Abstract
Cobalamin (B12) typically functions as an enzyme cofactor but can also regulate gene expression via RNA-based riboswitches. B12-directed gene regulatory mechanisms via protein factors have, however, remained elusive. Recently, we reported down-regulation of a light-inducible promoter in the bacterium Myxococcus xanthus by two paralogous transcriptional repressors, of which one, CarH, but not the other, CarA, absolutely requires B12 for activity even though both have a canonical B12-binding motif. Unanswered were what underlies this striking difference, what is the specific cobalamin used, and how it acts. Here, we show that coenzyme B12 (5′-deoxyadenosylcobalamin, AdoB12), specifically dictates CarH function in the dark and on exposure to light. In the dark, AdoB12-binding to the autonomous domain containing the B12-binding motif foments repressor oligomerization, enhances operator binding, and blocks transcription. Light, at various wavelengths at which AdoB12 absorbs, dismantles active repressor oligomers by photolysing the bound AdoB12 and weakens repressor–operator binding to allow transcription. By contrast, AdoB12 alters neither CarA oligomerization nor operator binding, thus accounting for its B12-independent activity. Our findings unveil a functional facet of AdoB12 whereby it serves as the chromophore of a unique photoreceptor protein class acting in light-dependent gene regulation. The prevalence of similar proteins of unknown function in microbial genomes suggests that this distinct B12-based molecular mechanism for photoregulation may be widespread in bacteria.
Keywords: carotenogenesis, Thermus thermophilus, antirepressor, MerR, TtCarH
B12 is an essential vitamin in humans and other animals, and the only vitamin synthesized exclusively by microorganisms (1–5). It is also the largest and most complex of nonpolymeric biomolecules, whose central feature is a tetrapyrrole-derived cobalt-containing corrin moiety. Two distinct B12 forms are used in vivo (4, 6): one (AdoB12) has 5′-deoxyadenosine and the other (methylcobalamin, MeB12) has a methyl coordinated to the corrin cobalt. The stable but nonfunctional vitamin B12 or cyanocobalamin (CNB12), with a cyano–cobalt bond, must be converted to AdoB12 or MeB12 for intracellular use. Typically, B12 functions as an essential cofactor of enzymes such as isomerases, methyltransferases, and reductases (6, 7). B12 has also been implicated in directly regulating gene expression via the B12 riboswitch, an RNA-based mechanism widespread in bacteria (8–11). Here, specific binding of AdoB12 to the 5′-untranslated messenger RNA leader sequence of a target gene or operon (usually involved in B12 synthesis or transport) allosterically modulates RNA structure to regulate gene expression. In contrast to this RNA-based switch, B12-directed gene regulation via a protein factor is virtually uncharted. We recently reported that B12 is absolutely required by a transcriptional repressor to down-regulate a light-inducible promoter in the bacterium Myxococcus xanthus (12). However, the molecular mechanism for this B12-dependent gene regulation has remained enigmatic.
Blue light is known to trigger a well studied regulatory cascade in M. xanthus that culminates in the synthesis of carotenoids, which protect cells against photooxidative damage (13). Cells usually sense light, a key environmental factor, via photoreceptor proteins containing small-molecule chromophores, and six such photoreceptor families have been cataloged in bacteria (14–16). However, none of these have been implicated in M. xanthus light-induced carotenogenesis, in which available data suggest that light is sensed by a photosensitizer molecule, protoporphyrin IX, rather than by a dedicated photoreceptor protein (13, 17). This essentially leads to the release of the extracytoplasmic function σ-factor CarQ from its membrane-bound anti–σ-factor CarR. Freed CarQ then associates with core RNA polymerase and, together with a number of other accessory factors, drives the expression of one carotenogenic gene, crtIb, as well as of the regulatory carQRS operon, which encodes CarS besides CarQ and CarR (Fig. S1A) (13). CarS is an antirepressor with an SH3-domain topology that mimics operator DNA to specifically target the DNA recognition helix of the MerR-type DNA-binding winged-helix domain of two paralogous repressors, CarA and CarH, to counteract their binding to operator (18, 19). In the dark, these two repressors down-regulate the promoter PB that drives expression of the carB–carA gene cluster, where the remaining carotenogenic genes are grouped (Fig. S1A). CarA and CarH have a similar two-domain architecture (Fig. 1A and Fig. S1B). Besides their autonomously stable, N-terminal DNA-binding domains (CarANt and CarHNt, respectively) that recognize the same operator (12, 19, 20), CarA and CarH have C-terminal domains (CarACt and CatHCt, respectively) with a B12-binding motif of the type found in enzymes like methionine synthase (6, 12, 21–23). However, only CarH requires B12 for its repressor activity (12).
Fig. 1.
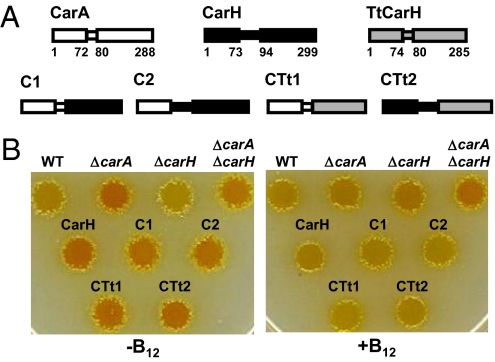
A single module confers AdoB12-dependent activity in vivo. (A) Schematic representations of CarA, CarH, TtCarH, and the chimeras derived from them. Wider rectangles represent N- and C-terminal domains, the narrower one the linker between them, with regions in white, black, and gray corresponding to CarA, CarH, and TtCarH, respectively. Numbers correspond to residues delimiting the indicated segment. (B) Color phenotype in the presence or absence of 1 μM CNB12 (“B12”) in the dark of M. xanthus WT strain and those with the genotype indicated above the cell spots. C1, C2, CTt1, CTt2, or CarH (as positive control) are expressed from a heterologous site in the ΔcarA ΔcarH strain.
Why CarH, and not CarA, depends on B12, what is the B12 form involved, and how it is linked to a light response are studied here. We show that it is AdoB12 that specifically directs CarH activity. Our studies in vivo and in vitro uncover an ability of AdoB12 to induce repressor oligomerization and markedly enhance operator DNA binding to bring about gene repression in the dark. Furthermore, the photosensitivity of AdoB12 (24–26) is exploited in light-induced disassembly of active repressor oligomers to diminish operator binding and relieve PB repression in vivo, thus linking B12 to the light response. AdoB12 did not produce similar effects with CarA, thereby providing a rationale for its B12-independent action. The AdoB12-based mechanism serves as a direct route to PB photoregulation by CarH, distinct from the one involving CarS described previously. More importantly, our findings reveal a functional role of AdoB12: that as a chromophore of broad spectral sensitivity in a unique type of photoreceptor protein. Given the numerous CarA/CarH-like proteins of unknown functions being identified in other bacterial species from genome data (Table S1), it is likely that the AdoB12-based photoregulation mechanism we describe here is widely used in bacteria.
Results
A Single Autonomous Module Confers B12-Dependent Activity in Vivo.
To address why CarH but not CarA requires B12 for activity, we first asked if replacing CarACt by CarHCt can confer B12-dependent repressor activity. For this, we constructed two chimeric proteins, C1 and C2, in which CarANt is linked to CarHCt by the shorter CarA “linker” (C1) or the longer CarH one (C2; Fig. 1A and Fig. S1B). C1 and C2 were then introduced into a strain lacking both CarA and CarH, so that neither of the endogenous proteins can mask the function of the introduced chimera, which was directly assessed by the colony color phenotype.
WT M. xanthus cells are yellow in the dark and turn red in the light from the carotenoids produced by expressing all of the structural genes (Car+ phenotype; Fig. 1B and Fig. S1 A and C). In the dark, cells lacking CarH exhibit the Car+ phenotype (yellow) because CarA represses PB. By contrast, those lacking CarA are yellow when B12 (CNB12, AdoB12, or MeB12) is present in the growth medium and CarH can repress PB, but are orange when B12 is absent, as carB, though not crtIb, is expressed [Car(+) phenotype] (12). Cells lacking CarA and CarH are always orange in the dark (CarC) because carB is not repressed (Fig. 1B). In the light, all three mutant strains become red, like the WT, as both carB and crtIb are turned on (Fig. S1C). A plasmid construct with the gene for C1 or C2 (or CarH, as a control) expressed from a constitutive promoter (12, 20) was introduced into the M. xanthus strain lacking endogenous CarA and CarH, where it integrates at an unlinked site in the chromosome. Unlike the CarC recipient strain, the resulting transformants were Car(+): yellow in the dark only in the presence of B12 and red in the light, just like the control strain with CarH (Fig. 1B and Fig. S1C). Thus, C1 and C2 behave like CarH, indicating that swapping CarACt for CarHCt (regardless of the linker) confers B12-dependent repressor activity.
Although native CarA, CarANt, and CarACt have been purified, as has CarHNt, the impossibility of obtaining active CarH or CarHCt has thwarted in vitro studies aimed at unraveling its B12 dependence. As noted earlier, CarA/CarH homologues of unknown functions are present in many bacterial species. That one such homologue exists in Thermus thermophilus (whose function is unknown) is particularly advantageous as thermophilic proteins are usually soluble, easily purified, and amenable to biochemical/structural analysis. Also, M. xanthus and T. thermophilus genomes have similar GC contents (69–70%). Hence, we constructed two more chimeras, CTt1 and CTt2, in which the C-terminal domain of the T. thermophilus homologue was fused to CarANt and CarHNt, respectively, via their corresponding linkers (Fig. 1A). As with C1 and C2 earlier, CTt1 or CTt2 were introduced into the M. xanthus strain lacking endogenous CarA and CarH, and again the resulting transformants were yellow in the dark only when B12 was present and became red in the light (Fig. 1B and Fig. S1C). Hence, CTt1 and CTt2 also function like CarH. This indicates that, as with CarHCt, the C-terminal domain of the T. thermophilus homolog (henceforth TtCarH) can confer B12-dependent activity.
AdoB12 Specifically Enhances Operator DNA Binding in Vitro.
We could purify native CTt1 and CTt2 and thus examine their binding to the CarA/CarH operator in vitro and their dependence on a specific B12 form. CarA binds cooperatively to its operator, first to a high-affinity, palindromic site upstream of PB and then to a low-affinity site straddling the −35 region of PB to block access to RNA polymerase (Fig. S2A) (27). This is observed in EMSA by the progressive appearance of a lower band followed by an upper band at higher CarA concentrations (Fig. S2B). Although CTt1 has the same DNA-binding domain as CarA, it appeared to bind operator less tightly: even at concentrations 15-fold higher than CarA, two diffuse bands were formed rather than the single, well defined low-mobility complex seen with CarA (Fig. 2A). This difference must therefore stem from the distinct C-terminal domains in CTt1 and CarA, and probably results from CarA, but not CTt1, binding cooperatively to DNA. Operator affinity for CTt2 was even lower, with hardly perceptible binding even at high protein concentrations (Fig. 2A). That CTt2 binds less tightly than CTt1 is likely because CarANt has a greater affinity for operator DNA than CarHNt (12).
Fig. 2.
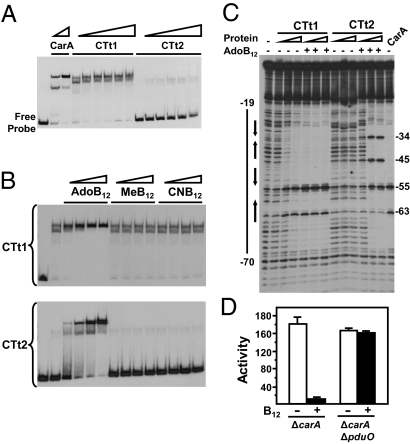
AdoB12 enhances DNA binding in vitro and is required for CarH-dependent PB repression in vivo. (A) EMSA with CarA, CTt1, or CTt2 and a 130-bp probe containing the CarA/CarH operator (CCR130). Protein concentrations increase from left to right in order: 30, 60, 110, 220, 450, and 900 nM for CTt1 and CTt2, and 20 and 40 nM for CarA. (B) EMSA with CCR130 and 30 nM of CTt1 (Top) or CTt2 (Bottom) in the presence of increasing concentrations (16, 31, 63, and 125 nM from left to right) of AdoB12, MeB12, or CNB12, as indicated. (C) DNase I footprinting with CCR130 and CTt1 or CTt2 in the presence or absence of AdoB12. Protein concentrations for CTt1 and CTt2 increase from left to right in order: 60, 110, and 220; for CarA, 60 nM was used. AdoB12 was added at a fivefold molar excess relative to protein. The solid line on the left (labeled −19 to −70) is the segment protected against DNase I by CarA (rightmost lane), which is also observed with CTt1 or CTt2 when AdoB12 is present. pI and pII, the two operator subsites, are each shown by a pair of arrows facing one another, and the hypersensitive sites are numbered on the right. (D) Reporter PB-lacZ expression levels in the absence and in the presence of 1 μM CNB12 for the ΔcarA and ΔcarA ΔpduO strains. The mean of three independent measurements and the SEM for the specific β-gal activities (“Activity”) are shown.
Both CTt1 and CTt2 required B12 to function in vivo. Hence, we probed if any of the different B12 forms affected DNA binding in vitro. Given the light sensitivity of cobalamins (24), special care was taken to carry out these experiments in the dark. Neither MeB12 nor CNB12 affected operator binding by CTt1 or CTt2 (Fig. 2B). Also, no effect was seen with hemin (Fig. S2C), which has been reported to bind to some proteins involved in redox-light sensing that have a B12-binding motif (28–30). By contrast, AdoB12 enhanced DNA binding by CTt1 and, even more dramatically, by CTt2: a single, well defined, low-mobility retarded complex was now formed, with little or no free probe detected (Fig. 2B). Consistent with its action being B12-independent in vivo, CarA DNA-binding in vitro was unaffected by AdoB12, MeB12, or CNB12 (Fig. S2D). In DNase I footprinting, CarA protects a stretch extending from the −19 to −70 positions relative to the transcription start site, with hypersensitivities at −55 and −63 (27). AdoB12 enabled CTt1 and, more markedly, CTt2 to protect against DNase I the same operator stretch mapped for CarA (Fig. 2C). DNase I hypersensitive sites indicate DNA distortion on repressor binding. The two additional hypersensitive sites (−34 and −45) seen for CTt2 possibly reflect variations in DNA binding between CarHNt and CarANt. Altogether, these data demonstrate that AdoB12 specifically enhances binding by CTt1 or CTt2 to the CarA/CarH operator in vitro.
AdoB12 Is Essential for PB Repression by CarH in Vivo.
Eliminating AdoB12 should abolish CarH-mediated PB repression in vivo if, as suggested by the in vitro analysis described here, AdoB12 is required to enhance operator binding. AdoB12 biosynthesis or its generation in vivo from inactive forms like CNB12 requires ATP:corrinoid adenosyltransferase (ATR) activity (4, 31). Hence, knocking out ATR would eliminate intracellular AdoB12. Three ATR classes (CobA, PduO, and EutT) are implicated in converting inactive cobalamins to AdoB12 (31–33). The M. xanthus genome lacks genes for de novo AdoB12 biosynthesis and has one ATR gene, whose product is similar to the PduO-type human ATR (Fig. S3). We therefore deleted this pduO gene in the ΔcarA M. xanthus strain (to avoid CarA masking CarH activity). Despite endogenous CarH being available, reporter PB-lacZ expression in a strain lacking both pduO and carA remained high with or without B12, unlike in the ΔcarA control (Fig. 2D). Thus, CarH cannot repress PB expression if AdoB12 is not generated in vivo.
CarH and TtCarH, but Not CarA, Require AdoB12 for Oligomerization.
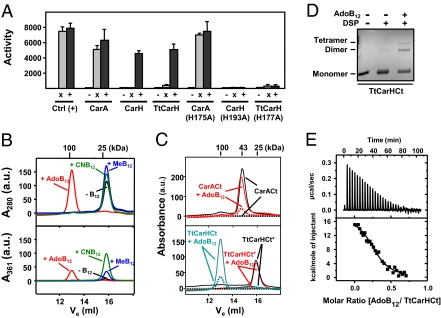
The results described here demonstrate that AdoB12 enhances operator binding in vitro and determines PB repression by CarH in vivo. However, how exactly does AdoB12 modulate repressor function? CarA oligomerization via CarACt is crucial for efficient DNA binding in vitro and repression of PB in vivo (20). We therefore examined if AdoB12 acts at the level of repressor oligomerization by using different protein–protein interaction assays. To study such interactions in vivo, we chose an Escherichia coli–based two-hybrid system (34), as this organism is incapable of de novo B12 synthesis but can generate AdoB12 from exogenously supplied B12 (2, 5). Two-hybrid analysis revealed that CarHCt or the TtCarH C-terminal domain (TtCarHCt) self-interact only in the presence of B12, in clear contrast to CarACt that self-interacts whether or not B12 is present (Fig. 3A and Fig. S4A). Moreover, mutating to A the conserved H of the B12-binding motif (Fig. S1B) in CarHCt (H193) or TtCarHCt (H177) eliminated their B12-dependent oligomerization, but an equivalent change in CarA (H175A) had no effect (Fig. 3A and Fig. S4A). This is consistent with our previous observation that the CarH H193A mutation abolished B12-dependent repression of PB in vivo, whereas the H175A mutation in CarA had no effect on its function (12). Thus, CarH and TtCarH, although not CarA, require B12 and an intact B12-binding motif to oligomerize via their C-terminal domains.
Fig. 3.
AdoB12 binding drives CarHCt and TtCarHCt oligomerization. (A) Two-hybrid analysis in E. coli for self-interactions in the dark of the CarA, CarH, or TtCarH C-terminal domain and their variants with the conserved H of the B12-binding motif mutated to A. Interactions correlate with β-gal specific activities (“Activity”; mean and SEM from three independent measurements are shown) from reporter lacZ expression. Ctrl (+), positive control using the GCN4 leucine zipper; −, negative control expressing only one fusion protein. Cells expressing both fusion proteins were grown in the absence (x; gray bars) or presence (+; black bars) of 1 μM CNB12. (B) Elution profiles off a Superdex200 analytical gel filtration column tracked by the absorbance (in arbitrary units) at 280 nm (Top) or 361 nm (Bottom) for 45 μM TtCarHCt alone (black lines) or with fivefold excess of AdoB12 (red lines), CNB12 (green lines), or MeB12 (blue lines). Mr (in kDa) for each peak maximum is marked at the top. (C) Elution profile in the presence (red lines) or absence (black lines) of fivefold excess AdoB12 tracked by the absorbance (in arbitrary units) at 280 nm (solid lines) or at 361 nm (dotted lines) for CarACt (Top) and the TtCarHCt H177A mutant, TtCarHCt* (Bottom). The latter includes, for comparison, the elution profile for TtCarHCt in the presence of AdoB12 tracked at 280 and 361 nm (solid and dotted lines in cyan, respectively). (D) Dithiobis(succinimidylpropionate) cross-linking of TtCarHCt in the presence and absence of AdoB12. (E) ITC of 18 μM TtCarHCt titrated with 78 μM AdoB12 at 25 °C. Top: Heat change with each injection. Bottom: Corresponding integrated heat normalized and corrected for the heat of dilution versus molar ratio, the line being the best fit of the data to a single-site binding model. Parameters from four independent experiments are indicated in the text.
Concordant results were obtained in size-exclusion chromatography (SEC) performed in the dark. TtCarHCt alone, with CNB12, or MeB12, eluted with an apparent molecular weight (Mr) of 24.4 ± 0.7 kDa, close to the measurement of 22.6 kDa calculated for a monomer (Fig. 3B). A coincident peak at 361 nm, where only B12 absorbs (35), when CNB12 or MeB12 was present indicated that these can bind TtCarHCt (Fig. 3B, Bottom). Remarkably, with AdoB12, a tetrameric species was observed (Mr = 99.2 ± 2 kDa). The absorption spectrum of this peak fraction was similar to that of free AdoB12 (Fig. S4B, Top), and estimates of its protein and AdoB12 contents (SI Materials and Methods) indicated a 1:1 stoichiometry. Similarly, CTt2 alone, with CNB12, or MeB12, was a monomer but formed an AdoB12-bound tetramer (Fig. S4C). By comparison, CarACt with or without AdoB12 was a stable dimer (Mr = 41.3 ± 1 kDa; calculated monomer value = 22.9 kDa; Fig. 3C, Top), and the TtCarHCt H177A mutant was a monomer with no detectable B12 binding (TtCarHCt*; Fig. 3C, Bottom). The photosensitivity of the complex (as described later) precluded use of light-based methods such as analytical ultracentrifugation or dynamic light scattering to independently check AdoB12-driven tetramer formation. Nonetheless, and in agreement with SEC, chemical cross-linking experiments revealed a band that would correspond to a TtCarHCt tetramer only when AdoB12 was present (Fig. 3D). AdoB12 also markedly increased the intensity of the band for the TtCarHCt dimer, presumably because it is more probable to simultaneously cross-link two subunits than four. Isothermal titration calorimetry (ITC) detects heat changes associated with binding and provides values for the thermodynamic parameters describing this binding. We detected no heat change on titrating TtCarHCt with CNB12 or MeB12 at 25 °C. By contrast, for the same conditions, with AdoB12 there was net heat uptake. These ITC data, when fit to a single-site binding model, yielded a binding enthalpy (ΔH) of 15.2 ± 0.9 kcal/mol, entropy (TΔS) of 23.5 ± 1.4 kcal/mol, and equilibrium Kd of 832 ± 240 nM, but underestimated the stoichiometry (N) as 0.34 ± 0.10 (Fig. 3E), possibly because the single-site binding model does not consider cooperativity effects from AdoB12-induced tetramerization. A more realistic treatment of these complexities must await future work, but ITC does reveal tight, entropically favored binding of AdoB12 to TtCarHCt. In sum, specific binding of AdoB12 to the C-terminal domain of CarH or TtCarH, but not of CarA, drives their higher-order assembly, thus providing a molecular rationale for why only the first two depend on B12 for function.
Light Impairs AdoB12-Driven Oligomerization and DNA Binding.
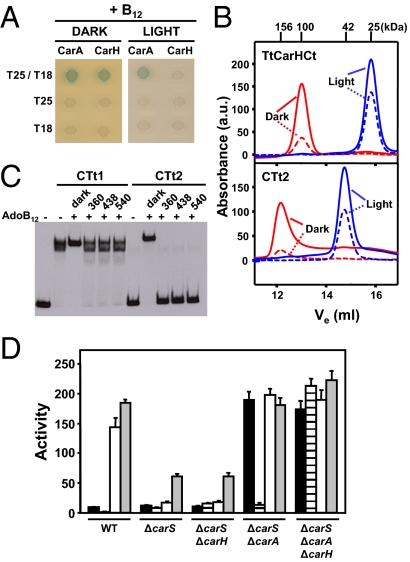
Light-induced homolytic cleavage of the AdoB12 Co-C bond is very rapid (24–26). Hence, extreme care was taken to avoid light in the aforementioned experiments. However, given the implication of AdoB12 in a cellular response to light, its photosensitivity could have direct physiological relevance. This prompted us to check how light affects AdoB12-induced oligomerization, DNA binding in vitro, and PB repression in vivo. In two-hybrid analysis, light impaired B12-mediated oligomerization of CarHCt (Fig. 4A). Furthermore, AdoB12-bound TtCarHCt or CTt2 exposed to light (white or at wavelengths centered at 360, 438, or 540 nm, at which AdoB12 absorbs) eluted in SEC as monomers, not as the tetramers observed in the dark (Fig. 4B). Moreover, the absorption spectrum of the eluted peak had a maximum at 358 nm (Fig. S4B, Bottom), which is characteristic of hydroxocobalamin (OHB12) produced on aerobic AdoB12 photolysis (26). Also, no heat change was detected in ITC titrations of TtCarHCt with light-exposed AdoB12. In EMSA, irradiating AdoB12-bound CTt1 or CTt2 samples at the three wavelengths mentioned earlier caused the well defined retarded complex formed in the dark to revert to that observed with no AdoB12 (Fig. 4C). This did not occur with red light, at a wavelength at which AdoB12 does not absorb (Fig. S5A). Thus, light over a wide spectral range (UV, blue, and green, but not red) antagonizes AdoB12-induced oligomerization and DNA binding that occurs in the dark.
Fig. 4.
Light disrupts AdoB12-mediated oligomerization, DNA binding in vitro, and PB repression in vivo. (A) Two-hybrid cell spot assay for CarACt and CarHCt in the dark and in the light in the presence of CNB12. Cells expressing the indicated C-terminal domain fused to T25 and to T18 (T25/T18) or to just one of these as negative controls (T25 or T18 in the figure) were spotted on plates containing X-gal. Interaction correlates with the blue color intensity of the spot. (B) Elution profiles off a Superdex200 analytical gel filtration column for 45 μM TtCarHCt (Top) or CTt2 (Bottom) with AdoB12 tracked by absorbance (in arbitrary units) at 280 nm (solid lines) or at 361 nm (dashed lines) in the dark (red lines), and after exposure to light (blue lines). Mr (in kDa) for each peak maximum is marked at the top. (C) Effect of light on DNA binding in vitro. EMSA with samples containing CCR130 and 30 nM CTt1 or CTt2, and with or without fivefold molar excess AdoB12 relative to protein that were incubated in the dark (35 min) or irradiated with light (5 min) at the wavelength (in nm) indicated at the top after 30 min incubation in the dark. (D) Reporter PB-lacZ expression (in terms of specific β-gal activity) in the dark (black bars), in the dark with 1 μM CNB12 (striped bars), in the light (white bars), and in the light with 1 μM CNB12 (gray bars) for each of the strains indicated. The mean of three independent measurements and SEM for the specific β-gal activities (“Activity”) are shown.
These data strongly suggest that TtCarH activity in T. thermophilus would be AdoB12-dependent and light-sensitive. Interestingly, TtcarH is adjacent to genes encoding a carotenogenic enzyme (crtB) and a photolyase (phr), but is transcribed in the opposite sense (36) (Fig. S6A). If, like CarH, TtCarH regulates carotenogenesis, it may recognize a site upstream of crtB. To address this, we purified TtCarH and examined its binding in vitro to a probe containing the crtB–TtcarH intergenic region (Fig. S6A). In EMSA, significant levels of free probe were observed even at high protein concentrations in the absence of AdoB12 (Fig. S6B). Including AdoB12 considerably enhanced DNA binding and, moreover, led to the formation of a well defined lower-mobility complex that was not observed even at high TtCarH concentrations when AdoB12 was absent. Consistent with this, TtCarH yielded a DNase I footprint only in the presence of AdoB12 (Fig. S6C). Interestingly, inspection of the sequence corresponding to the DNase I footprint indicated elements akin to the CarA/CarH operator (Fig. S6A). Moreover, light at wavelengths at which AdoB12 absorbs counteracted this binding (Fig. S6D). As the identified DNA-binding site lies in the intergenic region, which would contain promoter elements for both crtB and TtcarH, it is conceivable that TtCarH regulates crtB as well as its own expression and, more importantly, that it employs the AdoB12-based photoregulatory mechanism.
AdoB12–CarH Complex Provides a Distinct Photosensory Pathway for Light-Induced Carotenogenesis in M. xanthus.
We next examined the functional relevance of AdoB12 photosensitivity on PB expression. For this, we used a ΔcarS M. xanthus genetic background, to separate the direct effect of light on AdoB12-CarH from antirepression by CarS (12, 18). Consistent with CarA being B12-independent and with the need for CarS to counteract its activity, the strain in which PB is repressed only by CarA (ΔcarS ΔcarH) remained yellow in the light with or without B12 (Fig. S5B), and PB-lacZ expression was always low, as in the ΔcarS strain (Fig. 4D). In contrast, the strain with PB repressed solely by AdoB12-CarH (ΔcarS ΔcarA) turned from yellow to red when exposed to white light, and PB-lacZ expression was induced approximately 10-fold, to levels comparable to those for WT under the same conditions or for the ΔcarS ΔcarA ΔcarH strain (which expresses PB constitutively). By contrast, and consistent with the data in vitro, red light did not relieve PB repression by CarH in vivo (Fig. S5 B and C). Therefore, its intrinsic photosensitivity enables AdoB12 to serve, via CarH, as both an “off” and an “on” switch that couples gene regulation to the light signal. This provides a distinct photosensory pathway, which is independent of CarS, for light-induced carotenogenesis in M. xanthus. Most importantly, it reveals a use of AdoB12 in a unique photoreceptor design to achieve light-dependent gene regulation.
Discussion
The principal discovery of this study is the finding that AdoB12, best known as an enzyme cofactor, determines a light-responsive switch that modulates gene expression via a transcriptional factor (Fig. 5). In the only other B12-based mechanism for gene regulation, the B12 riboswitch, RNA and not protein binds tightly (Kd of approximately 300 nM) and specifically to AdoB12 to adopt a distinct structure that down-regulates gene expression (10). Our studies indicate that AdoB12 is also the form that specifically modulates CarH (and TtCarH) activity by fomenting higher-order assembly to enhance specific binding to operator DNA and bring about repression of target genes. Association of AdoB12 to a repressor to induce tetramer formation for functionally effective binding to a two-site operator echoes a frequently exploited strategy by several other bacterial transcriptional factors. In these, binding of a specific ligand is allosterically coupled to cooperative binding to operator (usually also bipartite) to achieve tightly regulated function (37). That the CarH paralogue, CarA, despite having a canonical B12-binding site, does not require the cofactor for oligomerization nor for effective operator binding, thus underlies the striking difference between the two proteins. Our domain-swap and mutational analyses show that the AdoB12 dependence is conferred by CarHCt or TtCarHCt, and that the conserved H of the consensus B12-binding motif in this domain is crucial for AdoB12-induced oligomerization. Future high-resolution studies could provide further insights into the fine details of how AdoB12 directs the oligomerization and activity of CarH and TtCarH, yet not of CarA.
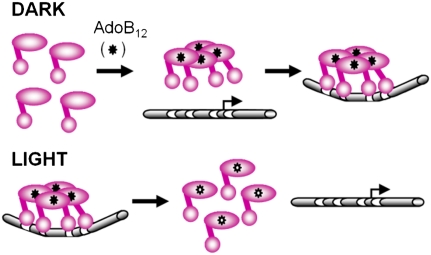
Fig. 5.
Model for the light-responsive AdoB12-based regulatory switch. The schematic depicts how specific-binding of AdoB12 (filled asterisk) to CarH favors formation of tetramers that can then bind to the operator and repress PB. Light activates PB expression in a CarS-independent pathway by disrupting the photosensitive AdoB12-repressor complex to diminish operator-binding and allow RNA polymerase access to PB. The two-domain repressor organization is shown by the small sphere for the N-terminal DNA-binding domain, a large oval for the C-terminal AdoB12-binding domain, and a line for the linker between the two domains. DNA, represented as a tube with each consecutive pair of white strips representing one site of the bipartite operator, is shown bent when bound by active repressor. AdoB12 photolysis to OHB12 is represented by the unfilled asterisk.
A major finding in this work is that, besides fomenting repressor assembly, AdoB12 enables the repressor to serve a photosensory role in a more direct signaling route for light-induced carotenogenesis in M. xanthus, an alternative to the CarS-mediated pathway that is also light-activated. This ability is based on photolysis of the repressor-bound AdoB12, which can be evoked by light over a broad wavelength range. As a consequence, repressor oligomers are disassembled and operator binding is impaired. AdoB12 is therefore one more in the list of chromophores such as flavin mononucleotide, flavin adenine dinucleotide, retinal, p-coumaric acid, and linear tetrapyrroles (bilins and biliverdins) that confer light sensing ability to photoactive proteins (14–16). The predominant trigger for carotenogenesis in M. xanthus is blue light, as this alone can activate the carQRS operon (via the distinct protoporphyrin IX-mediated pathway) to produce CarS and neutralize PB repression by CarA and CarH (13). Blue light is also within the broad wavelength range in which the AdoB12–CarH switch can operate. However, although the CarA-CarS–mediated pathway is B12-independent, AdoB12 must be available for CarH to operate. This suggests that the CarA-CarS mechanism may have evolved to bypass conditions of limited B12 availability, as M. xanthus, unable to synthesize B12 de novo, can only gain access to this cofactor from other soil microorganisms upon which it preys. Retention of the AdoB12–CarH switch in M. xanthus may be an evolutionary vestige given the very ancient origins of B12 and B12-mediated pathways (2). It may also reflect a selective advantage that could be related to the ability of this switch to sense wavelengths distinct from blue light and/or to control the expression of yet-unknown genes not subject to CarA-CarS regulation.
Our discovery of an AdoB12-based photosensor has wider implications. It could underlie the unknown functions of many proteins with a CarA/CarH-like domain architecture that have come to light as a result of large-scale genome sequencing, TtCarH being one example. A database search for B12-binding, MerR family proteins identifies more than 100 nonredundant entries in various bacterial genomes (Table S1). Most of these (except for M. xanthus and two others) contain only one version of the protein. Strikingly, the corresponding gene is often located among those encoding putative photolyases or carotenogenic enzymes, typically linked to a light response. Some of these homologues may, like CarH, depend on B12 for function, whereas others, like CarA, may not. It is worth noting that, other than in Stigmatella aurantiaca (a myxobacterium very closely related to M. xanthus), CarS homologues are not found (12, 18). Although factors resembling CarS in function, but not in sequence, may exist for on/off control of some of the newly identified CarA/CarH homologues, a more straightforward possibility is that the activities of many of these homologues are modulated solely by a light-responsive AdoB12 switch, like the one identified in this study.
Known photoreceptor proteins are typically of a modular design, with light-sensing entrusted to an independent domain (14–16). As a result, the light-sensing input module can be found linked to various types of effector or output domains for use in diverse processes (14–16). Our finding that B12-dependent photosensing is also housed in a single, autonomous module suggests that, other than in MerR-type DNA-binding factors or conventional B12-dependent enzymes, it may also occur in association with other effector domains. A search in the genome database produced hits in which the B12-binding module is found associated with sensor histidine kinases, response regulators and, curiously, even as stand-alone proteins, all with functions as yet undefined (Table S1). It is conceivable that the AdoB12-driven higher-order assembly and light-sensing mechanism described in this study is an underlying feature of the functions of a number of these newly identified proteins. Also, the finding that the AdoB12-dependent light-sensing module is autonomous and can thus be readily transplanted would be advantageous for designing novel, synthetic light-responsive factors.
Materials and Methods
Strains, Plasmids, and Growth Conditions.
Table S2 lists the bacterial strains and plasmids used in this study. Growth conditions and strain constructions are detailed in SI Materials and Methods.
Protein Purification and Protein–Protein, Protein–DNA, and Protein–Cobalamin Interactions.
Procedures for protein purification and their analysis are described in SI Materials and Methods.
Database Analysis.
Proteins with a CarA/CarH-like B12-binding domain associated to a MerR-type DNA-binding domain or other effector domains, or present as standalone modules in the nonredundant protein and microbial genome databases, were identified by using the BLAST suite of programs (38).
Supplementary Material
Acknowledgments
We thank Dr. Francisco García-Heras, José Antonio Madrid, and Dr. Cesar Flores-Flores for technical assistance. This study was funded by Ministerio de Ciencia e Innovación–Spain Grants BFU2009-12445-C02-01 (to M.E.-A.) and BFU2009-12445-C02-02 (to S.P.), and by Fundación Séneca–Murcia Grant 08748/PI/08 (to F.J.M.). J.M.O.-G. was supported by a Consejo Superior de Investigaciones Cientificas Predoctoral fellowship (Spain).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018972108/-/DCSupplemental.
References
- 1.Croft MT, Lawrence AD, Raux-Deery E, Warren MJ, Smith AG. Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature. 2005;438:90–93. doi: 10.1038/nature04056. [DOI] [PubMed] [Google Scholar]
- 2.Roth JR, Lawrence JG, Bobik TA. Cobalamin (coenzyme B12): Synthesis and biological significance. Annu Rev Microbiol. 1996;50:137–181. doi: 10.1146/annurev.micro.50.1.137. [DOI] [PubMed] [Google Scholar]
- 3.Taga ME, Larsen NA, Howard-Jones AR, Walsh CT, Walker GC. BluB cannibalizes flavin to form the lower ligand of vitamin B12. Nature. 2007;446:449–453. doi: 10.1038/nature05611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warren MJ, Raux E, Schubert HL, Escalante-Semerena JC. The biosynthesis of adenosylcobalamin (vitamin B12) Nat Prod Rep. 2002;19:390–412. doi: 10.1039/b108967f. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Rodionov DA, Gelfand MS, Gladyshev VN. Comparative genomic analyses of nickel, cobalt and vitamin B12 utilization. BMC Genomics. 2009;10:78. doi: 10.1186/1471-2164-10-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ludwig ML, Matthews RG. Structure-based perspectives on B12-dependent enzymes. Annu Rev Biochem. 1997;66:269–313. doi: 10.1146/annurev.biochem.66.1.269. [DOI] [PubMed] [Google Scholar]
- 7.Banerjee R, Ragsdale SW. The many faces of vitamin B12: Catalysis by cobalamin-dependent enzymes. Annu Rev Biochem. 2003;72:209–247. doi: 10.1146/annurev.biochem.72.121801.161828. [DOI] [PubMed] [Google Scholar]
- 8.Mandal M, Breaker RR. Gene regulation by riboswitches. Nat Rev Mol Cell Biol. 2004;5:451–463. doi: 10.1038/nrm1403. [DOI] [PubMed] [Google Scholar]
- 9.Nahvi A, Barrick JE, Breaker RR. Coenzyme B12 riboswitches are widespread genetic control elements in prokaryotes. Nucleic Acids Res. 2004;32:143–150. doi: 10.1093/nar/gkh167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nahvi A, et al. Genetic control by a metabolite binding mRNA. Chem Biol. 2002;9:1043–1049. doi: 10.1016/s1074-5521(02)00224-7. [DOI] [PubMed] [Google Scholar]
- 11.Vitreschak AG, Rodionov DA, Mironov AA, Gelfand MS. Regulation of the vitamin B12 metabolism and transport in bacteria by a conserved RNA structural element. RNA. 2003;9:1084–1097. doi: 10.1261/rna.5710303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pérez-Marín MC, Padmanabhan S, Polanco MC, Murillo FJ, Elías-Arnanz M. Vitamin B12 partners the CarH repressor to downregulate a photoinducible promoter in Myxococcus xanthus. Mol Microbiol. 2008;67:804–819. doi: 10.1111/j.1365-2958.2007.06086.x. [DOI] [PubMed] [Google Scholar]
- 13.Elías-Arnanz M, Fontes M, Padmanabhan S. Carotenogenesis in Myxococcus xanthus: A complex regulatory network. In: Whitworth DE, editor. Myxobacteria: Multicellularity and Differentiation. Washington, DC: ASM Press; 2008. pp. 211–225. [Google Scholar]
- 14.Losi A, Gärtner W. Bacterial bilin- and flavin-binding photoreceptors. Photochem Photobiol Sci. 2008;7:1168–1178. doi: 10.1039/b802472c. [DOI] [PubMed] [Google Scholar]
- 15.Purcell EB, Crosson S. Photoregulation in prokaryotes. Curr Opin Microbiol. 2008;11:168–178. doi: 10.1016/j.mib.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 16.van der Horst MA, Key J, Hellingwerf KJ. Photosensing in chemotrophic, non-phototrophic bacteria: Let there be light sensing too. Trends Microbiol. 2007;15:554–562. doi: 10.1016/j.tim.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Burchard RP, Dworkin M. Light-induced lysis and carotenogenesis in Myxococcus xanthus. J Bacteriol. 1966;91:535–545. doi: 10.1128/jb.91.2.535-545.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.León E, et al. A bacterial antirepressor with SH3 domain topology mimics operator DNA in sequestering the repressor DNA recognition helix. Nucleic Acids Res. 2010;38:5226–5241. doi: 10.1093/nar/gkq277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Navarro-Avilés G, et al. Structural basis for operator and antirepressor recognition by Myxococcus xanthus CarA repressor. Mol Microbiol. 2007;63:980–994. doi: 10.1111/j.1365-2958.2006.05567.x. [DOI] [PubMed] [Google Scholar]
- 20.Pérez-Marín MC, López-Rubio JJ, Murillo FJ, Elías-Arnanz M, Padmanabhan S. The N terminus of Myxococcus xanthus CarA repressor is an autonomously folding domain that mediates physical and functional interactions with both operator DNA and antirepressor protein. J Biol Chem. 2004;279:33093–33103. doi: 10.1074/jbc.M405225200. [DOI] [PubMed] [Google Scholar]
- 21.Cervantes M, Murillo FJ. Role for vitamin B12 in light induction of gene expression in the bacterium Myxococcus xanthus. J Bacteriol. 2002;184:2215–2224. doi: 10.1128/JB.184.8.2215-2224.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drennan CL, Huang S, Drummond JT, Matthews RG, Ludwig ML. How a protein binds B12: A 3.0 A X-ray structure of B12-binding domains of methionine synthase. Science. 1994;266:1669–1674. doi: 10.1126/science.7992050. [DOI] [PubMed] [Google Scholar]
- 23.Ludwig ML, Drennan CL, Matthews RG. The reactivity of B12 cofactors: The proteins make a difference. Structure. 1996;4:505–512. doi: 10.1016/s0969-2126(96)00056-1. [DOI] [PubMed] [Google Scholar]
- 24.Bond CM, Lees KA, Enever RP. Photolytic decomposition of 3 cobalamins: A quantitative study. J Pharm Pharmacol. 1972;24(suppl):143. [PubMed] [Google Scholar]
- 25.Chen E, Chance MR. Nanosecond transient absorption spectroscopy of coenzyme B12. Quantum yields and spectral dynamics. J Biol Chem. 1990;265:12987–12994. [PubMed] [Google Scholar]
- 26.Schwartz PA, Frey PA. 5′-Peroxyadenosine and 5′-peroxyadenosylcobalamin as intermediates in the aerobic photolysis of adenosylcobalamin. Biochemistry. 2007;46:7284–7292. doi: 10.1021/bi700077v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.López-Rubio JJ, et al. Operator design and mechanism for CarA repressor-mediated down-regulation of the photoinducible carB operon in Myxococcus xanthus. J Biol Chem. 2004;279:28945–28953. doi: 10.1074/jbc.M403459200. [DOI] [PubMed] [Google Scholar]
- 28.Han Y, Meyer MH, Keusgen M, Klug G. A haem cofactor is required for redox and light signalling by the AppA protein of Rhodobacter sphaeroides. Mol Microbiol. 2007;64:1090–1104. doi: 10.1111/j.1365-2958.2007.05724.x. [DOI] [PubMed] [Google Scholar]
- 29.Moskvin OV, Gilles-Gonzalez MA, Gomelsky M. The PpaA/AerR regulators of photosynthesis gene expression from anoxygenic phototrophic proteobacteria contain heme-binding SCHIC domains. J Bacteriol. 2010;192:5253–5256. doi: 10.1128/JB.00736-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moskvin OV, Kaplan S, Gilles-Gonzalez MA, Gomelsky M. Novel heme-based oxygen sensor with a revealing evolutionary history. J Biol Chem. 2007;282:28740–28748. doi: 10.1074/jbc.M703261200. [DOI] [PubMed] [Google Scholar]
- 31.Yamanishi M, Vlasie M, Banerjee R. Adenosyltransferase: An enzyme and an escort for coenzyme B12? Trends Biochem Sci. 2005;30:304–308. doi: 10.1016/j.tibs.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 32.Johnson CL, et al. Functional genomic, biochemical, and genetic characterization of the Salmonella pduO gene, an ATP:cob(I)alamin adenosyltransferase gene. J Bacteriol. 2001;183:1577–1584. doi: 10.1128/JB.183.5.1577-1584.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mera PE, Escalante-Semerena JC. Multiple roles of ATP:cob(I)alamin adenosyltransferases in the conversion of B12 to coenzyme B12. Appl Microbiol Biotechnol. 2010;88:41–48. doi: 10.1007/s00253-010-2773-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karimova G, Ullmann A, Ladant D. A bacterial two-hybrid system that exploits a cAMP signaling cascade in Escherichia coli. Methods Enzymol. 2000;328:59–73. doi: 10.1016/s0076-6879(00)28390-0. [DOI] [PubMed] [Google Scholar]
- 35.Firth RA, Hill HA, Pratt JM, Williams RJ, Jackson WR. The circular dichroism and absorption spectra of some vitamin B12 derivatives. Biochemistry. 1967;6:2178–2189. doi: 10.1021/bi00859a040. [DOI] [PubMed] [Google Scholar]
- 36.Henne A, et al. The genome sequence of the extreme thermophile Thermus thermophilus. Nat Biotechnol. 2004;22:547–553. doi: 10.1038/nbt956. [DOI] [PubMed] [Google Scholar]
- 37.Lu D, et al. Crystal structure of TtgV in complex with its DNA operator reveals a general model for cooperative DNA binding of tetrameric gene regulators. Genes Dev. 2010;24:2556–2565. doi: 10.1101/gad.603510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Altschul SF, et al. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.