Abstract
Recombinant adenoviruses (rAds) based on types 5 (rAd5) and 35 (rAd35) have emerged as important vaccine delivery vectors in clinical testing for a variety of pathogens. A major difference between these vectors is their binding to cellular receptors used for infection. Whereas rAd5 binds coxsackie-adenovirus receptor (CAR), rAd35 binds the complement regulatory protein CD46. Although rAd35 infected and phenotypically matured human blood dendritic cells (DCs) more efficiently than rAd5, we show here that rAd35 markedly suppressed DC-induced activation of naive CD4+ T cells. rAd35 specifically blocked both DCs and anti-CD3/CD28 mAb-induced naive T-cell proliferation and IL-2 production. This effect was also observed in CD4+ memory T cells but to a lesser extent. The suppression occurred by rAd35 binding to CD46 on T cells and was independent of infection. CD46 engagement with mAb mimicked the effects of rAd35 and also led to deficient NF-κB nuclear translocation. In contrast, rAd5 and rAd35 vectors with ablated CD46 binding did not inhibit T-cell activation. Our findings provide insights into the basic biology of adenoviruses and indicate that CD46 binding may have an impact on the generation of primary CD4+ T-cell responses by Ad35.
Recombinant adenoviruses (rAds) have emerged as potential vaccine delivery vehicles for a variety of human pathogens (1). Of the many adenovirus types, species C adenovirus type 5 (Ad5) has been the most widely used. A lower seroprevalent species B adenovirus type 35 (Ad35) has also been tested in preclinical trials (2) and is currently being tested in numerous clinical trials as a vaccine candidate against HIV-1, Plasmodium, and Mycobacterium tuberculosis. Ad5 and Ad35 are distinguished by their use of cellular receptors. Ad5 interacts with coxsackie-adenovirus receptor (CAR) (3) and several soluble factors, including lactoferrin (4, 5) and coagulation factor X (6, 7), which enhance binding to host cells. In contrast, Ad35 binds CD46 with high affinity and avidity (8). Adenovirus receptor use strongly influences cellular and tissue tropism (9) and also likely plays a role in mediating viral recognition by immune cells. We previously reported that rAd35 infects human dendritic cells (DCs) in a CD46-dependent manner and induces DC phenotypic maturation and IFN-α in plasmacytoid DCs (PDCs) (10). In contrast, rAd5 infects DCs less efficiently and does not induce maturation. Whereas CAR is associated with epithelial cell tight junctions, CD46 is expressed on most nucleated human cells, including immune cells in peripheral blood. A complement regulatory protein with cofactor activity, CD46 protects cells from autologous complement lysis by C3b (11). It also serves as a primary receptor for multiple human pathogens (12), including measles virus (13, 14), human herpesvirus 6 (15), Neisseria gonorrhoeae (16), and Streptococcus pyogenes (17). CD46 couples with intracellular signaling pathways through putative sequence motifs encoded within its cytoplasmic tail (18) to regulate human T-cell function in vitro and in vivo (19, 20). Thus, CD46 ligation can modulate CD4+ T-cell proliferation and cytokine production following T-cell receptor (TCR)-dependent activation (21).
The activation of naive CD4+ T cells following infection or vaccination is crucial for generating helper T-cell responses. In this study, we compared how rAd5 and rAd35 influence the activation of naive CD4+ T cells via their differential receptor use. Using multiple human DC subsets isolated from blood or immobilized CD3/CD28 mAb to activate sorted naive CD4+ T cells in vitro, we found that rAd35 suppressed proliferation and IL-2 production. CD46 engagement with mAb mimicked the inhibitory effect of rAd35 and also led to defective nuclear translocation of NF-κB following T-cell activation. In contrast, rAd5 vectors and chimeric rAd35 vectors with ablated CD46 binding did not alter T-cell activation. Collectively, these data suggest that Ad35 can actively modulate T-cell function via CD46 binding, and thus provide unique insights into the basic biology of CD46-binding adenoviruses.
Results
rAd35 Vectors More Efficiently Infect Human DCs and Induce Phenotypic Maturation.
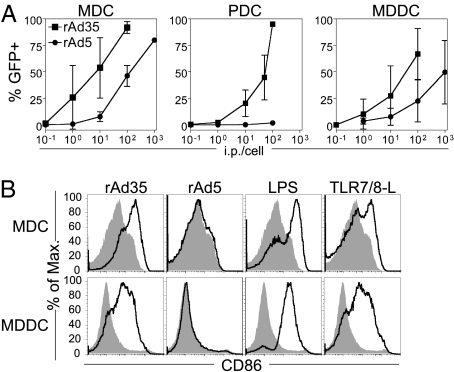
To determine whether rAd5 and rAd35 differentially affect DC-mediated activation of primary CD4+ T cells, we first measured the capacity of these replication-deficient vectors to infect and activate human DCs. Freshly isolated immature CD11c+ myeloid DCs (MDCs), CD123+ PDCs, and CD1a+ monocyte-derived DCs (MDDCs) were exposed to rAd5 or rAd35 encoding a GFP transgene over a range of infectious particles per cell (ip/cell). The frequency of GFP+ cells was used as a measure of infection after 24 h. In agreement with our previous reports (4, 10), rAd35 infected all DC subsets more efficiently than rAd5 (Fig. 1A). rAd35, in contrast to rAd5, induced up-regulation of multiple phenotypic maturation markers, including CD86 (Fig. 1B), CD40, and CD80 (10) (Fig. S1), comparable to LPS or Toll-like receptor 7/8-ligand (TLR7/8-L) stimulation. rAd35 exposure also led to increased HLA-DR on MDDCs but not on MDCs, where it was already expressed at high levels (Fig. S1).
Fig. 1.
rAd35 vectors infect and activate DCs. (A) MDCs, PDCs, or MDDCs were exposed to rAd5-GFP (●) or rAd35-GFP (■) at doses from 0.1 to 1,000 ip/cell for 24 h, at which time the frequency of GFP+ cells was measured by flow cytometry. Graphs depict mean ± SD for rates of infection assessed on DCs from at least six donors. (B) MDCs or MDDCs were exposed to rAd5 (100 ip/cell), rAd35 (10 ip/cell), or TLR ligands for 24 h. CD86 expression of all DCs was measured by flow cytometry. Histograms depict mock (gray-filled) or stimulated (black line) DCs for one representative donor of three independent experiments.
rAd35 Suppresses DC Activation of Naive CD4+ T Cells.
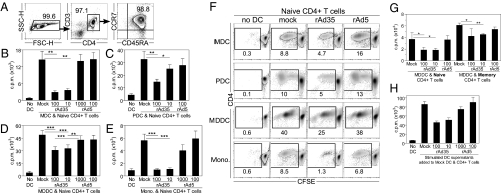
DC activation of naive CD4+ T cells plays a critical role in priming helper T-cell responses. We have previously shown that human DCs exposed to rAd5 and rAd35 express and present rAd-encoded antigen to antigen-specific CD4+ and CD8+ memory T cells (10). In fact, DCs, exposed to rAd35, activated insert-specific polyfunctional memory T cells equivalently to DCs pulsed with antigen-matched peptides. Here, we expanded these studies by assessing how rAd-exposed DCs influence activation of naive CD4+ T cells. DCs or monocytes were exposed for 24 h to either rAd5 or rAd35 and then cocultured with sorted allogeneic naive CD4+ T cells (Fig. 2A). Proliferation was measured on day 5 with 3H-thymidine (Fig. 2 B–E) or carboxyfluorescein succinimidyl ester (CFSE) (Fig. 2F). Contrary to rAd5, rAd35 significantly suppressed DC and monocyte-induced naive CD4+ T-cell proliferation. We observed a similar effect, although to a lesser extent, on the memory subset compared with donor-matched naive CD4+ T cells (Fig. 2G). When we transferred the supernatant from rAd-exposed DCs to unexposed DCs that were then added to allogeneic CD4+ T cells, only supernatants from rAd35-exposed DCs inhibited CD4+ T-cell proliferation (Fig. 2H). This indicates that a soluble factor, either cell-derived or residual virus from the initial inoculum, played a dominant role in suppressing proliferation. The rAd35-mediated inhibitory effects were not the result of MHC II down-regulation because this marker was either unchanged or up-regulated on DCs (Fig. S1). Thus, despite inducing phenotypic maturation of DCs, rAd35 interferes with DC-mediated activation of naive CD4+ T cells.
Fig. 2.
rAd35-exposed DCs or monocytes suppress allogeneic naive CD4+ T-cell proliferation. (A) Purity of naive CD4+ T cells was assessed by flow cytometry from a representative donor. Immature MDCs (B), PDCs (C), MDDCs (D), or monocytes (E) were exposed to rAd5 or rAd35 for 24 h and then transferred to sorted allogeneic naive CD4+ T cells at a ratio of 1 to 10. Proliferation was measured on day 5 by 3H-thymidine incorporation. Graphs depict mean ± SD for MDCs (n = 4), PDCs (n = 3), MDDCs (n = 16), and monocytes (n = 4). Wilcoxon signed-rank test: *P < 0.05; **P < 0.005; ***P < 0.0005. (F) DCs or monocytes were exposed to either rAd5 (100 ip/cell) or rAd35 (10 ip/cell) for 24 h and then transferred to sorted allogeneic CFSE-labeled naive CD4+ T cells. CFSE dilution was evaluated on day 5 by flow cytometry. Flow plots are from one representative donor of three independent experiments. (G) Sorted donor-matched naive and memory CD4+ T-cell proliferation induced by mock- or rAd-infected MDDCs. Proliferation was measured on day 5 with 3H-thymidine. Graphs depict mean ± SD (n = 4). Paired t test: *P < 0.05, **P < 0.005. (H) MDDCs were exposed to either rAd5 or rAd35 for 24 h, and the supernatants were harvested and transferred to untreated DCs cocultured with sorted allogeneic CD4+ T cells. Proliferation was measured on day 5 with 3H-thymidine. One representative donor is shown.
rAd35 Exposure Leads to Decreased CD46 Expression on Naive CD4+ T Cells.
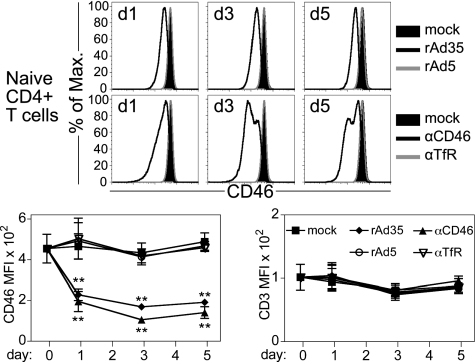
Although freshly isolated T cells express the rAd35 receptor CD46, no GFP+ cells are detected following rAd35 exposure (10). Because rAd35 binds CD46 on T cells, however, we hypothesized that rAd35 may directly regulate T-cell activation via binding to CD46. The Ad35 knob proteins bind with high affinity to the extracellular N-terminal short consensus repeat 1 (SCR1) and SCR2 of CD46 (22). We first asked whether rAd35 influences CD46 expression on naive CD4+ T cells by exposing the cells to immobilized rAd35 or rAd5. Immobilized adenovirus particles were unable to infect susceptible cells such as DCs (Fig. S2). We also tested an anti-CD46 mAb (clone 13/42) that binds the SCR1 domain of CD46 (23, 24). rAd35 and anti-CD46 mAb decreased surface CD46 expression by up to 75% after 3 and 5 d, whereas none of the treatments affected CD3 levels (Fig. 3). Immobilized anti-CD46 mAb clone M177, which binds SCR2 (23), caused similar CD46 down-regulation as clone 13/42 (Fig. S3). Both mAbs have overlapping binding regions on CD46 with rAd35 and block infection (10, 24). Neither immobilized rAd35 nor anti-CD46 mAb interfered with the anti-CD46 staining mAb (Fig. S4). In summary, these results indicate that direct binding of rAd35 to naive CD4+ T cells induces down-regulation of CD46 and that SCR1- and SCR2-specific anti-CD46 mAbs mimic rAd35 binding.
Fig. 3.
rAd35 binding leads to lower CD46 expression on naive CD4+ T cells. Naive CD4+ T cells were mock-treated or exposed to immobilized rAd5 or rAd35 (100 ip/cell), anti-CD46 mAb, or anti-TfR mAb. Surface CD46 expression on live cells (Annexin-V−/7-AAD−) was evaluated by flow cytometry after 1–5 d as indicated. Histograms depict CD46 expression on mock and treated naive CD4+ T cells of one representative donor from three independent experiments. Graphs are a summary (mean ± SD) of CD46 and CD3 mean fluorescence intensity data of three donors. Paired t test: **P < 0.01.
CD46 Engagement Blocks CD3/CD28-Induced CD4+ T-Cell Proliferation.
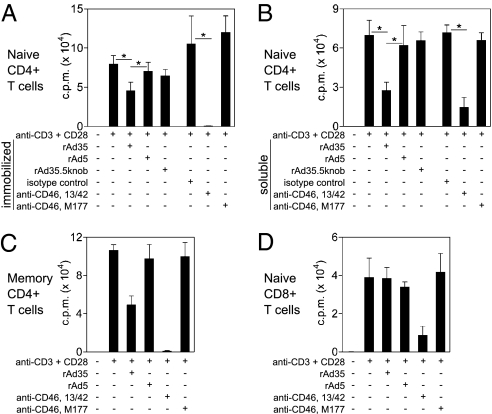
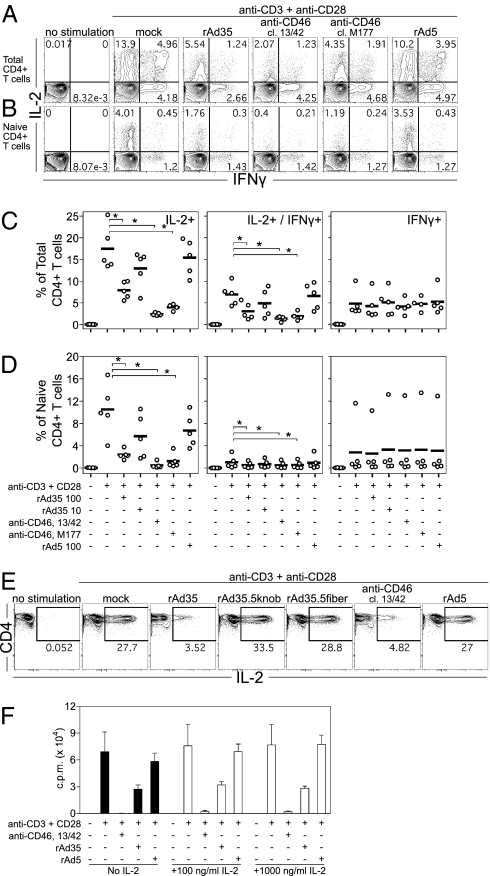
Because rAd35 directly engaged naive T cells, we next assessed whether rAd35 could inhibit naive T-cell proliferation independent of potential DC-mediated effects. To this end, we treated naive CD4+ T cells with immobilized anti-CD3 and anti-CD28 mAbs. Costimulation was required to activate naive T cells and was relevant, because rAd35 induced up-regulation of CD28 ligands (CD80/86) on DCs. Such activation excludes any effect that rAd infection may have on DC function. The effect of rAd35 exposure on T-cell proliferation was again compared with SCR1- and SCR2-binding anti-CD46 mAbs (13/42 and M177, respectively). To determine whether binding of rAd35 knob to CD46 was required to suppress proliferation, we also tested a mutant chimeric rAd35 that does not bind CD46, because the fiber-knob was exchanged with the Ad5 knob (rAd35.5knob) (25). The rAd35.5knob was used at equivalent total particle units to rAd35. As expected, CD3/CD28 induced proliferation of naive CD4+ T cells. Exposure to rAd35 or the SCR1 targeting anti-CD46 mAb (13/42), both in immobilized (Fig. 4A) and soluble form (Fig. 4B), significantly inhibited T-cell proliferation. CD3/CD28-induced proliferation of memory CD4+ T cells was also negatively affected but not as strongly (Fig. 4C). In contrast, the SCR2 targeting anti-CD46 mAb (M177), rAd5, and rAd35.5knob mutant did not have an impact on proliferation (Fig. 4 A–C). Further, proliferation of naive CD8+ T cells was reduced only by anti-CD46 mAb (13/42) and not by rAd35 (Fig. 4D). Thus, rAd35 and SCR1 targeting anti-CD46 mAb caused preferential inhibition of naive CD4+ T-cell proliferation.
Fig. 4.
rAd35 and CD46 engagement blocks proliferation of CD3/CD28-activated naive CD4+ T cells. Naive CD4+ T cells were activated with immobilized anti-CD3/CD28 mAb with or without immobilized (A) or soluble (B) rAd5, rAd35 (200 ip/cell), or anti-CD46 mAb. A chimeric rAd35 vector with rAd5 knobs (rAd35.5knob) was also used. Graphs are a summary (mean ± SEM) of data from three donors. Paired t test: *P < 0.05. (C) Memory CD4+ T cells were activated with anti-CD3/CD28 with or without rAd (200 ip/cell) or anti-CD46 mAb. The graph is a summary (mean ± SEM) of data from two donors. (D) Naive CD8+ T cells were activated with anti-CD3/CD28 with or without rAd (200 ip/cell) or anti-CD46 mAb. The graph is a summary (mean ± SEM) of data from four donors. Proliferation was evaluated in triplicate on day 5 with 3H-thymidine.
Because CD46 has been implicated in cellular survival signaling pathways (26), we assessed the survival kinetics of naive CD4+ T cells following CD46 engagement. Neither immobilized anti-CD46 mAb nor rAd vectors adversely affected naive T-cell survival or responsiveness to IL-2 over 6 d (Fig. S5). CD46 engagement may therefore block T-cell activation by suppressing proliferation rather than inducing T-cell apoptosis or death.
CD46 Engagement Blocks TCR-Dependent IL-2 Production in CD4+ T Cells.
It has been reported that CD46 engagement regulates IL-2 in CD46-transgenic murine CD4+ T cells (21) and blocks CD3/CD28-induced IFN-γ in CD8+ T cells (27). As such, we evaluated early IL-2 and IFN-γ production in CD3/CD28-activated total or naive CD4+ T cells. Immobilized rAd35 or anti-CD46 mAb reduced the frequency of total (Fig. 5 A and C) and naive (Fig. 5 B and D) CD4+ T cells producing IL-2 alone. In contrast, no effect on the IFN-γ single-producing cells was observed with any treatment. For both total and naive CD4+ T cells, there was a significant reduction in the IL-2/IFN-γ–coexpressing cells (Fig. 5 A–D). These effects were specific for rAd35 and anti-CD46 mAb (both SCR1 and SCR2 targeting), because rAd5 (Fig. 5 A–D) and isotype-matched anti-transferrin receptor (TfR) mAb had no such effects (Fig. S6A). Contrary to CD3/CD28 stimulation, CD46 engagement had no effect on phorbol 12-myristate 13-acetate (PMA) and ionomycin-induced IL-2 and IFN-γ, suggesting that TCR-dependent activation is specifically targeted (Fig. S6B). The requirement of CD46 binding by rAd35 for inhibition of IL-2 was also examined using two mutant chimeric rAd35 vectors deficient in CD46 binding: the previously described rAd35.5knob and the rAd35.5fiber that was transposed with the entire Ad5 fiber, including knob. The chimeric vectors were used at equivalent particle units per cell and ip/cell doses, respectively, as rAd35. We found that the inhibition of IL-2 in CD3/CD28-activated naive CD4+ T cells by rAd35 and CD46 mAb was not evident with rAd5, rAd35.5knob, or rAd35.5fiber vectors (Fig. 5E). Thus, rAd35-mediated IL-2 suppression in naive CD4+ T cells was dependent on rAd35 knob interactions with CD46 and not other rAd35-binding properties. To test whether it was the CD46-mediated suppression of IL-2 that led to lower proliferation of naive CD4+ T cells (Fig. 4 A and B), we performed similar proliferation experiments with the addition of increasing concentrations of IL-2 (Fig. 5F). We found that excess IL-2 did not restore proliferation blocked by anti-CD46 mAb or rAd35, indicating that lower IL-2 production caused by CD46 ligation did not directly cause lower proliferation. An expanded analysis of CD4+ T-cell function showed that CD46 ligation also inhibited TNF production (Fig. S7). Mip-1β production was, in turn, only modestly affected by anti-CD46 mAb clone 13/42 but not by rAd35 and anti-CD46 mAb clone M177 (Fig. S7). Taken together, these findings suggest that CD46-mediated suppression preferentially targets certain T-cell functions and that the specificity and strength of CD46 binding may determine the functions and cell types affected.
Fig. 5.
rAd35 and CD46 ligation inhibit CD3/CD28-induced IL-2 in CD4+ T cells. (A–F) Sorted total or naive CD4+ T cells were activated with CD3/CD28 with or without immobilized rAd5, rAd35 (100 ip/cell), anti-CD46 mAb, or anti-TfR mAb for 5 h. Intracellular IL-2 and IFN-γ production was measured by flow cytometry. Flow plots depict one representative donor of five for sorted total (A) or naive (B) CD4+ T cells. Graphs summarize the mean from five donors for frequencies of single-positive IL-2–, double-positive IL-2/IFN-γ–, and IFN-γ–producing total (C) and naive (D) CD4+ T cells. Wilcoxon signed-rank test: *P = 0.031. (E) Flow cytometry plots show naive CD4+ T cells stimulated with anti-CD3/CD28 mAb with or without rAd5 (100 ip/cell), rAd35 (10 ip/cell), rAd35.5knob (10 ip/cell), rAd35.5fiber (100 ip/cell), or anti-CD46 mAb. (F) Proliferation of anti-CD3/CD28–activated naive CD4+ T cells was measured on day 5 with 3H-thymidine. Cells were stimulated with or without rAd (200 ip/cell) or anti-CD46 mAb in the presence of increasing concentrations of exogenous IL-2. The graph is a summary (mean ± SEM) of data from two donors.
Deficient NF-κB Activation Following CD46 Engagement.
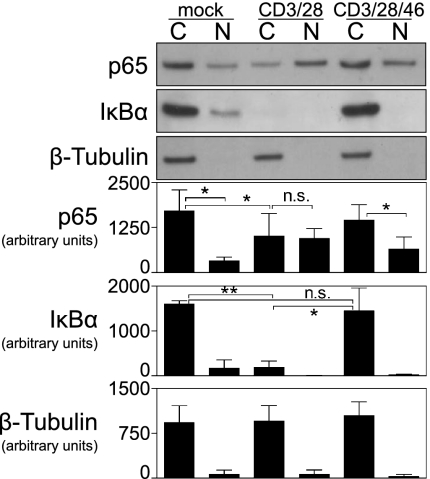
Finally, because IL-2 was reduced in CD46-ligated T cells and the IL-2 gene is a major target of NF-κB transcription factor activity, we evaluated NF-κB activation. CD4+ T cells were stimulated with CD3/CD28 for 4 h with or without immobilized anti-CD46 mAb (13/42). The cytoplasmic and nuclear protein contents were fractionated and analyzed by Western blot. β-tubulin staining confirmed the fractionate purity (Fig. 6). In mock-treated T cells, the p65-subunit of NF-κB and its regulatory component IκBα were present predominantly in the cytoplasm. CD3/CD28 activated the NF-κB pathway, because IκBα was degraded in the cytoplasm and more p65 was present in the nucleus compared with mock-treated cells (Fig. 6). In contrast, CD46 mAb caused a greater proportion of p65 to remain in the cytoplasm and IκBα was not degraded. Together, these findings indicate that CD46 engagement leads to impaired nuclear translocation of NF-κB in CD3/CD28-activated CD4+ T cells, which may explain the reduced IL-2 and proliferation.
Fig. 6.
CD46 engagement suppresses NF-κB activation in CD4+ T cells. SDS/PAGE and Western blot analysis for p65, IκBα, and β-tubulin in lysed C and N fractions of sorted CD4+ T cells after 4 h of no stimulation (mock) or stimulation by immobilized anti-CD3/CD28 mAb with or without anti-CD46 mAb (13/42). C, cytoplasmic; N, nuclear. Densitometry measurements are summarized (mean ± SD) for three donors. Paired t test: *P < 0.05; **P < 0.005; or not significant (n.s.) for P > 0.05.
Discussion
In this study, we assessed the impact of two major rAd types used in clinical vaccine trials, rAd5 and rAd35, on the activation of naive CD4+ T cells, which is a critical step in generating transgene-specific immunity. Professional antigen-presenting cells, such as DCs, play an essential role in activating naive CD4+ T cells to develop helper T-cell responses. rAd35 vectors, however, which more efficiently infect and activate DCs compared with rAd5, show less potent immunogenicity in nonhuman primates (10, 25, 28, 29). Here, we demonstrate that rAd35 but not rAd5 inhibits human naive CD4+ T-cell proliferation independent of DC infection. rAd35 exposure led to the down-regulation of CD46 on naive CD4+ T cells, suggesting that rAd35 interacts directly with these cells. Anti-CD46 mAb with binding regions overlapping rAd35 also blocked proliferation in a similar manner, whereas rAd5 and chimeric rAd35 particles with ablated CD46 binding had no effect. Only an SCR1-binding CD46 mAb blocked proliferation, whereas SCR1- and SCR2-directed mAbs blocked IL-2 production. These findings suggest that rAd35 exerts its effects on T cells via specific engagement of CD46, although the mechanisms explaining the difference in binding the SCR1 and SCR2 domains remain unclear. Importantly, the inhibitory effects of CD46 engagement were not attributable to down-regulated CD3 because this marker was unchanged by rAd35 or mAb. The CD46 ligation effects were not specific for the naive subset, because proliferation and cytokine production were also curtailed in memory and total CD4+ T cells, respectively. CD46-mediated suppression of cytokine production was notably specific for TCR-dependent activation as it had no effect on PMA/ionomycin stimulation.
Our data showing that CD46 engagement regulates immune cell function are supported by an expanding body of work (20). The cytoplasmic tail-1 (cyt-1) isoform of CD46 contains a putative tyrosine phosphorylation site for protein kinase C and casein kinase 2, whereas the cyt-2 isoform is tyrosine-phosphorylated by src kinases in T cells (18). Because human cells contain both isoforms, these data establish that CD46 has signaling capacity (30). For example, CD46 ligation inhibits IL-12 in monocytes (31) and induces autophagy (30). Structural and biochemical analyses have shown that each of the 12 rAd35 knob trimeric proteins may bind up to three CD46 monomers, which indicates that rAd35 may form multivalent complexes with its receptor similar to mAb and induce signaling (22, 32, 33). CD46 ligation with submaximal CD3/CD28 activation (i.e., using equivalent mAb concentrations as used here) causes defective Akt and Survivin signaling that leads to reduced proliferation of CD4+ T cells (26). We also show that suppression of T-cell function by CD46 binding was associated with reduced activation of NF-κB, as evidenced by an absence of IκBα degradation and nuclear translocation of p65. CD46 engagement modulates cytokine production and proliferation in CD4+ T cells in transgenic mice expressing human CD46 (21). In that study, CD46 ligation of double-transgenic (expressing cyt-1 and cyt-2 isoforms) CD4+ T cells also inhibited IL-2 but not IFN-γ following TCR-mediated activation. It was recently reported that CD46 ligation induces nuclear translocation of inducible cAMP early repressor/cAMP response element modulator (ICER/CREM), which negatively regulates IL-2 gene transcription in human CD4+ T cells (19). This mechanism supports the ablation of IL-2 production following activation of the naive CD4+ T cells reported here, although questions remain regarding the temporal regulation of IL-2 by CREM/ICER. It remains to be determined why CD46 engagement blocks IL-2 and TNF but has less of an effect on IFN-γ and Mip-1β. CD46 engagement is also involved in the differentiation of human CD4+ T cells into type 1 regulatory T cells that produce IL-10 and suppress bystander T-cell proliferation (34). Although these cells may play an accessory role in our cultures, the deficient NF-κB activation, together with the finding that exogenous IL-2 does not restore CD46-suppressed proliferation, suggests that naive CD4+ T cells are directly affected by CD46 engagement. The specific modulation of TCR-induced proliferation and IL-2 production by rAd35 binding to CD46 was also not attributable to increased cell death or apoptosis. The suppressive effects were specific to Ad35 binding CD46, because no effect was observed with rAd5 or CD46 binding deficient mutant rAd35 particles. Contrary to naive CD4+ T cells, CD3/CD28-induced proliferation of naive CD8+ T cells was not affected by rAd35. CD46 ligation with anti-CD46 mAb (13/42) did reduce proliferation of these cells, however. It is possible that CD8+ naive T cells are less directly affected by CD46 ligation than CD4+ naive T cells because of different thresholds of activation or isoform expression of cytoplasmic tails. It will be important to determine whether effects reported on immune synapse polarization of CD8+ T cells with CD46 mAb are also linked to reduced proliferation after TCR activation (27).
CD46-mediated immune modulation of human CD4+ T cells was recently demonstrated ex vivo, where patients with rheumatoid arthritis had specific defects in CD46 signaling (19). Studying the role of CD46 in vivo in the context of rAd35 inoculation is currently limited by the lack of a small animal model, because mice do not express CD46 on somatic cells and a murine protein with similar function to human CD46 has not been described. Thus, such in vivo studies must be performed in humans, nonhuman primates, or transgenic mice expressing human CD46. A drawback with the CD46 transgenic mouse is that it is unknown how intact the CD46 signaling pathway is, because the downstream signaling molecules are of murine origin and expression of the cytoplasmic tail isoforms may vary.
CD46 engagement by rAd35 may represent one mechanism to explain why rAd35 vectors show lower immunogenicity as vaccine delivery vehicles. It is plausible that rAd interacts with T cells in the periphery or travels via lymphatic vessels to encounter T cells in lymph nodes. Although the findings described here may represent one potential mechanism of immune suppression by rAd35, the conditions in vivo after vector injection may be more complex. With regard to this, we found that rAd35 and CD46 mAb led to lower surface CD46 expression on naive CD4+ T cells, a hallmark of CD46 engagement. Others have previously shown receptor down-regulation on other cell types by many CD46-using pathogens (13–16, 35, 36). In lymphoid cells, CD46 internalization does not occur constitutively but is induced on ligation (37, 38). Because CD46 down-regulation renders cells more susceptible to complement-mediated lysis (11), CD4+ T-cell viability may also be adversely affected by complement activation following exposure to rAd35 in vivo. Future studies should clarify if complement activation upon rAd35 exposure affects T-cell activation, although it is important to note that C3b binds CD46 SCR3/4 domains rather than SCR1/2 domains, which Ad35 binds (39). In summary, our findings indicate that Ad35 may use CD46 not solely because it is ubiquitously expressed but also because engagement induces CD46 down-regulation and preferentially suppresses naive CD4+ T-cell activation. The findings reported here highlight important biological properties of rAd35 that may guide its use as a gene delivery or vaccine vector in humans.
Materials and Methods
Cells.
This study was approved by the Ethical Review Boards at the Karolinska Institutet and National Institutes of Health. All cells were cultured in complete media [CM; containing RPMI 1640, 10% (vol/vol) FBS, l-glutamine, penicillin, and streptomycin; Sigma–Aldrich] at 37 °C. Primary human MDCs and PDCs were isolated using anti-CD1c and anti–BDCA-4 microbead kits, respectively, on an Automacs (Miltenyi) and cultured as previously described (4, 10, 40). Monocytes were isolated or differentiated into CD1a+/CD14− MDDCs over 6 d in CM with human IL-4 and GM-CSF as described previously (4). Buffy coats or elutriated lymphocytes were treated with CD4+ or CD8+ T-cell enrichment mixtures (RosetteSep; StemCell), followed by centrifugation on a Ficoll-Plaque (General Electric) density gradient. Purity was >95% for CD4+ and >90% for CD8+ total T cells. Naive T cells were sequentially sorted by magnetic depletion with CD45RO microbeads (Miltenyi) and were >95% CD45RA+/CCR7+ for CD4+ T cells (Fig. 2A) and >90% for CD8+ T cells as determined by flow cytometry. Memory T cells were from the CD45RO+ fraction.
Abs and Flow Cytometry.
In total, 4 × 105 cells in polystyrene round-bottomed tubes (Becton Dickinson) were washed with PBS with 2% FBS and stained for surface phenotype [CD3, CD4, CD8, CD1a, CD11c, CD123, CD14, HLA-DR, CD80, and CD86 (Becton Dickinson); CD46 (eBioscience); CD45RA (Beckman Coulter); and CCR7 (R&D Systems)]. After staining, cells were washed again and evaluated on a FACSCalibur or LSR II flow cytometer (Becton Dickinson). Data were analyzed using FlowJo software v8 (Tree Star).
Intracellular Cytokine Staining.
Staining of intracellular cytokines was performed as previously described (10). T cells stimulated in the presence of Brefeldin A (10 μg/mL; Sigma–Aldrich) were washed and stained for surface markers and LIVE/DEAD fixable dead cell stains (Invitrogen). Cells were then fixed and permeabilized with the cytofix/cytoperm kit; costained for IL-2, IFN-γ, TNF, or Mip-1β; washed again with perm/wash buffer; and analyzed by flow cytometry (all from Becton Dickinson).
Preparation of rAd Vectors.
Construction, preparation, and characterization of replication-deficient rAd vectors expressing GFP and deleted of E1 and E3 genes were previously described (41).
Infection and Stimulation of DCs.
DC or monocytes (2–4 × 105) were exposed to a range of ip/cell doses of rAd5-GFP or rAd35-GFP for 24 h in CM, and the frequency of GFP-expressing cells was measured by flow cytometry as previously described (4, 10). DCs were otherwise stimulated with 1 ng/mL LPS from Escherichia coli 055:B5 (Sigma–Aldrich) or 1 μg/mL TLR7/8-L (3M) (40).
Allogeneic DC Activation of T Cells.
DC or monocytes were exposed to rAd for 24 h and then transferred with supernatant to 1 × 105 allogeneic sorted naive CD4+ T cells at a DC/T-cell ratio of 1:10 and cultured in CM for 5 d in round-bottomed 96-well plates (Corning) or round-bottomed polystyrene tubes. Alternatively, only supernatants from stimulated DCs were transferred along with mock-stimulated DCs as indicated. Proliferation of T cells was measured in triplicate with 1 μCi of 3H-thymidine (Amersham) incorporation over the final 16 h of culture, or, alternatively, 4 × 105 CFSE-labeled (Invitrogen) naive CD4+ T cells were used in coculture experiments and CFSE dilution was evaluated using flow cytometry as previously described (40). Radioactivity of incorporated 3H-thymidine is expressed as counts per minute.
Activation of T Cells.
Anti-CD3 ε-chain (HIT3a, 1 μg/mL; Becton Dickinson), anti-CD28 (CD28.2, 1 μg/mL; Becton Dickinson), anti-CD46 (13/42, 5 μg/mL; BMA), anti-CD46 (M177, 5 μg/mL; Hycult) mAb or rAd vectors (10–200 ip/cell) in PBS were immobilized overnight at 4 °C onto 96-well MaxiSorp flat-bottomed plates (Nunc). Isotype-matched anti-TfR (OKT9) or irrelevant mouse IgG1 mAb was used as a mAb control (eBioscience). Immobilization solution was removed, and T cells were added. rAd vectors or anti-CD46 mAb was otherwise added in soluble form. Alternatively, T cells were stimulated with 1 μg/mL PMA and 1 μM ionomycin (Sigma–Aldrich) with or without immobilized anti-CD46 mAb or rAd. Brefeldin A was added after 30 min of activation for 5 h to measure intracellular cytokines, or cells were analyzed for proliferation with 3H-thymidine. In total, 4 × 105 cells or 4 × 104 cells were used for the cytokine assay or proliferation assay, respectively. Recombinant human IL-2 (Roche) was used in proliferation experiments at indicated concentrations.
Viability and CD46 Expression.
rAd5, rAd35, anti-CD46 mAb (13/42 or M177), or anti-TfR mAb (100 ip/cell) was immobilized as described above. In total, 4 × 105 naive CD4+ T cells were then added with or without 100 ng/mL human IL-2 (Peprotech). At indicated intervals (0–6 d) of exposure time, cells were stained with anti-CD3, anti-CD46, Annexin-V, and 7-AAD (eBioscience) and analyzed by flow cytometry.
Cytoplasmic and Nuclear Extraction and Western Blot Analysis.
CD4+ T cells were stimulated with immobilized mAb as described above and centrifuged immediately to ensure simultaneous activation. After 4 h, NE-PER nuclear and cytoplasmic extraction reagents (Pierce) were used to extract cytoplasmic and nuclear fractions. Denatured proteins and standards (Bio-Rad) were separated by 4–12% (vol/vol) SDS/PAGE and transferred to HyBond-P membranes (Amersham). Membranes were blocked with PBS containing 5% (wt/vol) powdered milk and 0.1% (vol/vol) Tween-20 and blotted with goat anti-p65 or rabbit anti-IκBα polyclonal Ab (Santa Cruz Biotech) or with mouse anti–β-tubulin mAb (Sigma–Aldrich). HRP-conjugated secondary Abs were obtained from Santa Cruz Biotech. Protein chemiluminescence was measured using ECL detection reagents and film (General Electric) on a Curix 60 developer (AGFA). ImageJ software v1.42q (National Institutes of Health) was used for densitometry.
Statistical Analysis.
Significance was determined using either the Student's paired t test or Wilcoxon signed-rank matched-pair tests with Prism software v4 (GraphPad).
Supplementary Material
Acknowledgments
The authors are grateful to Kai Eng, Kerrie Sandgren, Peter Sahlström, Matthew Johnson, and the Department of Transfusion Medicine (Karolinska Hospital) for technical assistance. This study was supported by the Åke Wiberg's foundation, the Swedish Society for Medical Research, Vetenskapsrådet, Jeansson's Foundation, and the Swedish Society for Medicine.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1017146108/-/DCSupplemental.
References
- 1.Barouch DH, Nabel GJ. Adenovirus vector-based vaccines for human immunodeficiency virus type 1. Hum Gene Ther. 2005;16:149–156. doi: 10.1089/hum.2005.16.149. [DOI] [PubMed] [Google Scholar]
- 2.Barouch DH, et al. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J Immunol. 2004;172:6290–6297. doi: 10.4049/jimmunol.172.10.6290. [DOI] [PubMed] [Google Scholar]
- 3.Bergelson JM, et al. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 4.Adams WC, et al. Adenovirus serotype 5 infects human dendritic cells via a coxsackievirus-adenovirus receptor-independent receptor pathway mediated by lactoferrin and DC-SIGN. J Gen Virol. 2009;90:1600–1610. doi: 10.1099/vir.0.008342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johansson C, et al. Adenoviruses use lactoferrin as a bridge for CAR-independent binding to and infection of epithelial cells. J Virol. 2007;81:954–963. doi: 10.1128/JVI.01995-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalyuzhniy O, et al. Adenovirus serotype 5 hexon is critical for virus infection of hepatocytes in vivo. Proc Natl Acad Sci USA. 2008;105:5483–5488. doi: 10.1073/pnas.0711757105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waddington SN, et al. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell. 2008;132:397–409. doi: 10.1016/j.cell.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 8.Gaggar A, Shayakhmetov DM, Lieber A. CD46 is a cellular receptor for group B adenoviruses. Nat Med. 2003;9:1408–1412. doi: 10.1038/nm952. [DOI] [PubMed] [Google Scholar]
- 9.Arnberg N. Adenovirus receptors: Implications for tropism, treatment and targeting. Rev Med Virol. 2009;19:165–178. doi: 10.1002/rmv.612. [DOI] [PubMed] [Google Scholar]
- 10.Loré K, et al. Myeloid and plasmacytoid dendritic cells are susceptible to recombinant adenovirus vectors and stimulate polyfunctional memory T cell responses. J Immunol. 2007;179:1721–1729. doi: 10.4049/jimmunol.179.3.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oglesby TJ, Allen CJ, Liszewski MK, White DJ, Atkinson JP. Membrane cofactor protein (CD46) protects cells from complement-mediated attack by an intrinsic mechanism. J Exp Med. 1992;175:1547–1551. doi: 10.1084/jem.175.6.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cattaneo R. Four viruses, two bacteria, and one receptor: Membrane cofactor protein (CD46) as pathogens’ magnet. J Virol. 2004;78:4385–4388. doi: 10.1128/JVI.78.9.4385-4388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartz R, et al. Mapping amino acids of the measles virus hemagglutinin responsible for receptor (CD46) downregulation. Virology. 1996;224:334–337. doi: 10.1006/viro.1996.0538. [DOI] [PubMed] [Google Scholar]
- 14.Schnorr JJ, et al. Measles virus-induced down-regulation of CD46 is associated with enhanced sensitivity to complement-mediated lysis of infected cells. Eur J Immunol. 1995;25:976–984. doi: 10.1002/eji.1830250418. [DOI] [PubMed] [Google Scholar]
- 15.Santoro F, et al. CD46 is a cellular receptor for human herpesvirus 6. Cell. 1999;99:817–827. doi: 10.1016/s0092-8674(00)81678-5. [DOI] [PubMed] [Google Scholar]
- 16.Gill DB, Koomey M, Cannon JG, Atkinson JP. Down-regulation of CD46 by piliated Neisseria gonorrhoeae. J Exp Med. 2003;198:1313–1322. doi: 10.1084/jem.20031159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okada N, Liszewski MK, Atkinson JP, Caparon M. Membrane cofactor protein (CD46) is a keratinocyte receptor for the M protein of the group A streptococcus. Proc Natl Acad Sci USA. 1995;92:2489–2493. doi: 10.1073/pnas.92.7.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang G, Liszewski MK, Chan AC, Atkinson JP. Membrane cofactor protein (MCP; CD46): Isoform-specific tyrosine phosphorylation. J Immunol. 2000;164:1839–1846. doi: 10.4049/jimmunol.164.4.1839. [DOI] [PubMed] [Google Scholar]
- 19.Cardone J, et al. Complement regulator CD46 temporally regulates cytokine production by conventional and unconventional T cells. Nat Immunol. 2010;11:862–871. doi: 10.1038/ni.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kemper C, Atkinson JP. T-cell regulation: With complements from innate immunity. Nat Rev Immunol. 2007;7:9–18. doi: 10.1038/nri1994. [DOI] [PubMed] [Google Scholar]
- 21.Marie JC, et al. Linking innate and acquired immunity: Divergent role of CD46 cytoplasmic domains in T cell induced inflammation. Nat Immunol. 2002;3:659–666. doi: 10.1038/ni810. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, et al. Identification of CD46 binding sites within the adenovirus serotype 35 fiber knob. J Virol. 2007;81:12785–12792. doi: 10.1128/JVI.01732-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buchholz CJ, et al. Mapping of the primary binding site of measles virus to its receptor CD46. J Biol Chem. 1997;272:22072–22079. doi: 10.1074/jbc.272.35.22072. [DOI] [PubMed] [Google Scholar]
- 24.Fleischli C, et al. The distal short consensus repeats 1 and 2 of the membrane cofactor protein CD46 and their distance from the cell membrane determine productive entry of species B adenovirus serotype 35. J Virol. 2005;79:10013–10022. doi: 10.1128/JVI.79.15.10013-10022.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nanda A, et al. Immunogenicity of recombinant fiber-chimeric adenovirus serotype 35 vector-based vaccines in mice and rhesus monkeys. J Virol. 2005;79:14161–14168. doi: 10.1128/JVI.79.22.14161-14168.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meiffren G, Flacher M, Azocar O, Rabourdin-Combe C, Faure M. Cutting edge: Abortive proliferation of CD46-induced Tr1-like cells due to a defective Akt/Survivin signaling pathway. J Immunol. 2006;177:4957–4961. doi: 10.4049/jimmunol.177.8.4957. [DOI] [PubMed] [Google Scholar]
- 27.Oliaro J, et al. Ligation of the cell surface receptor, CD46, alters T cell polarity and response to antigen presentation. Proc Natl Acad Sci USA. 2006;103:18685–18690. doi: 10.1073/pnas.0602458103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ophorst OJ, et al. An adenoviral type 5 vector carrying a type 35 fiber as a vaccine vehicle: DC targeting, cross neutralization, and immunogenicity. Vaccine. 2004;22:3035–3044. doi: 10.1016/j.vaccine.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 29.Shiver JW, Emini EA. Recent advances in the development of HIV-1 vaccines using replication-incompetent adenovirus vectors. Annu Rev Med. 2004;55:355–372. doi: 10.1146/annurev.med.55.091902.104344. [DOI] [PubMed] [Google Scholar]
- 30.Joubert PE, et al. Autophagy induction by the pathogen receptor CD46. Cell Host Microbe. 2009;6:354–366. doi: 10.1016/j.chom.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Karp CL, et al. Mechanism of suppression of cell-mediated immunity by measles virus. Science. 1996;273:228–231. doi: 10.1126/science.273.5272.228. [DOI] [PubMed] [Google Scholar]
- 32.Nemerow GR, Pache L, Reddy V, Stewart PL. Insights into adenovirus host cell interactions from structural studies. Virology. 2009;384:380–388. doi: 10.1016/j.virol.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pache L, Venkataraman S, Nemerow GR, Reddy VS. Conservation of fiber structure and CD46 usage by subgroup B2 adenoviruses. Virology. 2008;375:573–579. doi: 10.1016/j.virol.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 34.Kemper C, et al. Activation of human CD4+ cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature. 2003;421:388–392. doi: 10.1038/nature01315. [DOI] [PubMed] [Google Scholar]
- 35.Lövkvist L, et al. CD46 Contributes to the severity of group A streptococcal infection. Infect Immun. 2008;76:3951–3958. doi: 10.1128/IAI.00109-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakurai F, Akitomo K, Kawabata K, Hayakawa T, Mizuguchi H. Downregulation of human CD46 by adenovirus serotype 35 vectors. Gene Ther. 2007;14:912–919. doi: 10.1038/sj.gt.3302946. [DOI] [PubMed] [Google Scholar]
- 37.Crimeen-Irwin B, et al. Ligand binding determines whether CD46 is internalized by clathrin-coated pits or macropinocytosis. J Biol Chem. 2003;278:46927–46937. doi: 10.1074/jbc.M308261200. [DOI] [PubMed] [Google Scholar]
- 38.Yant S, Hirano A, Wong TC. Identification of a cytoplasmic Tyr-X-X-Leu motif essential for down regulation of the human cell receptor CD46 in persistent measles virus infection. J Virol. 1997;71:766–770. doi: 10.1128/jvi.71.1.766-770.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Persson BD, et al. Structure of the extracellular portion of CD46 provides insights into its interactions with complement proteins and pathogens. PLoS Pathog. 2010;6:e1001122. doi: 10.1371/journal.ppat.1001122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Douagi I, et al. Human B cell responses to TLR ligands are differentially modulated by myeloid and plasmacytoid dendritic cells. J Immunol. 2009;182:1991–2001. doi: 10.4049/jimmunol.0802257. [DOI] [PubMed] [Google Scholar]
- 41.Cheng C, et al. Mechanism of ad5 vaccine immunity and toxicity: Fiber shaft targeting of dendritic cells. PLoS Pathog. 2007;3:e25. doi: 10.1371/journal.ppat.0030025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.