Abstract
Constitutive Hedgehog (HH) signaling underlies several human tumors, including basal cell carcinoma (BCC). Recently, Bijlsma et al (Bijlsma MF, et al. (2006) PLoS Biol 4: 1397–1410) reported a new biologic function for vitamin D3 in suppressing HH signaling in an in vitro model system. Based on that work, we have assessed effects of vitamin D3 on HH signaling and proliferation of murine BCCs in vitro and in vivo. We find that indeed in BCC cells, vitamin D3 blocks both proliferation and HH signaling as assessed by mRNA expression of the HH target gene Gli1. These effects of vitamin D3 on Gli1 expression and on BCC cell proliferation are comparable to the effects of cyclopamine, a known inhibitor of the HH pathway. These results are specific for vitamin D3, since the precursor 7-dehydrocholesterol and the downstream products 25-hydroxy vitamin D3 [25(OH)D] and 1,25-dihydroxy vitamin D3 [1,25(OH)2D] are considerably less effective in reducing either Gli1 mRNA or cellular proliferation. Moreover, these effects seem to be independent of the vitamin D receptor (VDR) since shRNA knock down of VDR does not abrogate the anti HH effects of D3 despite reducing expression of the VDR target gene 24-hydroxylase. Finally, topical vitamin D3 treatment of existing murine BCC tumors significantly decreases Gli1 and Ki67 staining. Thus, topical vitamin D3 acting via its HH inhibiting effect may hold promise as an effective anti-BCC agent.
Introduction
In 1941, Apperly (1) noted that the incidence of colon cancer in the United States is considerably higher in the North than in the South, and the data favoring this “latitudinal gradient” remain strong for several cancers including in particular those of the colon, breast, and prostate. Four decades later, Garland and Garland noted that the differences in cancer incidence in different locales are inversely related to the amount of sunlight they receive and proposed that this gradient might be explained by an anti-cancer effect of varying amounts of vitamin D made in sun-exposed skin (2, 3). This proposal has been seminal, and 6000 papers have been published touching on vitamin D and cancer. Approaches taken to investigate this putative relationship include comparisons between cancer risk and sun exposure and/or dietary vitamin D intake; assessments of circulating 25(OH)D, the most readily available measurement of body vitamin D status, in patients with cancers and controls; comparisons of DNA polymorphisms in the genes encoding the vitamin D receptor (VDR) and the enzyme responsible for the catabolism of the VDR ligand: 1,25(OH)2D; and studies of the cancer preventive effects of supplemental dietary vitamin D. The latter include one very large prospective study of the effects of 400 IU of vitamin D3/day, which showed no effects on cancer incidence or mortality (4), albeit the compliance rate in this study was poor, and one considerably smaller prospective study of 1,100 IU vitamin D3/day, which found a statistically significant reduction of cancer incidence in those taking the vitamin supplement (5). Taken together, the positive correlation of cancer incidence and latitude of residence seems strong and the inverse correlation of sunlight exposure and cancer seems moderately strong, but the mechanistic importance of any inverse correlation of cancer incidence and vitamin D3 and the anti-cancer efficacy of vitamin D3 supplementation remain uncertain (6–8).
The most studied mechanism of the effect of vitamin D3 is the 1,25(OH)2D induced transcriptional activation of the VDR with resultant changes in cell behavior including enhanced differentiation and reduced proliferation of skin keratinocytes (9–11). By contrast, Bijlsma and colleagues (12) recently proposed a new biologic function for unhydroxylated vitamin D3 - the inhibition of hedgehog (HH) signaling. They found that D3 binds to Smo specifically and thereby inhibits Gli reporter activity in C3H/10T1/2 fibroblasts in vitro. In addition, D3 treatment of zebrafish in vivo mimicked the Smo−/− phenotype. In fact, Bijlsma and colleagues (12) propose that Ptch1 protein accomplishes its inhibition of HH signaling by transporting vitamin D3 to Smo protein.
HH signaling was identified initially as a pathway crucial to development but more recently has come to be seen as a potentially important stimulator of carcinogenesis when dysregulated. This can occur via mutations in the genes encoding components of the pathway or by excess production of HH ligand by the tumor or stromal cells (13). Indeed, the first in man inhibitor of HH signaling, GDC-0449, is now in clinical trials for at least eight human cancers [clinicaltrials.gov], and several other HH inhibitors are in varying stages of clinical development.
Of the human cancers with mutations in HH signaling pathway components, the best studied tumor-HH relationship in humans and mice is that found in basal cell carcinomas (BCCs), and inhibition of HH signaling with small molecule drugs can have dramatic inhibitory effects on human BCCs (14). BCCs are the most common of all human cancers, affecting approximately 1 million Americans per year (15). The pivotal molecular abnormality in BCCs is constitutive activation of the HH signaling pathway, in 10–20% of tumors by mutational activation of SMO and in the great majority of the others associated with mutational inactivation of PTCH1 (16–19) (20–23). In addition to mutational activation of the HH pathway, human BCCs also frequently have mutations in p53 (22, 24). Ptch1+/− mice develop BCCs after mutational insults, and the addition of conditional loss of keratinocyte p53 greatly accelerates murine BCC carcinogenesis
Based on the known role of HH signaling in BCC carcinogenesis and on this newly reported HH-inhibitory function of vitamin D3, we have studied whether vitamin D3, its precursor, and its hydroxylated derivatives can inhibit cellular proliferation and down-regulate HH signaling in established murine BCC cell lines and in murine BCCs in vivo. We then investigated whether the anti-BCC effects of vitamin D3 are mediated via the classic VDR pathway.
RESULTS
In vitro Studies
Vitamin D3 inhibits proliferation of BCC cell lines
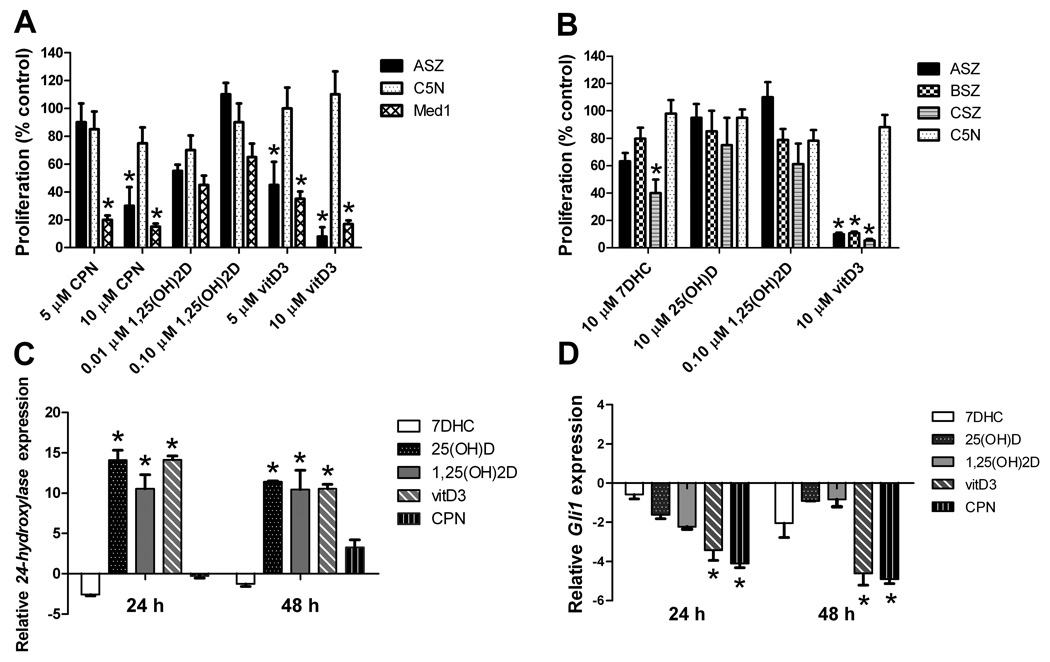
We treated an established murine BCC cell line (ASZ), a murine non-tumorigenic keratinocyte cell line (C5N), and a murine medulloblastoma cell line (Med1) with upregulated HH signaling (25) with cyclopamine or vitamin D3 and assessed cellular proliferation after 48 hours. As we found previously, the classical HH inhibitor cyclopamine decreases Med1 and ASZ proliferation at 5 µM and 10 µM, respectively, but does not decrease C5N cell proliferation (Fig.1A) (26). Vitamin D3 similarly inhibits the proliferation of ASZ and Med1 cells by more than 80% while not significantly inhibiting the proliferation of C5N cells (Fig.1A). Vitamin D3 also inhibits the proliferation of two other BCC cell lines (BSZ and CSZ) that were established from BCC tumors from Ptch1+/− mice with keratinocyte deletion of p53 (Fig. 1B). Neither the vitamin D3 precursor 7-dehydrocholesterol (7DHC) nor the hydroxylated forms 25(OH)D and 1,25(OH)2D consistently inhibit proliferation of BCC cells. We tested lower concentrations of 1,25(OH)2D because of the non-specific cell toxicity of concentrations greater than 1 µM (27). Thus, vitamin D3 specifically inhibits the proliferation of all three BCC cell lines tested.
Fig. 1.
Vitamin D3 is a potent inhibitor of cellular proliferation and Gli1 mRNA (n = 3 experiments). (A) Cellular proliferation studies were conducted in a BCC cell line (ASZ), non-tumorigenic keratinocytes (C5N), and a medulloblastoma cell line (Med1) incubated for 48 h with cyclopamine (CPN) versus 1,25(OH)2D and vitamin D3. Mean±SEM, *P < 0.01 compared to C5N. (B) Cellular proliferation was assayed in BCC cell lines (ASZ, BSZ, CSZ) and non-tumorigenic keratinocytes (C5N) 48 h after treatment. Mean±SEM, *P < 0.01 compared to C5N. (C) 24-hydroxylase mRNA relative expression in ASZ cells treated with 7DHC (10 µM), 25(OH)D (10 µM), 1,25(OH)2D (0.1 µM), vitamin D3 (10 µM), and CPN (10 µM) at 24 and 48 h. Mean±SEM, *P < 0.01 compared to control (DMSO or EtOH). (D) Gli1 mRNA relative expression in ASZ cells treated with 7DHC (10 µM), 25(OH)D (10 µM), 1,25(OH)2D (0.1 µM), vitamin D3 (10 µM), and CPN (10 µM) at 24 and 48 h. Mean±SEM, *P < 0.01 compared to control (DMSO or EtOH).
Vitamin D3 decreases Gli1 mRNA expression in BCC cell lines
To address the mechanism by which vitamin D3 inhibits ASZ proliferation, we next measured mRNA levels of the HH target gene Gli1 and of the VDR target gene 25-Hydroxyvitamin D-24-hydroxylase (24-hydroxylase, CYP24) in ASZ cells incubated with vitamin D3, 7DHC, 25(OH)D, or 1,25(OH)2D for 24 or 48 hours. Vitamin D3, 25(OH)D, and 1,25(OH)2D each increased 24-hydroxylase mRNA levels. This was expected as normal human keratinocytes and BCCs can convert vitamin D3 to 25(OH)D and then to the VDR-activating1,25(OH)2D (9, 28) (Fig.1C). In contrast, 7DHC fails to increase 24-hydroxylase levels, since UV is required to convert 7DHC to vitamin D3. As expected, cyclopamine does not affect 24-hydroxylase mRNA levels. Similar to prior reports (26), cyclopamine at 10 µM decreases Gli1 mRNA by 3–5 fold at 24 hours and 48 hours after incubation (Fig. 1D). Vitamin D3 treatment at the same concentration (10 µM) also decreases Gli1 mRNA by 3–4 fold at 24 hours and 48 hours. In contrast, 7DHC (10µM), 25(OH)D (10 µM), and 1,25(OH)2D (0.10 µM) each fail to reduce Gli1 mRNA significantly. Our finding that 25(OH)D and 1,25(OH)2D can activate VDR (as indicated by increased expression of the VDR target gene 24-hydroxylase), without significantly reducing Gli1 mRNA is consistent with Bijlsma et al’s data in other test systems indicating that vitamin D3’s inhibition of the HH pathway is independent of VDR. Importantly, our finding that concentrations of 25(OH)D and of 1,25(OH)2D that did not affect murine BCC cell growth did up-regulate VDR target gene expression is consistent with the effect on proliferation being independent of the VDR.
Vitamin D3 decreases Gli1 mRNA expression independent of the vitamin D3 receptor
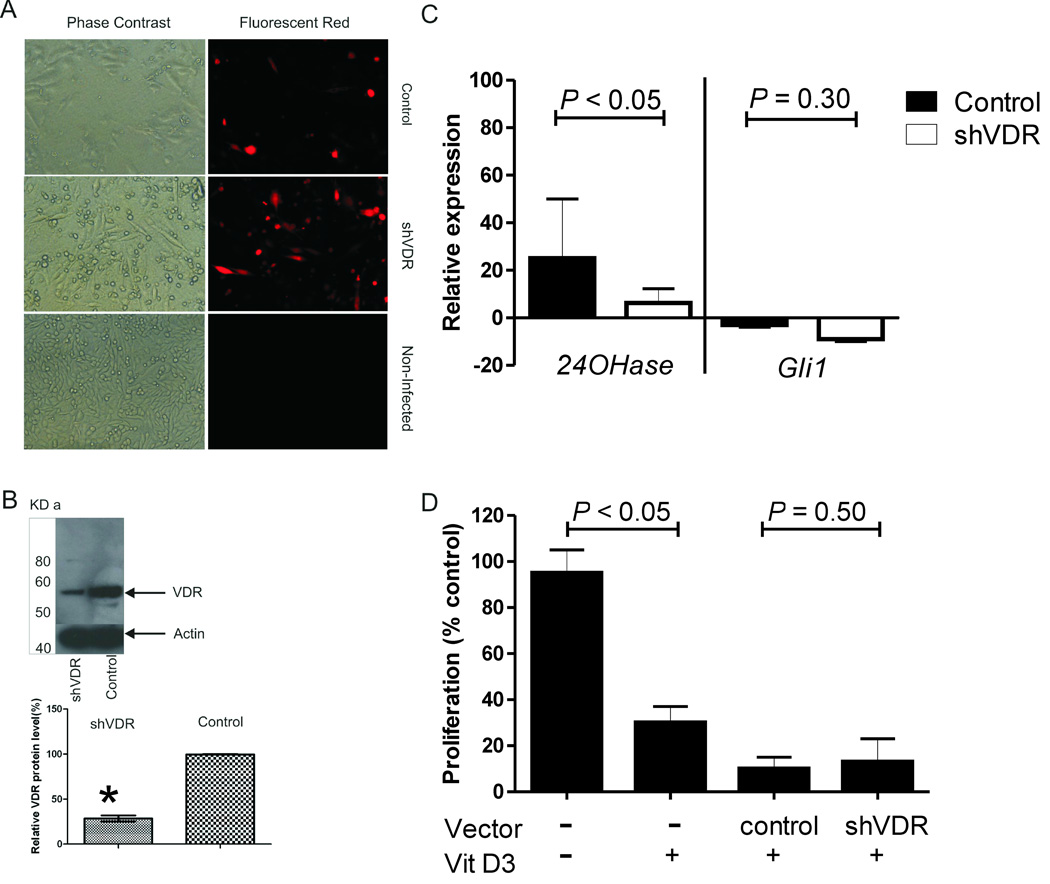
To investigate further the requirement of VDR signaling for the effect of vitamin D3, we incubated ASZ cells with a viral construct expressing shRNA against VDR (shVDR) for 3 days, and then treated these cells with 5 µM of vitamin D3 for 24 hours (n = 3 experiments). This construct infected approximately 80–90% of cells as measured by the expression of red fluorescence (Fig. 2A). Western blot analysis indicated that shVDR infection reduced VDR protein levels by approximately 75% as compared to VDR protein levels in cells infected with a virus expressing a random oligo (Fig. 2B). To assess the effectiveness of shVDR on VDR function, we measured 24-hydroxylase mRNA levels by qPCR in cells treated with vitamin D3. Vitamin D3 increased 24-hydroxylase mRNA by at least 20-fold in ASZ cells previously treated with the control shRNA. Vitamin D3 increased 24-hydroxylase levels in ASZ cells pre-treated with shVDR by a much lower amount (Fig. 2C). By contrast, pre-treatment with shVDR failed to affect vitamin D3’s decrease in Gli1 mRNA levels (Fig. 2C). Furthermore, vitamin D3 inhibited proliferation of ASZ cells pretreated with shVDR or control vector (Fig. 2D). These results were similar when shVDR was knocked down in a second cell line (BSZ, data not shown).
Fig. 2.
Anti-BCC effects mediated by vitamin D3 are intact when vitamin D3 receptor is blocked (n =3 experiments). (A) Phase contrast and fluorescence images for ASZ cells treated with control, shVDR, or no treatment for 72 h. (B) Upper panel: western blot showing VDR and β-actin protein levels in ASZ cells treated with shVDR or control. Lower panel: Quantitation of VDR protein level relative to β-actin in ASZ cells treated with shVDR or control. Mean±SEM. (C) Comparison of relative 24-hydroxylase and Gli1 mRNA in ASZ cells pretreated with control vector or shVDR, and then exposed to vitamin D3 (5 µM) for 24 h. Mean±SD. Note: the y-axis scale is different from Figure 1C, D. (D) Cellular proliferation in ASZ cells pretreated with no treatment, control vector, shVDR, and then exposed to vitamin D3 at 5µM for 24 h. Proliferation was assessed as % of control ASZ cells without vitamin D3 treatment. Mean±SD.
In vivo Studies
Vitamin D3 decreases proliferation but does not affect differentiation in BCC tumors in vivo
To test the in vivo effects of vitamin D3 on BCC tumors, we next utilized our murine model in which treatment with ionizing radiation (IR) at age 8 weeks induces BCC carcinogenesis. Specifically, our IR-treated Ptch1+/− K14-CreER2 p53 fl/fl mice treated with tamoxifen at age 6 weeks to activate Cre and thereby delete keratinocyte p53 develop multiple visible BCC tumors starting at age 5–6 months. These murine BCC tumors histologically resemble nodular human BCCs (Fig. 3-1A); and similar to BCCs in Ptch1+/− p53 wild type mice (16) these tumors express the basal cell marker keratin 14 (Fig. 3-1B) but not the suprabasalar differentiation marker keratin 10 (Fig. 3-1C), and have a high level of proliferation as measured by positive staining for Ki67 (Fig.3-1E, F). As expected for cells lacking p53, they do not express the apoptosis marker cleaved caspase 3 (CC3) (Fig. 3-1D).
Fig. 3.
Fig. 3.1. Histology of BCCs treated topically with acetone (control) for 30 days (A–F) or with topical 1.3 mg/kg vitamin D3 for 30 days (G–L). β-gal and hematoxylin and eosin staining for acetone or vitamin D3 treated BCC (Panel A, G). BCCs from Ptch1+/− K14-Cre-ER p53fl/fl mice stain blue due to β-galactosidase activity which is encoded by the lacZ gene that was inserted to replace the wildtype Ptch1 gene. Keratinocyte markers of differentiation, K14 (Panel B, H) and K10 (Panel C, I) are shown as well as CC3, a marker of apoptosis (Panel D, J) and Ki67 for proliferation (Panel E, F, K, L). Scale bars, 100 µm. Fig. 3.2. Levels of Ki67 staining in BCC tumors treated with either acetone (control, n = 7) or vitamin D3 (1.3 mg/kg, n = 10) for 30 days (P < 0.05).
We applied acetone without (n = 7) or with 1.3 mg/kg vitamin D3 (n = 10) to visible BCCs daily for approximately 30 days (Fig. 3-1, G–L). Treated BCCs continue to express keratin14 (Fig. 3-1H) but not keratin10 (Fig. 3-1I), suggesting that vitamin D3 does not induce differentiation of BCC cells. However topical vitamin D3 treatment markedly decreases cellular proliferation as shown by reduction of Ki67 staining (P = 0.02) (Fig. 3-1K, L; Fig. 3-2). Topical D3 did not affect apoptosis as assessed by the low number of brown staining CC3 positive cells relative to background staining (Fig. 3-1 D vs. J). These topical applications of vitamin D3 to BCCs and their surrounding skin were sufficient to increase circulating levels of 25(OH)D by approximately 4-fold (210 ± 160 vs. 50 ± 12 ng/ml, P < 0.01).
Topical vitamin D3 decreases Gli1 mRNA level in BCC tumors in vivo
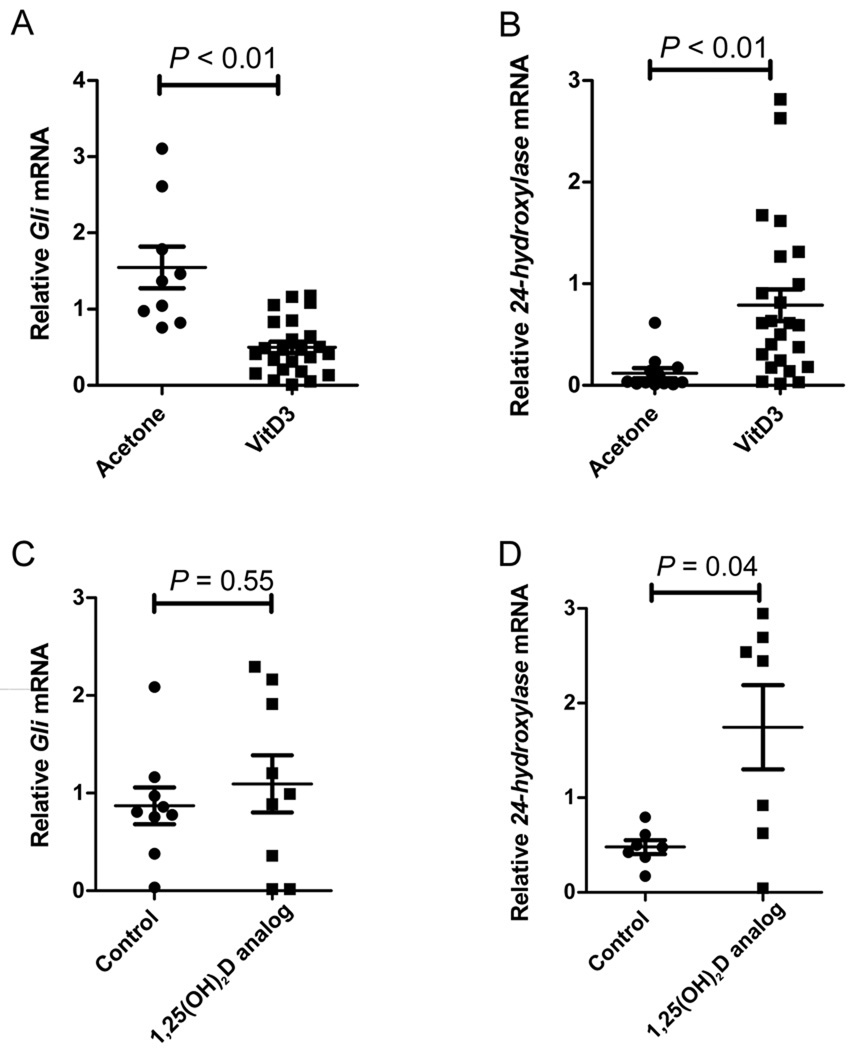
We also treated murine nodular BCCs with topical acetone (n = 9) or with topical vitamin D3 (2.6 mg/kg) (n = 24) daily for 4 days and measured changes in mRNA expression of Gli1 and 24-hydroxylase. Topical vitamin D3 reduced Gli1 mRNA levels (Fig. 4A) and induced significant increases in skin 24-hydroxylase mRNA (Fig. 4B). We found no correlation between suppression of Gli1 mRNA levels and increase of 24-hydroxylase mRNA levels (R = 0.2, P = 0.28), consistent with the idea that vitamin D3 inhibition of HH signaling is independent of VDR. Topical application of a 1,25(OH)2D analog (calcipotriene, Dovonex cream, Leo Pharmaceuticals) used clinically for treatment of psoriasis did not significantly alter Gli1 mRNA but induced significant increases in skin 24-hydroxylase mRNA (Fig. 4C, D). However, calcipotriene cream, similar to topical vitamin D3, also decreases Ki67 staining in BCC tumor cells (1.7 ± 1.0 vs. 2.8 ± 1.0, calcipotriene vs. control cream, respectively, P = 0.02).
Fig. 4.
Vitamin D3 decreases Gli1 and increases 24-hydroxylase in murine BCCs in vivo. (A) Relative mRNA level of Gli1 in total RNA from BCC tumors treated with either acetone (n = 9) or vitamin D3 (2.6 mg/kg) (n = 24) for 4 days. (B) Relative mRNA level of 24-hydroxylase in total RNA from BCC tumors treated with either acetone (n = 12) or vitamin D3 (2.6 mg/kg) (n=24). (C) Relative mRNA level of Gli1 in total RNA from BCC tumors treated with either control cream (n = 9) or 1,25(OH)2D analog cream, calcipotriene (n = 9) for 4 days. (D) Relative mRNA level of 24-hydroxylase in total RNA from BCC tumors treated with either control (n = 7) cream or 1,25(OH)2D analog cream, calcipotriene (n = 7).
Discussion
We have assessed the effects of vitamin D3 on carcinogenesis using BCCs because this is the tumor in which aberrant HH signaling is most clearly the pivotal molecular abnormality. Our findings in BCC tumor cell lines in vitro and in BCC tumors in vivo are highly consistent with findings in non-cancer assays used by Bijlsma and colleagues (29) to identify anti-HH effects of vitamin D3 that are independent of the VDR. They suggest that non-hydroxylated vitamin D3 may have therapeutic potential as an inhibitor of BCC carcinogenesis - as a chemopreventive and/or a chemotherapeutic agent. Others have noted the structural similarity between the Smo inhibitor cyclopamine and sterols such as vitamin D3 (30).
Furthermore, our findings suggest a more complex role for UV radiation in BCC carcinogenesis than has been considered previously. The evidence that sunlight enhances BCC risk is well accepted. In fact, a significant proportion of PTCH1 and p53 gene mutations in human BCCs are of the UVB-signature type (31–33). As an additional mechanism by which sunlight might enhance BCC carcinogenesis, UV radiation is known to be immunosuppressive, and there is some evidence for a role for the immune system in protecting against BCC carcinogenesis (34). However, details of the relationship between sunlight and BCC risk have been confusing, unlike the more straightforward relationship between sunlight and squamous cell carcinoma (SCC) risk. Thus the relative risk for BCCs peaks at a lifetime exposure of 10,000–35,000 hours whereas that for SCCs, which are not known to be driven by HH activation, continues to rise with more hours of sunlight (35, 36). Were UVB not only to stimulate BCC carcinogenesis via mutagenesis and immunosuppression but also to increase cutaneous vitamin D3, thus blocking hedgehog signaling and thereby inhibiting BCC growth, this might provide at least a partial explanation for the less than straightforward relationship between sun exposure and BCC risk. However, other mechanisms also may contribute to the complex relationship. Thus despite our knockdown data, the VDR itself, even without the 1,25(OH)2D ligand may affect BCC carcinogenesis, as illustrated by the development of BCCs in VDR−/− mice treated with DMBA (37). One possible mechanism for this could be the non-1,25(OH)2D dependent effect of VDR on Wnt signaling, which appears to function downstream of HH signaling in BCCs (38, 39). Our data do not exclude possible anti-cancer effects of vitamin D3 acting via the canonical VDR pathway in tumor and/or stromal cells since the topical 1,25(OH)2D analog also decreases Ki67 measured tumor proliferation.
Collectively, our data demonstrate that pharmacologic doses of vitamin D3 can block proliferation of murine BCC cell lines in vitro and block proliferation in BCC tumors in vivo. We do not know the ultimate effect of vitamin D3 on long term tumor proliferation studies or tumor induction, and we do not know the role of endogenous physiologic doses of vitamin D3 in the HH pathway or BCC development. However, our findings suggest a mechanism underlying the latitudinal gradient for cancers of the colon, breast, and prostate and even a non-sunlight approach to their prevention. Indeed, we are testing this possibility in mouse models of visceral cancer as well as extending our murine BCCs studies.
Materials and Methods
Cell lines
Use of the murine BCC cell lines (ASZ, BSZ, and CSZ), the mouse medulloblastoma cell line Med-1, and the immortalized non-tumorigenic murine keratinocyte cell line C5N were as described previously (So et al, 2006, 2008). The ASZ cell line was generated from a nodular BCC tumor from an IR-exposed Ptch1+/− mouse (26). The BSZ and CSZ cell lines were generated from nodular BCCs from Ptch1+/− K14-CreER p53 fl/fl mice treated with tamoxifen to delete p53. A murine medulloblastoma cell line (Med1) was maintained and propagated in 154-CF medium (Cascade Biologics, Portland, OR) and the C5N cell line was generated from murine epidermis and maintained in DME H-21medium (UCSF Cell Culture Facility, San Francisco, CA) (25).
Ptch1+/− K14CreER p53 fl/fl mice
We bred mice with the K14-Cre-ER transgene (40) and mice with a floxed p53 allele (41) with our Ptch1+/− mice to generate Ptch1+/− K14-Cre-ER p53fl/fl mice. We treated these mice intraperitoneally at age 6 weeks with 100 µg/day of tamoxifen for three consecutive days. At 8 weeks of age, Ptch1+/− K14-Cre-ER p53fl/fl mice were exposed to 4 Gy of ionizing radiation (IR). Mice were fed a 1% calcium Teklad Global 18% Protein Rodent Diet 2918 Irradiated, containing 1.5 IU/g Vitamin D3 from Harlan Laboratories (Teklad Diets, Madison WI). Mice were housed in a barrier facility with 12 hour light/dark cycles using fluorescent bulbs emitting undetectable UV.
Vitamin D3 treatments
Vitamin D3, 7DHC, 25(OH)D or 1,25(OH)2D (Sigma Aldrich, St. Louis, MI) were solubilized in 100% ethanol for cell culture experiments. Stocks were stored at −80°C for up to 2 months at 33mM. KAAD-cyclopamine (Toronto Research Chemicals Inc., Toronto, Canada; catalog # K171000) was dissolved in 100% DMSO and stored at −80°C for up to 2 months at 10 mM. For topical treatment of BCC tumors in vivo, 1.3–2.6 mg/kg (equivalent to 1,500–3,000 IU) of vitamin D3 was applied daily to the tumors. Approximately 200 µl of the vitamin D3/acetone solution was applied to visible BCCs on 20 cm2 area of dorsal back skin for 5 days per week for 30 days (1.3 mg/kg for changes in immunohistochemistry studies, Ki67) or 2.6 mg/kg daily for 4 days (for changes in Gli1 mRNA). Commercially available 1,25(OH)2D analog cream (Dovonex, calcipotriene 0.005%) was donated by Leo Pharmaceuticals, Dublin, Ireland.
Cell proliferation assay
Cell numbers were assayed with the WST-1 cell proliferation assay (Roche Applied Science, Indianapolis, IN). 7 × 103 cells were seeded in 96-well plates and cultured overnight to 60–70 % confluency and then serum starved overnight. Cells were incubated with vitamin D3, 7DHC, 1,25(OH)2D or cyclopamine at various concentrations for 48 hours and colorimetric readings were measured at 450 nM on a microplate reader, SpectraMax 340PC (Molecular Devices, Sunnyvale, CA) as previously described (26).
Quantitative PCR for Gli1 and 24hydroxylase mRNA expression
RNA was collected using the PureLink RNA mini kit (Invitrogen, Carlsbad, CA). Reverse transcription was performed using the Taqman reverse transcription kit (Applied Biosystems, Foster City, CA) and qPCR was performed on cDNA using TaqMan premixed primer probes and reagents from Applied Biosystems (Foster City, CA). Ribosomal 18S or GAPDH was used as a normalization control for all experiments.
Adenoviral infection of shRNA to knock-down the vitamin D3 receptor
The target sequences of shRNAs produced from adenoviral constructs are VDR 5’-CCATTGAGGTCATCATGTT-3’and the non-silencing control 5’-TGCGTTGCTAGTACCAACT-3’(42). 1.5 × 105 ASZ cells were seeded in Ti-12.5 flasks and infected 4 hours later at a rate of 100 infectious units/cell and grown for 3 days in 154-CF medium containing 2% chelexed fetal bovine serum, 1X penicillin and streptomycin and 0.05 mM calcium chloride (42). Cells were then serum starved overnight and exposed to vitamin D3 at 5 µM for 24 hours.
Western blotting
Proteins were extracted with Lysis-M reagent (Roche, Mannheim, Germany) and protein concentration was measured by using the micro BCA protein kit (Thermo science Pierce, Rockford, IL). Proteins were resolved using 4–20% pre-cast gradient SDS/PAGE gels (Invitrogen, Carlsbad, CA), semi-dry transferred to PVDF membrane (Millipore, Billerica, MA), blocked with 5% milk-TBST, and hybridized overnight at 4°C by using VDR-specific (1:300) (Santa Cruz Biotech, Santa Cruz, CA) or β-actin (1:15000) (Sigma, St, Louise, MI) polyclonal antibody. After incubation with secondary antibody conjugated with HRP (1:2000) (Cell Signaling, Beverly, MA) for 1 hour at room temperature, signals were detected by ECL using the Supersignal West Femto maximum Sensitivity Substrate Kit (Thermo Scientific Pierce, Rockford, IL). Relative protein levels were quantified using ImageJ software.
B-gal staining and immunohistochemistry
LacZ-encoded bacterial β-galactosidase was detected by incubation of glutaraldehyde and formalin fixed tissue with X-gal (Roche Applied Science, Indianapolis, IN) (43). Mouse tissues were fixed in 10% buffered formalin, embedded in paraffin, and cut into 5 um sections. Sections were deparaffinized in xylene and antigen retrieval was carried out in Trilogy solution (Cell Marque, Rocklin, CA, USA) by heating. Endogenous peroxidase was blocked with 3% hydrogen peroxide and endogenous biotin was blocked by Avidin Biotin blocking system (Vector Laboratories, Burlingame, CA, USA). Sections were also blocked with normal Goat Serum (Vector Laboratories, Burlingame, CA, USA). Biotinylated goat anti rabbit (Vector Laboratories, Burlingame, CA, USA) was used to detect the primary antibody and was followed by incubation with the Vectastain ABC kit (Vector Laboratories, Burlingame, CA, USA) and visualized with liquid DAB and substrate chromogen system (Dako, Carpinteria, CA, USA). Antibodies used included control rabbit immunoglobulin (1:250 dilution) and rabbit anti mouse polyclonal antibody against K10 (1:500 dilution) (Covance, Princeton, NJ, USA), rabbit anti mouse polyclonal antibody against K14 (1:2000 dilution) (Covance, Princeton, NJ, USA), rabbit anti mouse polyclonal antibody against cleaved caspase-3 (CC3) (1:800 dilution) (Pharmingen, San Diego, CA) (overnight at 4°C) and rabbit anti mouse polyclonal antibody against Ki67 (1:400) (Thermo Scientific, Waltham, MA, USA) (60 minutes at room temperature). Ki67 staining per tumor was ranked from lowest to highest (0–4) by two independent readers blinded to the treatment group.
Statistical analysis
Nonparametric t-test’s and Spearman’s correlation test were used to compare the difference in mean values and correlations of Gli1 and 24-hydroxylase mRNA levels, respectively. All P values reported are two-sided.
Acknowledgements
We thank Allan Balmain for the C5N cell line, Pierre Chambon and Anton Berns for supplying the K14-CreER2 and p53 fl alleles, and Loretta Chan for her assistance with the hematoxylin–eosin staining and immunohistochemistry staining. This work was supported by the National Institute of Health [CA81888 and CN-95116 to E.E] and the NIH/NCRR/OD UCSF-CTSI KL2 RR024130, NIH/NIAMS K23 AR056736-01 and Prevent Cancer Foundation to J.T.
Abbreviations
- HH
Hedgehog
- BCC
Basal cell carcinoma
- VDR
vitamin D receptor
Footnotes
Conflict of Interest
The authors state no conflict of interest
References
- 1.Apperly F. The relation of solar radiation to cancer mortality in North America. Cancer Res. 1941;1:191–195. doi: 10.1158/0008-5472.CAN-15-3169. [DOI] [PubMed] [Google Scholar]
- 2.Garland F, Garland C, Gorham E, Young J. Geographic variation in breast cancer mortality in the United States: a hypothesis involving exposure to solar radiation. Prev Med. 1990;19:614–622. doi: 10.1016/0091-7435(90)90058-r. [DOI] [PubMed] [Google Scholar]
- 3.Garland C, Garland F, Gorham E, et al. The role of vitamin D in cancer prevention. Am J Public Health. 2006;96:252–261. doi: 10.2105/AJPH.2004.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wactawski-Wende J, Kotchen JM, Anderson GL, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354:684–696. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- 5.Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85:1586–1591. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 6.Garland C, Shekelle RB, Barrett-Connor E, Criqui MH, Rossof AH, Paul O. Dietary vitamin D and calcium and risk of colorectal cancer: a 19-year prospective study in men. Lancet. 1985;1:307–309. doi: 10.1016/s0140-6736(85)91082-7. [DOI] [PubMed] [Google Scholar]
- 7.Mokady E, Schwartz B, Shany S, Lamprecht SA. A protective role of dietary vitamin D3 in rat colon carcinogenesis. Nutr Cancer. 2000;38:65–73. doi: 10.1207/S15327914NC381_10. [DOI] [PubMed] [Google Scholar]
- 8.Spina CS, Tangpricha V, Uskokovic M, Adorinic L, Maehr H, Holick MF. Vitamin D and cancer. Anticancer Res. 2006;26:2515–2524. [PubMed] [Google Scholar]
- 9.Bikle DD. Vitamin D receptor, UVR, and skin cancer: a potential protective mechanism. J Invest Dermatol. 2008;128:2357–2361. doi: 10.1038/jid.2008.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holick MF. Calcium plus vitamin D and the risk of colorectal cancer. N Engl J Med. 2006;354:2287–2288. doi: 10.1056/NEJMc060753. author reply -8. [DOI] [PubMed] [Google Scholar]
- 11.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 12.Bijlsma MF, Spek CA, Zivkovic D, van de Water S, Rezaee F, Peppelenbosch MP. Repression of Smoothened by Patched-Dependent (Pro-)Vitamin D3 Secretion. PLoS Biol. 2006;4:1397–1410. doi: 10.1371/journal.pbio.0040232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nolan-Stevaux O, Lau J, Truitt M, et al. GLI1 is regulated through Smoothened-independent mechanisms in neoplastic pancreatic ducts and mediates PDAC cell survival and transformation. Genes Dev. 2009;23:24–36. doi: 10.1101/gad.1753809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Von Hoff DD, LoRusso PM, Rudin CM, et al. Inhibition of the Hedgehog Pathway in Advanced Basal-Cell Carcinoma. N Engl J Med. 2009;361:1164–1172. doi: 10.1056/NEJMoa0905360. [DOI] [PubMed] [Google Scholar]
- 15.Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol. 1994;30:774–778. doi: 10.1016/s0190-9622(08)81509-5. [DOI] [PubMed] [Google Scholar]
- 16.Aszterbaum M, Epstein J, Oro A, et al. Ultraviolet and ionizing radiation enhance the growth of BCCs and trichoblastomas in patched heterozygous knockout mice. Nat Med. 1999;5:1285–1291. doi: 10.1038/15242. [DOI] [PubMed] [Google Scholar]
- 17.Hahn H, Wicking C, Zaphiropoulous PG, et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85:841–851. doi: 10.1016/s0092-8674(00)81268-4. [DOI] [PubMed] [Google Scholar]
- 18.Johnson RL, Rothman AL, Xie J, et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668–1671. doi: 10.1126/science.272.5268.1668. [DOI] [PubMed] [Google Scholar]
- 19.Epstein E. Basal cell carcinomas: attack of the hedgehog. Nature Reviews Cancer. 2008;8:743–754. doi: 10.1038/nrc2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacob L, Lum L. Hedgehog signaling pathway. Sci STKE. 2007;2007:cm6. doi: 10.1126/stke.4072007cm6. [DOI] [PubMed] [Google Scholar]
- 21.Tang JY, So PL, Epstein EH., Jr Novel Hedgehog pathway targets against basal cell carcinoma. Toxicol Appl Pharmacol. 2007;224:257–264. doi: 10.1016/j.taap.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Epstein EH. Basal Cell Carcinoma-Attack of the Hedgehog. Nature Reviews Cancer. 2008 doi: 10.1038/nrc2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, McMahon AP, Allen BL. Shifting paradigms in Hedgehog signaling. Curr Opin Cell Biol. 2007;19:159–165. doi: 10.1016/j.ceb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Rady P, Scinicariello F, Wagner RF, Jr, Tyring SK. p53 mutations in basal cell carcinomas. Cancer Res. 1992;52:3804–3806. [PubMed] [Google Scholar]
- 25.So PL, Fujimoto MA, Epstein EH., Jr Pharmacologic retinoid signaling and physiologic retinoic acid receptor signaling inhibit basal cell carcinoma tumorigenesis. Mol Cancer Ther. 2008;7:1275–1284. doi: 10.1158/1535-7163.MCT-07-2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.So PL, Langston AW, Daniallinia N, et al. Long-term establishment, characterization and manipulation of cell lines from mouse basal cell carcinoma tumors. Exp Dermatol. 2006;15:742–750. doi: 10.1111/j.1600-0625.2006.00465.x. [DOI] [PubMed] [Google Scholar]
- 27.Pillai S, Bikle DD. Adenosine triphosphate stimulates phosphoinositide metabolism, mobilizes intracellular calcium, and inhibits terminal differentiation of human epidermal keratinocytes. J Clin Invest. 1992;90:42–51. doi: 10.1172/JCI115854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bikle DD. Vitamin D regulated keratinocyte differentiation. J Cell Biochem. 2004;92:436–444. doi: 10.1002/jcb.20095. [DOI] [PubMed] [Google Scholar]
- 29.Bijlsma MF, Peppelenbosch MP, Spek CA. (Pro-)vitamin D as treatment option for hedgehog-related malignancies. Med Hypotheses. 2008;70:202–203. doi: 10.1016/j.mehy.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Corcoran RB, Scott MP. Oxysterols stimulate Sonic hedgehog signal transduction and proliferation of medulloblastoma cells. Proc Natl Acad Sci U S A. 2006;103:8408–8413. doi: 10.1073/pnas.0602852103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aszterbaum M, Rothman A, Johnson RL, et al. Identification of mutations in the human PATCHED gene in sporadic basal cell carcinomas and in patients with the basal cell nevus syndrome. J Invest Dermatol. 1998;110:885–888. doi: 10.1046/j.1523-1747.1998.00222.x. [DOI] [PubMed] [Google Scholar]
- 32.Reifenberger J, Wolter M, Knobbe CB, et al. Somatic mutations in the PTCH, SMOH, SUFUH and TP53 genes in sporadic basal cell carcinomas. Br J Dermatol. 2005;152:43–51. doi: 10.1111/j.1365-2133.2005.06353.x. [DOI] [PubMed] [Google Scholar]
- 33.Ling G, Ahmadian A, Persson A, et al. PATCHED and p53 gene alterations in sporadic and hereditary basal cell cancer. Oncogene. 2001;20:7770–7778. doi: 10.1038/sj.onc.1204946. [DOI] [PubMed] [Google Scholar]
- 34.Vogt A, Chuang PT, Hebert J, et al. Immunoprevention of basal cell carcinomas with recombinant hedgehog-interacting protein. J Exp Med. 2004;199:753–761. doi: 10.1084/jem.20031190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kricker A, Armstrong BK, English DR, Heenan PJ. Does intermittent sun exposure cause basal cell carcinoma? A case-control study in western Australia. Int J Cancer. 1995;60:489–494. doi: 10.1002/ijc.2910600411. [DOI] [PubMed] [Google Scholar]
- 36.Rosso S, Zanetti R, Martinez C, et al. The multicentre south European study 'Helios' II: different sun exposure patterns in the aetiology of basal cell and squamous cell carcinomas of the skin. British Journal of Cancer. 1996;73:1447–1454. doi: 10.1038/bjc.1996.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zinser GM, Sundberg JP, Welsh J. Vitamin D(3) receptor ablation sensitizes skin to chemically induced tumorigenesis. Carcinogenesis. 2002;23:2103–2109. doi: 10.1093/carcin/23.12.2103. [DOI] [PubMed] [Google Scholar]
- 38.Palmer HG, Anjos-Afonso F, Carmeliet G, Takeda H, Watt FM. The Vitamin D Receptor Is a Wnt Effector that Controls Hair Follicle Differentiation and Specifies Tumor Type in Adult Epidermis. PLoS ONE. 2008;3:e1483. doi: 10.1371/journal.pone.0001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang S, Andl T, Grachtchouk V, et al. Pathological responses to oncogenic Hedgehog signaling in skin are dependent on canonical Wnt/[beta]-catenin signaling. Nat Genet. 2008;40:1130–1135. doi: 10.1038/ng.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Metzger D, Li M, Chambon P. Targeted somatic mutagenesis in the mouse epidermis. Methods Mol Biol. 2005;289:329–340. doi: 10.1385/1-59259-830-7:329. [DOI] [PubMed] [Google Scholar]
- 41.Jonkers J, Meuwissen R, van der Gulden H, Peterse H, van der Valk M, Berns A. Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat Genet. 2001;29:418–425. doi: 10.1038/ng747. [DOI] [PubMed] [Google Scholar]
- 42.Hawker NP, Pennypacker SD, Chang SM, Bikle DD. Regulation of human epidermal keratinocyte differentiation by the vitamin D receptor and its coactivators DRIP205, SRC2, and SRC3. J Invest Dermatol. 2007;127:874–880. doi: 10.1038/sj.jid.5700624. [DOI] [PubMed] [Google Scholar]
- 43.So PL, Lee K, Hebert J, et al. Topical tazarotene chemoprevention reduces Basal cell carcinoma number and size in Ptch1+/− mice exposed to ultraviolet or ionizing radiation. Cancer Res. 2004;64:4385–4389. doi: 10.1158/0008-5472.CAN-03-1927. [DOI] [PubMed] [Google Scholar]