Abstract
Human papillomavirus (HPV) is the most common sexually transmitted infection. Vaccines for HPV infection can reduce the risk of cervical cancer. To further improve such vaccines and to explore other methods of preventing or treating viral infection, longitudinal studies in experimental animals are desirable. Here, we describe a newly-developed multi-color endoscopic fluorescence imaging system to visualize early HPV infection with fluorescent protein-encoded pseudoviruses (PsV) in the female genital tract of living mice. With this imaging method, the course of HPV PsV infection, and the effects of intervention to prevent infection can be monitored in a single mouse over time. Female immunocompetent or athymic mice were pretreated with a vaginal spermicide, and then HPV PsV containing an authentic viral capsid and encapsidating green or red fluorescent protein (GFP or RFP) reporter gene was intravaginally instilled. Expression of GFP or RFP was detected 1 day after PsV challenge, and peaked after 2 or 3 days, decreasing thereafter. No fluorescence was detected in vaccine-treated immunocompetent mice. By using serial infection of the same PsV type (HPV 16) encoding either GFP or RFP, different infection patterns of repeated exposure can be monitored. This method offers the ability to monitor experimental virus infections before and after intervention thereby accelerating the development of appropriate prevention and therapy.
Keywords: fluorescence imaging, endoscopy, viral infection, papilloma virus
Introduction
Human papillomavirus (HPV) is usually acquired by sexual contact, and up to 80% of sexually active women show evidence of HPV infection at some stage of their lives. Although HPV infection is usually asymptomatic, more than 90% of cervical cancers are associated with HPV infection (1, 2). HPV16 is detected in approximately 50% of cervical cancers worldwide. The recently developed and FDA approved HPV vaccines, Gardasil® (Merck, Whitehouse Station, NJ) and Cervarix™ (GlaxoSmithKline, Rixensart, Belgium), markedly reduce the risk of infection by the vaccine-targeted HPV types, thereby preventing cervical pre-cancerous lesions, provided the vaccination occurs prior to HPV exposure (3, 4). In order to develop the next generation of vaccines with maximal potency and to explore interventions that prevent infection after exposure to HPV, studies using large numbers of mice, euthanized at various time points, must be conducted. Alternatively, a smaller number of animals can be serially studied over time, using each mouse as its own control. While both methods are valid, the latter approach may be more cost effective and realistic. We have developed a multi-color fluorescence mini-endoscopic imaging system with a highly sensitive charge coupled device (CCD) camera and multi-color fluorescence imaging capabilities for real-time monitoring of the viral infection with HPV pseudovirus (PsV) encapsidating GFP or RFP. Animals were observed longitudinally after experimental interventions to determine the effectiveness in reducing or preventing infection.
Materials and Methods
Reagents
Depo-Provera was purchased from Pfizer (La Jolla, CA) and diluted to a final concentration of 30mg/ml in phosphate buffered saline (PBS). Carboxymethylcellulose (CMC) was purchased from Sigma-Aldrich (St. Louis, MO) and dissolved in deionized water. Conceptrol, which contains 4% of nonoxynol-9 (N-9), was purchased from McNeil-PPC Inc. (Skillman, NJ). Gardasil was purchased from Merck. Intron A was purchased from Schering-Plough (Kenilworth, NJ) and dissolved in distilled water. It was mixed with CMC to make a final concentration of 2% CMC.
Pseudovirus
HPV16 PsV with green fluorescent protein (GFP) or tdTomato (RFP) fluorescent reporter constructs were prepared as previously described (5–7).
HPV PsV challenge in vivo
All procedures were conducted in compliance with the Guide for the Care and Use of Laboratory Animal Resources (1996), National Research Council, and approved by the local Animal Care and Use Committee. Six- to eight-week-old female Balb/c or homozygote athymic mice were purchased from Charles River (NCI-Frederick, Frederick, MD). During the procedure, mice were anesthetized with isoflurane. HPV PsV challenge was performed according to a previous report (5) with slight modification. In brief, mice were injected subcutaneously with 3 mg of Depo-Provera 4 days before HPV PsV challenge. For chemical disruption, mice were pretreated with intravaginal administration of 50 µl of N-9. For mechanical disruption, a commercially available model BF XP-60 bronchoscope (Olympus Co., Tokyo, Japan), 2.8 mm in diameter with a single biopsy channel, was inserted into the mice vagina with gentle insufflation of carbon dioxide. With white light endoscopic imaging (CLV-180, Olympus Co.), a cervical specimen was disrupted using biopsy forceps (FB-56D-1, Olympus Co.). Four hours after chemical or mechanical disruption, a HPV PsV titer of ~2.5 × 107 infectious units in 20 µl of 2% CMC was administered intravaginally. For vaccination, Balb/c mice were injected intramuscularly with 6 µg of Gardasil once or twice, three weeks apart. Seven days after the last immunization, mice received HPV PsV challenge. For IFN treatment, 1 MIU of Intron A dissolved in 20 µl of 2% CMC was administered to Balb/c or homozygote athymic mice intravaginally every day starting 3 days before HPV PsV challenge.
Fluorescence endoscopy and image analysis
Mice were examined with fluorescence endoscopy every day for up to 14 days after HPV PsV challenge. A model BF XP-60 bronchoscope system (Olympus Co.) was inserted intravaginally while the animal was under anesthesia, cervicovaginal epithelium was observed with white light imaging and dual fluorescence imaging by switching back and forth between the blue (465 to 500 nm) or green (530 to 555 nm) excitation filters. Endoscopic images were obtained via a dichroic splitter, where the excitation light images were displayed using the image processor (OTV-S7, Olympus Co.) and the fluorescence images were filtered by in-house designed multi-color emission filters (516 to 556 nm band-pass for GFP and 570 to 630 nm band-pass for RFP) prior to reaching the EM-CCD camera (Texas Instruments, Dallas, TX). Both images were displayed side by side on the PC monitor with DualView 2 software (RGB Spectrum, Alameda, CA). Real-time images of both white light and fluorescence images were recorded with hard disk recorder (Fig. 1A). Camera gain, exposure time and binning for the fluorescence images were held constant in each fluorescent protein throughout the study.
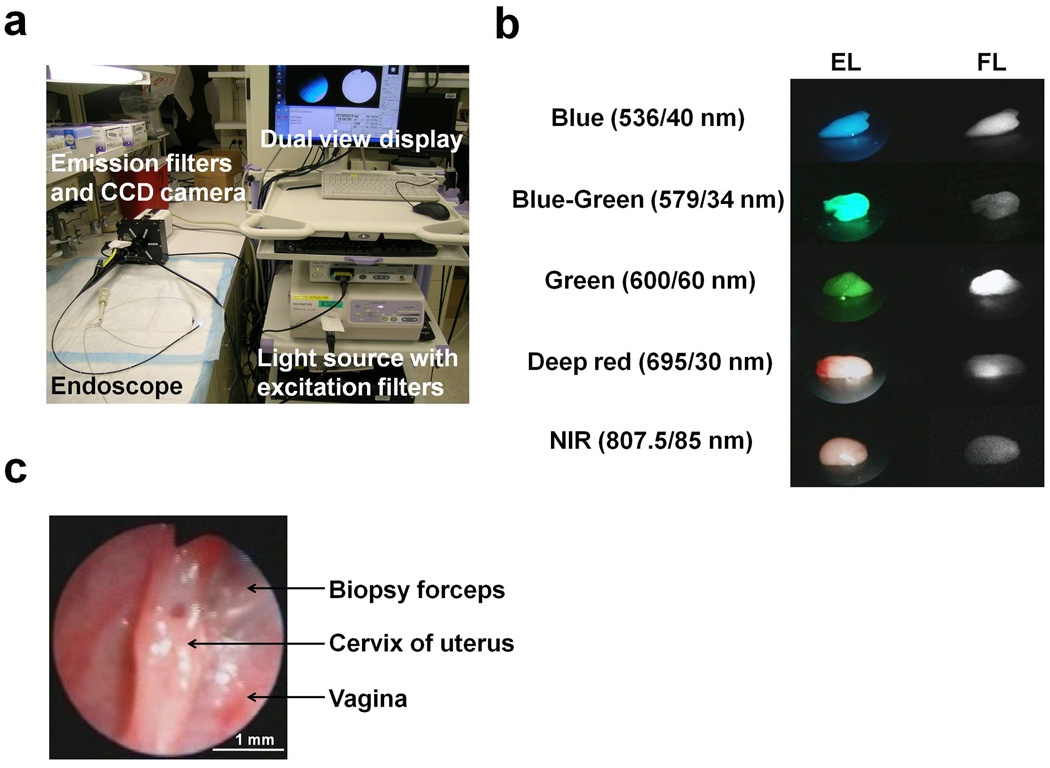
Figure 1. Multi-color fluorescence mini-endoscopic imaging of the mouse cervix.
(A) Multi-color fluorescence imaging system is based on a clinically available fiber optic endoscope and light source. Excitation light is provided by in-house designed excitation filters, which are switchable to either the visible light filter (white light) or band pass filter. Endoscopic images were obtained through a dichroic splitter, wherein the excitation light images were displayed with an image processor, and the fluorescence images were filtered by multi-color emission filters prior to reaching the EM-CCD camera. Both images are displayed side by side on the monitor. (B) Ex-vivo images of the phantoms (lymph nodes) incorporating fluorescent tracers, (Rhodamine green (close to GFP), TAMRA, RhodamineX (close to RFP), Cy5.5, ICG) showed the capability of multi-color fluorescence detection. Excitation light image (EL) and the fluorescence image (FL) were simultaneously displayed on the screen side by side. The emission filter spectrums are indicated. (C) White light endoscopic imaging of the mouse cervix. Biopsy forceps (tip = 1 mm diameter) were used to gauge the distance to the cervix which was held constant.
In order to compare fluorescence intensities during different HPV PsV challenges, the distance between the cervical opening and the endoscope head was maintained using the biopsy forceps as a guide. Snap shot images obtained from the hard disk recorder were used for calculating fluorescence intensities. All fluorescence images were analyzed with Image J software (http://rsbweb.nih.gov/ij/). Circular regions of interest (ROIs) were placed in the same regions of cervical epithelium in each mouse to determine the change of fluorescence intensities over time. The pixel intensities, varying between 0–255 were obtained from each ROI. Normal background signal was determined by measuring signal intensity prior to HPV PsV challenge in order to remove autofluorescence and camera noise.
Validation of HPV PsV infection
Mice were examined with fluorescence endoscopy 2 days after HPV PsV challenge. Using the green fluorescence filter set, real-time biopsies were performed with biopsy forceps. Smears were prepared from the fresh biopsy specimens, and examined with fluorescence microscopy (BX51, Olympus America, Melville, NY) equipped with the following filters: excitation wavelength, 450 to 490 nm; emission wavelength, 500 to 550 nm. Transmitted light differential interference contrast (DIC) images were also acquired.
Statistical analysis
Data are expressed as mean ± s.e.m. Analyses were carried out using a statistics program (GraphPad Instat, version 3.06; GraphPad Software, La Jolla, CA). For comparison of groups, the Mann-Whitney rank sum test was used. A level of P < 0.05 was considered statistically significant.
Results
Monitoring the HPV PsV infection by fluorescence mini-endoscopy in vivo
Quantitative analysis of the initial phase of HPV has previously been performed by intravaginally infecting mice with HPV PsV encapsidating plasmids encoding genes for RFP. In order to assess the kinetics of gene expression, large numbers of animals were required to perform ex vivo imaging of the vaginal tracts over time (5). Here we sought to repeatedly monitor localized HPV PsV infection kinetics in the same animal by employing a multi-color mini-endoscope based on a commercial clinical endoscope, which allows for the independent detection of GFP and RFP fluorescence. This mini-endoscope uses a clinically available light source and fiber optic endoscope in addition to in-house designed excitation filters, a dichroic splitter, emission filters, and two cameras; a color camera for white light imaging and a highly sensitive electron multiplying-CCD (EM-CCD) camera for detecting fluorescence. This enabled the simultaneous, side by side imaging of white, or excitation, light imaging and fluorescence imaging of the cervicovaginal epithelium (Fig. 1A). This system, therefore, has the ability to image green, red and near infrared (NIR) fluorescence with the appropriate filter set (Fig. 1B). For quantitating fluorescence intensity over time, the distance between the cervical opening and the endoscope head was held constant at 3.0 mm (Fig. 1C).
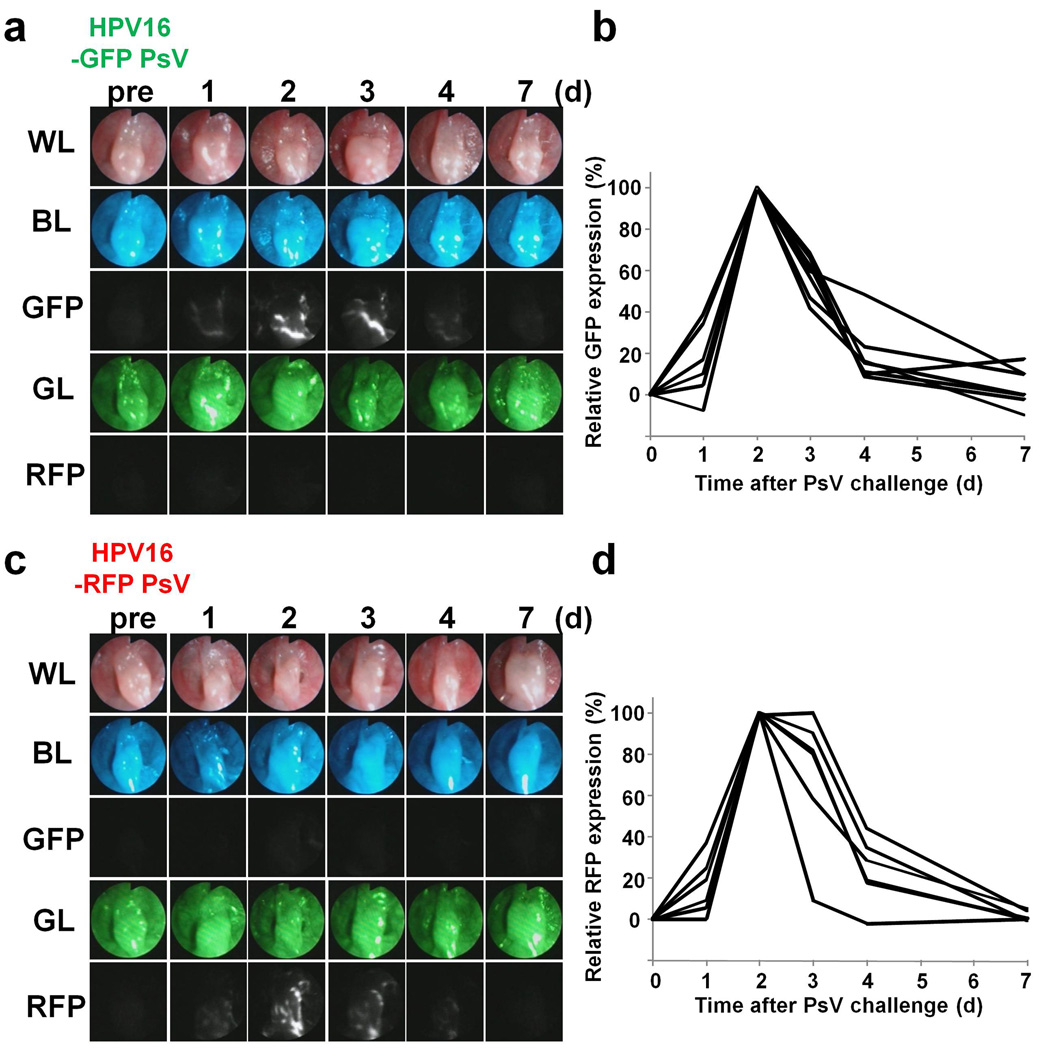
After pre-treatment with a vaginal spermicide, N-9 , a n H P V 1 6 P s V encapsidating either a GFP or a RFP expression plasmid was administered to the animals intravaginally (5). Expression of GFP or RFP was detected as early as 1 day after PsV challenge (n = 6 mice each), peaked at 2 or 3 days, and then decreased thereafter to the pre-infected baseline (Fig. 2, A–D). Both GFP and RFP encoding HPV PsVs behaved in the same manner. Settings of camera gain, exposure time and binning for the fluorescence images were held constant throughout the study. There was almost no background signal arising from the cervicovaginal epithelium as only minimal autofluorescence could be detected prior to infection. In addition, no cross talk was observed between GFP and RFP fluorescence and the sensitivity of the fluorescence did not vary significantly between the two (Fig. 2, A and C). Moreover, HPV PsV, which did not encapsidate a fluorescence reporter plasmid, or no HPV PsV challenge showed no detectable fluorescence (Supplementary Fig. S1). These results indicate that it is possible to detect and monitor HPV infection over time in the cervicovaginal epithelium in living mice using this fluorescence mini-endoscopy system (Supplementary video S1, A and B).
Figure 2. Monitoring the HPV16 PsV infection over time.
(A) Time course observation of GFP encoding HPV PsV infection (see also video S1, A). GFP expression indicates HPV PsV infection could be serially monitored in each mouse. White light image (WL), blue excitation light image (BL), green fluorescence image (GFP), green excitation light image (GL) and tdTomato fluorescence image (RFP) were obtained every day after PsV challenge. (B) Quantitation of relative GFP expression in each mouse (n = 6 mice). Fluorescence intensity was normalized to the baseline intensity prior to infection. Peak GFP expression was considered as 100% expression. (C and D) Similar experiments were performed with RFP encoding HPV PsV (see also video S1, B) (n = 6 mice).
To assess the infection efficiency of HPV PsV, simultaneous co-infection with approximately equal titers of GFP and RFP encoding HPV PsVs was performed. Both GFP and RFP expression were detected by day 1 and peaked on day 2, indicating that there were no significant differences in infectivity between the GFP- and RFP-expressing PsVs (Supplementary Fig. S2A). Merged green and red fluorescent images obtained 2 days after infection revealed co-localization of GFP and RFP fluorescence (n = 3 mice) (Supplementary Fig. S2B). Next, biopsy forceps were used to mechanically disrupt the cervical epithelium, thereby promoting infection with HPV PsV. During endoscopy, the dorsal cervix was selectively disrupted, and then mice received the HPV PsV challenge. Fluorescence was observed only in the rim and surrounding mucosa of the disrupted lesion, and peak expression was observed on day 2 after the HPV PsV challenge, which was similar to the results with chemical disruption (n = 3 mice) (Supplementary Fig. S3). These results confirm the findings in Roberts et al. that epithelial abrasion, whether induced chemically or mechanically, is required for HPV PsV infection (5) (Supplementary Fig. S2 and S3).
Next, biopsies were obtained from fluorescing lesions of the cervicovaginal epithelium during endoscopy (n = 3). Fluorescent lesions were selectively sampled using biopsy forceps. Cells exhibiting GFP expression at the site of biopsy were confirmed with fluorescence microscopy (Supplementary Fig. S4 and video S2).
Detection of HPV PsV infection after intervention
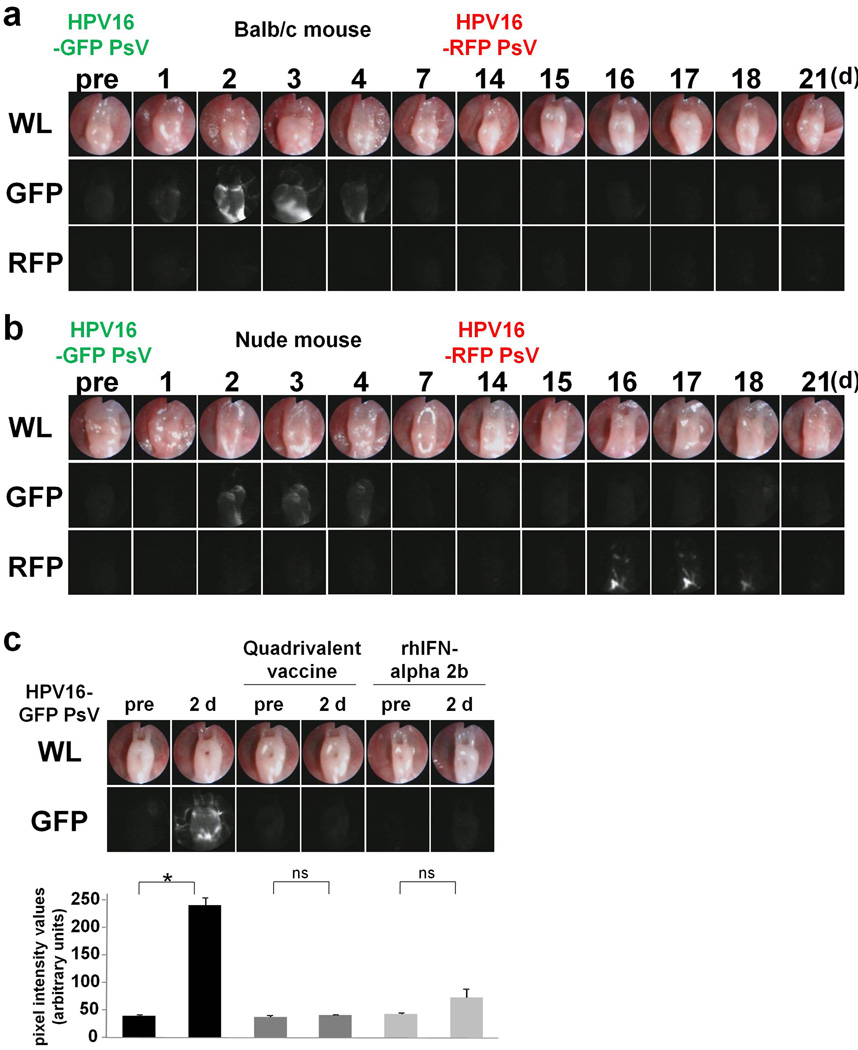
Previous studies report that intramuscular vaccination with native L1 virus-like particles (VLPs) can induce antibody-mediated, type-restricted protection against experimental papillomavirus infection (8, 9). To test whether intravaginal instillation of HPV capsids can generate protective immunity, 14 days after the initial vaginal infection, immunocompetent Balb/c and immunocompromized homozygote athymic mice were vaginally infected using an HPV PsV encapsidating the opposite fluorescent reporter plasmid from what was employed during the first HPV PsV challenge (e.g. GFP HPV PsV followed by RFP HPV PsV and vice versa). The use of two different probes allowed the second infection to be distinguished from the first but also abrogated the possibility of immunity generated against the first fluorescent protein. There was no detectable fluorescence after the second HPV PsV challenge in immunocompetent Balb/c mice (Fig. 3A), whereas fluorescence could be detected in immunocompromised athymic mice (Fig. 3B) (n = 3 mice each), suggesting that acquired immunity against the HPV capsid played a role in the inhibition of HPV PsV infection.
Figure 3. Detection of HPV PsV infection after second challenge, vaccination and interferon.
(A and B) Fourteen days following initial HPV PsV challenge with one fluorescent reporter, the mice were challenged with a second HPV PsV encoding a different fluorescent reporter. No detectable fluorescence was observed after the second HPV PsV challenge in immunocompetent Balb/c mice, whereas fluorescence could be detected in immunocompromized homozygote athymic mice (n = 3 mice each). (C) Pretreatment with quadrivalent vaccine or recombinant human IFN alpha-2b suppressed GFP expression. No apparent fluorescence was found in Balb/c pretreated with vaccine, and fluorescence was greatly reduced with IFN even 2 days after PsV challenge (n = 4 mice each, *P = 0.0079, Mann-Whitney rank sum test).
Next, Balb/c mice were immunized once or twice at an interval of three weeks with 6 µg of Gardasil, a quadrivalent vaccine composed of L1 VLPs from HPV types 6, 11, 16 and 18, to confirm the effect of vaccination. Seven days after the last immunization, mice received an HPV16 PsV challenge. Consistent with recent studies showing immunization with HPV VLPs protects mice from vaginal PsV challenge (10, 11), there was no apparent fluorescence after HPV PsV challenge at any time point in both vaccination groups (n = 4 mice each), indicating that the vaccine induced protection from infection (Fig 3C; shows results for mice vaccinated twice).
Topical interferon (IFN) was then tested as an antiviral therapy. One million international units (MIU) of Intron A®, a recombinant human IFN alpha-2b, which induces antiviral activity by suppressing the transcription and translation of viral genes, were administered into Balb/c or athymic mice intravaginally every day starting 3 days before HPV PsV challenge. Fluorescence was greatly reduced in both immunocompentent and immunocompromised mice pre-treated with IFN (n = 4 mice), indicating that topical IFN inhibits HPV PsV infection and/or suppresses the transcription of the reporter gene (Fig. 3C).
Collectively these results suggest that various strategies to inhibit HPV PsV cervicovaginal infection can be assessed in vivo using HPV PsVs encapsidating different fluorescent protein genes. The imaging technique described here allows for the monitoring of the early stages of HPV infection over time using minimally invasive fluorescence endoscopy in the cervicovaginal tract of living immunocompetent or immunocompromised mice.
Discussion
The pathological consequences of HPV infection are strictly species and tissue restricted to humans. However, the early events of binding, entry, and genome release appear not to be species restricted and therefore, cervicovaginal infection with HPV PsV in mice provides a platform for the examination of the initial phase of papillomavirus infection. Infection is promoted by trauma of the cervicovaginal epithelium, which exposes the basement membrane and the basal epithelial cells to virus. Fluorescent proteins are used as a robust tool for investigating the viral infection as well as the cancer research in vivo (12–16). A previous study showed that the spermicide N-9 increased the likelihood of HPV infection which was demonstrated with ex vivo optical fluorescence utilizing GFP and RFP encapsidated by the PsV (5). This experiment required multiple mice at each time point in order to reach statistical endpoints. The key technological advance reported herein is the development of a mini-endoscope that permits fluorescence imaging using a highly sensitive EM-CCD camera. By changing the filter sets, multi-color imaging becomes possible. The advantages of this are 1.) the infection in a single living mouse can be followed over time, and 2.) multi-color fluorescence signals can be analyzed to separately test the impact of a second HPV PsV infection using a PsV encapsidating a different reporter gene. Furthermore, use of this method provides a means to accurately induce and image pinpoint infections by creating specific mini-abrasions or sample the infected tissue with fluorescence-guided biopsy under endoscopic guidance (Supplementary Fig. S3 and video S2).
As there was no cross talk between GFP and RFP fluorescence (17), preventive and early therapeutic interventions could be performed to determine resistance to re-infection using serial infections with different HPV PsVs. Therefore, infecting with the same type of HPV PsV but with different color reporters can be useful in the investigation of the duration and intensity of the immune response against the primary infection or vaccination (Fig. 3, A and B). For instance, in this study infection was prevented or ameliorated by prior vaccination with Gardasil and topical administration of recombinant human IFN (Fig. 3C), as demonstrated by longitudinal observations of reduced fluorescent gene expression after intervention.
In vivo observation of HPV PsV infection has also been successfully performed using a bioluminescence reporter imaging system (Supplementary Fig. S5) (18, 19). There are two technical advantages of our method over the bioluminescence imaging, 1.) real-time multiple color capability 2.) direct access to the tissue for localizing infection or sampling infected cells resulting in much higher resolution imaging. From the photo-physical point of view, the endoscope can minimize light scattering and absorbance caused by overlapping tissue resulting in more accurate depiction of the lesion, and theoretically, allows better quantitative measurements of the infection. Moreover this method does not require administration of an exogenous substrate, luciferin, which is necessary for bioluminescence (20). Furthermore, GFP and RFP are readily translated to ex vivo histopathology while this is more difficult with bioluminescence.
Until recently it has not been possible to directly visualize HPV PsV infection in vivo. Improvements in optical technology allow placement of mini-endoscopes in small cavities with full fluorescence capabilities in real-time. These tools provide an opportunity to investigate the effects of preventive and therapeutic interventions for HPV PsV infections in small rodents, much as they would be investigated in humans.
Supplementary Material
Acknowledgments
Grant Support: This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 2.zur Hausen H. Papillomavirus infections--a major cause of human cancers. Biochim Biophys Acta. 1996;1288:F55–F78. doi: 10.1016/0304-419x(96)00020-0. [DOI] [PubMed] [Google Scholar]
- 3.Siddiqui MA, Perry CM. Human papillomavirus quadrivalent (types 6, 11, 16, 18) recombinant vaccine (Gardasil) Drugs. 2006;66:1263–1271. doi: 10.2165/00003495-200666090-00008. discussion 72-3. [DOI] [PubMed] [Google Scholar]
- 4.Bryan JT. Developing an HPV vaccine to prevent cervical cancer and genital warts. Vaccine. 2007;25:3001–3006. doi: 10.1016/j.vaccine.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 5.Roberts JN, Buck CB, Thompson CD, et al. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med. 2007;13:857–861. doi: 10.1038/nm1598. [DOI] [PubMed] [Google Scholar]
- 6.Buck CB, Pastrana DV, Lowy DR, Schiller JT. Efficient intracellular assembly of papillomaviral vectors. J Virol. 2004;78:751–757. doi: 10.1128/JVI.78.2.751-757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buck CB, Thompson CD, Pang YY, Lowy DR, Schiller JT. Maturation of papillomavirus capsids. J Virol. 2005;79:2839–2846. doi: 10.1128/JVI.79.5.2839-2846.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breitburd F, Kirnbauer R, Hubbert NL, et al. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J Virol. 1995;69:3959–3963. doi: 10.1128/jvi.69.6.3959-3963.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suzich JA, Ghim SJ, Palmer-Hill FJ, et al. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc Natl Acad Sci U S A. 1995;92:11553–11557. doi: 10.1073/pnas.92.25.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caldeira JD, Medford A, Kines RC, et al. Immunogenic display of diverse peptides, including a broadly cross-type neutralizing human papillomavirus L2 epitope, on virus-like particles of the RNA bacteriophage PP7. Vaccine. 2010 doi: 10.1016/j.vaccine.2010.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuburu N, Kweon MN, Hervouet C, et al. Sublingual immunization with nonreplicating antigens induces antibody-forming cells and cytotoxic T cells in the female genital tract mucosa and protects against genital papillomavirus infection. J Immunol. 2009;183:7851–7859. doi: 10.4049/jimmunol.0803740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang M, Baranov E, Moossa AR, Penman S, Hoffman RM. Visualizing gene expression by whole-body fluorescence imaging. Proc Natl Acad Sci U S A. 2000;97:12278–12282. doi: 10.1073/pnas.97.22.12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao M, Yang M, Baranov E, et al. Spatial-temporal imaging of bacterial infection and antibiotic response in intact animals. Proc Natl Acad Sci U S A. 2001;98:9814–9818. doi: 10.1073/pnas.161275798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffman RM, Zhao M. Whole-body imaging of bacterial infection and antibiotic response. Nat Protoc. 2006;1:2988–2994. doi: 10.1038/nprot.2006.376. [DOI] [PubMed] [Google Scholar]
- 15.Tran Cao HS, Kaushal S, Lee C, et al. Fluorescence laparoscopy imaging of pancreatic tumor progression in an orthotopic mouse model. Surg Endosc. 2011;25:48–54. doi: 10.1007/s00464-010-1127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffman RM, Yang M. Color-coded fluorescence imaging of tumor-host interactions. Nat Protoc. 2006;1:928–935. doi: 10.1038/nprot.2006.119. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman RM. The multiple uses of fluorescent proteins to visualize cancer in vivo. Nat Rev Cancer. 2005;5:796–806. doi: 10.1038/nrc1717. [DOI] [PubMed] [Google Scholar]
- 18.Johnson KM, Kines RC, Roberts JN, Lowy DR, Schiller JT, Day PM. Role of heparan sulfate in attachment to and infection of the murine female genital tract by human papillomavirus. J Virol. 2009;83:2067–2074. doi: 10.1128/JVI.02190-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kines RC, Thompson CD, Lowy DR, Schiller JT, Day PM. The initial steps leading to papillomavirus infection occur on the basement membrane prior to cell surface binding. Proc Natl Acad Sci U S A. 2009;106:20458–20463. doi: 10.1073/pnas.0908502106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keyaerts M, Verschueren J, Bos TJ, et al. Dynamic bioluminescence imaging for quantitative tumour burden assessment using IV or IP administration of D: -luciferin: effect on intensity, time kinetics and repeatability of photon emission. Eur J Nucl Med Mol Imaging. 2008;35:999–1007. doi: 10.1007/s00259-007-0664-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.