:345–381. doi: 10.1016/S0065-308X(05)62010-6. [DOI] [PubMed] [Google Scholar]
- 2.Lafferty KD. The ecology of climate change and infectious diseases. Ecology. 2009;90:888–900. doi: 10.1890/08-0079.1. [DOI] [PubMed] [Google Scholar]
- 3.McMichael AJ, Woodruff RE, Hales S. Climate change and human health: Present and future risks. Lancet. 2006;367:859–869. doi: 10.1016/S0140-6736(06)68079-3. [DOI] [PubMed] [Google Scholar]
- 4.Sutherst RW. Global change and human vulnerability to vector-borne diseases. Clin Microbiol Rev. 2004;17:136–173. doi: 10.1128/CMR.17.1.136-173.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson K. Global warming and the spread of disease: The debate heats up. Trends Ecol Evol. 2000;15:488. doi: 10.1016/s0169-5347(00)02011-5. [DOI] [PubMed] [Google Scholar]
- 6.Johansson MA, Dominici F, Glass GE. Local and global effects of climate on dengue transmission in Puerto Rico. PLoS Negl Trop Dis. 2009;3:e382. doi: 10.1371/journal.pntd.0000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gething PW, et al. Climate change and the global malaria recession. Nature. 2010;465:342–345. doi: 10.1038/nature09098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paaijmans KP, Read AF, Thomas MB. Understanding the link between malaria risk and climate. Proc Natl Acad Sci USA. 2009;106:13844–13849. doi: 10.1073/pnas.0903423106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paaijmans KP, et al. Influence of climate on malaria transmission depends on daily temperature variation. Proc Natl Acad Sci USA. 2010;107:15135–15139. doi: 10.1073/pnas.1006422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Randolph SE, Rogers DJ. Fragile transmission cycles of tick-borne encephalitis virus may be disrupted by predicted climate change. Proc Biol Sci. 2000;267:1741–1744. doi: 10.1098/rspb.2000.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Randolph SE. Perspectives on climate change impacts on infectious diseases. Ecology. 2009;90:927–931. doi: 10.1890/08-0506.1. [DOI] [PubMed] [Google Scholar]
- 12.Thomas MB, Blanford S. Thermal biology in insect-parasite interactions. Trends Ecol Evol. 2003;18:344–350. [Google Scholar]
- 13.Kramer LD, Ebel GD. Dynamics of flavivirus infection in mosquitoes. Adv Virus Res. 2003;60:187–232. doi: 10.1016/s0065-3527(03)60006-0. [DOI] [PubMed] [Google Scholar]
- 14.Blanford S, Read AF, Thomas MB. Thermal behaviour of Anopheles stephensi in response to infection with malaria and fungal entomopathogens. Malar J. 2009;8:72. doi: 10.1186/1475-2875-8-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delatte H, Gimonneau G, Triboire A, Fontenille D. Influence of temperature on immature development, survival, longevity, fecundity, and gonotrophic cycles of Aedes albopictus, vector of chikungunya and dengue in the Indian Ocean. J Med Entomol. 2009;46:33–41. doi: 10.1603/033.046.0105. [DOI] [PubMed] [Google Scholar]
- 16.Tun-Lin W, Burkot TR, Kay BH. Effects of temperature and larval diet on development rates and survival of the dengue vector Aedes aegypti in north Queensland, Australia. Med Vet Entomol. 2000;14:31–37. doi: 10.1046/j.1365-2915.2000.00207.x. [DOI] [PubMed] [Google Scholar]
- 17.Bates M, Roca-Garcia M. The development of the virus of yellow fever in haemagogus mosquitoes. Am J Trop Med Hyg. 1946;26:585–605. doi: 10.4269/ajtmh.1946.s1-26.585. [DOI] [PubMed] [Google Scholar]
- 18.Chamberlain RW, Sudia WD. The effects of temperature upon the extrinsic incubation of eastern equine encephalitis in mosquitoes. Am J Hyg. 1955;62:295–305. doi: 10.1093/oxfordjournals.aje.a119780. [DOI] [PubMed] [Google Scholar]
- 19.Kilpatrick AM, Meola MA, Moudy RM, Kramer LD. Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. PLoS Pathog. 2008;4:e1000092. doi: 10.1371/journal.ppat.1000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kramer LD, Hardy JL, Presser SB. Effect of temperature of extrinsic incubation on the vector competence of Culex tarsalis for western equine encephalomyelitis virus. Am J Trop Med Hyg. 1983;32:1130–1139. doi: 10.4269/ajtmh.1983.32.1130. [DOI] [PubMed] [Google Scholar]
- 21.Reisen WK, Fang Y, Martinez VM. Effects of temperature on the transmission of west nile virus by Culex tarsalis (Diptera: Culicidae) J Med Entomol. 2006;43:309–317. doi: 10.1603/0022-2585(2006)043[0309:EOTOTT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 22.Watts DM, Burke DS, Harrison BA, Whitmire RE, Nisalak A. Effect of temperature on the vector efficiency of Aedes aegypti for dengue 2 virus. Am J Trop Med Hyg. 1987;36:143–152. doi: 10.4269/ajtmh.1987.36.143. [DOI] [PubMed] [Google Scholar]
- 23.Turell MJ, Lundström JO. Effect of environmental temperature on the vector competence of Aedes aegypti and Ae. taeniorhynchus for Ockelbo virus. Am J Trop Med Hyg. 1990;43:543–550. doi: 10.4269/ajtmh.1990.43.543. [DOI] [PubMed] [Google Scholar]
- 24.Endy TP, et al. Spatial and temporal circulation of dengue virus serotypes: A prospective study of primary school children in Kamphaeng Phet, Thailand. Am J Epidemiol. 2002;156:52–59. doi: 10.1093/aje/kwf006. [DOI] [PubMed] [Google Scholar]
- 25.Nisalak A, et al. Serotype-specific dengue virus circulation and dengue disease in Bangkok, Thailand from 1973 to 1999. Am J Trop Med Hyg. 2003;68:191–202. [PubMed] [Google Scholar]
- 26.Anderson KB, et al. Burden of symptomatic dengue infection in children at primary school in Thailand: A prospective study. Lancet. 2007;369:1452–1459. doi: 10.1016/S0140-6736(07)60671-0. [DOI] [PubMed] [Google Scholar]
- 27.Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11:480–496. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koenraadt CJ, et al. Spatial and temporal patterns in pupal and adult production of the dengue vector Aedes aegypti in Kamphaeng Phet, Thailand. Am J Trop Med Hyg. 2008;79:230–238. [PubMed] [Google Scholar]
- 29.Strickman D, Kittayapong P. Dengue and its vectors in Thailand: Calculated transmission risk from total pupal counts of Aedes aegypti and association of wing-length measurements with aspects of the larval habitat. Am J Trop Med Hyg. 2003;68:209–217. [PubMed] [Google Scholar]
- 30.Scott TW, et al. Longitudinal studies of Aedes aegypti (Diptera: Culicidae) in Thailand and Puerto Rico: Population dynamics. J Med Entomol. 2000;37:77–88. doi: 10.1603/0022-2585-37.1.77. [DOI] [PubMed] [Google Scholar]
- 31.Sheppard PM, Macdonald WW, Tonn RJ, Grab B. The dynamics of an adult population of Aedes aegypti in relation to dengue haemorrhagic fever in Bangkok. J Anim Ecol. 1969;38:661–702. [Google Scholar]
- 32.Focks DA, Daniels E, Haile DG, Keesling JE. A simulation model of the epidemiology of urban dengue fever: Literature analysis, model development, preliminary validation, and samples of simulation results. Am J Trop Med Hyg. 1995;53:489–506. doi: 10.4269/ajtmh.1995.53.489. [DOI] [PubMed] [Google Scholar]
- 33.Parton WJ, Logan JA. A model for diurnal variation in soil and air temperature. Agric Meteorol. 1981;23:205–216. [Google Scholar]
- 34.Garrett-Jones C. Prognosis for interruption of malaria transmission through assessment of the mosquito's vectorial capacity. Nature. 1964;204:1173–1175. doi: 10.1038/2041173a0. [DOI] [PubMed] [Google Scholar]
- 35.Macdonald G. The Epidemiology and Control of Malaria. London: Oxford Univ Press; 1957. [Google Scholar]
- 36.Diallo M, et al. Vector competence of Aedes aegypti populations from Senegal for sylvatic and epidemic dengue 2 virus isolated in West Africa. Trans R Soc Trop Med Hyg. 2008;102:493–498. doi: 10.1016/j.trstmh.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 37.Lambrechts L, et al. Genetic specificity and potential for local adaptation between dengue viruses and mosquito vectors. BMC Evol Biol. 2009;9:160. doi: 10.1186/1471-2148-9-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hurlbut HS. The effect of environmental temperature upon the transmission of St. Louis encephalitis virus by Culex pipiens quinquefasciatus. J Med Entomol. 1973;10:1–12. doi: 10.1093/jmedent/10.1.1. [DOI] [PubMed] [Google Scholar]
- 39.Black WC, IV, et al. Flavivirus susceptibility in Aedes aegypti. Arch Med Res. 2002;33:379–388. doi: 10.1016/s0188-4409(02)00373-9. [DOI] [PubMed] [Google Scholar]
- 40.Reisen WK, Meyer RP, Presser SB, Hardy JL. Effect of temperature on the transmission of western equine encephalomyelitis and St. Louis encephalitis viruses by Culex tarsalis (Diptera: Culicidae) J Med Entomol. 1993;30:151–160. doi: 10.1093/jmedent/30.1.151. [DOI] [PubMed] [Google Scholar]
- 41.Scott TW, et al. Longitudinal studies of Aedes aegypti (Diptera: Culicidae) in Thailand and Puerto Rico: Blood feeding frequency. J Med Entomol. 2000;37:89–101. doi: 10.1603/0022-2585-37.1.89. [DOI] [PubMed] [Google Scholar]
- 42.Lambrechts L, Chavatte JM, Snounou G, Koella JC. Environmental influence on the genetic basis of mosquito resistance to malaria parasites. Proc Biol Sci. 2006;273:1501–1506. doi: 10.1098/rspb.2006.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.