Abstract
The objective of this review is to provide a broad overview of the advantages and limitations of carbon-based nanomaterials with respect to analytical chemistry. Aiming to illustrate the impact of nanomaterials on the development of novel analytical applications, developments reported in the 2005–2010 period have been included and divided into sample preparation, separation, and detection. Within each section, fullerenes, carbon nanotubes, graphene, and composite materials will be addressed specifically. Although only briefly discussed, included is a section highlighting nanomaterials with interesting catalytic properties that can be used in the design of future devices for analytical chemistry.
Keywords: Carbon-based nanomaterials, Separations, Detection, Sample preparation
1. Introduction
Nanomaterials, materials with sizes or features ranging from 1 to 100 nm in one or more dimensions [1,2], are the core of an emerging technological revolution. The main advantages of these materials are unique thermal, mechanical, electronic, and biological properties not found in conventional materials [3–7]. Combining these unique properties with their remarkable recognition capabilities [8] has resulted in systems with significantly improved performance [9] and novel applications across physics, chemistry, biology, engineering, and computer science [10]. Apart from high mechanical strength and low weight, most of the exceptional characteristics of nanomaterials are linked to their surface properties (area, roughness, energetics, and electron distributions) [11], which enable improved interactions with many biological entities [12]. Such interactions depend not only on the fabrication method, but also on the size and specific geometry of the nanoparticles [13]. As expected, these characteristics combined with the ability to form hydrogen bonds, π-π stacking, dispersion forces, dative bonds, and hydrophobic interactions can affect the stability and selectivity of nanomaterials [14]. Consequently, the distinctive properties of nanomaterials have sparked interest in analytical chemistry and have been used to develop innovative applications in sample preparation [15–18], separation [19–22], and sensing [23–28].
Considering the aforementioned properties of nanomaterials, the objective of this review is to provide a broad snapshot of the applications of carbon-based nanomaterials to analytical chemistry reported during the 2005–2010 period, aiming to perform a critical evaluation of the characteristics and performance of these nanomaterials. Several significant contributions published prior to 2005 have been included, but only briefly discussed. It is also worth mentioning recent reviews that focus on different aspects of the application of various nanomaterials to analytical chemistry [2,28–45]. Although outside the scope of the present review, it is important to note that several techniques are currently available for the analysis of nanoparticles [46] including electrophoresis [47,48], liquid chromatography [49,50], electrical cross-flow filtration [51], and gel permeation [52]. Also outside the scope of the present review are the potential toxic effects of nanomaterials [53–57]. In this regard, it is critical to emphasize that researchers must be aware of the detrimental effects that these novel materials could have on human health and adopt the appropriate safety precautions.
2. Relevant characteristics of carbon-based nanomaterials
Carbon-based nanoparticles have been extensively used in analytical applications. Although the reasons for the selection of one particular allotrope over another are still imprecise (and largely rely on previous experiences and availability), a wide variety of carbon-based materials are available and have been applied to analytical procedures. While the use of nanodiamonds, nano-onions, peapods, nanofibers, nanorings, and nanotubules has been reported, recent applications mainly focus on the use of fullerenes and nanotubes (CNT) [14]. In both cases, the basic structure is composed of a layer of sp2-bonded carbon atoms, where each atom is connected to three other carbon atoms in the x–y plane and by a weakly delocalized π-electron cloud along the z-axis. This configuration, which resembles that of graphene, is responsible for the good electrical conductivity, the capability to form charge-transfer complexes when in contact with electron donor groups [14], and the π-plasmon resonance observed in some of these particles [58]. Furthermore, this configuration is also responsible for the development of strong van der Waals’ forces that significantly hamper the dispersion and solubility of carbon-based nanoparticles. To overcome these limitations different pretreatment methods have been proposed [59–63], though the addition of polar groups (oxygen-, hydroxyl-, polyvinylpyrrolidone, and phenyl-) [64,65] and surface defects typically affect the stability [66,67] as well as the mechanical, magnetic, optical [61,68–70], and electrical properties [42,71,72].
Another interesting aspect stemming from the simple structure of most carbon-based nanomaterials is that the reactivity of atoms situated in the plane is different than those at the edges. In this regard, Compton et al. found that for a number of biologically important compounds, the electrochemistry of different CNT is comparable to that of different planes of graphite [73] and that metallic impurities contained within were responsible for some of the electrochemical catalytic properties of CNT [74]. Pumera et al. also found that non-metallic impurities (nanograhite) can also affect the electrochemical activity of CNT [75,76]. Furthermore, the same group showed evidence supporting that the electroanalytical parameters (repeatability, sensitivity, linearity of the analytical response, and selectivity) of single-, double-, and multi-walled CNT are inferior with respect to surfaces of glassy carbon and edge-plane pyrolytic graphite electrodes [77].
Although not as popular as CNT, graphene is another material that shows great promise in the future of analytical chemistry. Similar to CNT, graphene consists of a one atom thick carbon (sp2 hybridized) sheet composed of six-member rings [78] providing an exposed surface area that is nearly twice as large as that of single-walled carbon nanotubes [75]. Other advantages of this material that make it attractive for analytical applications include its high mechanical strength, high elasticity, high thermal conductivity [75] and the absence of metallic impurities that can affect the accuracy of a sensor [79]. Graphene can also be interlinked with CNT for the fabrication of high performance transparent flexible electrodes, resulting in films with conductivities and optical properties comparable to commercial indium-tin oxide (ITO) [67]. As stated by Lim et al. [80], the electrochemical activity of crystalline graphene is markedly different in chemical composition and structure from reduced graphene oxide flakes. To investigate the effect of edge plane defects on the electrochemical and biosensing activities, a systematic study of the heterogeneous charge transfer rate as a function of defect density on EG was carried out. Interestingly, it was determined that the electrochemistry of EG converges with that of reduced GO flakes following an anodization treatment (see Figure 1), and that anodized graphene is able to resolve the anodic peaks of all four nucleic acid bases in double stranded and single stranded nucleic acids, a performance unmatched by other electrodes..
Figure 1.
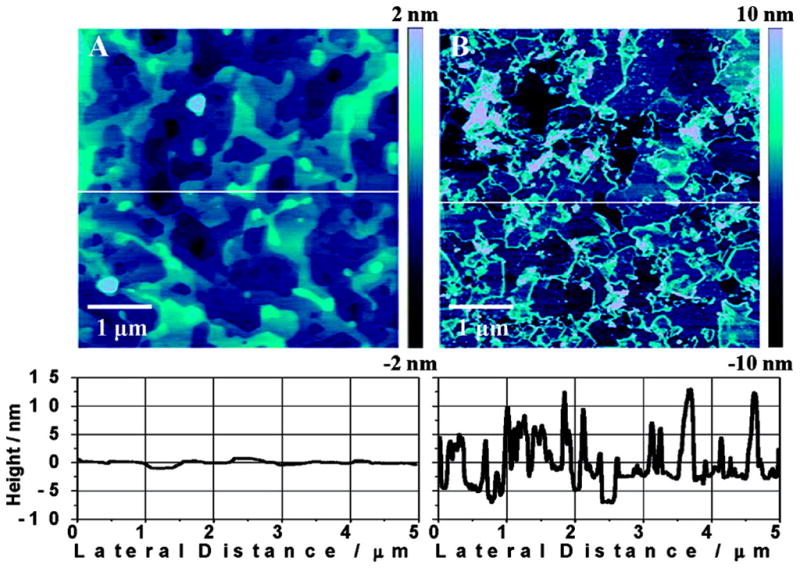
AFM topographical images and cross sections for (A) pristine EG and (B) anodized EG surfaces. Edge defects are generated on the anodized EG surface, leading to high electrochemical activity [80].
Although commercial availability of graphene and graphene platelets is quite limited, they can be fabricated using a variety of methods, the first of which was published in 2004 [81]. As reported, Novoselov et al. was able to achieve high quality films that were stable under ambient conditions in up to 10 μm lengths by mechanical exfoliation (repeated peeling) of small patches of highly ordered pyrolytic graphite [81]. Since that time, many other fabrication methods have been developed, including unzipping multi-wall CNT to form graphene ribbons [82,83], substrate independent methods using micromolding inside a capillary [84], and spray deposition of graphene oxide (GO)–hydrazine dispersions [85]. While fabrication methods have certainly become more widely available, it is important to note that although it may be possible to fabricate single graphene sheets, these sheets have a tendency to stick together forming multi-layer nanostructures [86]. Additionally, it is important to mention that very careful and thorough characterization needs to be carried out when working with these materials because while many articles report the use of graphene, upon closer examination the material is technically graphene with a multi-layer structure [75].
Carbon-based nanomaterials also offer the possibility of combining other types of nanomaterials to form nanocomposites, merging different properties in a single new material. There is nearly an infinite number of possibilities when designing nanocomposites: fullerene-Pd nanocrystals [87], poly(2,5-dimethylaniline)-CNT [27], ceramic-CNT [88], and teflon-CNT [89] are just a few examples. Other examples of nanocomposites and their advantages are discussed in later sections.
Though not typically regarded as having remarkable properties resulting from the presence of nanofeatures, thin carbon films (in the nm range) can be made by either pyrolyzing photoresist or methods (plasma deposition, arc deposition, ion sputtering, and laser evaporation) [90,91]. Probably the main advantages associated to these films is that they are transparent (due to the thickness) [92,93], amenable to standard microfabrication procedures [94,95], and their properties can be (relatively) easily tailored by selecting the appropriate starting material [96].
While most researchers would agree with R. Feynman that there is plenty of room at the bottom, not everything is perfect at the nanoscale. Some of the most commonly cited drawbacks of the use of carbon-based nanomaterials include the cost, the poor batch-to-batch reproducibility of the fabrication and purification processes, and the difficulty of obtaining a comprehensive description of the materials from most manufacturers. A direct consequence is the limited number of reliable commercial sources available for these materials. Another problem associated with the use of carbon-based materials is their tendency to form stable complexes with organic molecules (particularly if an oxidation step is involved). This observation is supported by the small number of papers reporting reusability of the substrates.
In general, the use of carbon-based nanomaterials in analytical chemistry is advantageous. When properly selected, these new materials have the potential to produce significant improvements in all of the classical analytical processes: sample preparation, separation, and detection. Examples of the use of carbon-based nanomaterials are herein discussed.
3. Recent developments
3.1. Sample preparation
Fullerenes are composed of a thermodynamically-stable carbon shell ~1 nm in diameter that can withstand heat, pressure and radiation but, due to their unique electron-hybridization pattern of sp2 bonds, are also highly configurable [97]. Fullerenes display a relatively high electron affinity [98], and a hydrophobic surface [14] that increases their adsorption capacity towards organic molecules, as well as their permeability through lipid membranes [99]. In addition, these compounds have a high surface/volume ratio which makes them ideal for extraction procedures, as demonstrated by the examples herein discussed. In 2006, Agrawal found that uranium (VI) could be extracted from human blood serum, natural water, seawater, standard samples, and monazite sand with a detection limit of 0.1 ng·mL−1 by utilizing N-phenyl-(1,2-methano-fullerene C60)-61 formohydroxamic acid [100]. Jin and co-workers observed that inserting fullerenes into certain hydrophobic polymer membranes (Figure 2) can adsorb estrogenic pollutants from surface and treated waters [101]. This technique was found to be ideal for removing contaminants like estrone, estradiol, and ethinylestradiol which have high hydrophobicity and low volatility.
Figure 2.
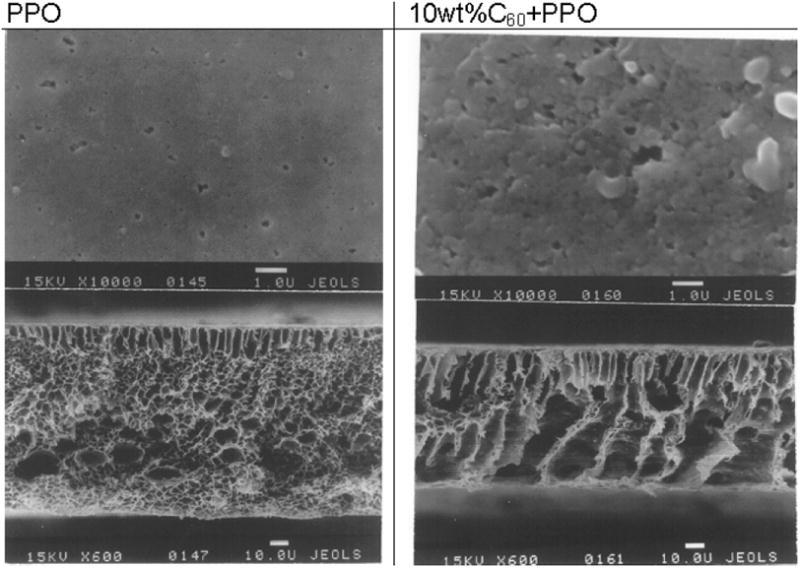
SEM images of fullerenes inserted in hydrophobic polymer membranes for the preconcentration of estrogenic compounds [101].
Compounds like flavonoids, proteins, peptides, and hydrophilic small molecules like phosphopeptides can be extracted by solid phase extraction (SPE) using C60-fullerene bound to silica particles [102]. Aromatic and non-aromatic amines can be discriminated using SPE with a combination of two columns, one with fullerene C60 and the other with Merck LiChrolut EN [103]. Through this novel method, aromatic amines could be retained in the fullerene column while the non-aromatic amines remain in the commercial column. Jurado-Sanchez and co-workers compared the SPE efficiencies of different sorbents when extracting several types of amines [104]. Fullerenes proved to be adequate for the extraction of amines (retention efficiency of 65%). Additionally, SPE of tryptic peptides from human serum albumin and fibrinogen can be obtained using silica gels derivatized with C60-fullerenes [105].
CNT have also shown strong adsorption affinity towards organic and inorganic molecules (particularly hydrophobic ones), relative non-porosity, and ability to develop π-π electrostatic interactions with other molecules [14,106]. In this regard, Zhou and co-workers developed a multi-wall CNT phase as SPE for the determination of the water contaminants metalaxyl, diethofencarb, myclobutanil, prometryn, and tebuconazole [107]. Zhou’s group produced a fast, sensitive, and simple method that allowed the detection of these analytes at concentrations as low as 3 ng·L−1. Wang et al. proposed a similar technique for the extraction (with CNT as the sorbent material), analysis, and quantification of pesticides in water samples [108]. In this case, Wang used gas chromatography-mass spectrometry (GC-MS) to analyze the mixture and obtained a linearity of all 12 pesticides over a range of 0.04–4 μg·L−1 with detection limits reaching 0.01–0.03 μg·L−1. Wang’s findings demonstrated that several pesticides could be analyzed simultaneously at a low cost and short analysis time. Alternatively, Al-Degs and co-workers observed that determining the presence of contaminants in water samples can be done without the need for chromatographic separations; instead, the simultaneous determination of pesticides can be performed using CNT-SPE with multivariate calibration [108,109]. However, after a critical comparison of the enrichment efficiency of CNT against C18 and activated carbon, the same group of researchers advised investigators to try activated carbon before using significantly more expensive sorbents (such as CNT or C18 silica) [110]. Multi-wall CNT as SPE can be used for the determination of several organophosphorus pesticides and thiadiazine in forestal, ornamental, and agricultural soils by employing GC with nitrogen phosphorus detection [106]. The same group also demonstrated the advantages of this approach by measuring organophosphorus contaminants (ethoprophos, diazinon, chlorpyriphos methyl, fenestration, malathion, chlorpyriphos, fenamiphos, and buprofezin) in fruit juice [111]. This method yielded mean recovery values beyond 73% and detection limits ranging from 1.85 to 7.32 μg·L−1 for the previously mentioned pesticides. A different approach was presented by Lopez-Feria et al. when they packed a commercially available PTFE-SPE cartridge with multi-wall CNT or carboxylated single-wall CNT for the determination of pesticides in two monovarietal and one ecologic commercial extra virgin olive oil samples [112]. In their paper, Lopez-Feria’s group showed that SPE could be done in one single-preconcentration-elution step that allowed an analysis time of less than 8 min with detection limits of 1 to 3 μg·L−1. Single-wall CNT can also be used to preconcentrate metals like Co, Cu, Pb, and Ni [113]. Additionally, preconcentration and SPE of heavy metal ions like Cu(II), Co(II), Ni(II), and Pb(II) present in environmental samples can be performed by the addition of a complexing reagent, like o-cresolphthalein complexone, to the above mentioned technique [114].
Determination of parabens in cosmetic products is also achievable via multi-wall CNT-SPE using a corona-charged aerosol detector as demonstrated by Marquez-Sillero and co-workers [115]. This technique proved to be successful in determining the concentration of different parabens with detection limits in the range of 0.5–2 mg·L−1. Alkylbenzene sulfonates can also be extracted using carboxyl modified multi-wall CNT as SPE adsorbent, and later detected by employing HPLC [116]. Analytical scale membrane extractions are made possible by immobilizing functionalized CNT into membranes, facilitating the solute exchange and extraction from the donor to the acceptor phase [117].
Although adopting different nomenclatures, a number of variants to SPE have been developed incorporating CNT as sorbent materials. Among other examples, a novel method called solid phase membrane tip extraction (SPMTE) was developed by See et al. [118]. This new approach consisted of a membrane-protected multi-wall CNT SPE utilized for microextractions which was integrated in a semi-automated dynamic mode. This method resulted in good detection limits (0.2–0.5 μg·L−1) and good recoveries (95%–101%) for triazine herbicides. Another example is the modification of solid phase microextraction (SPME) with single-wall CNT to extract environmental pollutants like methyl tert-butyl ether, ethyl tert-butyl ether, and methyl tert-amyl ether from human urine [119] or the fabrication of a micro-solid-phase extraction (μ-SPE) in the needle of a syringe using single-wall CNT and multi-wall CNT as the sorbent materials [120]. The main advantage of the latter is the possibility of integrating sampling, analyte enrichment, and sample introduction into a single device.
Nanocomposites have also proven to be appropriate for the extraction of several analytes. Multi-wall CNT-polyaniline nanocomposites have been used to extract and detect phenolic compounds through GC analysis [121]. Du and co-workers developed this technique and showed that the nanocomposites could be used more than 250 times without losing their efficiency and were able to achieve a detection limit as low as 2 ng·L−1. Multi-wall CNT-Sudan IV molecularly imprinted polymers were used for the extraction of Sudan IV from chili powders [122].
3.2. Separation
Although different nanomaterials have been applied to a wide variety of separation techniques [40], the field seems to be dominated by methods requiring small amounts of the selected nanomaterials (capillary electrophoresis, microchip capillary electrophoresis, and some chromatographic techniques). In this regard, Moliner-Martínez et al. used fullerenes (C60) coated with surfactants as a pseudostationary phase [123,124] and concluded that while the presence of fullerenes increase the migration time of selected analytes (β-lactams antibiotics, amphenolicols and anti-inflammatory drugs), they did not significantly improve sensitivity.
Carbon nanotubes have been extensively used as buffer additives in CE (pseudostationary phases). In this regard, Xiong et al. [125] improved the separation of purine and pirimidine bases in yeast by adding multi-wall CNT to the background electrolyte. The results of this work showed that the nanotubes provided greater resolution than TX-100 alone. Xu et al. [126] improved the separation of DNA fragments by adding a mixture of polyvinylpyrrolidone (PVP) and multi-wall CNT to the running buffer. The authors hypothesized that a synergistic effect between the network generated by PVP and nanotubes was responsible for the improvements. Moreover, Na et al. in 2006 proposed an effective technique to separate the two enantiomers of clembuterol using CNT coated with β-cyclodextrins. The authors stated that the tubular structure of the CNT allowed a better attachment of β-cyclodextrins to the nanotube surface. The system also enabled the enantiomeric separation of ephedrine [127].
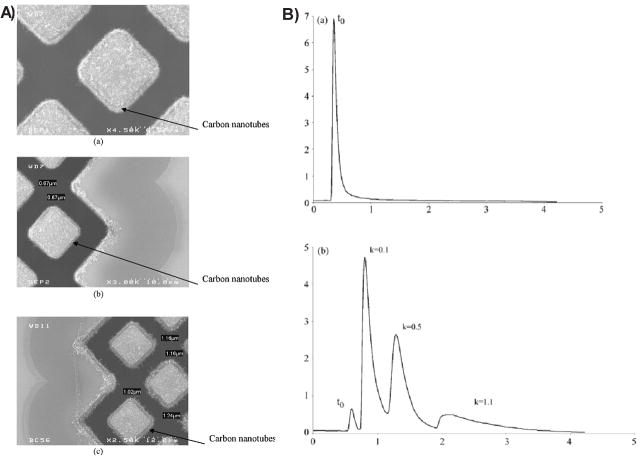
An alternative use of nanomaterials in separation protocols is to immobilize them onto a solid support. Sombra et al. [128] demonstrated that coating the capillary wall with oxidized multi-wall CNT (immobilized through covalent modification of fused capillaries) allowed the separation of eight non-steroidal anti-inflammatory drugs, β-lactams antibiotics, and chloramphenicol with high resolution and without band-broadening or distortion of the baseline. The authors also stated that single-wall CNT immobilized by the same procedure did not render comparable results. Single- wall carbon nanohorns have been used as a stationary phase in CE for the separation of five water-soluble vitamins [129]. The electrochromatographic features obtained when the nanohorns were immobilized in the capillary showed good separation efficiencies and higher retention factors than those obtained with a bare fused-silica capillary. Furthermore, the results were comparable to those obtained with single-wall CNT immobilized onto the capillary and showed significant improvements in resolution. Moreover, Stege et al. [130] implemented a method for the determination of melatonin in complex food matrices by capillary electrochromatography with immobilized carboxylic multi-walled CNT as a stationary phase. The results showed high electrochromatographic resolution, good capillary efficiencies, and improved sensitivity with respect to those obtained with conventional capillaries. Alternatively, the microfabrication of a liquid chromatographic column made out of silicon, structured by a perfectly ordered two-dimensional array of squared micropillars and modified by in situ synthesized CNT was presented by Fonverne et al. [131]. As shown in Figure 3, CNT were grown on the surface of the pillars via chemical vapor deposition and used to separate uracil, phenol, N,N-diethyl-m-toluamide, and toluene. This phase could be reused several times without variation of the results, indicating good adhesion of the CNT and high compatibility with the microfluidic application.
Figure 3.
A) SEM images of the CNT growth with the final process before the sealing of the cover plate: (a) with a 20 nm nickel film, (b) with a 50 nm nickel film, and (c) with a 100 nm nickel film. B) HPLC analysis of the reversed phase test mixture (uracil, phenol, N,N-diethyl-m-toluamide, toluene), isocratic eluent water:acetonitrile (80:20), at a flow rate of 700 nL·min−1 with a UV detection at 254 nm (a) on the reference column (C18 reversed phase coating) and (b) on the CNT column. Reprinted with permission from Ref [131].
André et al. [132] investigated the preparation and chromatographic characteristics of a silica column that was chemically-modified with amino groups, and then functionalized with CNT. In this work, the authors separated eight aromatic compounds and four terpenes, obtaining good resolution and reproducibility. In agreement with other reports [14], the authors proposed that dipole-dipole interactions, hydrogen bonds, π-π stacking, dispersion forces, dative bonds, hydrophobic interactions, and steric effects were involved in the separations. Also, they inferred that the planar geometry of the analytes, the substitution in the ortho position of polychlorinated biphenyls, as well as the hydroxyl position in the terpenes played an important role in the interaction with CNT. The synthesis of a novel stationary phase, based on classic swelling polymerization methods, was presented by Zhong et al. [133]. The novel composite, made with polystyrene and multi-wall CNT, was used as a stationary phase for HPLC with a wide tolerance to pH values and a long lifetime.
Immobilizing nanoparticles is another viable option for controlling the retention of analytes in gas chromatography (GC). A novel stationary phase was developed using self-assembled single-wall CNT and used to separate various classes of compounds [134]. The authors stated that the addition of single-wall CNT enabled obtaining good separation efficiency, classical chromatography behavior, and high-resolution separations. The high surface area of the CNT allowed separations of gases. At the same time, the high thermal stability of the CNT permitted separations of compounds with higher molecular weights at higher temperatures, extending the range of conditions to be applied on the same column.
3.3. Detection
One of the properties that arises when particle size reaches the nanometer scale (and upon the interaction with electromagnetic radiation) is the development of the so-called surface plasmon resonance. This collective excitation of the electrons on the particle’s surface depends on the chemistry, size, and shape of the particle [135] leading to the design of a wide variety of optical probes. Among other nanomaterials, CNT display unique optical properties [58,61,68,136] that include small band-gaps and photoluminescence in the near-infrared (NIR). Taking advantage of such properties, Chen et al. reported the use of single-wall CNT as macromolecular Raman labels for highly-sensitive and selective protein detection with 1000-fold greater sensitivity than fluorescence. The strong Raman intensity of CNT tags was applied to the detection of human auto-antibodies against proteinase 3 in serum, a biomarker for Wegener’s granulomatosis [137]. Because single-wall CNT exhibit a sharp absorption peak in the UV–Vis–NIR range when they are individually dispersed in aqueous solutions, Cao et al. was able to develop CNT-based molecular probes by conjugating single-stranded DNA (ssDNA) with single-wall CNT to study hybridization events. Hybridization on the sidewall of the CNT resulted in systematic red shifts of the absorption spectra of semiconducting nanotubes, demonstrating that ssDNA–CNT probes could potentially be used to detect specific kinds of DNA oligonucleotides as optical nano-biosensors [138]. Song et al. described a sensor to determine Cu2+ ions with magnetic silica nanoparticles attached to multi-wall CNT using click chemistry. In this work, the authors proposed a hybrid nanomaterial that presented peroxidase-like color activity [139].
The synthesis of aligned CNT/polymer composite films with high optical transparency, robust flexibility, and excellent conductivity was reported by Peng et al. These composite films showed many potential applications, such as flexible conductors for optoelectronic devices [140]. In addition, it has been reported that CNT can be used to enhance the electrogenerated chemiluminescence (ECL) of CdS quantum dots (QD) film by reducing the injection barrier of electrons to the QD [141].
Many of the advantages of electrochemical detection can be enhanced by the use of conductive nanoparticles. For this reason, several authors have used a variety of nanomaterials (inorganic, organic, and composites) to modify conventional detection electrodes. In most cases, the nanoparticles increased the electrode area (and consequently the sensitivity) [142], enhanced the electron transfer between the surface and redox centers in analytes, and/or acted as catalysts to increase the efficiency of electrochemical reactions. Consequently, a rich body of literature has emerged supporting the advantages of fullerenes [143–145] and CNT [146–151] towards the detection of both inorganic and organic species. Among them, Deng et al. used multi-wall CNT to modify carbon paste electrodes in the presence of alizarin violet (AV) for the determination of molybdenum (VI) traces by anodic adsorptive stripping voltammetry [152]. Besides the analytical advantages of this approach, the authors stated that this method avoids the use of toxic and expensive mercury electrodes. A sensitive method for the analysis of heavy metals using l-cysteine-functionalized multi-wall CNT has been described [153]. Aiming to improve the analytical performance of electrodes, composites of multi-wall CNT/bismuth/Nafion were deposited and examined for sensitivity towards trace Pb(II) and Cd(II) by anodic stripping voltammetry [154,155].
The application of CNT to the electrochemical detection of organic molecules has also been heavily investigated. Lui et al. studied the electrochemical behavior of hydroquinone (HQ) using cyclic voltammetry (CV) with a glassy carbon electrode modified with a gel containing multi-wall CNT and an ionic liquid at room-temperature. With the modified electrode, the authors obtained a pair of quasi-reversible redox peaks for HQ and stated that the reported cathodic peak current (Ipc = 9.608×10−4 A) was 43 times larger than the current of the bare GCE, and 11 times larger than that of the Ipc obtained with the multi-wall CNT/GCE [156]. Erdem et al. reported the advantages of using a graphite pencil electrode modified with CNT for the detection of nucleic acids and DNA hybridization based on enhancement of the guanine signal using differential pulse voltammetry [157]. A sensor for the detection of methimazole, an emergent contaminant and disruptor of the endocrine system, was developed by Martinez et al. [158]. The authors obtained a low detection limit of 0.056 μmol·L−1 and high throughput, processing as many as 25 samples per hour. Similarly, Ghalkhani et al. performed voltammetric studies of sumatriptan on the surface of a pyrolytic graphite electrode modified with multi-wall CNT decorated with AgNP. The modified electrode was successfully used for the accurate determination of trace amounts of sumatriptan in pharmaceutical preparations [159]. Multi-wall CNT have also been used for many other applications in food science. In this regard, it is worth mentioning the excellent work performed by Escarpa’s group in the determination of flavonoids and antioxidant profiles [160,161] and the work from Compton’s group for the determination of capsaicin, the chemical responsible for the hot taste of chilli peppers [162]. Kachoosangi et al. also developed a composite electrode composed of multi-wall CNT and the ionic liquid n-octylpyridinum hexafluorophosphate. This electrode showed improved electrochemical performance (sensitivity and stability) with respect to other conventional electrodes using graphite and mineral oil [163]. Moreover, the mediator tris(2,2′-bipyridyl)cobalt(III) (Co(bpy)33+) which was incorporated into the multi-wall CNT–Nafion composite film via a simple ion-exchange route was developed by Chen et al. [164]. Then, AuNP were attached onto Co(bpy)33+/multi-wall CNT–Nafion film via electrostatic interactions between the negatively charged AuNP and the positively charged Co(bpy)33+. M. Chicharro et al. used a glassy carbon electrode modified with carbon nanotubes dispersed in PEI for the amperometric detection of phenolic pollutants (phenol, 3-chlorophenol, 2,3-dichlorophenol and 4-nitrophenol) and herbicides (amitrol, asulam, diuron, fenuron, monuron and chlortoluron) in micellar electrokinetic capillary chromatography (MEKC) separations [165].
CNT also have the potential to promote the attachment of biorecognition elements [166], the retention of catalytic activity [167], and the electron transfer rate (in some cases even with a direct and quasi-reversible redox process [166–170]). For these reasons, CNT are still one of the most popular substrates to prepare biosensors. However, one of the critical challenges (especially when the electrode area is limited), is to understand the driving forces and consequences of the interaction between CNT and proteins [149,171]. This issue is critical for maximizing the activity of the biological entity immobilized onto the surface. Valenti et al. [172] investigated the kinetics of the adsorption–desorption process of a model protein (BSA) to CNT. The authors stated that BSA molecules arriving at the CNT surface may adopt a preferred orientation with the positive and non-polar patches of the protein facing the hydrophobic sorbent surface, resulting in an attachment-controlled adsorption process. Later, Mora et al. investigated the relationship between the interaction phenomena (adsorption/desorption kinetics and amount) and the activity of adsorbed D-amino acid oxidase (DAAO) [173]. They found that the adsorption of DAAO to CNT is controlled by a combination of hydrophobic and electrostatic forces, and observed that the activity of the sensor was influenced not only by the adsorbed amount but also by the conformation adopted by the enzyme on the CNT surface. Carot et al. studied the adsorption mechanism of short chain 20-mer pyrimidinic homo ss-DNA (oligodeoxyribonucleotide, ODN: polyC20 and polyT20) onto CNT [174]. Felhofer et al. recently demonstrated that the activity of a model enzyme (catalase) adsorbed to thin-films of CNT depended not only on the adsorbed amount but also on the initial adsorption rate [175]. Using a similar experimental set-up Nejadnik et al. showed that in some cases, enzymes (e.g. glucose oxidase) can be adsorbed to the interior of the CNT film, obtaining nanocomposites with higher catalytic activities [176] (see. These results support the hypothesis that adsorption can be effectively used to immobilize enzymes to the surface of CNT. Besides being simpler and faster, this route also avoids the use of chemicals (such as 3-(3- dimethylaminopropyl)carbodiimide [177]) that may not only increase the cost of the work, but also may lead to some loss of protein activity resulting from the handling in the process and the possible formation of cross-linked protein aggregates. Although examples of the use of CNT as substrates for the development of biosensors abound in literature, it is worth highlighting a report describing the benefits of using vertically aligned carbon nanotubes (CNT) [178].
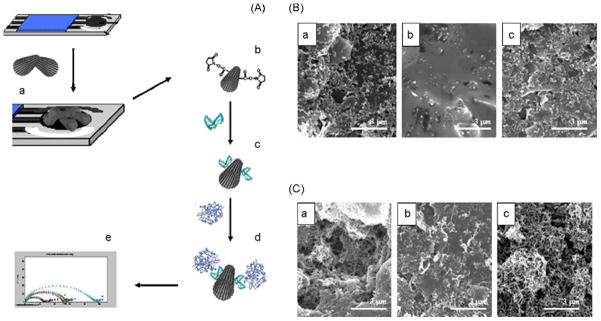
Aiming to improve the sensitivity of electrochemical detection of proteins, Liu et al. developed an aptasensor based on CNT [179]. In this work, authors used thrombin as a model target analyte and thrombin-binding aptamer as a molecular recognition element. The authors stated that the sensor enabled the amplification of the electrochemical signal and significant improvements of the sensitivity, reaching a limit of detection of 5×10−13 mol·L−1 for thrombin. Kara et al. described a label-free aptasensor (Figure 4) designed for direct protein analysis at multi-wall CNT-enhanced screen-printed carbon electrode surfaces [180]. The 5′amino-linked aptamer sequence was immobilized onto the modified screen printed electrode and then the binding of thrombin to aptamer sequence was monitored by electrochemical impedance spectroscopy in the presence of 5 mmol·L−1 [Fe(CN)6]3−/4−. A detection limit of 105 pmol·L−1 was obtained.
Figure 4.
(A) Schematic representation of the experimental procedure followed for the obtaining of the analytical signal: (a) multi-wall CNT modification of the SPCEs; (b) surface modification with covalent agents; (c) aptamer binding; (d) α-thrombin interaction; (e) EIS detection. (B) SEM images of the working surface area of bare SPCES after electrochemical pretreatment (a), after aptamer immobilization (b) and after its interaction with thrombin (c). (C) SEM images of the working surface area of MWCNT modified SPCEs after the same modifications detailed in (B). SEM resolution: 3 μm [180].
Stege et al. modified screen-printed electrodes with multi-wall CNT for the determination of arylsulphatase and phosphatase activities in soil. The authors proposed that this method could be applied for the screening of microbial activities in real matrices thus and could have a significant application in the agricultural industry [181]. Moreover, screen-printed immunosensors modified with CNT were integrated to both continuous-flow systems and microfluidic systems to determine Botrytis cinerea and prostate specific antigen, respectively [182,183]. In both cases, the authors stated that the sensitivity of the electrochemical signal was greater than the one obtained without CNT. Zhao et al. have developed a novel Shigella flexneri immunosensor based on HRP-labeled antibodies against S. flexneri (HRP–anti-S. flexneri) immobilized by physical adsorption on the multi-wall CNT/sodium alginate composite-modified screen-printed electrode surface. The analytical performance of the immunosensor towards S. flexneri was investigated by CV with a linear range of 104 to 1011 CFU·mL−1 and a detection limit of 3.1 × 103 CFU·mL−1 [184]. Other examples of CNT based immunosensors applied for the detection of bacteria and viruses have also been recently reported [185].
In order to perform mechanistic studies of the immobilized proteins or to fabricate electrochemical biosensors, various nanocomposites of CNT with MnO2 [186], NiO [187], TiO2 [188], Pt [189], or Au [148,190] have also been prepared. Chen et al. developed an amperometric glucose biosensor based on a MnO2/multi-wall CNT electrode [186]. MnO2 was homogeneously coated on vertically aligned multi-wall CNT by electrodeposition and showed high resistance towards fouling by chloride ions. In addition, interference from other species was avoided. A matrix of NiO/multi-wall CNT, for the immobilization of protein and biosensing was developed by Qiu et al. [187]. The modified electrode showed excellent electrocatalytic activity towards the reduction of H2O2 without the help of an electron mediator. Moreover, CNT-modified titania nanotube arrays, prepared by vapor-growing CNT inside of the titania nanotube have been used by Pang et al. [188]. Pt nanoparticles of 3 nm in diameter were uniformly deposited on TiO2/CNT electrodes, showing remarkably improved catalytic activities for the oxidation of H2O2. The consequent glucose biosensor fabricated by modifying a TiO2/CNT/Pt electrode with glucose oxidase yielded a high sensitivity with a response time of less than 3 s and a detection limit of 6 μmol·L−1. Also, Wang et al., have used HRP incorporated into a multi-wall CNT/thionine/Au (MTAu) composite film via electrostatic interactions between positively charged HRP and negatively charged MTAu [190]. The resulting composite was able to retain the electrocatalytic activity of HRP and showed good direct electron transfer behavior. These hybrid nanomaterials exhibited a desirable microenvironment for protein immobilization and great facilitation of the electron transfer reaction.
CNT have also been studied as a way to improve the performance of other detection platforms including a ratiometric pH sensor to measure real water samples [191] and a field-effect biosensor to measure penicillin [150]. In addition, CNT have been used to develop a gas ionization sensor [192] and a prototype detector for the determination of radiation [193]. Li et al. reported a hydrogen sensor with high sensitivity and selectivity based on a composite of single-wall CNT and chitosan. The improvement in the detection was attributed to the active binding of hydrogen gas to the amino and hydroxyl functional groups in chitosan [194]. Wongchoosuk et al. reported an electronic nose based on hybridized CNT–SnO2 gas sensors prepared by electron beam evaporation (see Figure 5). This device was used to detect methanol, a contaminant in whiskeys [195].
Figure 5.
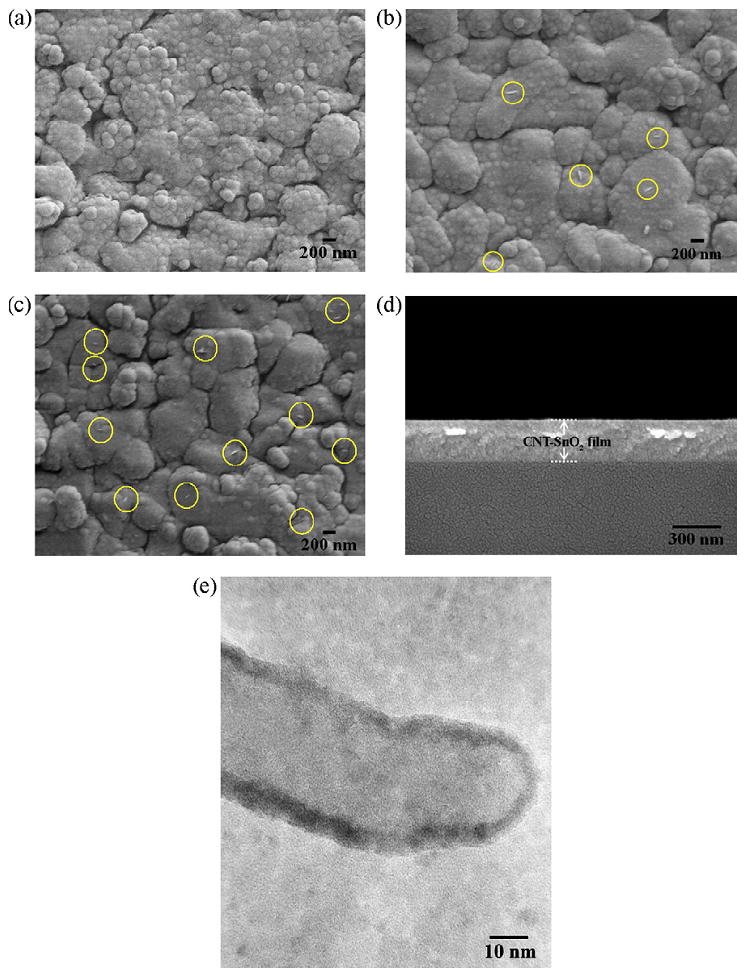
SEM images of sensing films; (a) undoped SnO2 film, (b) 0.5 wt% CNT–SnO2 film and (c) 1 wt% CNT–SnO2 film. The yellow circles in (b) and (c) indicate CNT fragments, (d) typical cross-sectional SEM image of CNT–SnO2 film and (e) typical HRTEM image of CNT–SnO2 film [195].
As previously stated, graphene has unique electronic properties [196] and therefore can enable the development of revolutionary technological applications [78]. Several sensors based on graphene have also been reported, mainly in combination with other materials [197]. For example, a new and highly enhanced sensing platform based on a Nafion–graphene nanocomposite film was established, enhancing the sensitivity for Cd2+ determinations [198]. Graphene has also been used as support for Pt-Ru NPs for the electro-oxidation of methanol [199]. Kang also demonstrated that graphene (deposited on GCE) showed excellent electrocatalytic activity towards paracetamol [200]. More recently, a highly sensitive and specific fluorescence resonance energy transfer (FRET) aptasensor for thrombin detection was developed based on the dye-labeled aptamer assembled graphene.[201]
4. Nanomaterials for Future Applications in Analytical Chemistry
Although some papers presented are beyond the scope of this review, the purpose of this section is to highlight interesting catalytic properties of carbon-based nanomaterials that have the potential to be used to develop a wide variety of new analytical applications.
As stated in the introduction of this review, fullerenes are known for their electronic properties and high electron affinity. These properties make them good catalytic materials for a variety of reactions. For instance, a mixture of polyhydroxy fullerenes (PHF) and titanium dioxide improves the photocatalytic degradation of organic dyes due to hydroxylation and concomitant suspension of fullerenes, as shown by Krishna and co-workers [202]. Titanium oxide, coupled with fullerenes, is a common combination for the catalysis of certain reactions. Krishna et al. proved that a mixture of TiO2 and PHF increased the concentration of hydroxyl radicals by up to 60% when compared to the concentrations obtained without PHF, a fact that is consistent with the above- mentioned enhancement of dye degradation and other microbial inactivation experiments performed by the same group [203]. A nanotube array with the same combination of materials and catalytic properties allowed the photoelectric catalytic degradation of nonbiodegradable azodyes with very high efficiency rates [204]. In addition to TiO2, γ-Al2O3 can also be coupled with fullerenes to achieve catalysis [205]. In this case, photocatalysis of the oxidation of organic compounds under an oxygen atmosphere was possible in temperatures up to 200 °C. Tzirakis and co-workers used the photo-oxygenation of 2-methyl-2-heptene as a probe reaction and obtained an increased catalytic activity in the presence of fullerenes (up to 3% w/w). Depleted fullerene soot (with fullerene content of about 2–3%) can be used as a support for the catalytic the reduction of NO with CO [206]. The production of bimetallic copper-cobalt and copper- manganese oxides supported on DFS yielded high activity in the reduction of NO with CO below 150 °C. Other combinations also illustrate catalytic possibilities for fullerenes. Yu and co-workers demonstrated that hydrophilic C60-derived nanostructures have catalytic effects in antitumoral and antibacterial applications [207].
CNT are great catalysts due to their wide variety of surface properties, including high surface area. Multi-wall CNT can catalyze the redox reaction of chloroauric acid and reductive drugs for the production of AuNP [208]. These particles can later be used for the analysis of tetracycline hydrochloride (a reductive drug) by light scattering. Detection of methane in environmental samples by a nickel electrode modified with multi-wall CNT, Nafion, and nickel hydroxide was developed by Qiao et al. [209]. This detection was possible due to the catalytic effects that the modified electrode had on the oxidation of methane. Nitrogen-containing CNT immobilized to platinum electrodes promoted the electrocatalytic oxidation of methanol in direct methanol fuel cells [210].
CNT can also be used to support catalytic metal-based nanoparticles. GCE modified with multi-wall CNT, coated with TiO2 nanoparticles, were found to display electrocatalytic properties in the reduction of H2O2 [211]. The same composite was used in the photo-electro-catalytic degradation of methylene blue [212]. Another type of composite that can be used in catalytic reactions is Pd-nanoparticles supported on carboxylic functionalized CNT for the electro-oxidation of ethanol on a GCE [213]. The oxidation of ethanol was achieved in alkaline medium and the results obtained show promise for the development of methanol fuel cells and ethanol sensors. The electro-oxidation of methanol is also possible by employing Pt nanoparticles immobilized to CNT modified with polyimide materials [214] and PtRu nanoparticles supported on nitrogen-doped CNT [215].
5. Conclusions
A wide number of novel applications of carbon-based nanomaterials have been recently reported. Besides chemical composition, size and shape are probably the most important variables identified. In addition, the hydrophobicity of the surface (affected by the number of functional groups) plays a key role in the optical, electrochemical, and adsorptive properties of carbon-based nanomaterials and should be carefully evaluated. Typically, hydrophobic particles can be used to enhance non-specific interactions (via increases in surface area) with organic molecules. On the other hand, highly derivatized carbon-based surfaces provide excellent platforms to develop applications based on electrostatic and specific interactions.
Although there is an incredible volume of literature supporting the use of carbon-based nanomaterials, researchers should carefully asses the properties of such materials before claiming exceptional behavior. Additionally, while the main focus of reports published in past years has been the use of carbon nanotubes, it is the authors’ opinion that this trend may change as researchers develop new types of carbon-based materials. Among them, graphene was identified as one emerging allotrope that could play a fundamental role in the preparation of future sensors. All things considered, the use of carbon-based nanoparticles in analytical chemistry has been obviously advantageous and has enabled the integration of analytical chemistry with a large number of fields. Although today the use of nanotechnology in analytical chemistry has a fairly young approach that mixes art, intuition, and science; many researchers around the world have recognized the utility of nanomaterials. We believe that the analytical applications of carbon-based nanomaterials will continue to rise and will soon develop into a mature and independent field.
Acknowledgments
Financial support was provided in part by The University of Texas at San Antonio, the National NIH Grant number 1SC3GM081085 (Institute of General Medical Sciences), NIH Grant Number 2G12RR013646-11 (National Center for Research Resources), the National Council of Scientific and Technical Research (CONICET-Argentina), the National Agency for Promotion of Science and Technology (FONCYT, Argentina; PICT-BID) and the National University of San Luis (Argentina). K. Scida thanks the financial support from the Welch Foundation Departmental Research Grant AX-0026.
Biographies

Dr. Garcia received his BS in Biochemistry and PhD in Chemistry from the National University of Cordoba (Argentina) in 1996 and 2001, respectively. From 2002 to 2004, he was a postdoctoral fellow at Mississippi State University and Colorado State University under the supervision of Dr. W. Wilson and Dr. Charles Henry, respectively. In September of 2004 he joined the faculty at The University of Texas at San Antonio as an Assistant Professor of Analytical Chemistry. He was promoted to Associate professor September 2010. Currently, his group is focused on the study of interactions of proteins with nanostructured surfaces and their use in analytical chemistry. Additionally, he is developing microfluidic devices to monitor biologically active compounds.

Gabrielle G. Haby joined UTSA in 2007 as part of the PhD program after graduating from Sul Ross State University. She is interested in developing lab-on-a-chip devices for forensic applications. She is also very interested in chemical education, particularly linked to Analytical Chemistry.

Dra. Patricia W. Stege is a PhD in Chemistry of the National University of San Luis. She is a PhLic in Molecular Biology and a Bachelor in Biochemistry. She is a TA in Physical Chemistry and researcher at the INQUISAL-CONICET. Her research interests are the introduction of new material as a stationary and pseudo-stationary phase in capillary electrophoresis and the development of new methods for the measurement of the interaction between biological molecules.

Dr. Germán A. Messina received his PhD in Analytical Chemistry in 2006 at Universidad Nacional de San Luis, Argentina. He is currently a Professor in Analytical Chemistry at the Universidad Nacional de San Luis. Dr. Messina’s research interest comprises the development of news analytical biosensors for clinical and environmental applications.

Karen Scida graduated from UT San Antonio in August of 2010. She is now pursuing graduate studies in Chemistry at UT Austin. Her interests include the design of bipolar electrodes and their analytical applications in microfluidic devices.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tretyakov YD, Goodilin EA. Russ Chem Rev. 2009;78:801–820. [Google Scholar]
- 2.He L, Toh C-S. Anal Chim Acta. 2006;556:1–15. doi: 10.1016/j.aca.2005.08.042. [DOI] [PubMed] [Google Scholar]
- 3.Cheng M-D. J Envirom Sci Health. 2005;39:2691–2705. doi: 10.1081/ese-200027028. [DOI] [PubMed] [Google Scholar]
- 4.Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. J Biomed Mater Res. 2000;51:475–483. doi: 10.1002/1097-4636(20000905)51:3<475::aid-jbm23>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 5.Cheng F-Y, Wang SP-H, Su C-H, Tsai T-L, Wu P-C, Shieh D-B, Chen J-H, Hsieh PC-H, Yeh C-S. Biomaterials. 2008;29:2104–2112. doi: 10.1016/j.biomaterials.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Chung Y-C, Chen IH, Chen C-J. Biomaterials. 2008;29:1807–1816. doi: 10.1016/j.biomaterials.2007.12.032. [DOI] [PubMed] [Google Scholar]
- 7.Deng C, Chen J, Chen X, Xiao C, Nie L, Yao S. Biosens Bioelectron. 2008;23:1272–1277. doi: 10.1016/j.bios.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Yoshimatsu K, Ye L, Lindberg J, Chronakis IS. Biosens Bioelectron. 2008;23:1208–1215. doi: 10.1016/j.bios.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Engel E, Michiardi A, Navarro M, Lacroix D, Planell JA. Trends Biotechnol. 2008;26:39–47. doi: 10.1016/j.tibtech.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Murray RW. Anal Chem. 2009;81:1723–1723. doi: 10.1021/ac900238p. [DOI] [PubMed] [Google Scholar]
- 11.Liu H, Webster TJ. Biomaterials. 2007;28:354–369. doi: 10.1016/j.biomaterials.2006.08.049. [DOI] [PubMed] [Google Scholar]
- 12.Willner I, Willner B. Nano Lett. 2010;10:3805–3815. doi: 10.1021/nl102083j. [DOI] [PubMed] [Google Scholar]
- 13.Murray RW. Chem Rev. 2008;108:2688–2720. doi: 10.1021/cr068077e. [DOI] [PubMed] [Google Scholar]
- 14.Valcarcel M, Cardenas S, Simonet BM, Moliner-Martinez Y, Lucena R. TrAC, Trends Anal Chem. 2008;27:34–43. [Google Scholar]
- 15.Cai Y-q, Cai Y-e, Mou S-f, Lu Y-q. J Chromatogr A. 2005;1081:245–247. doi: 10.1016/j.chroma.2005.05.080. [DOI] [PubMed] [Google Scholar]
- 16.Xiao C, Han S, Wang Z, Xing J, Wu C. J Chromatogr A. 2001;927:121–130. doi: 10.1016/s0021-9673(01)01046-9. [DOI] [PubMed] [Google Scholar]
- 17.Sudhir P-R, Wu H-F, Zhou Z-C. Anal Chem. 2005;77:7380–7385. doi: 10.1021/ac051162m. [DOI] [PubMed] [Google Scholar]
- 18.Kabir A, Hamlet C, Soo Yoo K, Newkome GR, Malik A. J Chromatogr A. 2004;1034:1–11. doi: 10.1016/j.chroma.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 19.Kiyokatsu J, Kunihiko Y, John CF, Wilton RB. J Microcolumn Sep. 1992;4:187–190. [Google Scholar]
- 20.Ming-Feng H, Chih-Ching H, Huan-Tsung C. Electrophoresis. 2003;24:2896–2902. [Google Scholar]
- 21.Mari T, Katsuyama Y, Nogami K, Nagata H, Wakuda K, Fujimoto M, Nagasaki Y, Yoshikawa K, Kataoka K, Baba Y. Lab Chip. 2005;5:199–204. doi: 10.1039/b410498f. [DOI] [PubMed] [Google Scholar]
- 22.Gomes D, Nunes SP, Peinemann K-V. J Membr Sci. 2005;246:13–25. [Google Scholar]
- 23.Wang J, Chen G, Chatrathi MP, Musameh M. Anal Chem. 2004;76:298–302. doi: 10.1021/ac035130f. [DOI] [PubMed] [Google Scholar]
- 24.Sotiropoulou S, Gavalas V, Vamvakaki V, Chaniotakis NA. Biosens Bioelectron. 2003;18:211–215. doi: 10.1016/s0956-5663(02)00183-5. [DOI] [PubMed] [Google Scholar]
- 25.Glynou K, Ioannou PC, Christopoulos TK, Syriopoulou V. Anal Chem. 2003;75:4155–4160. doi: 10.1021/ac034256+. [DOI] [PubMed] [Google Scholar]
- 26.Park J, Kurosawa S, Watanabe J, Ishihara K. Anal Chem. 2004;76:2649–2655. doi: 10.1021/ac035321i. [DOI] [PubMed] [Google Scholar]
- 27.Bavastrello V, Stura E, Carrara S, Erokhin V, Nicolini C. Sens Actuators, B. 2004;98:247–253. [Google Scholar]
- 28.Haick HJ. Phys D: Appl Phys. 2007;40:7173–7186. [Google Scholar]
- 29.Costa-Fernandez JM, Pereiro R, Sanz-Medel A. TrAC, Trends Anal Chem. 2006;25:207–218. [Google Scholar]
- 30.Riu J, Maroto A, Rius FX. Talanta. 2006;69:288–301. doi: 10.1016/j.talanta.2005.09.045. [DOI] [PubMed] [Google Scholar]
- 31.Rivas GA, Rubianes MD, Rodríguez MC, Ferreyra NF, Luque GL, Pedano ML, Miscoria SA, Parrado C. Talanta. 2007;74:291–307. doi: 10.1016/j.talanta.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 32.Barsan N, Koziej D, Weimar U. Sens Actuators, B. 2007;121:18–35. [Google Scholar]
- 33.Nilsson C, Birnbaum S, Nilsson S. J Chromatogr A. 2007;1168:212–224. doi: 10.1016/j.chroma.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 34.Agui L, Yanez-Sedeno P, Pingarron JM. Anal Chim Acta. 2008;622:11–47. doi: 10.1016/j.aca.2008.05.070. [DOI] [PubMed] [Google Scholar]
- 35.Chen ZL, Owens G. Anal Chim Acta. 2008;607:1–14. doi: 10.1016/j.aca.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 36.Vairavapandian D, Vichchulada P, Lay MD. Anal Chim Acta. 2008;626:119–129. doi: 10.1016/j.aca.2008.07.052. [DOI] [PubMed] [Google Scholar]
- 37.Gómez-Hens A, Fernández-Romero JM, Aguilar-Caballos MP. TrAC, Trends Anal Chem. 2008;27:394–406. doi: 10.1016/j.trac.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biricova V, Laznickova A. Bioorg Chem. 2009;37:185–192. doi: 10.1016/j.bioorg.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 39.Dreizin EL. Prog Energ Combust. 2009;35:141–167. [Google Scholar]
- 40.Liu F-K. J Chromatogr A. 2009;1216:9034–9047. doi: 10.1016/j.chroma.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 41.Noguez C, Garzón IL. Chem Soc Rev. 2009;39:757–771. doi: 10.1039/b800404h. [DOI] [PubMed] [Google Scholar]
- 42.Qureshi A, Kang WP, Davidson JL, Gurbuz Y. Diam Relat Mater. 2009;18:1401–1420. [Google Scholar]
- 43.Sun L, Gibson RF, Gordaninejad F, Suhr J. Compos Sci Technol. 2009;69:2392–2409. [Google Scholar]
- 44.Tokonami S, Shiigi H, Nagaoka T. Anal Chim Acta. 2009;641:7–13. doi: 10.1016/j.aca.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 45.Justino CIL, Rocha-Santos TA, Duarte AC. TrAC Trends Anal Chem. 2010;29:1172–1183. [Google Scholar]
- 46.Yu L, Andriola A. Talanta. 2010;82:869–875. doi: 10.1016/j.talanta.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 47.Surugau N, Urban PLJ. Sep Sci. 2009;32:1889–1906. doi: 10.1002/jssc.200900071. [DOI] [PubMed] [Google Scholar]
- 48.Rodriguez MA, Armstrong DWJ. Chromatogr B. 2004;800:7–25. doi: 10.1016/j.jchromb.2003.09.060. [DOI] [PubMed] [Google Scholar]
- 49.Chen Z, Xu L, Liang Y, Wang J, Zhao M, Li YJ. Chromatogr A. 2008;1182:128–131. doi: 10.1016/j.chroma.2007.12.084. [DOI] [PubMed] [Google Scholar]
- 50.Liu F-K, Chang Y-CJ. Chromatogr A. 2010;1217:1647–1653. doi: 10.1016/j.chroma.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 51.Lin Y-T, Sung M, Sanders PF, Marinucci A, Huang CP. Sep Purif Technol. 2007;58:138–147. [Google Scholar]
- 52.Guerreiro AR, Chianella I, Piletska E, Whitcombe MJ, Piletsky SA. Biosens Bioelectron. 2009;24:2740–2743. doi: 10.1016/j.bios.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 53.Fischer HC, Chan WCW. Curr Opin Biotechnol. 2007;18:565–571. doi: 10.1016/j.copbio.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 54.Bystrzejewska-Piotrowska G, Golimowski J, Urban PL. Waste Manage. 2009;29:2587–2595. doi: 10.1016/j.wasman.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 55.Yang Z, Liu ZW, Allaker RP, Reip P, Oxford J, Ahmad Z, Ren GJR. Soc Interface. 2010;7:S411–S422. doi: 10.1098/rsif.2010.0158.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnston HJ, Hutchison G, Christensen FM, Peters S, Hankin S, Stone V. Crit Rev Toxicol. 2010;40:328–346. doi: 10.3109/10408440903453074. [DOI] [PubMed] [Google Scholar]
- 57.Fadeel B, Garcia-Bennett AE. Adv Drug Delivery Rev. 2009;62:362–374. doi: 10.1016/j.addr.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 58.Barnes TM, van de Lagemaat J, Levi D, Rumbles G, Coutts TJ, Weeks CL, Britz DA, Levitsky I, Peltola J, Glatkowski P. Phys Rev B. 2007;75:23541001–22354110. [Google Scholar]
- 59.Khabashesku VN, Margrave JL, Barrera EV. Diamond Relat Mater. 2005;14:859–866. [Google Scholar]
- 60.Popov VN. Mater Sci Eng, R. 2004;43:61–102. [Google Scholar]
- 61.Zhao J, Chen X, Xie JRH. Anal Chim Acta. 2006;568:161–170. doi: 10.1016/j.aca.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 62.Wooten M, Gorski W. Anal Chem. 2010;82:1299–1304. doi: 10.1021/ac902301b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bose S, Khare RA, Moldenaers P. Polymer. 2010;51:975–993. [Google Scholar]
- 64.Nakamura E, Isobe H. Accounts Chem Res. 2003;36:807–815. doi: 10.1021/ar030027y. [DOI] [PubMed] [Google Scholar]
- 65.Wang HZ, Huang ZP, Cai QJ, Kulkarni K, Chen CL, Carnahan D, Ren ZF. Carbon. 2010;48:868–875. [Google Scholar]
- 66.Jackson R, Domercq B, Jain R, Kippelen B, Graham S. Adv Funct Mater. 2008;18:2548–2554. [Google Scholar]
- 67.Guoqing X, et al. Nanotechnology. 2010;21:405201. [Google Scholar]
- 68.Soetedjo H, Mora MF, Garcia CD. Thin Solid Films. 2010;518:3954–3959. doi: 10.1016/j.tsf.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Murakami Y, Einarsson E, Edamura T, Maruyama S. Phys Rev Lett. 2005;94:087402–087404. doi: 10.1103/PhysRevLett.94.087402. [DOI] [PubMed] [Google Scholar]
- 70.Murakami Y, Einarsson E, Edamura T, Maruyama S. Carbon. 2005;43:2664–2676. doi: 10.1103/PhysRevLett.94.087402. [DOI] [PubMed] [Google Scholar]
- 71.Odom TW, Huang J-L, Kim P, Lieber CM. Nature. 1998;391:62–64. [Google Scholar]
- 72.Masheter AT, Abiman P, Wildgoose GG, Wong E, Xiao L, Rees NV, Taylor R, Attard GA, Baron R, Crossley A, Jones JH, Compton RG. J Mat Chem. 2007;17:2616–2626. [Google Scholar]
- 73.Banks CE, Davies TJ, Wildgoose GG, Compton RG. Chem Commun. 2005;7:829–841. doi: 10.1039/b413177k. [DOI] [PubMed] [Google Scholar]
- 74.Banks CE, Crossley A, Salter C, Wilkins SJ, Compton RG. Angew Chem Int Ed. 2006;45:2533–2537. doi: 10.1002/anie.200600033. [DOI] [PubMed] [Google Scholar]
- 75.Pumera M, Ambrosi A, Bonanni A, Chng ELK, Poh HL. TrAC Trends Anal Chem. 2010;29:954–965. [Google Scholar]
- 76.Ambrosi A, Pumera M. Chemistry – A European Journal. 2010;16:10946–10949. doi: 10.1002/chem.201001584. [DOI] [PubMed] [Google Scholar]
- 77.Scott CL, Pumera M. Electrochem Com. 2011;13:213–216. [Google Scholar]
- 78.Tkachev SV, Buslaeva EY, Gubin SP. Inorg Mater. 2011;47:1–10. [Google Scholar]
- 79.Pumera M, Miyahara Y. Nanoscale. 2009;1:260–265. doi: 10.1039/b9nr00071b. [DOI] [PubMed] [Google Scholar]
- 80.Lim CX, Hoh HY, Ang PK, Loh KP. Anal Chem. 2010;82:7387–7393. doi: 10.1021/ac101519v. [DOI] [PubMed] [Google Scholar]
- 81.Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA. Science. 2004;306:666–669. doi: 10.1126/science.1102896. [DOI] [PubMed] [Google Scholar]
- 82.Bai J, Huang Y. Mat Sci Eng R. 2010;70:341–353. [Google Scholar]
- 83.Kim WS, Moon SY, Bang SY, Choi BG, Ham H, Sekino T, Shim KB. Appl Phys Lett. 2009;95:083103–083103. [Google Scholar]
- 84.He Q, Sudibya HG, Yin Z, Wu S, Li H, Boey F, Huang W, Chen P, Zhang H. ACS Nano. 2010;4:3201–3208. doi: 10.1021/nn100780v. [DOI] [PubMed] [Google Scholar]
- 85.Pham VH, Cuong TV, Hur SH, Shin EW, Kim JS, Chung JS, Kim EJ. Carbon. 2010;48:1945–1951. [Google Scholar]
- 86.Ang PK, Wang S, Bao Q, Thong JTL, Loh KP. ACS Nano. 2009;3:3587–3594. doi: 10.1021/nn901111s. [DOI] [PubMed] [Google Scholar]
- 87.Kozlowski M, Diduszko R, Olszewska K, Wronka H, Czerwosz E. Vacuum. 2008;82:956–961. [Google Scholar]
- 88.Gong K, Zhang M, Yan Y, Su L, Mao L, Xiong S, Chen Y. Anal Chem. 2004;76:6500–6505. doi: 10.1021/ac0492867. [DOI] [PubMed] [Google Scholar]
- 89.Wang J, Musameh M. Anal Chem. 2003;75:2075–2079. doi: 10.1021/ac030007+. [DOI] [PubMed] [Google Scholar]
- 90.Kostecki R, Schnyder B, Alliata D, Song X, Kinoshita K, Kötz R. Thin Solid Films. 2001;396:36–43. [Google Scholar]
- 91.Schreiber M, Lutz T, Keeley GP, Kumar S, Boese M, Krishnamurthy S, Duesberg GS. Appl Surf Sci. 2010;256:6186–6190. [Google Scholar]
- 92.Donner S, Li H-W, Yeung ES, Porter MD. Anal Chem. 2006;78:2816–2822. doi: 10.1021/ac052244d. [DOI] [PubMed] [Google Scholar]
- 93.Dai Y, Swain GM, Porter MD, Zak Jerzy. Anal Chem. 2008;80:14–22. doi: 10.1021/ac085996r. [DOI] [PubMed] [Google Scholar]
- 94.Hebert NE, Snyder B, McCreery RL, Kuhr WG, Brazill SA. Anal Chem. 2003;75:4265–4271. doi: 10.1021/ac026425g. [DOI] [PubMed] [Google Scholar]
- 95.Gonzalez CF, Cropek DM, Henry CS. Electroanalysis. 2009;21:2171–2174. [Google Scholar]
- 96.Matsuo Y, Iwasa K, Sugie Y, Mineshige A, Usami H. Carbon. 2010;48:4009–4014. [Google Scholar]
- 97.Pycke BFG, Halden RU, Benn TM, Westerhoff P, Herckes P. TrAC Trends in Anal Chem. 2011;30:44–57. doi: 10.1016/j.trac.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Krishna V, Noguchi N, Koopman B, Moudgil BJ. Colloid Interface Sci. 2006;304:166–171. doi: 10.1016/j.jcis.2006.08.041. [DOI] [PubMed] [Google Scholar]
- 99.Bedrov D, Smith GD, Davande H, Li LJ. Phys Chem B. 2008;112:2078–2084. doi: 10.1021/jp075149c. [DOI] [PubMed] [Google Scholar]
- 100.Agrawal YK. Fuller Nanotub Car N. 2006;14:621–639. [Google Scholar]
- 101.Jin X, Hu JY, Tint ML, Ong SL, Biryulin Y, Polotskaya G. Desalination. 2007;214:83–90. [Google Scholar]
- 102.Vallant RM, Szabo Z, Bachmann S, Bakry R, Najam-ul-Haq M, Rainer M, Heigl N, Petter C, Huck CW, Bonn GnK. Anal Chem. 2007;79:8144–8153. doi: 10.1021/ac0712392. [DOI] [PubMed] [Google Scholar]
- 103.Jurado-Sanchez B, Ballesteros E, Gallego M. J Chromatogr A. 2009;1216:1200–1205. doi: 10.1016/j.chroma.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 104.Jurado-Sanchez B, Ballesteros E, Gallego M. Talanta. 2009;79:613–620. doi: 10.1016/j.talanta.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 105.Böddi K, Takátsy A, Szabó S, Marko L, Márk L, Wittmann I, Ohmacht R, Montskó G, Vallant RM, Ringer T, Bakry R, Huck CW, Bonn GK, Szabó Z. J Sep Sci. 2009;32:295–308. doi: 10.1002/jssc.200800462. [DOI] [PubMed] [Google Scholar]
- 106.Asensio-Ramos M, Hernández-Borges J, Borges-Miquel TM, RodrÌguez-Delgado MA. Anal Chim Acta. 2009;647:167–176. doi: 10.1016/j.aca.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 107.Zhou Q, Xiao J, Ding Y. Anal Chim Acta. 2007;602:223–228. doi: 10.1016/j.aca.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 108.Wang S, Zhao P, Min G, Fang G. J Chromatogr A. 2007;1165:166–171. doi: 10.1016/j.chroma.2007.07.061. [DOI] [PubMed] [Google Scholar]
- 109.Al-Degs YS, Al-Ghouti MA, El-Sheikh AH. J Hazard Mater. 2009;169:128–135. doi: 10.1016/j.jhazmat.2009.03.065. [DOI] [PubMed] [Google Scholar]
- 110.El-Sheikh AH, Sweileh JA, Al-Degs YS, Insisi AA, Al-Rabady N. Talanta. 2008;74:1675–1680. doi: 10.1016/j.talanta.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 111.Ravelo-Perez LM, Hernandez-Borges J, Rodriguez-Delgado MA. J Chromatogr A. 2008;1211:33–42. doi: 10.1016/j.chroma.2008.09.084. [DOI] [PubMed] [Google Scholar]
- 112.Lopez-Feria S, Cardenas S, Valcarcel M. J Chromatogr A. 2009;1216:7346–7350. doi: 10.1016/j.chroma.2009.02.060. [DOI] [PubMed] [Google Scholar]
- 113.Chen S, Liu C, Yang M, Lu D, Zhu L, Wang Z. J Hazard Mater. 2009;170:247–251. doi: 10.1016/j.jhazmat.2009.04.104. [DOI] [PubMed] [Google Scholar]
- 114.Duran A, Tuzen M, Soylak M. J Hazard Mater. 2009;169:466–471. doi: 10.1016/j.jhazmat.2009.03.119. [DOI] [PubMed] [Google Scholar]
- 115.Marquez-Sillero I, Aguilera-Herrador E, Cardenas S, Valcarcel M. J Chromatogr A. 2010;1217:1–6. doi: 10.1016/j.chroma.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 116.Guan Z, Huang Y, Wang W. Anal Chim Acta. 2008;627:225–231. doi: 10.1016/j.aca.2008.08.035. [DOI] [PubMed] [Google Scholar]
- 117.Hylton K, Chen Y, Mitra S. J Chromatogr A. 2008;1211:43–48. doi: 10.1016/j.chroma.2008.09.092. [DOI] [PubMed] [Google Scholar]
- 118.See HH, Marsin Sanagi M, Ibrahim WAW, Naim AA. J Chromatogr A. 2010;1217:1767–1772. doi: 10.1016/j.chroma.2010.01.053. [DOI] [PubMed] [Google Scholar]
- 119.Rastkari N, Ahmadkhaniha R, Yunesian M. J Chromatogr B. 2009;877:1568–1574. doi: 10.1016/j.jchromb.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 120.Sae-Khow O, Mitra S. J Chromatogr A. 2009;1216:2270–2274. doi: 10.1016/j.chroma.2009.01.037. [DOI] [PubMed] [Google Scholar]
- 121.Du W, Zhao F, Zeng B. J Chromatogr A. 2009;1216:3751–3757. doi: 10.1016/j.chroma.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 122.Zhang Z, Zhang H, Hu Y, Yao S. Anal Chim Acta. 2010;661:173–180. doi: 10.1016/j.aca.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 123.Moliner-Martínez Y, Cárdenas S, Valcárcel M. J Chromatogr A. 2007;1167:210–216. doi: 10.1016/j.chroma.2007.07.076. [DOI] [PubMed] [Google Scholar]
- 124.Moliner-Martínez Y, Barrios M, Cárdenas S, Valcárcel M. J Chromatogr A. 2008;1194:128–133. doi: 10.1016/j.chroma.2008.04.034. [DOI] [PubMed] [Google Scholar]
- 125.Xiong X, Ouyang J, Baeyens WRG, Delanghe JR, Shen X, Yang Y. Electrophoresis. 2006;27:3243–3253. doi: 10.1002/elps.200500870. [DOI] [PubMed] [Google Scholar]
- 126.Xu Y, Li SFY. Electrophoresis. 2006;27:4025–4028. doi: 10.1002/elps.200600270. [DOI] [PubMed] [Google Scholar]
- 127.Na N, Hu Y, Ouyang J, Baeyens WRG, Delanghe JR, Taes YEC, Xie M, Chen H, Yang Y. Talanta. 2006;69:866–872. doi: 10.1016/j.talanta.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 128.Sombra L, Moliner-Martínez Y, Cárdenas S, Valcárcel M. Electrophoresis. 2008;29:3850–3857. doi: 10.1002/elps.200800275. [DOI] [PubMed] [Google Scholar]
- 129.Jiménez-Soto JM, Moliner-Martínez Y, Cárdenas S, Valcárcel M. Electrophoresis. 2010;31:1681–1688. doi: 10.1002/elps.200900628. [DOI] [PubMed] [Google Scholar]
- 130.Stege PW, Sombra LL, Messina G, Martinez LD, Silva MF. Electrophoresis. 2010;31:2242–2248. doi: 10.1002/elps.200900782. [DOI] [PubMed] [Google Scholar]
- 131.Fonverne A, Ricoul F, Demesmay C, Delattre C, Fournier A, Dijon J, Vinet F. Sens Actuators, B. 2008;129:510–517. [Google Scholar]
- 132.André C, Gharbi T, Guillaume YCJ. Sep Sci. 2009;32:1757–1764. doi: 10.1002/jssc.200800683. [DOI] [PubMed] [Google Scholar]
- 133.Zhong Y, Zhou W, Zhang P, Zhu Y. Talanta. 2010;82:1439–1447. [Google Scholar]
- 134.Karwa M, Mitra S. Anal Chem. 2006;78:2064–2070. doi: 10.1021/ac052115x. [DOI] [PubMed] [Google Scholar]
- 135.Kelly KL, Coronado E, Zhao LL, Schatz GCJ. Phys Chem B. 2002;107:668–677. [Google Scholar]
- 136.Fanchini G, Miller S, Parekh BB, Chhowalla M. Nano Lett. 2008;8:2176–2179. doi: 10.1021/nl080563p. [DOI] [PubMed] [Google Scholar]
- 137.Chen Z, Tabakman SM, Goodwin AP, Kattah MG, Daranciang D, Wang X, Zhang G, Li X, Liu Z, Utz PJ, Jiang K, Fan S, Dai H. Nat Biotech. 2008;26:1285–1292. doi: 10.1038/nbt.1501. [DOI] [PubMed] [Google Scholar]
- 138.Cao C, Kim JH, Yoon D, Hwang E-S, Kim Y-J, Baik S. Mater Chem Phys. 2008;112:738–741. [Google Scholar]
- 139.Song Y, Qu K, Xu C, Ren J, Qu X. Chem Commun. 2010;46:6572–6574. doi: 10.1039/c0cc01593h. [DOI] [PubMed] [Google Scholar]
- 140.Peng H. J Am Chem Soc. 2008;130:42–43. doi: 10.1021/ja078267m. [DOI] [PubMed] [Google Scholar]
- 141.Jie G, Li L, Chen C, Xuan J, Zhu J-J. Biosens Bioelectron. 2009;24:3352–3358. doi: 10.1016/j.bios.2009.04.039. [DOI] [PubMed] [Google Scholar]
- 142.Liu S, Liu J, Wang L, Zhao F. Bioelectrochemistry. 2010;79:37–42. doi: 10.1016/j.bioelechem.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 143.Shiraishi H, Itoh T, Hayashi H, Takagi K, Sakane M, Mori T, Wang J. Bioelectrochemistry. 2007;70:481–487. doi: 10.1016/j.bioelechem.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 144.Lim IIS, Pan Y, Mott D, Ouyang J, Njoki PN, Luo J, Zhou S, Zhong C-J. Langmuir. 2007;23:10715–10724. doi: 10.1021/la701868b. [DOI] [PubMed] [Google Scholar]
- 145.Stefan-van Staden R-I. Talanta. 2011;81:865–870. doi: 10.1016/j.talanta.2010.01.030. [DOI] [PubMed] [Google Scholar]
- 146.Balasubramanian K, Burghard M. Anal Bioanal Chem. 2006;385:452–468. doi: 10.1007/s00216-006-0314-8. [DOI] [PubMed] [Google Scholar]
- 147.Tingry S, Innocent C, Touil S, Deratani A, Seta P. Mater Sci Eng C. 2006;26:222–226. [Google Scholar]
- 148.Manso J, Mena ML, Yáñez-Sedeño P, Pingarrón JJ. Electroanal Chem. 2007;603:1–7. [Google Scholar]
- 149.Sánchez S, Roldán M, Pérez S, Fàbregas E. Anal Chem. 2008;80:6508–6514. doi: 10.1021/ac7025282. [DOI] [PubMed] [Google Scholar]
- 150.Siqueira JR, Jr, Abouzar MH, Poghossian A, Zucolotto V, Oliveira ON, Jr, Schoning MJ. Biosens Bioelectron. 2009:497–501. doi: 10.1016/j.bios.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 151.Jacobs CB, Peairs MJ, Venton BJ. Anal Chim Acta. 2010;662:105–127. doi: 10.1016/j.aca.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 152.Deng P-H, Fei J-J, Feng Y-L. Sens Actuators, B. 2010;148:214–220. [Google Scholar]
- 153.Morton J, Havens N, Mugweru A, Wanekaya A. Electroanalysis. 2009;21:1597–1603. [Google Scholar]
- 154.Xu H, Zeng L, Xing S, Xian Y, Shi G, Jin L. Electroanalysis. 2008;20:2655–2662. [Google Scholar]
- 155.Jia X, Li J, Wang E. Electroanalysis. 2010;22:1682–1687. [Google Scholar]
- 156.Liu X, Ding Z, He Y, Xue Z, Zhao X, Lu X. Colloids Surf, B. 2010;79:27–32. doi: 10.1016/j.colsurfb.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 157.Erdem A, Papakonstantinou P, Murphy H. Anal Chem. 2006;78:6656–6659. doi: 10.1021/ac060202z. [DOI] [PubMed] [Google Scholar]
- 158.Martinez NA, Messina GA, Bertolino FA, Salinas E, Raba J. Sens Actuators, B. 2008;133:256–262. [Google Scholar]
- 159.Ghalkhani M, Shahrokhian S, Ghorbani-Bidkorbeh F. Talanta. 2009;80:31–38. doi: 10.1016/j.talanta.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 160.Crevillen AG, Pumera M, Gonzalez MC, Escarpa A. Lab on a Chip. 2009;9:346–353. doi: 10.1039/b809963d. [DOI] [PubMed] [Google Scholar]
- 161.Crevillen AG, Avila M, Pumera M, Gonzalez MC, Escarpa A. Anal Chem. 2007;79:7408–7415. doi: 10.1021/ac071247i. [DOI] [PubMed] [Google Scholar]
- 162.Kachoosangi RT, Wildgoose GG, Compton RG. Analyst. 2008;133:888–895. doi: 10.1039/b803588a. [DOI] [PubMed] [Google Scholar]
- 163.Kachoosangi RT, Musameh MM, Abu-Yousef I, Yousef JM, Kanan SM, Xiao L, Davies SG, Russell A, Compton RG. Anal Chem. 2008;81:435–442. doi: 10.1021/ac801853r. [DOI] [PubMed] [Google Scholar]
- 164.Chen S, Yuan R, Chai Y, Min L, Li W, Xu Y. Electrochim Acta. 2009;54:7242–7247. [Google Scholar]
- 165.Chicharro M, Arribas AS, Moreno M, Bermejo E, Zapardiel A. Talanta. 2007;74:376–386. doi: 10.1016/j.talanta.2007.07.034. [DOI] [PubMed] [Google Scholar]
- 166.Arun Prakash P, Yogeswaran U, Chen S-M. Talanta. 2009;78:1414–1421. doi: 10.1016/j.talanta.2009.02.033. [DOI] [PubMed] [Google Scholar]
- 167.Rahimi P, Rafiee-Pour H-A, Ghourchian H, Norouzi P, Ganjali MR. Biosens Bioelectron. 2010;25:1301–1306. doi: 10.1016/j.bios.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 168.Wang L, Wang J, Zhou F. Electroanalysis. 2004;16:627–632. [Google Scholar]
- 169.Zhou H, Lu T-H, Shi H-X, Dai Z-H, Huang X-H. J Electroanal Chem. 2008;612:173–178. [Google Scholar]
- 170.Salimi A, Noorbakhsh A, Ghadermarz M. Anal Biochem. 2005;344:16–24. doi: 10.1016/j.ab.2005.05.035. [DOI] [PubMed] [Google Scholar]
- 171.Karajanagi SS, Vertegel AA, Kane RS, Dordick JS. Langmuir. 2004;20:11594–11599. doi: 10.1021/la047994h. [DOI] [PubMed] [Google Scholar]
- 172.Valenti LE, Fiorito PA, Garcia CD, Giacomelli CEJ. Colloid Interface Sci. 2007;307:349–356. doi: 10.1016/j.jcis.2006.11.046. [DOI] [PubMed] [Google Scholar]
- 173.Mora MF, Giacomelli CE, Garcia CD. Anal Chem. 2009;81:1016–1022. doi: 10.1021/ac802068n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Carot ML, Torresi RM, Garcia CD, Esplandiu MJ, Giacomelli CEJ. Phys Chem B. 2010;114:4459–4465. doi: 10.1021/jp9085359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Felhofer JL, Caranto J, Garcia CD. Langmuir. 2010;26:17178–17183. doi: 10.1021/la103035n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Nejadnik MR, Deepak FL, Garcia CD. Electroanalysis. doi: 10.1002/elan.201000758. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gao Y, Kyratzis I. Bioconjugate Chem. 2008;19:1945–1950. doi: 10.1021/bc800051c. [DOI] [PubMed] [Google Scholar]
- 178.Luais E, Thobie-Gautier C, Tailleur A, Djouadi MA, Granier A, Tessier PY, Debarnot D, Poncin-Epaillard F, Boujtita M. Electrochimica Acta. 2010;55:7916–7922. [Google Scholar]
- 179.Liu X, Li Y, Zheng J, Zhang J, Sheng Q. Talanta. 2010;81:1619–1624. doi: 10.1016/j.talanta.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 180.Kara P, de la Escosura-Muñiz A, Maltez-da Costa M, Guix M, Ozsoz M, Merkoçi A. Biosens Bioelectron. 2010:1715–1718. doi: 10.1016/j.bios.2010.07.090. [DOI] [PubMed] [Google Scholar]
- 181.Stege PW, Messina GA, Bianchi G, Olsina RA, Raba J. Soil Biol Biochem. 2009;41:2444–2452. [Google Scholar]
- 182.Fernández-Baldo MA, Messina GA, Sanz MI, Raba J. Talanta. 2009;79:681–686. doi: 10.1016/j.talanta.2009.04.059. [DOI] [PubMed] [Google Scholar]
- 183.Panini NV, Messina GA, Salinas E, Fernández H, Raba J. Biosens Bioelectron. 2008;23:1145–1151. doi: 10.1016/j.bios.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 184.Zhao G, Zhan X, Dou W. Anal Biochem. 2010:53–58. doi: 10.1016/j.ab.2010.08.039. [DOI] [PubMed] [Google Scholar]
- 185.García-Aljaro C, Cella LN, Shirale DJ, Park M, Muñoz FJ, Yates MV, Mulchandani A. Biosens Bioelectron. 2010;26:1437–1441. doi: 10.1016/j.bios.2010.07.077. [DOI] [PubMed] [Google Scholar]
- 186.Chen J, Zhang W-D, Ye J-S. Electrochem Commun. 2008;10:1268–1271. [Google Scholar]
- 187.Qiu J-D, Cui S-G, Deng M-Q, Liang R-PJ. Appl Electrochem. 2010;40:1651–1657. [Google Scholar]
- 188.Pang X, He D, Luo S, Cai Q. Sens Actuators, B. 2009;137:134–138. [Google Scholar]
- 189.Kang X, Mai Z, Zou X, Cai P, Mo J. Talanta. 2008;74:879–886. doi: 10.1016/j.talanta.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 190.Wang Z, Li M, Su P, Zhang Y, Shen Y, Han D, Ivaska A, Niu L. Electrochem Commun. 2008;10:306–310. [Google Scholar]
- 191.Gao F, Tang L, Dai L, Wang L. Spectrochim Acta A. 2007;67:517–521. doi: 10.1016/j.saa.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 192.Hui G, Wu L, Pan M, Chen Y, Li T, Zhang X. Meas Sci Technol. 2006;17:2799. [Google Scholar]
- 193.Ambrosio A, Ambrosio M, Ambrosone G, Carillo V, Coscia U, Grossi V, Maddalena P, Passacantando M, Perillo E, Raulo A, Santucci S. Nucl Instrum Meth A. 2008;589:398–403. [Google Scholar]
- 194.Li W, Hoa ND, Kim D. Sens Actuators, B. 2010;149:184–188. [Google Scholar]
- 195.Wongchoosuk C, Wisitsoraat A, Tuantranont A, Kerdcharoen T. Sens Actuators, B. 2010;147:392–399. doi: 10.3390/s100807705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Shimizu T, Haruyama J, Marcano DC, Kosinkin DV, Tour JM, Hirose K, Suenaga K. Nat Nano. 2011;6:45–50. doi: 10.1038/nnano.2010.249. [DOI] [PubMed] [Google Scholar]
- 197.Yang W, Ratinac K, Ringer S, Thordarson P, Gooding J, Braet F. Angew Chem Int Edit. 2010;49:2114–2138. doi: 10.1002/anie.200903463. [DOI] [PubMed] [Google Scholar]
- 198.Li J, Guo S, Zhai Y, Wang E. Electrochem Commun. 2009;11:1085–1088. [Google Scholar]
- 199.Bong S, Kim Y-R, Kim I, Woo S, Uhm S, Lee J, Kim H. Electrochem Comm. 2010;12:129–131. [Google Scholar]
- 200.Kang X, Wang J, Wu H, Liu J, Aksay IA, Lin Y. Talanta. 2010;81:754–759. doi: 10.1016/j.talanta.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 201.Chang H, Tang L, Wang Y, Jiang J, Li J. Anal Chem. 2010;82:2341–2346. doi: 10.1021/ac9025384. [DOI] [PubMed] [Google Scholar]
- 202.Krishna V, Noguchi N, Koopman B, Moudgil B. J Colloid Interf Sci. 2006;304:166–171. doi: 10.1016/j.jcis.2006.08.041. [DOI] [PubMed] [Google Scholar]
- 203.Krishna V, Yanes D, Imaram W, Angerhofer A, Koopman B, Moudgil B. Appl Catal, B. 2008;79:376–381. [Google Scholar]
- 204.Lin J, Zong R, Zhou M, Zhu Y. Appl Catal, B. 2009;89:425–431. [Google Scholar]
- 205.Tzirakis MD, Vakros J, Loukatzikou L, Amargianitakis V, Orfanopoulos M, Kordulis C, Lycourghiotis AJ. Mol Catal, A. 2010;316:65–74. [Google Scholar]
- 206.Spassova I, Khristova M, Nickolov R, Mehandjiev D. J Colloid Interf Sci. 2008;320:186–193. doi: 10.1016/j.jcis.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 207.Yu C, Canteenwala T, Chiang LY, Wilson B, Pritzker K. Synthetic Met. 2005;153:37–40. [Google Scholar]
- 208.Hu P, Huang C, Zhang L. Sci China Ser B. 2008;51:866–871. [Google Scholar]
- 209.Qiao J, Tang S, Tian Y, Shuang S, Dong C, Choi MMF. Sens Actuators, B. 2009;138:402–407. [Google Scholar]
- 210.Maiyalagan T. Appl Catal, B. 2008;80:286–295. [Google Scholar]
- 211.Zhang L, Tian D-B, Zhu J-J. Bioelectrochemistry. 2008;74:157–163. doi: 10.1016/j.bioelechem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 212.Gao B, Peng C, Chen GZ, Li Puma G. Appl Catal, B. 2008;85:17–23. [Google Scholar]
- 213.Chen X-m, Lin Z-j, Jia T-t, Cai Z-m, Huang X-l, Jiang Y-q, Chen X, Chen G-n. Anal Chim Acta. 2009;650:54–58. doi: 10.1016/j.aca.2009.02.035. [DOI] [PubMed] [Google Scholar]
- 214.Zhang X, Shi X, Wang C. Catal Commun. 2009;10:610–613. [Google Scholar]
- 215.Chetty R, Kundu S, Xia W, Bron M, Schuhmann W, Chirila V, Brandl W, Reinecke T, Muhler M. Electrochim Acta. 2009;54:4208–4215. [Google Scholar]