Abstract
Vasectomy is a well accepted global contraceptive approach frequently associated with epididymal granuloma and sperm autoantibody formation. To understand the long-term sequelae of vasectomy, we investigated the early immune response in vasectomized mice. Vasectomy leads to rapid epithelial cell apoptosis and necrosis, persistent inflammation, and sperm granuloma formation in the epididymis. Vasectomized B6AF1 mice did not mount autoimmune response but instead developed sperm antigen-specific tolerance, documented as resistance to immunization-induced experimental autoimmune orchitis (EAO) but not experimental autoimmune encephalomyelitis. Strikingly, tolerance switches over to pathologic autoimmune state following concomitant CD4+CD25+Foxp3+ regulatory T cell (Treg) depletion: unilaterally vasectomized mice produce dominant autoantibodies to an orchitogenic antigen (zonadhesin), and develop CD4 T-cell– and antibody-dependent bilateral autoimmune orchitis. Therefore, (i) Treg normally prevents spontaneous organ-specific autoimmunity induction by persistent endogenous danger signal, and (ii) autoantigenic stimulation with sterile autoinflammation can lead to tolerance. Finally, postvasectomy tolerance occurs in B6AF1, C57BL/6, and A/J strains. However, C57BL/6 mice resisted EAO after 60% Treg depletion, but developed EAO after 97% Treg reduction. Therefore, variance in intrinsic Treg function—a possible genetic trait—can influence the divergent tolerogenic versus autoimmune response to vasectomy.
Keywords: vasoligation, testis autoantigen, granulomatous inflammation, innate response
Vasectomy is a global contraceptive approach with an annual rate of more than 0.5 million men in the United States (1); therefore, any significantly harmful effect attendant to vasectomy would pose a serious health hazard. Well documented are the development of sperm antibody response in vasectomized men and animals (2, 3), and autoimmune orchitis in vasectomized animals (4). More alarming is a positive statistical correlation with prostate cancer in long-term vasectomized humans (5, 6). Although not confirmed (4), the initial findings have continued to influence clinical decisions regarding vasectomy (7). Despite the uncertainties, studies on the basic mechanism of response to vasectomy have only been proposed (4) but not investigated (1). We now initiate a study with the premise that early immunological changes before sperm antibody detection can modify late events, and may unravel a mechanistic link to human systemic disease.
In animals and humans, sperm epididymal granuloma occurs commonly as a result of extravasated sperm (1), creating a localized endogenous danger signal (8, 9). The postvasectomy autoimmune response has likely resulted from continuous stimulation by exposed sperm antigens coming from the inflamed epididymis. However, the magnitude and incidence of the sperm antibody response vary greatly (30% to >80%), and the onset is often late (6–9 mo) (2, 3). We hypothesize that vasectomy may trigger an immunoregulatory process concomitant with an adaptive immune response to sperm antigens. This process may be T-cell–intrinsic (10) or T-cell–extrinsic, including the CD4+CD25+Foxp3+ regulatory T cells (Tregs) (11).
Treg cells are critical for peripheral tolerance (12). For internal organs, Treg cells may control tolerance in the regional lymph node (LN), where antigen-specific Treg continuously encounter tissue antigens (13–15). This idea has been advanced by two recent observations. Lathrope et al. reported a distinct TCR repertoire among the Tregs in individual LN, but a shared TCR repertoire among naive CD4 Foxp3-negative T cells (16). We showed that the Tregs from normal regional LN were 15 to 50 times more potent than those from nondraining LN in controlling autoimmune disease of the relevant target organs; in contrast, the CD4+CD25-naive T cells from different LNs induce tissue inflammation in the same organs (11). We postulate that LN-specific Tregs maintain tolerance by anticipating local danger signals, and prevent organ-specific autoimmunity. To test this hypothesis, we have investigated vasectomy as a clinically relevant localized danger signal.
The present study has two goals: (i) to elucidate the early immunopathologic events in the first 3 mo after vasectomy that may explain the long-term effects of vasectomy and (ii) to investigate the hypothesis that Tregs control pathogenic autoimmune response to localized endogenous danger signals.
Results
Vasectomy Rapidly Induces Epithelial Cell Necrosis and Tissue Inflammation in the Epididymis Without Sperm Antibody Response.
After vasectomy, sperm production in the testis is unperturbed (17), allowing millions of sperm per day to enter the epididymis, where dramatic changes are detectable (Fig. S1). Within hours, extensive epithelial cell apoptosis and necrosis were followed by sperm extravasation and sperm phagocytosis. By day 7, activated macrophages, dendritic cells, neutrophils, and T cells accumulated to form granuloma. Despite sperm antigen exposure and tissue inflammation, sperm antibodies were undetected in vasectomized (B6 × A/J)F1 (B6AF1), A/J, C57BL/6 (B6), or BALB/c mice for as long as 6 months. Because they are known responders to experimental autoimmune orchitis (EAO) induced by testis antigens in adjuvants (2), we question whether, in vasectomy, stimulation by epididymal sperm antigens (without an adjuvant injection) may elicit immune tolerance instead.
Vasectomy Induces Testis Antigen-Specific Tolerance Despite Sperm Antigen Exposure in the Context of Sterile Tissue Inflammation.
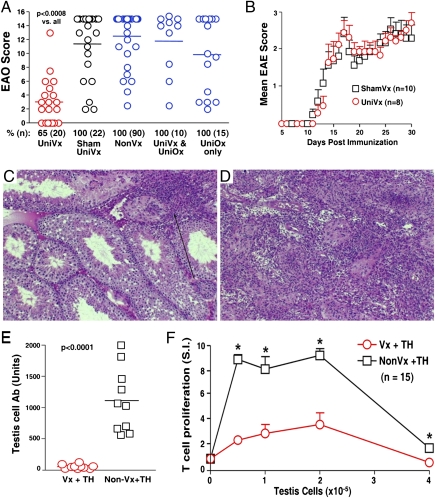
Indeed, unilaterally vasectomized (uni-vx) B6AF1 mice became highly resistant to EAO induction by immunization with testis antigen in adjuvant 3 wk later (Fig. 1A). Compared with control, the orchitis in contralateral testes was mild and infrequent (Fig. 1 A, C, and D); the serum antibody and T-cell response to testis antigens were profoundly reduced (Fig. 1 E and F). Tolerance is testis antigen-specific, as experimental autoimmune encephalomyelitis (EAE) induction was not affected (Fig. 1B). Tolerance is maintained by continuous sperm antigen exposure in the inflamed epididymis, as it was terminated by ipsilateral testis and epididymis ablation at 3 wk (Fig. 1A).
Fig. 1.
Testis antigen-specific tolerance in uni-vx mice. (A) Testicular pathology in mice immunized with testis antigen and adjuvant (TH); tolerance is terminated by ipsilateral testis and epididymis ablation at 3 wk (UniOx, unilateral orchiectomy). (B) Comparable EAE by myelin oligodendrocyte glycoprotein peptide 35–55 immunization in vasectomized or sham-vasectomized mice. Focal orchitis in TH-immunized vasectomized mice (C, arrow), and severe diffuse orchitis in TH-immunized, nonvasectomized mice (D). (H&E stain, magnification of 200×.) Testis antibody (E) and testis antigen-specific T-cell proliferation (F) were determined in TH-immunized uni-vx and nonvasectomized mice (data of three independent studies; *P < 0.02). EAO was graded as detailed in Table S1, and EAE clinical score was determined as described (38).
Concomitant Treg Depletion Terminates the Tolerance State and Induces Severe Bilateral Testicular Autoimmune Disease in Unilateral Vasectomy.
Tregs from normal mice prevent EAO that develops in day-3 thymectomized mice in an antigen-dependent and organ-specific manner (18). To investigate Tregs in postvasectomy tolerance, we depleted Tregs from uni-vx B6AF1 mice by CD25 monoclonal antibody at vasectomy. This led to 60% reduction in Foxp3+ Tregs in all LNs for 5 wk, and concomitant increase in activated Foxp3-negative effector T cells (Fig. S2A). That T-cell activation occurs exclusively in regional LN supports a testis antigen-specific effector T-cell response (Fig. S2B).
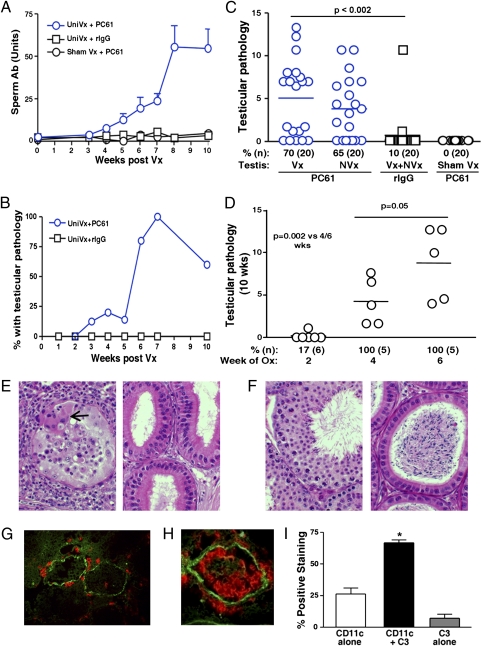
Serum antibodies to sperm and testis antigens were detected by 4 wk (Fig. 2A). Strikingly, left or right unilateral vasectomy was followed by severe bilateral orchitis 2 wk later (Fig. 2 B and C). This requires stimulation by endogenous antigens from the epididymis and testis in the first 3 to 4 wk, as their removal at 2 wk (but not 4 wk) prevented the response (Fig. 2D). Testicular pathology was characterized by multifocal infiltration of lymphocytes, granulocytes, dendritic cells, macrophages, and multinucleated giant cells that surrounded seminiferous tubules, penetrated the blood–testis barrier (BTB), and infiltrated tubular lumen (Fig. 2E, Left). In 85% of mice with orchitis, sufficient germ cell loss led to sperm depletion in the epididymis: a finding predictive of infertility (Fig. 2E, Right).
Fig. 2.
Bilateral EAO in uni-vx mice with Treg depletion. Sperm antibody response (A) and EAO progression (B) in uni-vx mice with Treg depletion (P < 0.04 from 4 to 10 wk; n = 4–10 mice per time point). (C): EAO in uni-vx mice with Treg depletion is bilateral (at 10 wk). (D) EAO is prevented by ablation of the contralateral vasectomized epididymis and testis at 2 wk but not at 4 or 6 wk after vasectomy. (E): Orchitis in uni-vx and Treg-depleted mice shows peritubular leukocytic infiltration inside aspermatogenic tubule (Left) and epididymal ducts without sperm (Right). (H&E stain, magnification of 400×; arrow indicates a giant cell) (F) uni-vx and rat IgG-treated mice have normal testis (Left) and sperm-filled epididymis (Right). (H&E stain, magnification of 400×.) (G–I) Peritubular immune complexes (complement C3, green) colocalize with CD4+ T cells (red) (G) and CD11c+ dendritic cells (red) (H) in the testis. (Magnification of ×400.) (I) Semiquantification by dual-color immunofluorescence microscopy shows colocalization of immune complex and CD11c+ cell clusters (*P = 0.008, n = 5). Polyclonal antibody to mouse IgG, κ-light chain, and complement C3 were used.
Pathogenic CD4+ T Cells Are Sufficient to Induce Postvasectomy Autoimmune Orchitis, and Autoantibody Has a Supportive Role.
Approximately 65% of the testis-infiltrating T cells expressed CD4 (Fig. S3 A and C); among them, 20% had potential to produce IFN-γ, and fewer than 2% produced IL-17 (Fig. S3 B and D). Orchitis was completely inhibited by depletion of CD4 T cells given after sperm autoantibody detection (Fig. S4A). Importantly, CD4+ T cells from mice with orchitis transferred severe EAO to syngeneic recipients, and no other pathologic state. Notably, only the CD4+ T cells from the testis-draining LNs were pathogenic (Fig. S4 B and C). Thus, the regional LNs are unique locations where pathogenic T cells respond to sperm antigen and preferentially accumulate.
In addition to immune cells, peritubular immune complexes were detected as patches of granular IgG and C3 (Fig. 2 G and H). Importantly, more than 70% of them were colocalized with clusters of CD11c+ dendritic cells and CD4 T cells at the BTB (Fig. 2 G–I). Indeed, serum antibody from uni-vx mice with Treg depletion, cotransferred with splenic CD4+ T cells, enhanced orchitis pathology (Fig. S4B). Therefore, although CD4+ T cells are necessary and sufficient to trigger postvasectomy autoimmune orchitis, orchitis severity is enhanced by autoantibody as complement-activating immune complexes.
Sperm-Specific Zonadhesin Is a Target Autoantigen with an Orchitogenic Polypeptide Domain.
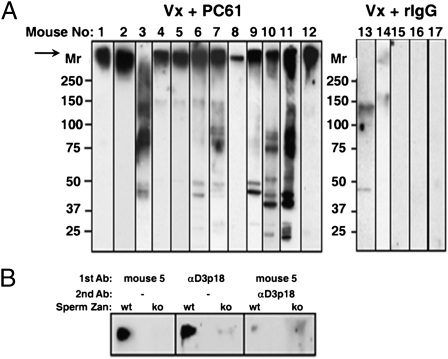
By Western blot on 4% to 8% SDS/PAGE and short chemiluminescence exposure, the serum antibody from many uni-vx B6AF1 mice with Treg depletion recognized a prominent, high-Mr (340 kDa) antigenic band (Fig. 3A, arrow). To verify our suspicion that this was zonadhesin (Zan) (19) (Fig. 3B), we showed that the serum antibody reacted with the 340-kDa protein in sperm of WT but not zan-null mice. In addition, reactivity of mouse serum antibody against the 340-kDa Zan antigen was blocked by preincubation with an affinity-purified rabbit antibody to ZanD3p18: a partial region of the D3p domain of Zan with B-cell epitope (20) (Fig. 3B and Fig. S5A). By detecting antibody binding to WT but not zan-null sperm extract, Zan antibody was detected in five of six (83%) Treg-depleted and uni-vx mice with EAO, but not in three mice without EAO or seven control mice (Fig. S5A). Therefore, uni-vx B6AF1 mice with Treg depletion mounted a dominant autoimmune response to Zan. We next showed that B6AF1 mice immunized with recombinant ZanD3p18 in adjuvant developed EAO (P = 0.01; Fig. S5 B and C); therefore, response to Zan is a likely mechanism of postvasectomy orchitis. Zan is expressed on the acrosomal membrane of spermatid and sperm, and can bind to zona pellucida and inhibit interspecies gamete interaction (20). Notably, Zan is the first murine orchitogenic antigen identified.
Fig. 3.
Serum antibody of uni-vx B6AF1 mice with Treg depletion targets the dominant sperm-specific Zan antigen. (A) Many sera from uni-vx (Vx) mice with Treg depletion react with a 340-kDa sperm protein band. (B) Serum antibody from mouse 5 (from A) reacts with the 340-kDa band of WT but not zan−/− (ko) sperm; and it inhibits the binding of D3p18-specific rabbit antibody to the D3p18 B-cell epitope of Zan (reproducible in three independent studies using sera from three mice).
Mouse Strain Variation in Immune Response to Vasectomy Is Due to Variance in Natural Treg Function.
To determine genetic control of postvasectomy response, we investigated the parenteral strains of B6AF1 mice. A/J and B6 mice developed epididymal inflammation and granuloma. Although both strains exhibited postvasectomy resistance to testis antigen immunization, the reduction in B6 mice was profound (Fig. S6 A and B). Also, after Treg depletion by CD25 antibody treatment, the uni-vx A/J mice responded but uni-vx B6 mice did not (Fig. S6 C and D). To determine whether this is explicable by a stronger Treg function in B6 mice, we studied the DEREG B6 mice with linked expression of diphtheria toxin receptor and Foxp3 (21). Indeed, 7 wk after 97% of Tregs were depleted by combined diphtheria toxin and CD25 antibody treatment, most DEREG B6 mice developed antibody response, severe EAO (Fig. S7 A, B, and D), and T-cell activation in the regional LN (Fig. S7C). Thus, the genetic variance is likely to be a result of the unique Treg function of B6 mice.
Discussion
We have investigated the mechanism of postvasectomy sperm-specific autoimmune response in uni-vx mice. Unlike other studies, we focused on the immunological events in the first 10 wk: long before sperm antibodies were detectable. We obtained unexpected results germane to the mechanism of Treg function and immune sequelae of vasectomy. First, vasectomized mice develop sperm-specific systemic tolerance despite sperm antigen presentation from an inflamed epididymis. Second, Treg depletion in vasectomy leads to spontaneous testis-specific autoimmune disease, invoked by the synergic effect between pathogenic CD4 T cells and autoantibody. Third, the antibody in B6AF1 mice dominantly targets the sperm-specific Zan, which is the first murine orchitogenic antigen identified. Fourth, the postvasectomy immune response is under genetic control, possibly dependent on intrinsic Treg function. We have shown that the well documented late postvasectomy autoimmune response is preceded by an early and Treg-controlled tolerance state, triggered by sperm antigens exposed in the inflamed epididymis soon after vasectomy.
Unilateral vasectomy alone does not cause autoimmunity unless Tregs are depleted. This supports the contention that a natural Treg function is the prevention of autoimmune disease induction by persistent endogenous danger. The CD4 T cells are pivotal in the pathogenesis of postvasectomy EAO: they respond to sperm antigens in the regional LN of the epididymis where they accumulate, and they synergize with immune complexes in the testis adjacent to the BTB to induce maximal orchitis.
Mice with vasectomy alone are resistant to immunization-induced EAO. The tolerance state is testis-specific, maintained by sperm antigens produced in the testis and released into interstitial tissue space of the inflamed epididymis. Therefore, tolerance can be induced by sperm antigens released from tissue with persistent inflammation. This finding is unexpected for vasectomy, but it is less unexpected from the viewpoint of the known divergent inflammatory responses to danger signals (22). Different local environments and different forms of cell death can determine the nature of an innate response. In turn, antigen presenting dendritic cells (23) and macrophages (24) with disparate functions are generated that may preferentially promote adaptive immunity or immune tolerance. Importantly, this divergent response can be regulated by Tregs that foster a tolerance state (25–28). Therefore, as a plausible mechanism, postvasectomy tolerance may depend on the feedback interaction of sperm antigen-specific Tregs with tolerogenic dendritic cells (27). Relevant to this consideration is the reported immune suppressive property of sperm (29).
Vasectomized mice are more resistant to EAO induced by testis antigen immunization than sham-vasectomized mice; therefore, postvasectomy tolerance actually exceeds the physiological level (Fig. 1A). This could be caused by a rapid response of sperm-specific Treg normally positioned in the testis-draining LNs, and the subsequent expansion or induction of Tregs that surpass effector T cells. However, this is possible only if the normal meiotic germ cell autoantigens can access the regional LN to stimulate the testis antigen-specific Treg (11).
According to prevailing dogma, the BTB formed by Sertoli cells in normal testis completely sequesters male meiotic germ cell antigens from immune recognition (2, 30) and these antigens are therefore more “foreign” than “self.” However, the validity of this paradigm is in dispute for the following reasons. The preleptotene spermatocytes that express autoimmunogenic antigens are located outside the BTB (31). To transfer orchitis, CD4 T cells and autoantibody would have to recognize cognate antigens outside the BTB. The Tregs of normal males surpass those of female donors in suppressing EAO induction (32). The female mice surpass male mice in their response to immunization with testis-specific lactate dehydrogenase 3 (LDH3; a sperm-specific antigen behind BTB) (33). Circulating LDH3 antibody was found to preferentially localize to the testis (34). Finally, with complete sequestration, the expectant antibody response to vasectomy should be highly diversified, targeting many “foreign” sperm antigens; instead, our finding is a dominant antibody response to Zan. Based on these experimental findings, we propose a “selective” antigen sequestration model: the nonsequestered germ cell antigens (i.e., LDH3) are protected by systemic tolerance in normal mice, and the sequestered and immunogenic antigens (i.e., Zan) would dominate the postvasectomy autoimmune response.
The postvasectomy EAO in mice with Treg depletion is under genetic control. According to our data, the resistance state of the B6 mice is a reflection of their strong intrinsic Treg function. This mechanism has been documented in autoimmune diabetes (35), and subsequently in autoimmune ovarian disease (36), autoimmune dacryoadenitis (37), and EAE (38). In all cases, disease resistance was mapped to the IL-2 locus in chromosome 3 (35), associated with enhanced production of IL-2 by activated T cells (35, 39).
The genetic data can explain the variable detection of sperm antibody response in humans and outbred animals, and are beginning to provide insight into the mechanism of systemic postvasectomy sequelae. Vasectomized individuals with stronger intrinsic Treg function (e.g., B6 mice) may develop tolerance and be less responsive to future sperm antigen challenge. Although this would minimize EAO development and undesirable outcomes in vasovasostomy, it could also impair testis antigen-specific immune surveillance. Along with persistent local tissue inflammation, this could favor the emergence of tumors expressing neoantigens shared with male germ cells (the cancer/testis antigens) (40). This consideration is consistent with a report on the significant increased incidence of malignant tumors in vasectomized BDF1 mice after 15 to 24 mo (41). To directly extrapolate this chain of events to humans is premature; nonetheless, our study has provided a framework for further investigation of immune perturbation associated with vasectomy.
Methods
Mice, Unilateral Vasectomy, and Treg Depletion.
Mice were purchased or bred in house. The zan-null B6 mouse (20) and B6-DEREG mice (21) were produced as previously described. Vasectomy was the occlusion and bissection of the vas deferens. To deplete Tregs, 250 μg of CD25 antibody (clone PC61) was injected on days −3, +3, and +7 (uni-vx, day 0); and 1 μg of diphtheria toxin (322326; Calbiochem) was injected in DEREG mice on days −1 and +1. Experiments followed guidelines of the Animal Care and Use Committees of University of Virginia, University of Vermont, and Texas Tech University.
Expression of Recombinant Zanp18 and Production of Rabbit Antibody.
Recombinant D3p18 domain (accession no. AAC26680) inclusive of amino acid Cys4502 to Lys4621 of mouse Zan transcript (accession no. U97068; nucleotides 13,669–14,050) were expressed and purified; the rabbit antibody against recombinant D3p18-GST was affinity-purified, devoid of GST reactivity (20).
EAO and EAE Induction.
EAO (31) and EAE (38) were induced as described, by using 100 μg ZanD3p18 fusion protein and 100 μg myelin oligodendrocyte glycoprotein peptide 35–55 per dose, respectively.
Sperm and Testis Cell Antibody and T-Cell Proliferation Assays.
Antibody was detected by ELISA or indirect immunofluorescence (31). Pooled LN and splenic T cells were stimulated by testis cell in the presence of irradiated splenocytes, and detected by cell-associated H3T (31).
SDS-Polyacrylamide Gel Electrophoresis and Western Blot.
Epididymal sperm proteins, extracted by Laemmli sample buffer (18), were analyzed in 4% to 8% gradient gels (disulphide-unreduced). After primary antibody (40 ng/mL), bound antibody was visualized by peroxidase-labeled antiserum to mouse or rabbit IgG, detected by chemiluminescence (Bio-Rad).
Statistical Analyses.
The Fisher exact test was used to compare incidences, and unpaired Mann–Whitney tests were used at all other times.
Supplementary Material
Acknowledgments
We thank Ruben Ter-Antonyan and Bill Dove for their guidance in mouse irradiation, and appreciate the excellent technical support provided by Yuefang Sun, Virginia Rubianes, and Joyce Nash. This study was supported by National Institutes of Heath Grants R01 AI 41236 and R01 AI 51420.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1017615108/-/DCSupplemental.
References
- 1.Adams CE, Wald M. Risks and complications of vasectomy. Urol Clin North Am. 2009;36:331–336. doi: 10.1016/j.ucl.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Tung KSK, Menge AC. Sperm and testicular autoimmunity. In: Rose NR, Mackay IR, editors. The Autoimmune Diseases. New York: Academic; 1985. pp. 537–590. [Google Scholar]
- 3.Alexander NJ, Anderson DJ. Vasectomy: Consequences of autoimmunity to sperm antigens. Fertil Steril. 1979;32:253–260. doi: 10.1016/s0015-0282(16)44228-7. [DOI] [PubMed] [Google Scholar]
- 4.Kaufman SC, Alexander DJ. Vasectomy: Autoimmunity and safety. In: Bronson RA, et al., editors. Reproductive Immunology. Boston: Blackwell Science; 1996. pp. 629–639. [Google Scholar]
- 5.Mettlin C, Natarajan N, Huben R. Vasectomy and prostate cancer risk. Am J Epidemiol. 1990;132:1056–1061. doi: 10.1093/oxfordjournals.aje.a115747. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg L, et al. Vasectomy and the risk of prostate cancer. Am J Epidemiol. 1990;132:1051–1055. doi: 10.1093/oxfordjournals.aje.a115746. [DOI] [PubMed] [Google Scholar]
- 7.Köhler TS, Fazili AA, Brannigan RE. Putative health risks associated with vasectomy. Urol Clin North Am. 2009;36:337–345. doi: 10.1016/j.ucl.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 9.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 11.Wheeler KM, Samy ET, Tung KSK. Cutting edge: Normal regional lymph node enrichment of antigen-specific regulatory T cells with autoimmune disease-suppressive capacity. J Immunol. 2009;183:7635–7638. doi: 10.4049/jimmunol.0804251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 13.Walker LS, Chodos A, Eggena M, Dooms H, Abbas AK. Antigen-dependent proliferation of CD4+ CD25+ regulatory T cells in vivo. J Exp Med. 2003;198:249–258. doi: 10.1084/jem.20030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisson S, et al. Continuous activation of autoreactive CD4+ CD25+ regulatory T cells in the steady state. J Exp Med. 2003;198:737–746. doi: 10.1084/jem.20030686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samy ET, Parker LA, Sharp CP, Tung KS. Continuous control of autoimmune disease by antigen-dependent polyclonal CD4+CD25+ regulatory T cells in the regional lymph node. J Exp Med. 2005;202:771–781. doi: 10.1084/jem.20041033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lathrop SK, Santacruz NA, Pham D, Luo J, Hsieh CS. Antigen-specific peripheral shaping of the natural regulatory T cell population. J Exp Med. 2008;205:3105–3117. doi: 10.1084/jem.20081359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flickinger CJ, Howards SS, Herr JC. Effects of vasectomy on the epididymis. Microsc Res Tech. 1995;30:82–100. doi: 10.1002/jemt.1070300107. [DOI] [PubMed] [Google Scholar]
- 18.Taguchi O, Nishizuka Y. Self tolerance and localized autoimmunity. Mouse models of autoimmune disease that suggest tissue-specific suppressor T cells are involved in self tolerance. J Exp Med. 1987;165:146–156. doi: 10.1084/jem.165.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardy DM, Garbers DL. Species-specific binding of sperm proteins to the extracellular matrix (zona pellucida) of the egg. J Biol Chem. 1994;269:19000–19004. [PubMed] [Google Scholar]
- 20.Tardif S, et al. Zonadhesin is essential for species specificity of sperm adhesion to the egg zona pellucida. J Biol Chem. 2010;285:24863–24870. doi: 10.1074/jbc.M110.123125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lahl K, et al. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J Exp Med. 2007;204:57–63. doi: 10.1084/jem.20061852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medzhitov R. Inflammation 2010: New adventures of an old flame. Cell. 2010;140:771–776. doi: 10.1016/j.cell.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 24.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 25.Maloy KJ, et al. CD4+CD25+ T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J Exp Med. 2003;197:111–119. doi: 10.1084/jem.20021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watanabe H, et al. Danger signaling through the inflammasome acts as a master switch between tolerance and sensitization. J Immunol. 2008;180:5826–5832. doi: 10.4049/jimmunol.180.9.5826. [DOI] [PubMed] [Google Scholar]
- 27.Darrasse-Jèze G, et al. Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. J Exp Med. 2009;206:1853–1862. doi: 10.1084/jem.20090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asquith M, Powrie F. An innately dangerous balancing act: intestinal homeostasis, inflammation, and colitis-associated cancer. J Exp Med. 2010;207:1573–1577. doi: 10.1084/jem.20101330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hurtenbach U, Shearer GM. Germ cell-induced immune suppression in mice. Effect of inoculation of syngeneic spermatozoa on cell-mediated immune responses. J Exp Med. 1982;155:1719–1729. doi: 10.1084/jem.155.6.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fijak M, Meinhardt A. The testis in immune privilege. Immunol Rev. 2006;213:66–81. doi: 10.1111/j.1600-065X.2006.00438.x. [DOI] [PubMed] [Google Scholar]
- 31.Yule TD, Montoya GD, Russell LD, Williams TM, Tung KS. Autoantigenic germ cells exist outside the blood testis barrier. J Immunol. 1988;141:1161–1167. [PubMed] [Google Scholar]
- 32.Taguchi O, Nishizuka Y. Experimental autoimmune orchitis after neonatal thymectomy in the mouse. Clin Exp Immunol. 1981;46:425–434. [PMC free article] [PubMed] [Google Scholar]
- 33.Marsh JA, O'Hern P, Goldberg E. The role of an X-linked gene in the regulation of secondary humoral response kinetics to sperm-specific LDH-C4 antigens. J Immunol. 1981;126:100–106. [PubMed] [Google Scholar]
- 34.Dutta RC, Goldberg E. Testis specific lactate dehydrogenase as target for immunoliposomes. Am J Reprod Immunol. 2008;60:26–32. doi: 10.1111/j.1600-0897.2008.00587.x. [DOI] [PubMed] [Google Scholar]
- 35.Yamanouchi J, et al. Interleukin-2 gene variation impairs regulatory T cell function and causes autoimmunity. Nat Genet. 2007;39:329–337. doi: 10.1038/ng1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teuscher C, Wardell BB, Lunceford JK, Michael SD, Tung KS. Aod2, the locus controlling development of atrophy in neonatal thymectomy-induced autoimmune ovarian dysgenesis, co-localizes with Il2, Fgfb, and Idd3. J Exp Med. 1996;183:631–637. doi: 10.1084/jem.183.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.del Rio R, Sun Y, Alard P, Tung KS, Teuscher C. H2 control of natural T regulatory cell frequency in the lymph node correlates with susceptibility to day 3 thymectomy-induced autoimmune disease. J Immunol. 2011;186:382–389. doi: 10.4049/jimmunol.1002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Butterfield RJ, et al. New genetic loci that control susceptibility and symptoms of experimental allergic encephalomyelitis in inbred mice. J Immunol. 1998;161:1860–1867. [PubMed] [Google Scholar]
- 39.del Rio R, et al. SNPs upstream of the minimal promoter control IL-2 expression and are candidates for the autoimmune disease-susceptibility locus Aod2/Idd3/Eae3. Genes Immun. 2008;9:115–121. doi: 10.1038/sj.gene.6364455. [DOI] [PubMed] [Google Scholar]
- 40.Simpson AJG, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer. 2005;5:615–625. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- 41.Anderson DJ, Alexander NJ, Fulgham DL, Palotay JL. Spontaneous tumors in long-term—vasectomized mice. Increased incidence and association with antisperm immunity. Am J Pathol. 1983;111:129–139. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.