Abstract
Nicotine is the primary psychoactive component of tobacco. Its reinforcing and addictive properties depend on nicotinic acetylcholine receptors (nAChRs) located within the mesolimbic axis originating in the ventral tegmental area (VTA). The roles and oligomeric assembly of subunit α4- and subunit α6-containing nAChRs in dopaminergic (DAergic) neurons are much debated. Using subunit-specific knockout mice and targeted lentiviral re-expression, we have determined the subunit dependence of intracranial nicotine self-administration (ICSA) into the VTA and the effects of nicotine on dopamine (DA) neuron excitability in the VTA and on DA transmission in the nucleus accumbens (NAc). We show that the α4 subunit, but not the α6 subunit, is necessary for ICSA and nicotine-induced bursting of VTA DAergic neurons, whereas subunits α4 and α6 together regulate the activity dependence of DA transmission in the NAc. These data suggest that α4-dominated enhancement of burst firing in DA neurons, relayed by DA transmission in NAc that is gated by nAChRs containing α4 and α6 subunits, underlies nicotine self-administration and its long-term maintenance.
Keywords: electrophysiology, lentivirus, nicotinic receptor, voltammetry, ventral striatum
Nicotine, the principal addictive component of tobacco smoke, is responsible for tobacco abuse, the leading cause of preventable morbidity and mortality, and referred to as an epidemic by the World Health Organization (1). More than 5 million people are expected to die every year from the consequences of nicotine addiction, and some 600,000 die from the consequences of secondhand smoke. The underlying neurobiological mechanisms of addiction are complex (2), and further work is required to identify novel smoking cessation targets (3). Nicotine exerts its reinforcing effects through its action on nicotinic acetylcholine receptors (nAChRs), a heterogeneous family of pentameric, ligand-gated ion channels (4, 5). nAChRs situated in the mesolimbic reward system mediate nicotine-induced dopamine (DA) release in the nucleus accumbens from midbrain dopaminergic (DAergic) neurons located in the ventral tegmental area (VTA) (6, 7). Among the different nAChRs expressed in this region, β2-containing nAChRs (β2* nAChRs) have been shown to play a crucial role in the positive rewarding properties of nicotine (8–10).
Nicotine modifies DA neuron excitability (11, 12) and switches activity from tonic to bursting, increasing striatal DA release (8, 13, 14). However, this somatic action in VTA is not exclusive. Nicotine also modulates striatal DA release probability by action on presynaptic nAChRs (15–18). However, there is little consensus about which of the varied array of possible oligomers of β2* nAChRs in DA soma and terminals, particularly those containing the α4 and α6 nAChR subunits, participate in nicotine reinforcement.
There is some evidence for roles of both α4*-containing (α4*) and α6-containing (α6*) nAChRs in vivo. In mice with α4 deletion (α4−/− mice), there is a 100% increase in basal striatal DA tone but a disappearance of both nicotine-elicited DA release, as measured by microdialysis (19), and i.v. self-administration (IVSA) (10). Modified α4* nAChRs that have a gain of function indicate an important role for this subunit in nicotine-induced reward, tolerance, and sensitization (20). However, there also is considerable evidence for dependence on α6 subunits. In α6−/− mice, IVSA is abolished (10), although, intriguingly, DA release in the nucleus accumbens (NAc) elicited by systemic nicotine is intact, as measured by microdialysis (21). In addition, α6* nAChRs within the NAc play a key role in regulating the sensitivity of DA release to presynaptic activation, measured with fast-scan cyclic voltammetry (15, 17). Recent studies in rats also suggest a role for α6* nAChRs in somatic (22) or terminal (23) regions during systemic self-administration of nicotine. Finally, modified α6 subunits, α6(L9′S), that have a gain of function suggest a potentially important role for the α6 subunit in the regulation of DA neuron firing and axonal release within dorsal striatum (24, 25). However, it has been unclear how these two key subunits, α4 and α6, in their native form, might be jointly important for nicotine dependence.
Results
Intracranial Self-Administration of Nicotine and Nicotine-Elicited Enhancement of DA Neuron Firing in the VTA Are Dependent on α4* but Not α6* nAChRs.
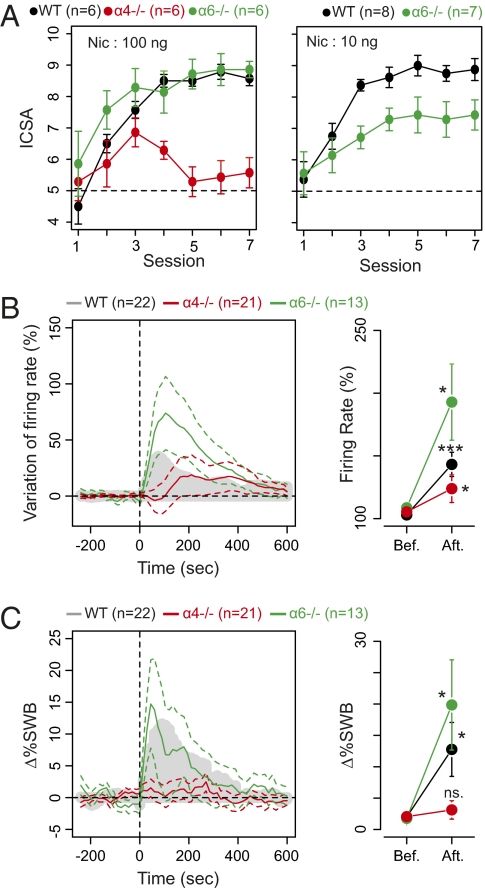
Mice lacking α6 or α4 subunits do not develop self-administration with IVSA (10). To address specifically the role of nAChRs at the level of DA somata, we compared the intracranial self-administration (ICSA) of nicotine into the VTA in WT mice and mice with targeted deletion of subunits. Sustained ICSA of nicotine (100 ng) into the VTA was readily acquired in WT mice over three sessions. Mice with α6 deletion (α6−/− mice) self-administered nicotine at this dose in a manner similar to WT (Fig. 1A Left). In α4−/− mice, however, the initial approach toward the nicotine-reinforced arm of the Y-maze did not lead to steady ICSA beyond session 3 and returned to chance level by session 4 (Fig. 1A Left). In contrast, α6−/− mice exhibited a WT-like ICSA response for the 100-ng nicotine dose. However, self-administration of the lower dose of nicotine (10 ng) was supported to a lesser extent than in WT mice (Fig. 1A Right).
Fig. 1.
Long-term nicotine ICSA is dependent on α4* nAChRs, and DA neurons in α4−/− mice display abolished bursting response to nicotine injection. (A) (Left) Intra-VTA nicotine self-administration in WT (black), α4−/− (red), and α6−/−green) mice: Number of self-administrations per daily session expressed as mean ± SEM. A1–A7 are nicotine self-administration trials, with 100 ng nicotine per self-administered dose. α6−/− mice exhibit normal self-administration [three-way ANOVA, genotype, drug, and session effects; genotype: F(3.561) = 0.544, P = 0.46; drug: F(354.073) = 54.089, df = 6, P < 0.001], whereas α4−/− mice do not maintain self-administration [three-way ANOVA, genotype, drug, and session effects; genotype: F(79.147) = 32.582, df = 6, P < 0.000]. (Right) Self-administration at 10 ng in WT and α6−/− mice. As on the left with 10 ng nicotine as salt per self-administered dose. (B) Firing frequency modification. (Left) Mean ± SEM of increased responses in firing frequency. (Right) Representation of the maximum (mean ± SEM) response in firing rate. In individual cells variation of the maximum firing rate from baseline ranged from −10 to 149%, with a mean of +40%, in WT mice (gray mask); from −18 to 203%, with a mean of +18%, in α4−/− mice (red trace), and from −31 to 340%, with a mean of +84%, in α6−/− mice (green trace). (C) Bursting: presentation as in B. Variation in percentage of spike within burst (%SWB; Materials and Methods) ranged from −3 to +53% with a mean of +10%. An increase in frequency was seen in 18 of 22 cells, and bursting activity was increased, or equal, in 18 of 22 cells. In cells from α4−/− mice variation in %SWB ranged from −12 to 16% with a mean of +1%. An increase in frequency was seen in 14 of 21 cells, whereas bursting activity was raised in 15 of 21 cells. In cell from α6−/− mice, variation in %SWB ranged from −6.6 to 60% with a mean of +18%. An increase in frequency was seen in 10 of 13 cells, whereas bursting activity was enhanced in 11 of 13 cells (Wilcoxon signed rank test comparison between baseline and nicotine response; *P < 0.05, ***P < 0.001).
We then used electrophysiological recordings to investigate how selective deletion of the α4 and α6 subunits modified nicotine-elicited changes in VTA neuron activity using electrophysiological recordings (see SI Materials and Methods for DA cell identification and activity analysis and Fig. S1 for spontaneous activity analysis). DA cells fired with slow and regular, irregular, or burst firing patterns (SI Materials and Methods). Bursts were identified as discrete events consisting of a sequence of spikes with (i) burst onset defined by two consecutive spikes within an interval <80 ms or (ii) burst termination defined by an interspike interval >160 ms (26, 27). Systemic administration of nicotine (30 μg/kg) in vivo resulted in a rapid and pronounced increase in firing rate in WT mice and in α6−/− mice (Fig. 1B). In α4−/− mice, nicotine-elicited increases in firing rate were delayed (∼100 s) and markedly attenuated compared with those in WT mice (Wilcoxon rank sum test; W = 147, P = 0.04163; Fig. 1B). Bursting activity (assessed as the percentage of spikes within a burst, %SWB) (SI Materials and Methods) increased after nicotine application in WT and α6−/− mice (Fig. 1C). There was no statistical difference between WT and α6−/− mice (Wilcoxon rank sum test; W = 163, P = 0.51 for firing rate; W = 172, P = 0.3277 for %SWB). However, in α4−/− mice, although firing rate increased after nicotine, there was no associated increase in %SWB, suggesting that the loss of ICSA in these animals correlates with a lack of burst firing activity and with reduced changes in average firing rate.
Both α6 and α4 Subunits Contribute to nAChR Control of DA Release in the NAc: Loss of Either Subunit Promotes Frequency Sensitivity of Release.
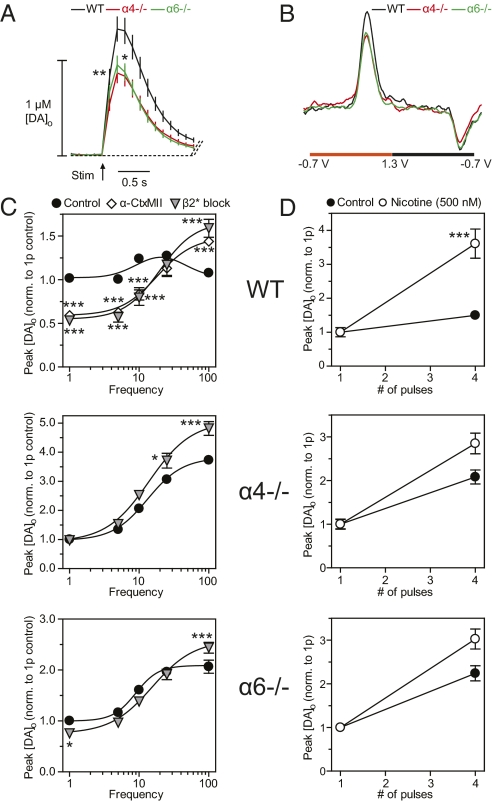
Besides their function in controlling DA neuron excitability in the VTA, α4 or α6 subunits also might play a major role in influencing DA release directly within the NAc through nAChRs on DA axons. In acute striatal slices of NAc, DA release probability is strongly modulated by endogenous ACh or nicotine acting at striatal nAChRs (15, 17, 18). The extracellular DA concentration ([DA]o) detected using fast-scan cyclic voltammetry at a carbon-fiber microelectrode after a single-pulse stimulus (0.2 ms) in the NAc was 1.35 ± 0.13 μM in WT mice (Fig. 2A), but was ∼30% lower in either α6−/− or in α4−/− mice (Fig. 2 A and B) (one-way ANOVA, Bonferroni t test; P < 0.05; n = 35). We explored the frequency sensitivity of DA release, using trains of four pulses at 1–100 Hz. In WT controls, DA release exhibited an inverted U-shaped dependence on frequency which was modified by an α6*nAChR antagonist, α-conotoxin MII (α-CtxMII; 30 nM), to promote significantly the contrast between DA signals released by low- vs. high-frequency stimuli (Fig. 2C Top), as reported previously (15). Antagonism of other nAChRs using a broad-spectrum antagonist (DHβE; 1 μM) did not further modify DA release. There were profound changes in the frequency sensitivity of DA transmission in control conditions in either α6−/− or α4−/− mice: DA release demonstrated a strong activity dependence that was similar to that seen in WT mice after full nAChR inhibition or desensitization by nicotine (Fig. 2C and refs. 15 and 17). Furthermore, inhibition of β2* nAChRs remaining in these knockout mice using DHβE (1 μM) had only a modest effect (Fig. 2C Middle and Bottom). We also tested whether nicotine itself (500 nM) could further promote the frequency sensitivity of DA release in α4−/− or α6−/− mice. In a simplified stimulus protocol that compared [DA]o evoked by one vs. four pulses at 100 Hz, nicotine (500 nM) in WT mice increased the contrast between [DA]o evoked by burst vs. single-pulse stimuli (Fig. 2D), a result that is consistent with an action via nAChR desensitization (17, 18). However, in both α4−/− and α6−/− mice, nicotine did not significantly promote the already strong activity dependence seen in control conditions (Fig. 2D). These data suggest that nAChR regulation of DA transmission in the NAc requires both α6 and α4 subunits together, i.e., α4α6* nAChRs. In other experiments, we explored whether a role for any α6(non-α4) or α4(non-α6) nAChRs remaining after subunit deletion could be revealed by boosting extracellular ACh. However, these experiments did not provide any evidence that independent α6* and α4* nAChRs can play significant roles (SI Materials and Methods: nAChR control of striatal DA transmission and Fig. S2).
Fig. 2.
In the NAc both the α4 and α6 subunits are necessary to maintain nicotine-sensitive cholinergic regulation of DA release. (A) Averaged profiles of extracellular [DA]o ± SEM evoked by a single pulse in WT (black), α4−/− (red), and α6−/− (green) mice from different recording sites (n = 35–36) in the NAc. One-way ANOVA for genotype, *P < 0.05, **P < 0.01. (B) Representative voltammograms used to identify DA following a single-stimulus pulse in A. DA is identified by characteristic oxidation and reduction peak potentials at 0.6 V and −0.2 V, respectively, vs. Ag/AgCl. (C) Mean peak [DA]o ± SEM vs. frequency during four-pulse trains (1–100 Hz) in WT (Top), α4−/− (Middle), and α6−/− mice (Bottom). Evoked [DA]o was recorded in drug-free control (●), α-CtxMI (◇), or nonspecific β2* block ( ). All data were normalized to one-pulse (1p) controls. (Post hoc t tests; *P < 0.05, ***P < 0.001 vs. controls; n = 9.) (D) Mean peak [DA]o ± SEM vs. pulse number for 100-Hz stimuli in the presence (○) or absence (●) of nicotine (500 nM) from recording sites (n = 32–36). All data were normalized to 1p. (Post hoc t tests; ***P < 0.001 vs. controls.)
). All data were normalized to one-pulse (1p) controls. (Post hoc t tests; *P < 0.05, ***P < 0.001 vs. controls; n = 9.) (D) Mean peak [DA]o ± SEM vs. pulse number for 100-Hz stimuli in the presence (○) or absence (●) of nicotine (500 nM) from recording sites (n = 32–36). All data were normalized to 1p. (Post hoc t tests; ***P < 0.001 vs. controls.)
Targeted Re-expression of α4 Restores Short-Term ICSA and Nicotine-Sensitive Bursting Properties of VTA DA Neurons.
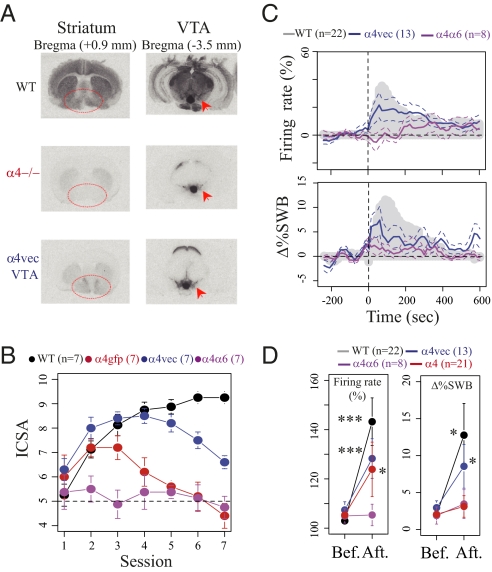
To explore further the dependence of ICSA on α4* nAChRs and the correlation with the ability of nicotine to elicit bursting activity, we used lentiviral re-expression of the α4 subunit in the VTA of α4−/− mice (denoted “α4vec”) to explore whether this treatment restored ICSA and/or the effect of nicotine on %SWB. Lentiviral re-expression was performed as described previously (8, 10). It restored nAChR binding (Fig. 3A) and nAChR control of DA release in the NAc (Fig. S3). Furthermore, re-expression of α4 in the VTA significantly prolonged ICSA. Nicotine self-administration rates fell only after session 5 (two-way ANOVA, effect of re-expression: F(2,20) = 12.07, P < 0.001; re-expression × session interaction: F(6,120) = 11.31, P < 0.001) (Fig. 3B). In addition, α4vec expression at least partially restored nicotine-elicited increases in firing rate after systemic injection (Δmean = 20.8%, Wilcoxon signed rank test, P < 0.001, n = 13) (Fig. 3 C and D). Re-expression also reduced the delay to maximum increase compared with α4−/− (Fig. 1B) and, importantly, significantly increased the %SWB (Δmean = 5.6%, Wilcoxon signed rank test, P = 0.032) (Fig. 3 C and D). These results demonstrate that although the VTA α4 subunit is not sufficient to restore ICSA completely in the long term, it is crucial to reward-relevant bursting activity of DA cells in the VTA. In the NAc, α4vec re-expression appeared to restore nAChR control of DA release. In particular, the contrast between [DA]o evoked by high- vs. low-frequency stimuli was significantly attenuated (Fig. S3); furthermore, α-CtxMII sensitivity was restored with no additive effect of DHβE, suggesting that the reintroduced α4 subunits reform native α6α4β2* nAChRs and that the function of α4β2* receptors without α6 subunits is limited, as in WT.
Fig. 3.
Re-expression of the α4 subunit in the VTA of α4−/− mice partially restored bursting and ICSA. (A) Re-expression of the α4 subunit in the VTA results in an increase in 125I-epibatidine binding in the NAc. Increased binding of 125I-epibatidine after α4 re-expression in α4−/− mice shown in the NAc (circled) compared with increased binding in the VTA (arrow). (B) Intra-VTA nicotine self-administration in WT (black), α4GFP−/− (red), α4vec−/− (blue), and α4α6−/− (magenta) mice. The number of self-administrations per daily session is expressed as mean ± SEM. A1–A7 are nicotine self-administration trials, with 100 ng nicotine as salt per self-administered dose. (C) Effect of nicotine injection on DA neurons of WT (gray mask), α4vec (blue trace), and α4α6−/− mice (magenta trace). Mean ± SEM of increased responses in firing frequency (Upper) and in percentage of spikes within burst (%SWB) (Lower). (D) Representation of the maximum (mean ± SEM) of the response in firing rate (Left) and in %SWB (Right). In α4vec mice, variation of the maximum firing rate from baseline in individual cells ranged from 0.6–65%, with a mean of +20%. The variation in %SWB ranged from −4 to 28% with a mean of +5.6%. An increase in frequency was seen in 13 of 13 cells, whereas bursting activity was enhanced in 12 of 13 cells. In α4α6−/− mice, variation of the maximum from baseline in individual cells ranged from −29 to 14%, with a mean of 0.4% in firing rate. The variation in %SWB ranged from −1 to 10% with a mean of +1.5%. Five of eight cells showed an increase in frequency, whereas bursting activity was enhanced in six of eight cells (Wilcoxon signed rank test; *P < 0.05; ***P < 0.001).
Finally, given the transient ability of α4−/− mice to self-administer nicotine in early training sessions (Figs. 1A and 3A) and the partial sensitivity of ICSA to the availability of the α6 subunit (Fig. 1B), we explored whether the behavioral and physiological effects of nicotine in α4−/− mice might be caused by the presence of α6 subunits by testing the effects of combined deletion of both α4 and α6 subunits. In α6α4−/− mice, both approach and learning phases of ICSA were abolished, and firing activity and changes to %SWB (n = 8) were completely insensitive to systemic application of nicotine (Figs. 3 B and D).
Discussion
Our results define distinct contributions of the α4 and α6 nAChR subunits in nicotine's reinforcing properties. We first show profound differences between intra-VTA and systemic nicotine self-administration in α4−/− and α6−/− mice. We propose that these differences may be related to the distinct contribution of α4* and α6* nAChRs in the soma vs. axon terminals (NAc) of VTA DA neurons. Indeed, we present evidence that the stability of intra-VTA nicotine self-administration is related to the bursting properties of DA cells and that α4* nAChRs in VTA neurons are critical for burst generation and long-term stability of ICSA, but α6* nAChRs are not. Finally, we show that nAChR control of striatal DA transmission in the NAc is dependent on a specific population of α6α4β2* nAChRs.
α6 Has a Modulatory Role in the Outcome of Nicotine's Action in the VTA.
The α6 subunit has been shown to be necessary for systemic IVSA (10), but during intra-VTA self-administration the role of α6* receptors revealed here is complex. ICSA was attenuated in α6−/− mice when presented with the lowest but not with higher doses of nicotine, but ICSA was eliminated in α4α6−/− mice, including an early phase of ICSA that did remain in α4−/− mice, presumably mediated via α6* nAChRs. These data suggest that native α6* nAChRs in VTA are not necessary for intra-VTA ICSA but nonetheless are consistent with the view that α6* nAChRs do have a role in nicotine reinforcement (22, 23). Our data correspondingly indicate slight, albeit not statistically significant, changes in the amplitude and duration of nicotine-evoked responses of VTA DA neurons in α6−/− mice as well as changes in the NAc in the dynamic frequency filtering of DA release probability.
The two nicotine self-administration protocols, IVSA and ICSA, necessarily involve nAChRs with different distributions. Systemic nicotine acts on nAChRs in the VTA and at α4α6* nAChRs on projections in the NAc, a key component in the dynamic frequency filtering of DA release probability. Furthermore, the published IVSA protocol is based on the ability to respond to nicotine by nose-poking during a 20-min session and, unlike the ICSA protocol used here, does not involve learning (28, 29). The single 20-min sessions involved in IVSA paradigms would be dominated by the acute psychomotor stimulant action of nicotine, in which the α6 subunit is required at the axon terminal level (24). We provide direct evidence using ICSA that the role of α6* nAChRs in nicotine reinforcement may be particularly important during the initial phase of reward, leading mice to approach a location or a cue associated with nicotine delivery (see below).
nAChR Control of Striatal DA Transmission in the NAc Is Dependent on a Population of α6α4β2* nAChRs.
We show that in the NAc both α4 and α6 subunits play a major role in influencing DA release by regulating the contrast in DA signals caused by burst vs. nonburst activity. It was demonstrated previously that this control is α6 dependent (15), and the apparent codependence on both α6 and α4 subunits revealed by the current study indicates that α6α4β2* nAChRs are those specifically involved. Interestingly, recent studies in the adjacent dorsal striatum, the caudate putamen (CPu), using expression of a nonnative α6(L9′S), which has gain of function, suggest that α6α4* nAChRs can be made to participate in the axonal control of DA release in the CPu (25). However, under native conditions, endogenous α6 subunits have been shown to play a more limited role in the control of DA transmission in the CPu than in the NAc (15).
In the NAc in α6−/− and α4−/− mice, there is enhanced frequency sensitivity of DA release, which also is thought to occur in WT genotypes after nAChR desensitization in the presence of systemic nicotine (17, 30) or in response to synchronized pauses in striatal cholinergic interneuron (ChI) activity caused by salient stimuli (31, 32). Under these conditions, changes in burst activity in the VTA will be relayed more faithfully by DA release from axons. Nicotine in the NAc, deletion of nAChR subunits in the NAc, or pauses in ChI firing therefore may have a permissive role in allowing DA to signal changes in DA neuron activity caused by nicotine in the VTA. Thus each of these scenarios ultimately might facilitate the DA-dependent striatal plasticity that underlies striatal learning. Therefore, subunit knockout in the NAc might not prevent but rather be permissive to (or promote) the acquisition of nicotine ICSA, provided that nicotine's action in the VTA is preserved. This scenario is seen in α6−/− mice. The data in α6−/− mice are consistent with previous observations showing that DA release is unaltered after systemic nicotine injections in α6−/− mice, when measured by microdialysis (33). It should be noted, however, that microdialysis measures of DA release may be an integrated function of net DA release and be poorly sensitive to discrete burst firing-induced release, unless uptake is blocked (34).
α4* nAChRs Are Specifically Involved in the Tonic-to-Phasic Transition Evoked by Nicotine.
Our results show that in the VTA, somatodendritic α4* nAChRs are specifically involved in the bursting mechanism of DA neurons and are required to provide bursting adaptation in the tonic/phasic transition that underpins reinforcement. DA neurons exhibit two distinct patterns of activity, bursting and regular spiking (35). Burst firing is associated with anticipatory and unexpected phases of reward (36, 37), and disruption of phasic DA impairs conditioned behavioral responses and learning about cues that predict salient events (38). In the context of nicotine addiction, it therefore is crucial to understand how nicotine switches DA cell activity from tonic to phasic. The increase in nicotine-elicited bursts disappears in α4−/− but not in α6−/− mice, and nicotine-elicited bursting in α4−/− mice is partially restored by reintroduction of α4 subunits (a4vec mice). These data support the notion that although a brief increase in firing rate can be the consequence of nicotine action at α6β2* nAChRs on DA cells, an increase in burst firing is caused by activation of α4β2* nAChRs. A current hypothesis posits that α4β2* nAChRs on GABAergic cells in the VTA are important in the regulation of DA neuron activity by nicotine through a mechanism of disinhibition (11). GABAergic neurons express mainly α4β2* nAChRs in WT mice and thus are supposed to be insensitive to nicotine in α4−/− mice. Because bursting is abolished in α4−/− mice, α4β2* nAChRs at the soma of DA and/or GABAergic cells then would be of importance for burst regulation. The re-expression of the α4 subunit in the VTA only partially restores responses to systemic nicotine: The percentage of burst increased but was not comparable to WT or β2-vec responses (8, 14). The differences might be caused by the distribution and/or subunit stoichiometry of the resulting nAChRs.
Contribution of α4* nAChRs in the Stability of the Development of Self-Administration.
The stability of nicotine self-administration relies upon α4* nAChRs, as demonstrated by the peculiar biphasic learning curve observed in α4−/− animals. These mice react to nicotine and are able to display approach behavior toward the nicotine-reinforced arm during the first three sessions. Subsequently, nicotine-seeking behavior is no longer observed, and random choices reappear. This two-phase phenomenon suggests at least two different steps in the normal role of reinforcement in learning. Immediately after stimulus detection, which itself is DA dependent (28), an approach is thought to occur that leads to cue inspection (39). Present in α4−/− mice, this behavior might depend on firing rate activation and/or be permitted by the heightened sensitivity to the activity of DA axon terminals. The following step, termed the “stamping-in” of the stimulus–response association, corresponds to the retroactive impact of reinforcement on behavior and also is thought to be DA dependent (29). Lost in α4−/− mice, this behavior then might depend on burst firing in DA neurons and perhaps on the consequently larger range in [DA]o released transiently in the striatum that would be expected during these higher frequencies.
In conclusion, these data support a differential role for the α4 and α6 subunits in the VTA and the NAc, with α4* nAChRs having a dominant role at the somatic level in burst response and nicotine-induced conditioning.
Materials and Methods
Animals.
Experiments were performed on WT mice (C57BL/6J strain), α4−/−, α6−/−, α4−/− × α6−/− and α4vec nAChR mice age 8–21 wk and weighing 25–35 g. α4−/− and α6−/− mice were generated as described previously (SI Materials and Methods and refs. 21 and 40).
In Vivo Electrophysiology: Extracellular Single-Cell Recordings.
Mice were anesthetized with chloral hydrate, 400 mg/kg i.p., supplemented as required to maintain optimal anesthesia throughout the experiment, and were positioned in a stereotaxic frame (David Kopf). Body temperature was kept at 37 °C by means of a thermostatically controlled heating blanket. All animals had a catheter inserted into the saphenous vein for i.v. administration of nicotine. Procedures for DA cell electrophysiological recording were described previously (14). After a baseline recording of 15–30 min, 10 μL saline (0.9% NaCl) was injected i.v. into the saphenous vein, and after 3–5 min, nicotine (30 μg/kg) was administered i.v. The dose was based on previous studies showing that nicotine can be i.v. self-administered at this dose in mice (41).
DA Cell Identification.
Extracellular identification of DA neurons was based on their location as well as on the set of unique electrophysiological properties that characterize these cells in vivo (SI Materials and Methods). We also labeled some cells with neurobiotin (Fig. 3B) to calculate the risk of misclassifying a non-DA cell as dopaminergic (SI Materials and Methods).
Slice Preparation and Voltammetry.
Coronal striatal slices, 300 μm thick, containing both NAc and CPu were prepared from mice brain of various genotypes using previously described methods (17, 42). [DA]o was monitored at 32 °C in bicarbonate-buffered artificial cerebrospinal fluid (containing 2.4 mM Ca2+) using fast-scan cyclic voltammetry with 10-μm carbon-fiber microelectrodes (tip length ∼50–100 μm, fabricated in-house) and a Millar Voltammeter (PD Systems) as described previously (17, 42). In brief, the scanning voltage was a triangular waveform (−0.7 V to +1.3V range vs. Ag/AgCl) at a scan rate of 800 V/s and a sampling frequency of 8 Hz. The evoked current signal was attributed to DA by comparing the potentials for peak oxidation and reduction currents with those of DA in calibration media (+500–600 and −200 mV vs. Ag/AgCl, respectively). Electrodes were calibrated in 2 μM DA in experimental media. Slice electrical stimulation is described in SI Materials and Methods.
Drugs.
The nicotine solution for in vivo measurements was prepared as follows: 0.5 mM of nicotine tartrate was dissolved in a 0.9% NaCl solution and adjusted to pH 7.2 using NaOH. A preliminary study showed that an i.v. injection of a control solution (0.9% NaCl/0.5 mM of KNa-tartrate) had no effect on the electrophysiological characteristics of DA neurons in WT animals.
Lentiviral Vector.
The lentiviral expression vectors are derived from the pHR′ expression vectors first described by Naldini et al. (43), with several subsequent modifications as indicated by Maskos et al. (8). In the lentivirus used in this study, the bicistronic expression of mouse WT α4 nAChR subunit cDNA and the EGFP cDNA is under the control of the mouse phosphoglycerate kinase promoter (10). For experiments with α4vec mice, controls were α4−/− and WT mice injected with a lentivirus expressing EGFP only. (Details of lentivirus stereotaxic injection are given in SI Materials and Methods.)
125I-Epibatidine Autoradiography.
Coronal sections (20 μm) were incubated at room temperature with 200 pM 125I-epibatidine (Perkin-Elmer) (specific activity 2,200 Ci/mmole) (Perkin-Elmer) in 50 mM Tris, pH 7.4, for 30 min. After incubation, sections were rinsed twice for 5 min in the same buffer and briefly in distilled water. Sections then were exposed to Kodak Biomax films overnight.
Self-Administration Protocol.
To assess the reinforcing effects of nicotine, we used a previously described mouse model of ICSA (8, 44). This model is based on a Y-maze discrimination task between a nicotine-reinforced arm and a neutral arm (SI Materials and Methods).
Statistical Analysis.
Statistical analyses were performed using R, a language and environment for statistical computing (http://www.r-project.org).
Firing pattern quantification.
DA cell firing in vivo was analyzed with respect to the average firing rate and the percentage of spikes within a burst (SI Materials and Methods). To quantify nicotine effect, each cell's activity was rescaled by its baseline value averaged during the 2.5 min before nicotine injection. We used a paired nonparametric Wilcoxon signed rank test to compare firing frequency and %SWB before and after nicotine injection. Nonpaired Wilcoxon tests were used to compare firing frequency and %SWB in two populations (SI Materials and Methods).
Voltammetric detection of dopamine.
Data are expressed as mean ± SEM, and the sample size, n, is the number of observations. Each data set represents results from three or more animals. Comparisons for differences in means were assessed by one- or two-way ANOVA, post hoc multiple comparison t tests (Bonferroni), or unpaired t tests using GraphPad Prism. Curve fitting and linear regressions were performed in GraphPad Prism or SigmaPlot.
Supplementary Material
Acknowledgments
This research was supported by the Institut Pasteur, Centre National de la Recherche Scientifique Unité de Recherche Associée 2182, Unité Mixte de Recherche 7102, and Unité Mixte de Recherche 5228; the Agence Nationale de Recherches programme Neuroscience, Neurology and Psychiatry (2005 and 2009); the Agence Nationale de Recherches Programme BLANC 2009; Medical Research Council (United Kingdom) Grant G0700932; Parkinson's Disease Society (United Kingdom) Grant G0808; and by the European Union Seventh Framework Programme under grant agreement HEALTH-F2-2008-202088 (NeuroCypres project). We also acknowledge financial support from the Network of European Neuroscience Institutes (ENI-NET), the Pierre Fabre Laboratories, and the Fondation Gilbert Lagrue (to N.M.) and the Bettencourt Foundation (to P.F.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1103000108/-/DCSupplemental.
References
- 1.World Health Organization . WHO Report on the Global Tobacco Epidemic. Geneva: WHO; 2009. [Google Scholar]
- 2.Changeux JP. Nicotine addiction and nicotinic receptors: Lessons from genetically modified mice. Nat Rev Neurosci. 2010;11:389–401. doi: 10.1038/nrn2849. [DOI] [PubMed] [Google Scholar]
- 3.Taly A, Corringer PJ, Guedin D, Lestage P, Changeux JP. Nicotinic receptors: Allosteric transitions and therapeutic targets in the nervous system. Nat Rev Drug Discov. 2009;8:733–750. doi: 10.1038/nrd2927. [DOI] [PubMed] [Google Scholar]
- 4.Changeux JP, Edelstein SJ. Nicotinic Acetylcholine Receptors: From Molecular Biology to Cognition. New York: Odile Jacob; 2005. [Google Scholar]
- 5.Corringer PJ, Le Novère N, Changeux JP. Nicotinic receptors at the amino acid level. Annu Rev Pharmacol Toxicol. 2000;40:431–458. doi: 10.1146/annurev.pharmtox.40.1.431. [DOI] [PubMed] [Google Scholar]
- 6.Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 1994;653:278–284. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- 7.Nisell M, Nomikos GG, Svensson TH. Systemic nicotine-induced dopamine release in the rat nucleus accumbens is regulated by nicotinic receptors in the ventral tegmental area. Synapse. 1994;16:36–44. doi: 10.1002/syn.890160105. [DOI] [PubMed] [Google Scholar]
- 8.Maskos U, et al. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature. 2005;436:103–107. doi: 10.1038/nature03694. [DOI] [PubMed] [Google Scholar]
- 9.Picciotto MR, et al. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- 10.Pons S, et al. Crucial role of alpha4 and alpha6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J Neurosci. 2008;28:12318–12327. doi: 10.1523/JNEUROSCI.3918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 12.Mansvelder HD, McGehee DS. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron. 2000;27:349–357. doi: 10.1016/s0896-6273(00)00042-8. [DOI] [PubMed] [Google Scholar]
- 13.Grenhoff J, Aston-Jones G, Svensson TH. Nicotinic effects on the firing pattern of midbrain dopamine neurons. Acta Physiol Scand. 1986;128:351–358. doi: 10.1111/j.1748-1716.1986.tb07988.x. [DOI] [PubMed] [Google Scholar]
- 14.Mameli-Engvall M, et al. Hierarchical control of dopamine neuron-firing patterns by nicotinic receptors. Neuron. 2006;50:911–921. doi: 10.1016/j.neuron.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Exley R, Clements MA, Hartung H, McIntosh JM, Cragg SJ. Alpha6-containing nicotinic acetylcholine receptors dominate the nicotine control of dopamine neurotransmission in nucleus accumbens. Neuropsychopharmacology. 2008;33:2158–2166. doi: 10.1038/sj.npp.1301617. [DOI] [PubMed] [Google Scholar]
- 16.Exley R, Cragg SJ. Presynaptic nicotinic receptors: A dynamic and diverse cholinergic filter of striatal dopamine neurotransmission. Br J Pharmacol. 2008;153(Suppl1):S283–S297. doi: 10.1038/sj.bjp.0707510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci. 2004;7:583–584. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- 18.Zhou FM, Liang Y, Dani JA. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nat Neurosci. 2001;4:1224–1229. doi: 10.1038/nn769. [DOI] [PubMed] [Google Scholar]
- 19.Marubio LM, et al. Effects of nicotine in the dopaminergic system of mice lacking the alpha4 subunit of neuronal nicotinic acetylcholine receptors. Eur J Neurosci. 2003;17:1329–1337. doi: 10.1046/j.1460-9568.2003.02564.x. [DOI] [PubMed] [Google Scholar]
- 20.Tapper AR, et al. Nicotine activation of alpha4* receptors: Sufficient for reward, tolerance, and sensitization. Science. 2004;306:1029–1032. doi: 10.1126/science.1099420. [DOI] [PubMed] [Google Scholar]
- 21.Champtiaux N, et al. Distribution and pharmacology of alpha 6-containing nicotinic acetylcholine receptors analyzed with mutant mice. J Neurosci. 2002;22:1208–1217. doi: 10.1523/JNEUROSCI.22-04-01208.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gotti C, et al. Nicotinic acetylcholine receptors in the mesolimbic pathway: Primary role of ventral tegmental area alpha6beta2* receptors in mediating systemic nicotine effects on dopamine release, locomotion, and reinforcement. J Neurosci. 2010;30:5311–5325. doi: 10.1523/JNEUROSCI.5095-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brunzell DH, Boschen KE, Hendrick ES, Beardsley PM, McIntosh JM. Alpha-conotoxin MII-sensitive nicotinic acetylcholine receptors in the nucleus accumbens shell regulate progressive ratio responding maintained by nicotine. Neuropsychopharmacology. 2010;35:665–673. doi: 10.1038/npp.2009.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drenan RM, et al. In vivo activation of midbrain dopamine neurons via sensitized, high-affinity a6* nicotinic acetylcholine receptors. Neuron. 2008;1:123–126. doi: 10.1016/j.neuron.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drenan RM, et al. Cholinergic modulation of locomotion and striatal dopamine release is mediated by alpha6alpha4* nicotinic acetylcholine receptors. J Neurosci. 2010;30:9877–9889. doi: 10.1523/JNEUROSCI.2056-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: Single spike firing. J Neurosci. 1984;4:2866–2876. doi: 10.1523/JNEUROSCI.04-11-02866.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: Burst firing. J Neurosci. 1984;4:2877–2890. doi: 10.1523/JNEUROSCI.04-11-02877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Redgrave P, Gurney K. The short-latency dopamine signal: A role in discovering novel actions? Nat Rev Neurosci. 2006;7:967–975. doi: 10.1038/nrn2022. [DOI] [PubMed] [Google Scholar]
- 29.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 30.Zhang H, Sulzer D. Frequency-dependent modulation of dopamine release by nicotine. Nat Neurosci. 2004;7:581–582. doi: 10.1038/nn1243. [DOI] [PubMed] [Google Scholar]
- 31.Cragg SJ. Meaningful silences: How dopamine listens to the ACh pause. Trends Neurosci. 2006;29:125–131. doi: 10.1016/j.tins.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 32.Morris G, Arkadir D, Nevet A, Vaadia E, Bergman H. Coincident but distinct messages of midbrain dopamine and striatal tonically active neurons. Neuron. 2004;43:133–143. doi: 10.1016/j.neuron.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 33.Champtiaux N, et al. Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J Neurosci. 2003;23:7820–7829. doi: 10.1523/JNEUROSCI.23-21-07820.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- 35.Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- 37.Tsai H-C, et al. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zweifel LS, et al. Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc Natl Acad Sci USA. 2009;106:7281–7288. doi: 10.1073/pnas.0813415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flagel SB, et al. An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: Implications for addiction. Neuropsychopharmacology. 2010;35:388–400. doi: 10.1038/npp.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marubio LM, et al. Reduced antinociception in mice lacking neuronal nicotinic receptor subunits. Nature. 1999;398:805–810. doi: 10.1038/19756. [DOI] [PubMed] [Google Scholar]
- 41.Picciotto MR, et al. Abnormal avoidance learning in mice lacking functional high-affinity nicotine receptor in the brain. Nature. 1995;374:65–67. doi: 10.1038/374065a0. [DOI] [PubMed] [Google Scholar]
- 42.Cragg SJ. Variable dopamine release probability and short-term plasticity between functional domains of the primate striatum. J Neurosci. 2003;23:4378–4385. doi: 10.1523/JNEUROSCI.23-10-04378.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naldini L, Blömer U, Gage FH, Trono D, Verma IM. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci USA. 1996;93:11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.David V, Besson M, Changeux JP, Granon S, Cazala P. Reinforcing effects of nicotine microinjections into the ventral tegmental area of mice: Dependence on cholinergic nicotinic and dopaminergic D1 receptors. Neuropharmacology. 2006;50:1030–1040. doi: 10.1016/j.neuropharm.2006.02.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.