Abstract
Background
Operative resection is the only curative treatment for pheochromocytomas. Inhibition of the phosphatidylinositol-3 kinase (PI3K)-Akt pathway has been shown to be an effective treatment of neuroendocrine (NE) tumors in vitro. We hypothesized that inhibition of the PI3K-Akt pathway would be a viable strategy to inhibit growth and hormonal secretion in pheochromocytoma cells.
Methods
Sixteen pheochromocytomas were analyzed for expression of phosphorylated Akt and the NE marker achaete scute complex-like 1 (ASCL1). Pheochromocytoma PC-12 cells were treated with up to 100 µM of the PI3K-specific inhibitor LY294002 for 48 h. Western blot analysis was used to measure phosphorylated Akt, total Akt, ASCL1, chromogranin A (CgA), and markers of apoptosis. Growth was assessed by a methylthiazolyldiphenyl-tetrazolium (MTT) bromide cellular proliferation assay for six days.
Results
Human pheochromocytomas expressed significant amounts of phosphorylated Akt, and there was a significant correlation between malignant pheochromocytomas and the amount of expressed ASCL1. LY294002 significantly inhibited the PI3K-Akt pathway. Treatment led to a dose-dependent decrease in both ASCL1 and CgA, indicating an alteration in the NE phenotype and hormonal suppression. Treatment decreased cellular proliferation, and cleavage of the apoptotic markers caspase-3 and PARP was observed.
Conclusions
Human pheochromocytoma tumor samples express high levels of phosphorylated Akt. LY294002 effectively inhibits the PI3K-Akt pathway, suppresses NE tumor markers, and decreases cellular proliferation via apoptosis in vitro. Inhibition of the PI3K pathway may represent a new strategy in the treatment of pheochromocytomas.
Introduction
Originating from neural crest cells, pheochromocytomas are considered neuroendocrine (NE) tumors. Pheochromocytomas are catecholamine-secreting neoplasms that typically arise from the adrenal medulla, but they also occur in extra-adrenal rests throughout the body [1]. These hormonally active tumors secrete vasoactive compounds, leading to the classic presentation of episodic headache, sweating, tachycardia, and hypertension [2, 3]. Pheochromocytoma is rare: less than 0.5% of patients with hypertensive symptoms have a pheochromocytoma [4], and the incidence of pheochromocytoma may be higher in patients with a history of cancer [5]. Surgical excision remains the only definitive cure for benign lesions, and unresectable or malignant disease has no effective treatment; therefore, novel approaches must be found.
Recently, advances have been made in targeted therapies of NE tumors such as pheochromocytoma. One of these pathways is the phosphatidylinositol 3′ kinase (PI3K)-Akt pathway. The PI3K-Akt pathway regulates normal cellular functions such as growth, survival, proliferation, and motility. PI3K is activated upon binding of growth factor receptor tyrosine kinases or G protein-coupled receptors. In turn, PI3K activates Akt (alternaively, protein kinase B) by phosphorylation at the threonine 308 and serine 473 residues. Activated Akt then interacts with a number of downstream targets including caspases, mTOR, Bad, AP-1, and transcription factors such as nuclear factor-kappa B (NF-κB) [6].
In many cancers, including NE tumors, the PI3K-Akt pathway is overexpressed [6, 7]. In PC-12 cells, which are used as a model for both neural development and pheochromocytoma, Akt was shown to be the effector of PI3K-mediated survival in the developing nervous system [8]. Nerve growth factor (NGF) can activate PI3K-Akt as a survival pathway, demonstrating that overexpression of this pathway is able to prevent apoptosis [9]. Under hypoxic conditions, pheochromocytoma cells upregulate Akt as a protective antiapoptotic response [10]. In a study of surgically obtained tumor samples, Fassnacht et al. found that Akt is highly phosphorylated in pheochromocytomas, whereas it was not as highly phosphorylated in benign adrenocortical tumors [11]. This led us to hypothesize that inhibition of the PI3K-Akt pathway could lower NE markers, suppress hormonal secretion, and inhibit growth in an in vitro model of pheochromocytoma.
Methods
Human tissue samples
After we obtained informed consent, we collected human pheochromocytoma tumor samples during surgical excision. The samples were frozen in liquid nitrogen and stored at −80°C. The identity of the tumors was verified by histologic review. Tumor cell lysates were prepared from the frozen samples by grinding in liquid nitrogen, and the samples were lysed as previously described [12]. The lysates were analyzed by Western blotting for phosphorylated Akt (pAkt), achaete scute complex-like 1 (ASCL1), and neuron-specific enolase (NSE), as described below. Relative amounts of protein expression were quantified using QuantityOne (version 4.6.3, BioRad Laboratories, Hercules, CA) standardized to the amount of protein loaded in each lane.
Cell culture
Rat pheochromocytoma PC-12 cells were obtained from American Type Culture Collection (ATCC, Manassas, VA) and were maintained in Ham’s F12K medium (ATCC) supplemented with 15% horse serum (Sigma Aldrich, St. Louis, MO), 2.5% fetal bovine serum (Sigma Aldrich), and 100 IU/ml penicillin plus 100 µg/ml streptomycin (Invitrogen, Carlsbad, CA), as previously described [13–15]. The cells were maintained in a humidified atmosphere of 5% CO2 at 37°C.
Western blot analysis
The PC-12 pheochromocytoma cells were treated with the PI3K-specific inhibitor LY294002 (Sigma Aldrich) in concentrations from 0 to 100 µM, and whole cell lysates were prepared as previously described [12]. An equal volume of dimethyl sulfoxide (DMSO; Sigma Aldrich) was used as a control. Total protein concentrations were quantified with a bicinchoninic acid assay kit (Pierce Biotechnology, Rockford, IL). Denatured cellular extracts were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred onto nitrocellulose membranes (Schleicher and Schuell, Keene, NH), blocked in milk, and incubated with appropriate antibodies.
The antibody dilutions were prepared as follows: 1:500 for chromogranin A (CgA; Zymed Laboratories, San Francisco, CA); 1:1,000 for ASCL1 (BD Biosciences, San Diego, CA), NSE (Research Diagnostics, Flanders, NJ), total Akt, poly-ADP ribose phosphate (PARP), cleaved caspase-3 (Cell Signaling Technology, Beverly, MA); 1:2,000 for pAkt (Cell Signaling Technology), and 1:10,000 for glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Trevigen, Gaithersburg, MD).
Horseradish peroxidase conjugated goat anti-rabbit IgG (1:2,000, Cell Signaling Technology) secondary antibody was used for cleaved caspase-3, CgA, GAPDH, PARP, pAkt, and total Akt, while goat anti-mouse IgG (1:200, Pierce Biotechnology) secondary antibody was used for ASCL1. For visualization of the protein signal, Immunstar (Bio-Rad Laboratories, Hercules, CA) was used for CgA, PARP, total Akt, and GAPDH. SuperSignal West Femto (Pierce Biotechnology) was used for ASCL1, pAkt, and cleaved caspase-3 according to the manufacturer’s instructions.
Cell proliferation assay
Cell proliferation was measured by the methylthiazolyldiphenyl-tetrazolium bromide (MTT; Sigma-Aldrich) rapid colorimetric assay. Cells were seeded in equal amounts in quadruplicate on 24-well plates and incubated overnight. The cells were then treated with LY in concentrations of 0, 25, 50, 75, and 100 µM and incubated for up to 6 days. Every 2 days, treatment medium was changed, and the MTT assay was performed by replacing the standard F12K medium with 250 µl of serum-free medium containing MTT (0.5 mg/ml) and incubating at 37°C. After 4 h of incubation, 750 µl DMSO was added to each well and mixed thoroughly. The absorbance of each well on the plates was then measured at 540 nm with a spectrophotometer (µQuant; Bio-Tek Instruments, Winooski, VT).
Statistical analysis
As appropriate to presented data, one-way analysis of variance (ANOVA) and the independent samples t-test were performed with SPSS (version 11; SPSS, Inc., Chicago, IL); P ≤ 0.05 was considered to be significant.
Results
Pheochromocytoma cells express high levels of phosphorylated Akt
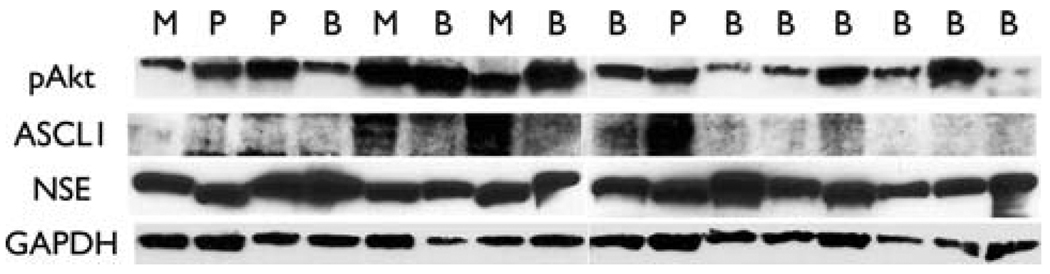
A total of 16 primary pheochromocytoma tumor samples were analyzed. Of the 16 tumors, 10 were benign, 3 were extra-adrenal abdominal paraganliomas, and 3 were malignant. Malignancy was defined as documented meta-static disease, either at the time of operation or from future metastases. All pheochromocytomas expressed high amounts of pAkt as measured by Western blot analysis (Fig. 1). Moreover, there was a significant association between malignant disease and relative levels of the proneuroendocrine transcription factor ASCL1 (P < 0.05, one-way ANOVA), suggesting that these tumors may express a stronger NE phenotype. Having confirmed that the PI3K-Akt pathway is active in pheochromocytoma tumors, we wanted to investigate its activity and effects in PC-12 cells.
Fig. 1.
The PI3K-Akt pathway is highly active in pheochromocytoma. There were 10 benign (B), 3 malignant (M), and 3 extra-adrenal paragangliomas (P). Surgically removed pheochromocytomas show a significant amount of active, phosphorylated Akt (pAkt). Moreover, there was a significant association between the levels of ASCL1 and malignant and extra-adrenal pheochromocytomas. The identity of these tumors was verified with NSE, a marker of NE tissue. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) is shown as a loading control. ASCL1 achate scute complex-like 1; NSE neuron-specific enolase; NE neuroendocrine
LY294002 inhibits phosphorylation of Akt in pheochromocytoma cells
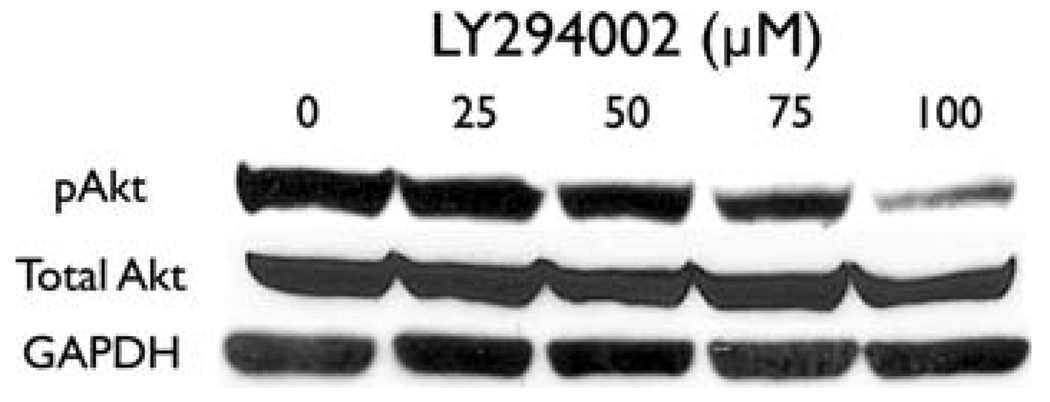
The PC-12 pheochromocytoma cells were treated with the Akt inhibitor LY294002 for two days. At baseline, PC-12 cells express high levels of pAkt at serine 473. This indicates that pheochromocytomas have a strongly activated PI3K-Akt pathway (Fig. 2). Treatment with LY294002 resulted in a dose-dependent decrease in pAkt, indicating inhibition of the pathway. There was no change in the total expression of Akt with increasing concentrations of LY294002. This inhibition is similar to an effect demonstrated in medullary thyroid cancer cells [16]. Thus, the PI3K-Akt pathway is highly active in pheochromocytoma cells, and it can be inhibited by LY294002.
Fig. 2.
The PI3K-specific inhibitor LY294002 inhibits Akt in pheochromocytoma cells. Treatment for 2 days with increasing concentrations of LY294002 decreases the amount of active, phosphorylated Akt (pAkt). The amount of total Akt is not affected by LY294002. GAPDH is shown as a loading control
Inhibition of Akt decreases neuroendocrine markers and suppresses hormonal secretion
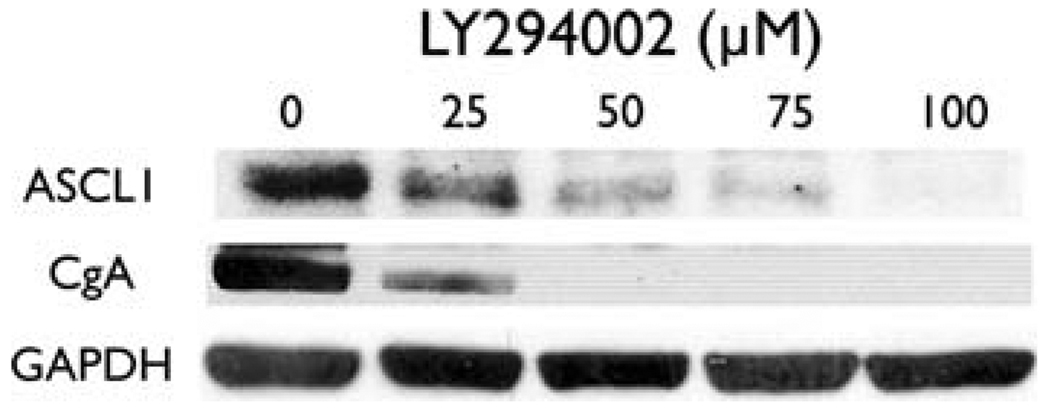
Treatment with LY294002 led to a dose-dependent decrease in ASCL1 (Fig. 3). Achaete scute complex-like 1 is a basic helix-loop-helix transcription factor that promotes neuronal differentiation and serves as a marker of the NE phenotype. It also downregulates CgA and limits hormonal secretion [17]. Chromogranin A is an acidic glycoprotein co-secreted with hormones by NE tumors. Reductions in CgA levels are correlated with decreases in hormonal secretion [13, 14]. We therefore wanted to see if LY294002 was also able to decrease CgA in PC-12 pheochromocytoma cells. As shown in Fig. 3, treatment of PC-12 cells with LY294002 decreased CgA, suggesting an overall decrease in hormonal secretion. Thus, inhibition of the PI3K-Akt pathway leads to an inhibition of the NE phenotype and a decrease in hormonal secretion.
Fig. 3.
Inhibition of the PI3K-Akt pathway reduces ASCL1 and CgA. Western blot analysis showed a decrease in levels of ASCL1, a proneuroendocrine transcription factor, and chromogranin A (CgA), a marker of hormonal secretion. Inhibition of the PI3K-Akt pathway leads to a decrease in the NE phenotypte and hormonal secretion
LY294002 significantly limits pheochromocytoma cell growth
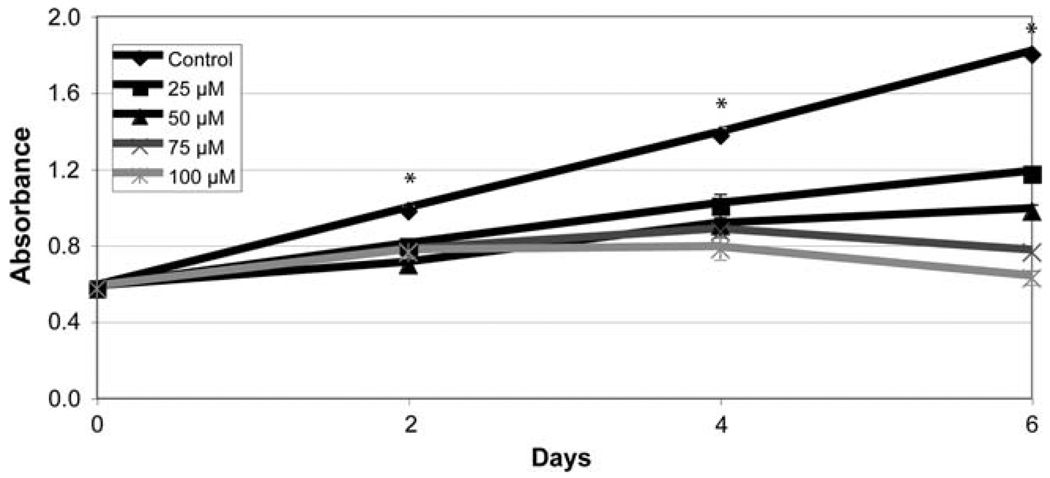
Our group has demonstrated growth suppression associated with PI3K-Akt inhibition in medullary thyroid cancer cells [16]. We wanted to see if inhibition of the PI3K-Akt similarly affected cellular proliferation of PC-12 cells. The MTT growth assay was used to determine the impact on pheochromocytoma cell growth. Growth was inhibited in a dose-dependent manner by LY294002 (Fig. 4). Moreover, growth was significantly suppressed after only 2 days by the lowest amount of LY294002 used (P < 0.001, one-way ANOVA). Therefore, blocking the PI3K-Akt pathway is a potent strategy for growth inhibition of pheochromocytoma cells.
Fig. 4.
LY294002 limits growth of PC-12 pheochromocytoma cells. PC-12 cells were treated with increasing amounts of LY294002 for up to 6 days, and cell viability was determined by a methylthiazolyldiph-enyltetrazolium bromide (MTT) colorimetric growth assay. Points represent mean ± SE. All treatments are significantly different from control after day 2 (* P < 0.001, one-way ANOVA)
Inhibition of the PI3K pathway leads to apoptosis in pheochromocytoma cells in vitro
Noticing significantly limited cellular proliferation with LY294002 treatment in PC-12 cells, we wanted to study the mechanism of this inhibition. We used a Western blot for PARP and caspase-3. Both of these proteins are well-known markers of the apoptotic pathway, and their cleavage is indicative of apoptosis. After 2 days of PI3K inhibition by LY294002, increased cleavage in both PARP and caspase-3 (Fig. 5) was noted. These increases were dose dependent and suggest that the cell death observed by MTT was due to apoptosis, which indicates that LY294002 causes growth inhibition via apoptosis. This concurs with other reports that overexpression of Akt prevents apoptosis in PC-12 cells [8–10, 18].
Fig. 5.
Inhibition of the PI3K-Akt pathway causes apoptosis. PC-12 cells were treated with the indicated concentrations of LY294002 for 2 days, and total cell lysates were prepared. An increase in the cleavage of poly-ADP ribose phosphate (PARP) and caspase-3 suggests that apoptosis induced cell death. GAPDH was used as a loading control. PARP
Discussion
Surgical resection is the only cure for benign pheochromocytomas. For unresectable or malignant disease, current medical treatment remains unsatisfactory. Thus there is an urgent need for targeted pharmacologic treatments of greater value and potency. In pheochromocytoma, these therapeutics could affect diverse targets such as the Notch1 [15], raf-1 [13], and GSK-3β [14] pathways. Targeting multiple pathways accomplishes two aims: it potentially increases the efficacy of treatments and it gives insight into how these tumors arise. We present here the results of the PI3K-Akt pathway inhibition in pheochromocytoma cells.
In this study, we demonstrate that human pheochromocytomas have high levels of pAkt at baseline. We also show that the inhibition of the PI3K-Akt pathway leads to a diminished NE phenotype, decreased hormonal secretion, and an inhibition of growth via apoptosis. This was accomplished with the use of the PI3K-specific inhibitor LY294002. Previous studies have demonstrated that the overexpression of Akt inhibits apoptosis and enhances survival of PC-12 cells [8–11, 18]. Our results make sense within this context, because PI3K-Akt pathway inactivation reverses the antiapoptotic effect of Akt overexpression. An important conclusion from this study is that the same pro-survival pathway in neuronal development can be exploited for growth inhibition in the treatment of pheochromocytoma.
Significantly, this study includes an analysis of human pheochromocytomas. From the tumor samples analyzed, it is clear that these tumors express significant amounts of phosphorylated Akt, which suggests that the pathway is active in pheochromocytomas and represents a therapeutic target.
Importantly, there was a significant association between the NE marker ASCL1 and malignant pheochromocytoma. It has been shown in knockout mice that ASCL1 is necessary for the development of chromaffin cells [19]. This is not limited only to differentiation of catecholamine-secreting ability, but affects the entire cell lineage in mice. Taken together, these findings imply that ASCL1 is an important marker in malignant pheochromocytoma.
We demonstrate that inhibition of the PI3K-Akt pathway causes a decrease in the NE phenotype and a suppression of hormonal secretion. A decrease in ASCL1 and CgA is also seen when the Notch1 [15], raf-1 [13], and GSK-3β [14] pathways are inhibited in pheochromocytoma cells. This would suggest that, at least downstream, these various targets might interact to affect the NE phenotype and promote the significant symptomatic burden on patients with pheochromocytoma. As these patients often suffer from symptoms such as sweating, headache, tachycardia, and hypertension, inhibition of the PI3K-Akt pathway could be therapeutically useful if it decreases hormonal secretion. This would also make the pathway an appealing target for future development.
The PI3K-Akt pathway is a commonly dysregulated pathway in human cancers [7]. This appears to also apply to NE tumors, including pheochromocytoma. In this study, we used LY294002, a known pharmacologic inhibitor of PI3K. However, LY294002 is not a viable therapeutic agent for use in humans because of its poor solubility and short half-life; current studies focus on pharmacologically viable inhibitors of these pathways, such as SF1126 [20]. Progression to clinical trials would require a more viable PI3K inhibitor.
In conclusion, satisfactory treatment of malignant or unresectable pheochromocytoma remains elusive. In this study, we examined the human tumor samples and the importance of targeting the pro-survival PI3K-Akt pathway in pheochromocytoma. Human tumor samples demonstrated an active PI3K-Akt pathway. Inhibition of the PI3K-Akt pathway with LY294002 in pheochromocytoma cells downregulated the NE phenotype, decreased hormonal secretion, and suppressed growth via apoptosis. Thus, targeting this pathway may represent a new treatment strategy for patients with unresectable or malignant pheochromocytoma.
Acknowledgments
Joel T. Adler is a Howard Hughes Medical Institute Research Training Fellow and is supported by the University of Wisconsin General Clinical Research Center. Additional support was provided by National Institutes of Health grants R21-CA117117, R01-CA121115, and R01-CA109053; the George H. A. Clowes, Jr., Memorial Research Career Development Award of the American College of Surgeons; a Carcinoid Cancer Foundation research award (to H.C.), and a Carcinoid Cancer Foundation Research Award (to M.K.).
Contributor Information
Joel T. Adler, Email: jtadler@wisc.edu.
Muthusamy Kunnimalaiyaan, Email: kunni@surgery.wisc.edu.
Herbert Chen, Email: chen@surgery.wisc.edu.
References
- 1.Adler JT, Meyer-Rochow GY, Chen H, et al. Pheochromocytoma: current approaches and future directions. Oncologist. 2008;13:779–793. doi: 10.1634/theoncologist.2008-0043. [DOI] [PubMed] [Google Scholar]
- 2.Manger WM. The vagaries of pheochromocytomas. Am J Hypertens. 2005;18:1266–1270. doi: 10.1016/j.amjhyper.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 3.Manger WM, Gifford JRW. Clinical and experimental pheochromocytoma. Cambridge: Blackwell Science; 1996. [Google Scholar]
- 4.Stein PP, Black HR. A simplified diagnostic approach to pheochromocytoma. A review of the literature and report of one institution’s experience. Medicine (Baltimore) 1991;70:46–66. doi: 10.1097/00005792-199101000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Adler JT, Mack E, Chen H. Isolated adrenal mass in patients with a history of cancer: remember pheochromocytoma. Ann Surg Oncol. 2007;14:2358–2362. doi: 10.1245/s10434-007-9426-4. [DOI] [PubMed] [Google Scholar]
- 6.Zhang X, Jin B, Huang C. The PI3K/Akt pathway and its downstream transcriptional factors as targets for chemoprevention. Curr Cancer Drug Targets. 2007;7:305–316. doi: 10.2174/156800907780809741. [DOI] [PubMed] [Google Scholar]
- 7.Bussink J, van der Kogel AJ, Kaanders JH. Activation of the PI3-K/AKT pathway and implications for radioresistance mechanisms in head and neck cancer. Lancet Oncol. 2008;9:288–296. doi: 10.1016/S1470-2045(08)70073-1. [DOI] [PubMed] [Google Scholar]
- 8.Dudek H, Datta SR, Franke TF, et al. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 9.Yao R, Cooper GM. Requirement for phosphatidylinositol-3 kinase in the prevention of apoptosis by nerve growth factor. Science. 1995;267:2003–2006. doi: 10.1126/science.7701324. [DOI] [PubMed] [Google Scholar]
- 10.Alvarez-Tejado M, Naranjo-Suarez S, Jimenez C, et al. Hypoxia induces the activation of the phosphatidylinositol 3-kinase/Akt cell survival pathway in PC12 cells: protective role in apoptosis. J Biol Chem. 2001;276:22368–22374. doi: 10.1074/jbc.M011688200. [DOI] [PubMed] [Google Scholar]
- 11.Fassnacht M, Weismann D, Ebert S, et al. AKT is highly phosphorylated in pheochromocytomas but not in benign adrenocortical tumors. J Clin Endocrinol Metab. 2005;90:4366–4370. doi: 10.1210/jc.2004-2198. [DOI] [PubMed] [Google Scholar]
- 12.Sippel RS, Carpenter JE, Kunnimalaiyaan M, et al. Raf-1 activation suppresses neuroendocrine marker and hormone levels in human gastrointestinal carcinoid cells. Am J Physiol Gastrointest Liver Physiol. 2003;285:G245–G254. doi: 10.1152/ajpgi.00420.2002. [DOI] [PubMed] [Google Scholar]
- 13.Kappes A, Vaccaro A, Kunnimalaiyaan M, et al. ZM336372, a Raf-1 activator, inhibits growth of pheochromocytoma cells. J Surg Res. 2006;133:42–45. doi: 10.1016/j.jss.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Kappes A, Vaccaro A, Kunnimalaiyaan M, et al. Lithium ions: a novel treatment for pheochromocytomas and paragangliomas. Surgery. 2007;141:161–165. doi: 10.1016/j.surg.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adler JT, Hottinger DG, Kunnimalaiyaan M, et al. Histone deacetylase inhibitors upregulate Notch-1 and inhibit growth in pheochromocytoma cells. Surgery. 2008;144:956–961. doi: 10.1016/j.surg.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kunnimalaiyaan M, Ndiaye M, Chen H. Apoptosis-mediated medullary thyroid cancer growth suppression by the PI3K inhibitor LY294002. Surgery. 2006;140:1009–1014. doi: 10.1016/j.surg.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 17.Sippel RS, Carpenter JE, Kunnimalaiyaan M, et al. The role of human achaete-scute homolog-1 in medullary thyroid cancer cells. Surgery. 2003;134:866–871. doi: 10.1016/s0039-6060(03)00418-5. [DOI] [PubMed] [Google Scholar]
- 18.Dai Y, Wei Z, Sephton CF, et al. Haloperidol induces the nuclear translocation of phosphatidylinositol 3′-kinase to disrupt Akt phosphorylation in PC12 cells. J Psychiatry Neurosci JPN. 2007;32:323–330. [PMC free article] [PubMed] [Google Scholar]
- 19.Huber K, Bruhl B, Guillemot F, et al. Development of chromaffin cells depends on MASH1 function. Development. 2002;129:4729–4738. doi: 10.1242/dev.129.20.4729. [DOI] [PubMed] [Google Scholar]
- 20.Garlich JR, De P, Dey N, et al. A vascular targeted pan phosphoinositide 3-kinase inhibitor prodrug, SF1126, with anti-tumor and antiangiogenic activity. Cancer Res. 2008;68:206–215. doi: 10.1158/0008-5472.CAN-07-0669. [DOI] [PubMed] [Google Scholar]