Abstract
The organization of neural progenitors in the developing mammalian neuroepithelium is marked by cadherin-based adherens junctions. Whereas RhoA, a founding member of the small Rho GTPase family, has been shown to play important roles in epithelial adherens junctions, its physiological roles in neural development remain uncertain due to the lack of specific loss-of-function studies. Here, we show that RhoA protein accumulates at adherens junctions in the developing mouse brain and colocalizes to the cadherin–catenin complex. Conditional deletion of RhoA in midbrain and forebrain neural progenitors using Wnt1-Cre and Foxg1-Cre mice, respectively, disrupts apical adherens junctions and causes massive dysplasia of the brain. Furthermore, RhoA-deficient neural progenitor cells exhibit accelerated proliferation, reduction of cell- cycle exit, and increased expression of downstream target genes of the hedgehog pathway. Consequently, both lines of conditional RhoA-deficient embryos exhibit expansion of neural progenitor cells and exencephaly-like protrusions. These results demonstrate a critical role of RhoA in the maintenance of apical adherens junctions and the regulation of neural progenitor proliferation in the developing mammalian brain.
Keywords: CNS development, cell adhesion
During brain development, proliferation, differentiation, and cell death of neural progenitor cells are tightly controlled to produce the organ of predetermined size (1–3). Apart from maintaining intercellular adhesion, adherens junctions that are preferentially located at the ventricular surface of the neuroepithelium are linked to several major signaling pathways to regulate the rate and mode of cell division in neural progenitors (4, 5). Classic cadherins are core components of adherens junctions. The extracellular regions of classic cadherins mediate adhesive recognition through homophilic interactions, whereas cytoplasmic domains associate with a set of cytoplasmic proteins collectively called catenins. Deletion of αE-catenin in the developing nervous system results in dysregulated proliferation of neural progenitor cells as well as disruption of adherens junctions (6). Furthermore, Lgl1-knockout mice and compound knockout mice for both Numb and Numbl also show disruption of adherens junctions together with abnormal proliferation of neural progenitor cells (7–9). These findings indicate that the cadherin–catenin complex has broader functions beyond cell–cell adhesion in mammalian neural progenitors.
Cadherin–catenin complexes interact with the actin cytoskeleton through α-catenin. Formation of adherens junctions is accompanied by profound changes in the actin cytoskeleton and accumulation of polymerized actin at the contact area, and both the formation and the maintenance of adherens junctions depend on the actin cytoskeleton (10). The Rho family of small GTPases, including RhoA, Cdc42, and Rac1, are key regulators of the actin cytoskeleton and coordinate junction assembly, stability, and function (11, 12). However, these functions are mainly deduced from studies in cell lines with overexpression of dominant-negative or constitutively active small Rho GTPases. However, the consequences of GTPase signaling alteration depend profoundly on cellular context and the specificity of the mutant proteins. The recent development of a conditional gene-targeting strategy has provided many new insights into the physiological functions of small Rho GTPases (13). For example, although Cdc42 and Rac1 are both implicated in epithelial apical junctions, conditional gene deletion in the telencephalon revealed that Cdc42, but not Rac1, is indispensable for the formation of apical adherens junctions in the developing brain (14–16). These findings underlie the importance of using conditional gene deletion to ascertain the biological functions of small Rho GTPases.
Among all small Rho GTPases, RhoA is one of the last remaining members whose in vivo gene-deletion consequences in the mammalian central nervous system are yet to be reported (13). In addition to RhoA, there are two other Rho isoforms in mammals, RhoB, and RhoC, which are highly homologous, and all three members induce stress fiber formation when overexpressed in fibroblasts (13). However, in knockout studies, RhoB-null and RhoC-null mice are viable and have normal development (17, 18). Recently, conditional deletion of RhoA in the developing skin was reported, but the mutant mice did not show any obvious phenotypes in vivo (19). Therefore, RhoA and its close isoforms, RhoB and RhoC, may have redundant functions in skin development. In mammalian epithelial cells, RhoA induces actomysin contractility to expand cell–cell adhesion (20). Furthermore, loss-of-function experiments using Drosophila embryos demonstrated that the Drosophila RhoA homolog, Rho1, is required for the organization of cadherin-based adherens junctions (21, 22). These studies indicate that mammalian RhoA may play a role in adherens junctions, but the physiological role of RhoA in adherens junctions in mammalian nervous system remains unknown.
In this study, we examined the role of RhoA in the developing mouse brain using a conditional gene-targeting strategy with two lines of Cre drivers (Wnt1-Cre for the midbrain and Foxg1-Cre for the forebrain mutation). These conditional gene deletions lead to similar phenotypes including the disruption of adherens junctions, massive expansion of neural progenitors, and disorganization of the brain. These findings uncover an essential and nonredundant role of RhoA in neural progenitor cells in the mouse central nervous system.
Results
Localization of RhoA Protein in the Developing Brain.
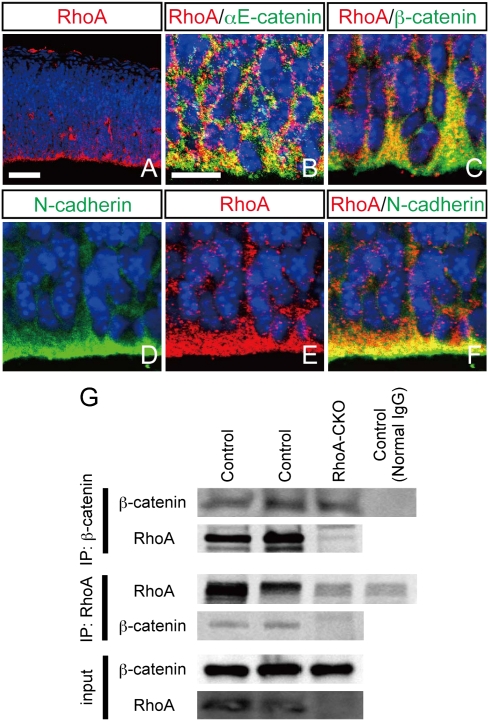
We first examined the spatial distribution of RhoA protein in the developing brain. RhoA was detected throughout the neuroepithelium and enriched at the apical portion of the ventricular zone at embryonic day 12.5 (E12.5) (Fig. 1A). Because the apical surface of the ventricular zone is also enriched with cadherin–catenin proteins, we compared the localization of RhoA with those of the adherens junction components. Double-immunofluorescence labeling showed that RhoA is extensively colocalized with αE-catenin, β-catenin, and N-cadherin at E13.5 (Fig. 1 B–F). Immunoprecipitation experiments further revealed the association of RhoA with β-catenin in the E13.5 midbrain (Fig. 1G). The specificity of protein–protein interaction was supported by the reciprocal use of anti-RhoA and anti–β-catenin antibodies in precipitation immunoblot but yielded the same result, as well as the loss of the interaction signal in conditional RhoA-null embryos (Fig. 1G; see below for conditional deletion of RhoA). Together, these data suggest that RhoA can be functionally associated with the cadherin–catenin complex.
Fig. 1.
RhoA accumulates at adherens junctions in the ventricular zone and interacts with β-catenin. (A) Immunohistochemistry for RhoA revealed that RhoA protein is enriched at the apical portion of the ventricular zone of the mesencephalon at E12.5. RhoA-positive signals colocalize with positive signals for αE-catenin (B), β-catenin (C), and N-cadherin (D–F) at E13.5. Nuclei are visualized by ToPro3. (Scale bars: A, 50 μm; B, 10 μm.) (G) Immunoprecipitation revealed the interaction of RhoA with β-catenin, and the interaction was not observed in conditional RhoA-null (RhoA-CKO) brain.
Deletion of RhoA in Mesencephalon Causes Exencephaly.
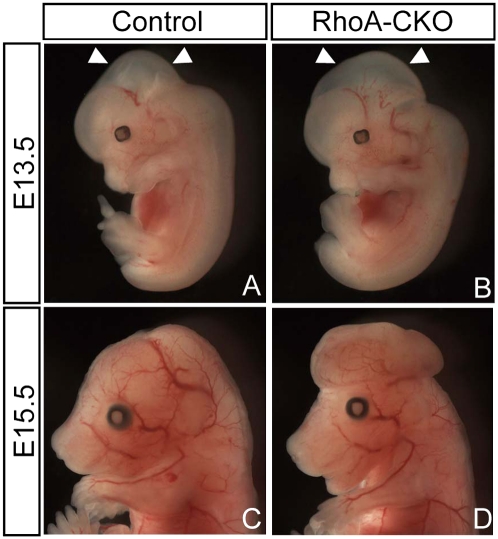
To determine the in vivo functions of RhoA in neural progenitors, we generated RhoA-floxed mice (Fig. S1) and crossed them with Wnt1-Cre mice that express Cre recombinase in the mesencephalon as well as in neural crest derivatives (23). By crossing with stop-floxed EGFP reporter mice, we detected the Wnt1-Cre–mediated Cre/loxP recombination in the mesencephalon at E9.5 (Fig. S2). In Wnt1-Cre; RhoAflox/flox (hereafter referred to as RhoA-CKO) embryos, we validated the absence of RhoA expression in the mesencephalon by immunostaining, as well as immunoblotting analysis (Fig. S3). Deletion of RhoA did not markedly affect the expression of other small Rho GTPase members (Fig. S3). At E13.5, RhoA-CKO embryos showed enlargement of the mesencephalon, compared with their littermates (Fig. 2 A and B, arrowheads), and at E15.5, they exhibited exencephaly-like protrusions (Fig. 2 C and D). About 80% of RhoA-CKO embryos exhibited exencephaly-like abnormalities from E15.5 to E16.5 (Table S1). Histologically, the protrusion consisted of a neuronal mass originating from the midbrain, which not only exposed dorsally but also pushed forebrain structures to the ventral side (Fig. S4). Because RhoA-CKO embryos did not exhibit exencephaly-like abnormalities before E12.5 (Table S1), we concluded that this anomaly was not caused by the defects of neural tube closure. Rather, it is more likely to be caused by abnormal expansion of neuronal cells.
Fig. 2.
Deletion of RhoA induces enlargement of the mesencephalon and subsequently leads to exencephaly-like protrusion formation. General appearance of control (A and C) and RhoA-CKO (B and D) embryos at E13.5 (A and B) and E15.5 (C and D).
RhoA Deficiency Disrupts Adherens Junctions at the Ventricular Surface.
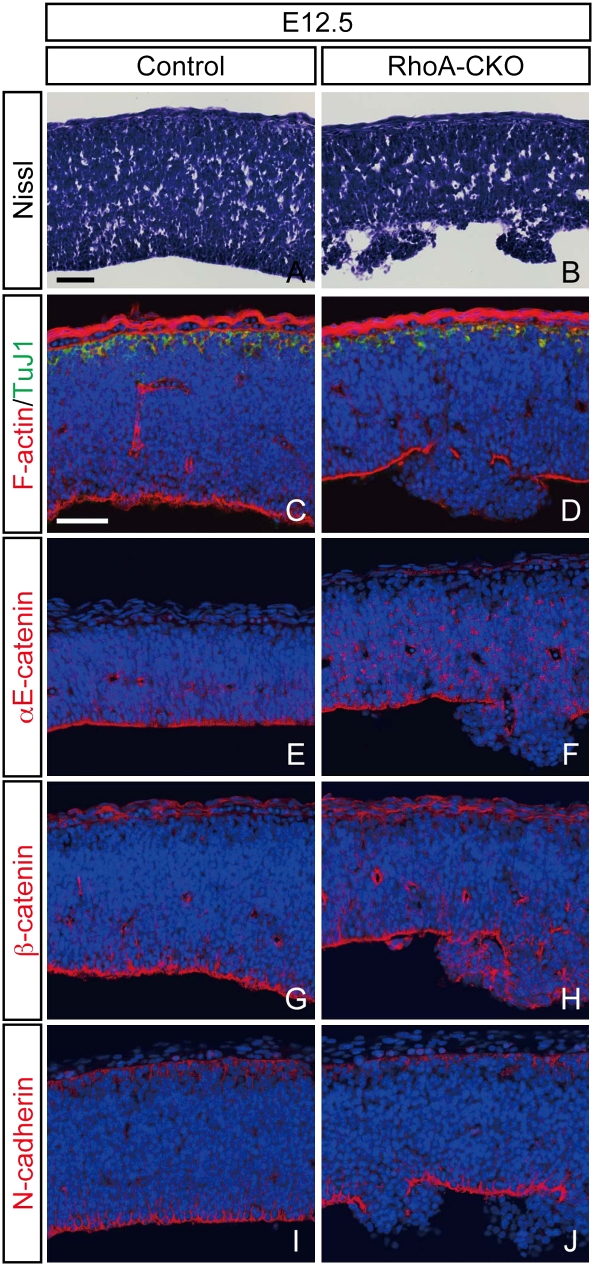
We next examined Nissl-stained sections from RhoA-CKO embryos and control littermates. The initial abnormality in the RhoA-deleted mesencephalon was detected as multiple nodules protruding from the apical surface around E11.5. This anomaly intensified by E12.5, and the cellular organization in the ventricular zone of the mesencephalon became highly irregular in RhoA-CKO embryos (Fig. 3 A and B). After E12.5, the dysplasia was observed in all of the RhoA-CKO embryos we examined. The apical F-actin staining was lost in dysplastic regions whereas the cellular organization at the basal side, including accumulation of early-born postmitotic neurons, appeared normal (Fig. 3 C and D), raising the possibility that RhoA deficiency may lead to disruption of the cadherin-based apical cell–cell adhesion. Consistent with this notion, we found that the localization of components of the cadherin–catenin complex, including αE-catenin, β-catenin, and N-cadherin, was all disrupted in the dysplastic region (Fig. 3 E–J). These results suggest that RhoA deletion leads to disruption of adherens junctions in the ventricular surface of the developing mesencephalon.
Fig. 3.
Disruption of adherens junctions in RhoA-deleted neural progenitor cells. (A and B) Nissl-stained sections through the developing mesencephalon from control (A) and RhoA-CKO (B) embryos at E12.5. The apical structure is partially disrupted in RhoA-CKO embryos, and neural progenitor cells are irregularly arranged and protruded to the ventricle in this region (B). (C and D) Double immunostaining for F-actin and βIII-tubulin of control (C) and RhoA-CKO (D) embryos. Apical F-actin staining is disrupted in the dysplastic region, whereas basal βIII-tubulin (TuJ1) staining remains unaffected (D). (E–I) Immunostaining for αE-catenin (E and F), β-catenin (G and H), and N-cadherin (I and J) of control (E, G, and I) and RhoA-CKO (F, H, and J) embryos at E12.5. Apical staining of these molecules is also disrupted in the dysplastic region. (Scale bars, 50 μm.)
Loss of RhoA Causes Massive Dysplasia in the Mesencephalon.
At E13.5, the dysplasia expanded throughout the mesencephalon and the cellular organization was markedly disorganized in RhoA-CKO embryos (Fig. S5 A and B). Nestin-positive neural progenitor/radial glial processes were also disorganized in RhoA-CKO embryos (Fig. S5 E and F). In addition, we frequently found neuroblast rosette-like structures that were also observed in αE-catenin and N-cadherin null mutants (6, 24) (Fig. S5 C and D). In the midbrain of control embryos, Ki67+ proliferative cells and βIII-tubulin+ postmitotic neurons were separated in the apical and basal compartments, respectively (Fig. 4A). However, these two cell populations were intermingled and the ratio of postmitotic neurons decreased in RhoA-CKO embryos [Fig. 4 B and C; control, 23.8 ± 3.4% (n = 3); RhoA-CKO, 16.5 ± 1.5% (n = 4); mean ± SD; P < 0.05 by Student's t test]. These results suggest that RhoA deficiency leads to massive dysplasia and expansion of neural progenitor cells in the developing mesencephalon.
Fig. 4.
Accelerated proliferation and failure in cell-cycle withdrawal result in expansion of neural progenitor cells in the RhoA-deficient mesencephalon. (A–C) Immunohistochemistry for Ki67 and βIII-tubulin revealed increased Ki67+ neural progenitor cells and decreased βIII-tubulin+ postmitotic neurons in RhoA-CKO embryos. The graph represents ratios of βIII-tubulin+ postmitotic neurons to the total neuronal cell number. (D–F) Immunostaining for p-H3 revealed increased mitosis in the RhoA-deficient mesencephalon. The graph shows ratios of mitotic cells to the total neuronal cell number. (G–I) Accelerated proliferation in RhoA-deficient neural progenitors. A higher percentage of RhoA-deficient progenitors (Ki67+) are labeled with BrdU after a 30-min pulse. The percentages of K67+ cells that incorporated BrdU are shown. (J–L) Cell-cycle withdrawal is compromised in RhoA-deficient neural progenitors. Pregnant mice were injected with BrdU 24 h before being killed. Cells reentering the cell cycle are BrdU+/Ki67+, whereas cells withdrawn from the cell cycle are BrdU+/Ki67−. Cell-cycle exit was determined as the ratio of cells that exited the cell cycle (BrdU+/Ki67−) to all cells that incorporated BrdU. All these analyses were carried out using E13.5 embryos. The graphs represent mean + SD. n = 3–6, *P < 0.05, **P < 0.01, Student's t test. (Scale bars, 50 μm.)
Accelerated Proliferation and Decreased Cell-Cycle Exit in RhoA-Deleted Neural Progenitor Cells.
To assess whether the loss of RhoA leads to changes in cell proliferation, we performed immunostaining with anti–phospho-histone H3 (p-H3) antibody to identify mitotic cells. In control embryos, p-H3–positive nuclei were localized close to the ventricular surface of the neuroepithelium (Fig. 4D). In contrast, in RhoA-CKO embryos, the p-H3–positive nuclei were dispersed throughout the brain (Fig. 4E). Quantitative analysis of the p-H3 staining demonstrated about a twofold increase in the number of mitotic cells among total neuronal cells in the mesencephalon in RhoA-CKO embryos [Fig. 4F; control, 2.8 ± 0.3% (n = 4); RhoA-CKO, 5.1 ± 0.8% (n = 4); P < 0.01].
Enlargement of the progenitor pool in RhoA-deficient midbrain may result from accelerated proliferation, decreased cell death, failure in cell-cycle exit, or any combination of these factors. First, to reveal potential changes in the proliferation, we counted the proportion of neural progenitor cells labeled by a 30-min pulse of BrdU and found a significant increase in the fraction of BrdU-labeled nuclei among all Ki67+ neural progenitor cells in RhoA-CKO embryos [Fig. 4 G–I; control, 52.9 ± 3.1% (n = 5); RhoA-CKO, 66.5 ± 4.6% (n = 3); P < 0.01]. The increase of BrdU+ fractions among Ki67+ cells suggests accelerated proliferation of neural progenitor cells. In addition, BrdU-positive nuclei of control embryos localized at the basal side of the ventricular zone (Fig. 4G), whereas those of RhoA-CKO embryos localized not only at the basal side but also at the apical side (Fig. 4H).
To examine whether loss of RhoA causes changes in cell-cycle withdrawal, we counted the proportion of cells that exit the cell cycle after 24 h labeling with BrdU, which was calculated as the ratio of BrdU+/Ki67− cells among all BrdU-labeled cells. This analysis revealed a significant decrease of the cell-cycle exit index in RhoA-CKO embryos, compared with control embryos [Fig. 4 J–L; control, 34.0 ± 2.1% (n = 6); RhoA-CKO, 22.9 ± 3.3% (n = 3); P < 0.01].
Finally, we compared the extent of apoptosis using the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) method and found that the number of apoptotic cells was not decreased but rather increased in RhoA-CKO embryos (Fig. S6). Thus, our results suggest that expansion of neural progenitor cells in the RhoA-deleted midbrain is caused by both accelerated proliferation and failure to exit the cell cycle.
Similar Defects in Adherens Junctions and Expansion of the Neural Progenitor Pool in Forebrain-Specific RhoA Deletion.
To examine whether RhoA has important neurodevelopmental functions outside the mesencephalon, we crossed RhoA-floxed mice with another Cre-driver line, Foxg1-Cre. In Foxg1-Cre mice, the Cre recombinase was expressed by telencephalic progenitors from E9.5 (25). Genotyping by PCR detected near-complete recombination of the floxed RhoA allele in the forebrain, but not the tail, of Foxg1-Cretg; RhoAflox/flox (hereafter referred to as Foxg1-RhoA-CKO) embryos at E13.5 (Fig. 5B). From E13.5 to E14.5, Foxg1-RhoA-CKO embryos exhibited an increasing incidence of the exencephaly phenotype (55% at E13.5 and 80% at E14.5, Fig. 5A and Table S1), similar to the Wnt1-Cre–mediated RhoA-CKO embryos. Histologically, the mass consisted of Pax6+ neural progenitors, which were greatly expanded compared with control embryos (Fig. 5 C and D). The ventricular surface was disorganized and the apical expression of αE-catenin became absent in Foxg1-RhoA-CKO embryos, Instead, there were rings of intense αE-catenin expression inside the brain mass in mutant embryos (Fig. 5 E and F). Ki67+ and Pax6+ progenitors either were extensively intermingled with βIII-tubulin+ postmitotic neurons (Fig. 5 G and H) or formed rosette-like clusters in E14.5 Foxg1-RhoA-CKO embryos, similar to those detected in Wnt1-Cre–derived embryos (Fig. S5 G and H). Together, the similar phenotypes in midbrain and forebrain suggest that RhoA may have important functions in neuroepithelial progenitors throughout the developing nervous system.
Fig. 5.
Forebrain RhoA deletion also causes disruption of adherens junctions and expansion of neural progenitor cells, leading to exencephaly-like protrusion formation. (A) Gross appearance of control (Left) and Foxg1-RhoA-CKO (Right) embryos at E14.5. (B) PCR analysis showed near-complete recombination of the floxed RhoA allele in the forebrain, but not the tail, of Foxg1-RhoA-CKO embryos at E13.5. (C and D) At E13.5, Foxg1-RhoA-CKO embryos exhibited expansion of the Pax6+ cell population, which formed masses in the developing cerebral cortex. (E and F) At E14.5, the apical/ventricular wall became disrupted and the localization of αE-catenin was lost in Foxg1-RhoA-CKO embryos. (G and H) Whereas Ki67+ neural progenitor cells and βIII-tubulin+ postmitotic neurons were completely segregated in control embryos (G), these two components were extensively intermingled and neural progenitor cells were expanded in Foxg1-RhoA-CKO embryos (H). (Scale bars: C and D, 100 μm; E–H, 50 μm.)
RhoA-Deleted Mesencephalon Shows Increased Expression of Downstream Targets of the Hedgehog Signaling Pathway.
Finally, we turned to the issues of signaling pathways responsible for the abnormality of neural progenitors in RhoA-CKO embryos. In this context, previous studies of mutants with a disrupted cadherin–catenin complex reported that αE-catenin null mice exhibit abnormal activation of the hedgehog pathway (6) and that deletion of Lgl1 causes loss of cell polarity and up-regulation of the Notch pathway (8). In addition, activation of Wnt/β-catenin signaling is also known to induce hyperproliferation in neural progenitor cells (26).
To compare the consequences of RhoA deletion with the previous studies, we examined the expression of transcriptional targets of hedgehog, Notch, and Wnt/β-catenin pathways by reverse transcription–quantitative PCR analysis. We found that the expression of Gli1 and Fgf15 was significantly elevated in the RhoA-deficient midbrain at E13.5 (Fig. 6A). The in situ hybridization analysis showed that Gli1 and Fgf15 transcripts were markedly up-regulated in neural progenitor cells of the RhoA-deficient midbrain at E13.5 (Fig. 6 B–E). We also found a mild but significant increase in the expression of Notch pathway target genes (Hes5 and Hes1) and a Wnt/β-catenin target gene (CyclinD1) (Fig. 6A). However, because these three genes are highly expressed in neural progenitor cells (27, 28) (Fig. 6 F and G), the slight increase in the expression of these genes may reflect the expansion of neural progenitor cells in RhoA-CKO embryos (Fig. 4 A–C). Indeed, the expression of Sox2, a marker for neural progenitor cells, was increased in RhoA-CKO embryos similar to that of Hes5, Hes1, and CyclinD1 (29) (Fig. 6A). In addition, because there were no significant changes in the expression of Axin2 and c-myc (Fig. 6 A, H, and I), the Wnt/β-catenin pathway is unlikely to be involved in the hyperproliferation of neural progenitor cells in RhoA-CKO embryos. Together, these results suggest that the hedgehog pathway is activated in RhoA-deficient neural progenitor cells.
Fig. 6.
Increased expression of downstream target genes of the hedgehog signaling pathway in the RhoA-deficient mesencephalon. (A) Reverse transcription–quantitative PCR analysis of Gli1, Fgf15, Hes5, Hes1, Axin2, c-myc, CyclinD1, and Sox2 in control and RhoA-deficient midbrains at E13.5. The graphs represent mean + SD. n = 5 for control and n = 7 for RhoA-CKO embryos, *P < 0.05, **P < 0.01, Student's t test. (B–I) In situ hybridization for Gli1 (B and C), Fgf15 (D and E), Hes5 (F and G), and Axin2 (H and I) of the mesencephalon from control (B, D, F, and H) and RhoA-CKO (C, E, G, and I) embryos at E13.5. (Scale bar, 50 μm.)
Discussion
RhoA, a founding member of the small Rho GTPase family, has been implicated in a host of cellular processes, but its physiological functions remain uncertain due to the lack of specific loss-of-function studies (13). In the present study using two lines of Cre drivers to delete RhoA in the midbrain or forebrain early in development, we showed that RhoA deficiency resulted in disruption of adherens junctions, hyperproliferation of neural progenitors, and brain dysplasia. These results demonstrated that RhoA has important and indispensable functions in mammalian brain development.
RhoA Is Indispensable for the Adherens Junction Formation in the Developing Brain.
The neuroepithelium of developing mammalian brains has a string of adherens junctions that connects neural progenitors at the ventricular surface, similar to apical epithelial junctions (4). The main building blocks of adherens junctions are cadherins and catenins, and in the developing mammalian brains, the critical components are N-cadherin, β-catenin, and αE-catenin, according to their unique gene-expression patterns and null-mutation phenotypes (5). In addition, cell-culture studies suggest that small Rho GTPases—especially Rac1, Cdc42, and RhoA—are important components of adherens junctions, regulating the interactions between catenins and the cytoskeleton (11, 12). In our study, we showed that RhoA is concentrated at the apical surface of developing brains and colocalized with cadherin–catenin proteins. RhoA-deleted embryonic brain showed disruption of adherens junctions and severe dysplasia, similar to the phenotypes of αE-catenin, β-catenin, and N-cadherin mutant mice (6, 24, 30). These results together with the finding that RhoA physically interacts with β-catenin strongly suggest that RhoA is an integral and indispensable component of adherens junctions in the developing mammalian brain.
Differential Roles of RhoA and Cdc42 in Adherens Junctions of Neural Progenitors.
Although Cdc42 deletions in previous reports also showed their essential roles in adherens junctions in the nervous system (14, 15), there are also significant differences in the phenotypes of RhoA and Cdc42 deletions, even when the same Cre driver (i.e., Foxg1-Cre mice) was used. In Foxg1-Cdc42 null embryos, the disappearance of apical cadherin–catenin proteins started as early as E9.5–E10.5, causing holoprosencephaly due to failure to expand the telencephalon (15). In contrast, RhoA deletion by Foxg1-Cre or Wnt1-Cre drivers caused a gradual disruption of apical cadherin–catenin protein localization between E11.5 and E14.5. Although we cannot exclude the possibility that the distinct phenotypes may be in part due to different amounts of residual RhoA and Cdc42 protein after Cre/loxP recombination, it is likely that Cdc42 has essential functions for the initial establishment of apical adherens junctions, whereas RhoA is essential for their maintenance in the developing mammalian brain. This interpretation is in keeping with the notion that different small Rho GTPases are involved in different steps of the adherens junction formation, expansion, and plasticity (11, 12).
Dysregulated Proliferation of RhoA-Deficient Neural Progenitor Cells.
During brain development, proliferation, differentiation, and cell death of neural progenitor cells are tightly regulated to produce the organ of predetermined size (1–3). Recent studies using mutant mice have shown the coincidence of defects in adherens junctions and hyperproliferation of neural progenitor cells, indicating the involvement of adherens junctions in this process (5). For example, deletion of αE-catenin in the developing cerebral cortex leads to defects in adherens junctions, massive disorganization of the neuroepithelium, and hyperproliferation of neural progenitor cells (6). Although the molecular mechanisms that link adherens junctions and proliferation control remain unclear, recent evidence suggests the involvement of major developmental signaling pathways as a cause of hyperproliferation (5). In αE-catenin mutant mice, the expression of Gli1 and Fgf15, targets of the hedgehog pathway, was up-regulated, and the activation of the hedgehog signaling pathway is likely to explain the hyperproliferation of neural progenitors (6). We found that the expression of Gli1 and Fgf15 was markedly increased in RhoA-CKO embryos, similar to that in the αE-catenin null mutants. Importantly, the hedgehog pathway plays a critical role in brain development and cancer, and Gli1 promotes proliferation of neural progenitor cells in the developing brain (31, 32). Thus, the hyperproliferation of neural progenitor cells in RhoA-CKO embryos may be caused, at least in part, by hyperactivation of the hedgehog pathway.
In conclusion, the present study identifies RhoA as a critical protein for the maintenance of adherens junctions and constrained proliferation of neural progenitors in the developing mammalian brain (Fig. S7).
Materials and Methods
Animals.
Mice harboring conditional RhoA alleles, in which exon 3 is flanked by loxP sites, were generated as described in Fig. S1.
Histological Analysis.
Nissl staining, in situ hybridization, and immunohistochemistry were performed on frozen sections. Brains of embryos were fixed in 4% paraformaldehyde, cryoprotected in 30% sucrose, embedded in OCT compound, and sectioned at 16 μm. Antibodies used in this study are listed in Table S2. To detect apoptotic cells, we carried out the TUNEL method using the ApopTag fluorescein in situ apoptosis detection kit (Millipore). We also used phalloidin-tetramethylrhodamine B isothiocyanate (Sigma) and ToPro3 or DAPI (Molecular Probes) to visualize F-actin and nuclei, respectively. Images were taken by an Axio Imager Z1 microscope and an LSM510 confocal microscope (Carl Zeiss).
Quantitation of Cell Differentiation, Mitosis, Proliferation, Cell-Cycle Exit, and Cell Death.
BrdU labeling and analysis of cell-cycle exit were performed as described (6, 8, 26). For quantitation of the data, immunohistochemical images were taken by an Axio Imager Z1 microscope (Carl Zeiss) and the total numbers of cells and cells stained with indicated antibodies were counted using AxioVision software (Carl Zeiss).
Immunoprecipitation.
Midbrains from E13.5 embryos were lysed in tissue lysis buffer [20 mM Tris-HCl (pH 7.6), 100 mM NaCl, 10 mM MgCl2, 1% Triton-X-100, 0.2% sodium deoxycholate, 2 mM phenylmethylsulphonyl fluoride, 100 nM okadaic acid, and a mixture of protease inhibitors]. Supernatants were exposed to the antibodies. Then, the immunocomplexes were captured by adding Protein A/G-agarose beads (Santa Cruz Biotechnology).
RNA Extraction and Reverse Transcription–Quantitative PCR.
Midbrains were dissected out from E13.5 embryos and total RNA was extracted using the RNeasy Micro Kit (Qiagen). Reverse transcription was performed with SuperScript III reverse transcriptase (Invitrogen). Real-time PCR was performed with QuantiTect (Qiagen) by the CFX96 Real-Time System (Bio-Rad). The intensity relative to β-actin was calculated, and the fold change relative to the relative intensity in control embryos is presented. Primers were designed by Primer3 (http://frodo.wi.mit.edu/primer3/).
Supplementary Material
Acknowledgments
The authors are grateful to Dr. Masato Nakafuku and Dr. Akira Nagafuchi for providing us with antibodies. The authors thank Dr. Fumiyasu Imai for critical comments on the manuscript. K.-i.K. is supported by Japan Society for the Promotion of Science Postdoctoral Fellowships for Research Abroad.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1101347108/-/DCSupplemental.
References
- 1.Haydar TF, Kuan CY, Flavell RA, Rakic P. The role of cell death in regulating the size and shape of the mammalian forebrain. Cereb Cortex. 1999;9:621–626. doi: 10.1093/cercor/9.6.621. [DOI] [PubMed] [Google Scholar]
- 2.Rakic P. Evolution of the neocortex: A perspective from developmental biology. Nat Rev Neurosci. 2009;10:724–735. doi: 10.1038/nrn2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joseph B, Hermanson O. Molecular control of brain size: Regulators of neural stem cell life, death and beyond. Exp Cell Res. 2010;316:1415–1421. doi: 10.1016/j.yexcr.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Farkas LM, Huttner WB. The cell biology of neural stem and progenitor cells and its significance for their proliferation versus differentiation during mammalian brain development. Curr Opin Cell Biol. 2008;20:707–715. doi: 10.1016/j.ceb.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Stepniak E, Radice GL, Vasioukhin V. Adhesive and signaling functions of cadherins and catenins in vertebrate development. Cold Spring Harb Perspect Biol. 2009;1:a002949. doi: 10.1101/cshperspect.a002949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lien WH, Klezovitch O, Fernandez TE, Delrow J, Vasioukhin V. alphaE-catenin controls cerebral cortical size by regulating the hedgehog signaling pathway. Science. 2006;311:1609–1612. doi: 10.1126/science.1121449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li HS, et al. Inactivation of Numb and Numblike in embryonic dorsal forebrain impairs neurogenesis and disrupts cortical morphogenesis. Neuron. 2003;40:1105–1118. doi: 10.1016/s0896-6273(03)00755-4. [DOI] [PubMed] [Google Scholar]
- 8.Klezovitch O, Fernandez TE, Tapscott SJ, Vasioukhin V. Loss of cell polarity causes severe brain dysplasia in Lgl1 knockout mice. Genes Dev. 2004;18:559–571. doi: 10.1101/gad.1178004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rasin MR, et al. Numb and Numbl are required for maintenance of cadherin-based adhesion and polarity of neural progenitors. Nat Neurosci. 2007;10:819–827. doi: 10.1038/nn1924. [DOI] [PubMed] [Google Scholar]
- 10.Mège RM, Gavard J, Lambert M. Regulation of cell-cell junctions by the cytoskeleton. Curr Opin Cell Biol. 2006;18:541–548. doi: 10.1016/j.ceb.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Samarin S, Nusrat A. Regulation of epithelial apical junctional complex by Rho family GTPases. Front Biosci. 2009;14:1129–1142. doi: 10.2741/3298. [DOI] [PubMed] [Google Scholar]
- 12.Harris TJ, Tepass U. Adherens junctions: From molecules to morphogenesis. Nat Rev Mol Cell Biol. 2010;11:502–514. doi: 10.1038/nrm2927. [DOI] [PubMed] [Google Scholar]
- 13.Heasman SJ, Ridley AJ. Mammalian Rho GTPases: New insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- 14.Cappello S, et al. The Rho-GTPase cdc42 regulates neural progenitor fate at the apical surface. Nat Neurosci. 2006;9:1099–1107. doi: 10.1038/nn1744. [DOI] [PubMed] [Google Scholar]
- 15.Chen L, et al. Cdc42 deficiency causes Sonic hedgehog-independent holoprosencephaly. Proc Natl Acad Sci USA. 2006;103:16520–16525. doi: 10.1073/pnas.0603533103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L, et al. Rac1 controls the formation of midline commissures and the competency of tangential migration in ventral telencephalic neurons. J Neurosci. 2007;27:3884–3893. doi: 10.1523/JNEUROSCI.3509-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu AX, Rane N, Liu JP, Prendergast GC. RhoB is dispensable for mouse development, but it modifies susceptibility to tumor formation as well as cell adhesion and growth factor signaling in transformed cells. Mol Cell Biol. 2001;21:6906–6912. doi: 10.1128/MCB.21.20.6906-6912.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hakem A, et al. RhoC is dispensable for embryogenesis and tumor initiation but essential for metastasis. Genes Dev. 2005;19:1974–1979. doi: 10.1101/gad.1310805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson B, et al. RhoA is dispensable for skin development, but crucial for contraction and directed migration of keratinocytes. Mol Biol Cell. 2011;22:593–605. doi: 10.1091/mbc.E09-10-0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamada S, Nelson WJ. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell-cell adhesion. J Cell Biol. 2007;178:517–527. doi: 10.1083/jcb.200701058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bloor JW, Kiehart DP. Drosophila RhoA regulates the cytoskeleton and cell-cell adhesion in the developing epidermis. Development. 2002;129:3173–3183. doi: 10.1242/dev.129.13.3173. [DOI] [PubMed] [Google Scholar]
- 22.Magie CR, Pinto-Santini D, Parkhurst SM. Rho1 interacts with p120ctn and alpha-catenin, and regulates cadherin-based adherens junction components in Drosophila. Development. 2002;129:3771–3782. doi: 10.1242/dev.129.16.3771. [DOI] [PubMed] [Google Scholar]
- 23.Hsu W, Mirando AJ, Yu HM. Manipulating gene activity in Wnt1-expressing precursors of neural epithelial and neural crest cells. Dev Dyn. 2010;239:338–345. doi: 10.1002/dvdy.22044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kadowaki M, et al. N-cadherin mediates cortical organization in the mouse brain. Dev Biol. 2007;304:22–33. doi: 10.1016/j.ydbio.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 25.Hébert JM, McConnell SK. Targeting of cre to the Foxg1 (BF-1) locus mediates loxP recombination in the telencephalon and other developing head structures. Dev Biol. 2000;222:296–306. doi: 10.1006/dbio.2000.9732. [DOI] [PubMed] [Google Scholar]
- 26.Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- 27.Ohtsuka T, et al. Visualization of embryonic neural stem cells using Hes promoters in transgenic mice. Mol Cell Neurosci. 2006;31:109–122. doi: 10.1016/j.mcn.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Glickstein SB, Alexander S, Ross ME. Differences in cyclin D2 and D1 protein expression distinguish forebrain progenitor subsets. Cereb Cortex. 2007;17:632–642. doi: 10.1093/cercor/bhk008. [DOI] [PubMed] [Google Scholar]
- 29.Ellis P, et al. SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Dev Neurosci. 2004;26:148–165. doi: 10.1159/000082134. [DOI] [PubMed] [Google Scholar]
- 30.Machon O, van den Bout CJ, Backman M, Kemler R, Krauss S. Role of beta-catenin in the developing cortical and hippocampal neuroepithelium. Neuroscience. 2003;122:129–143. doi: 10.1016/s0306-4522(03)00519-0. [DOI] [PubMed] [Google Scholar]
- 31.Ruiz i Altaba A, Palma V, Dahmane N. Hedgehog-Gli signalling and the growth of the brain. Nat Rev Neurosci. 2002;3:24–33. doi: 10.1038/nrn704. [DOI] [PubMed] [Google Scholar]
- 32.Palma V, Ruiz i Altaba A. Hedgehog-GLI signaling regulates the behavior of cells with stem cell properties in the developing neocortex. Development. 2004;131:337–345. doi: 10.1242/dev.00930. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.