Abstract
Inflammation contributes to the development of papillomas and squamous cell carcinomas in the well established DMBA/TPA model of skin carcinogenesis. Synthetic oligonucleotides (ODN) containing repetitive TTAGGG motifs have been shown to block deleterious inflammatory reactions in murine models of autoimmunity, pneumonitis and shock. This work examines whether treatment with suppressive (Sup) ODN can interfere with DMBA/TPA induced inflammation, thereby reducing papilloma formation. Results indicate that Sup ODN block TPA-dependent skin hyperplasia, edema and leukocytic infiltration. Sup ODN also inhibit the up-regulation of genes encoding pro-oncogenic chemokines and other markers of inflammation including CXCL2, CCL2, COX-2 and ODC. Of greatest import, Sup ODN reduce papilloma formation in a dose and sequence dependent manner. These findings suggest that Sup ODN may provide a novel means of preventing inflammation and associated oncogenesis.
Keywords: suppressive oligonucleotide, inflammation, prevention, tumorigenesis
Introduction
Inflammatory processes are associated with the development and/or progression of nearly one in five cancers (1). Ongoing inflammation has pleomorphic effects, including aiding in the proliferation and survival of malignant cells, promoting angiogenesis, facilitating metastasis, and subverting the host’s anti-tumor response. Limited data suggest that treatment with anti-inflammatory agents may reduce host susceptibility to cancer (1, 2).
One approach to down-regulating pro-inflammatory responses is through the use of immunosuppressive oligonucleotides (Sup ODN). These ODN express repetitive TTAGGG motifs patterned after the immunosuppressive domains present in mammalian telomeres (3). Previous studies established that Sup ODN can down-regulate injurious inflammatory reactions in diseases including arthritis, lupus, toxic shock and silicosis (3–8).
This work utilizes a well-established murine skin cancer model (9) to examine whether Sup ODN can block the inflammation associated with carcinogenesis. In this system, the “initiator” (DMBA) induces oncogenic transformation while the “promoter” (TPA) drives inflammation and tumorigenesis (9). Papillomas begin to appear 2–3 months after weekly TPA treatment, and a fraction of these undergo malignant transformation to form squamous cell carcinomas. This murine model shares important features of human multi-stage carcinogenesis, including well defined oncogenic changes at the cellular level and the necessity for inflammation to support papilloma and subsequent carcinoma development (9). Early markers of disease in this model include the up-regulation of pro-inflammatory cytokines and chemokines (including CXCL-2 and CCL-2). Carcinogenic transformation is preceded by the up-regulation of ODC and COX-2 (10, 11). Thus, one approach to determining the effect of novel therapies is by monitored changes in the activation state of these genes. Results from this work indicate that Sup ODN reduce TPA-dependent inflammation, and that this is associated with significantly decreased papilloma development.
Materials and Methods
Reagents
Phosphorothioate oligodeoxynucleotides (ODN) were synthesized at the CBER core facility (Bethesda, MD). The sequence of suppressive ODN A151 was ‘TTAGGGTTAGGGTTAGGGTTAGGG’ and of control ODN 1612 was ‘GCTAGATGTTAGCGT’. Previous studies showed that the effect of suppressive ODN was sequence but not length dependent, and that the length of the control ODN did not impact their activity (3). 7,12-dimethyl benz(a)anthracene (DMBA) and 12-O-tetradecanoyl-phorbal acetate (TPA) were obtained from Sigma Aldrich (St. Louis, MO).
Animals
Female CD-1 mice (5–6 weeks old) were obtained from the Charles River Laboratories (Frederick, MD) and acclimatized for 1 week prior to use. All experiments were conducted under Animal Care and Use Committee approved protocols.
Induction of skin papillomas
A 2 stage skin carcinogenesis protocol was followed (9). Briefly, hair was shaved from the dorsum of the mice. Only those animals in the resting phase of their hair cycle were used in this study. Tumor induction was initiated by topical application of 50 ug of 7,12-dimethylbenzanthracene (DMBA) in 200 ul of acetone. Tumor growth was promoted by weekly topical applications of 2.5 ug tetradecanoyl phorbol acetate (TPA) in 200 ul of acetone starting 2 wk after DMBA and continuing for 16 wk. ODN were also administered topically in acetone. To insure the absorption of topically administered agents, the stratum corneum was removed by “stripping” skin sites 5 times with Scotch Magic Tape 810 (3M, St. Paul, MN) immediately before application.
Analysis of TPA induced inflammation
CD-1 mice were shaved and treated with 2.5 ug TPA in 200 ul acetone ± 30 ug of ODN as described above. The animals were sacrificed 8 – 18 hr later. 8 mm punch biopsies were removed, weighed, embedded in paraffin, and stained. Infiltrating leukocytes were identified on sections stained with anti-myeloperoxidase Abs. Sections were analyzed using an Olympus IX50 microscope fitted with a digital camera. Cell numbers were determined by counting using Image J software in 3 randomly selected sites/slide and the results analyzed statistically.
Measurement of chemokine expression by RT-PCR
Skin biopsies were homogenized in TRIzol reagent (Invitrogen; Carlsbad, CA) and mRNA extracted using an RNeasy Minikit (Qiagen; Valencia, CA). Reverse transcription was performed using a QuantiTect Reverse Transcription Kit (Qiagen). ODC, COX-2 and chemokine mRNA levels were examined using the Applied Biosystems StepOne real time PCR system, in which primers obtained from the Gene Expression Assay set (Applied Biosystems, Foster City, CA) were amplified using the TaqMan Gene Expression Master Mix kit. Chemokine mRNA expression levels were then calculated by step-one software (Applied Biosystems) after correction for GAPDH expression independently for each sample.
Statistical analysis
All in vivo studies involving papilloma development used a minimum of 10 mice/group. Statistical significance was determined using Student’s T test and one-way analysis of variance followed by Scheffe's F test. A p value of less than 0.05 was considered to represent a statistically significant difference between group means.
Results
Suppressive ODN reduce TPA-induced skin inflammation
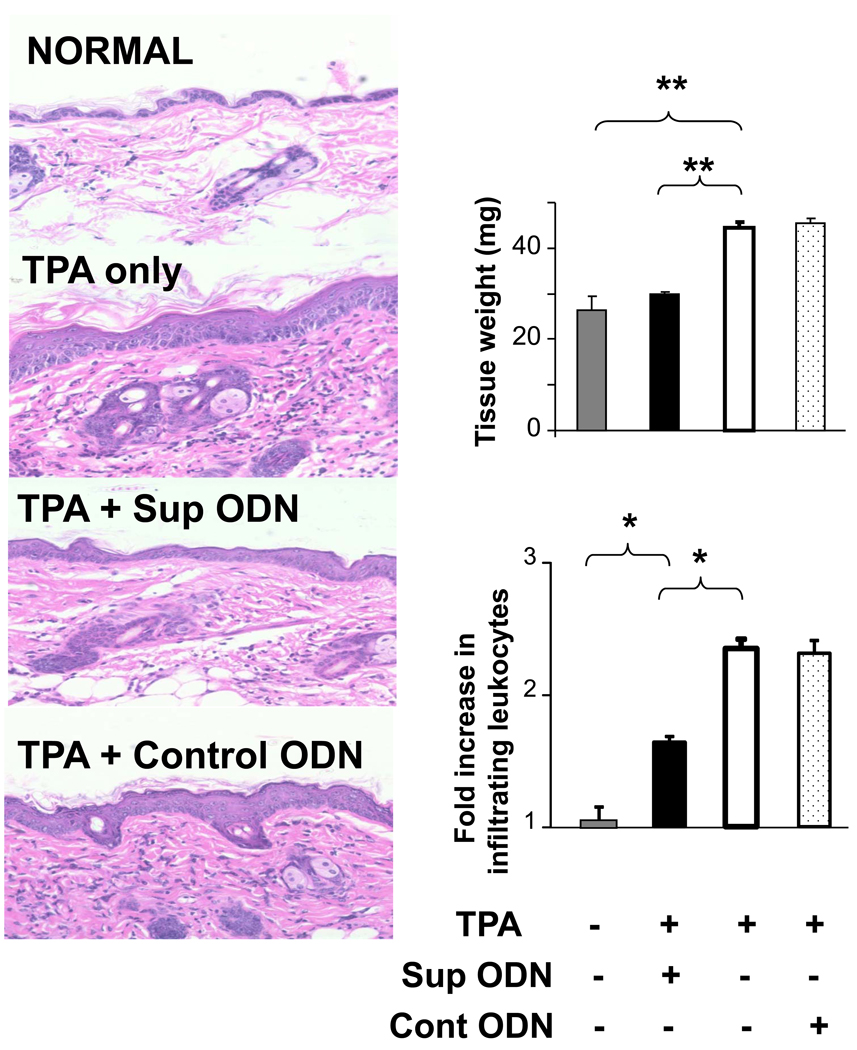
TPA administration causes the rapid onset of cutaneous edema accompanied by significant leukocyte infiltration (9, 12). To assess whether Sup ODN can block TPA dependent inflammation, areas of skin to which TPA had been administered were treated with Sup or control ODN. These skin sections were then biopsied and examined histologically. As seen in Fig 1, both the edema and cellular infiltration induced by TPA were significantly reduced by Sup ODN treatment (p < .05). These effects were sequence specific, as control and CpG ODN had no effect (Fig 1 and data not shown).
Figure 1. Effect of Sup ODN on TPA-induced skin inflammation.
CD-1 mice were treated topically with 2.5 ug of TPA ± 50 ug of ODN. Skin biopsies were taken 16 hr later and weighed (to assess skin edema). H&E stained tissue sections were used to measure of skin thickness (left panels) while the fold difference in the number of infiltrating leukocytes was determined in 3 high-powered fields/specimen using Image J software. Results reflect the mean ± SE from 3 independent experiments involving 5 independent mice/group/experiment. Note that TPA Rx also induced epidermal hyperplasia.
**; p < .01, *; p < .05,
Suppressive ODN prevent the development of DMBA/TPA-induced papillomas
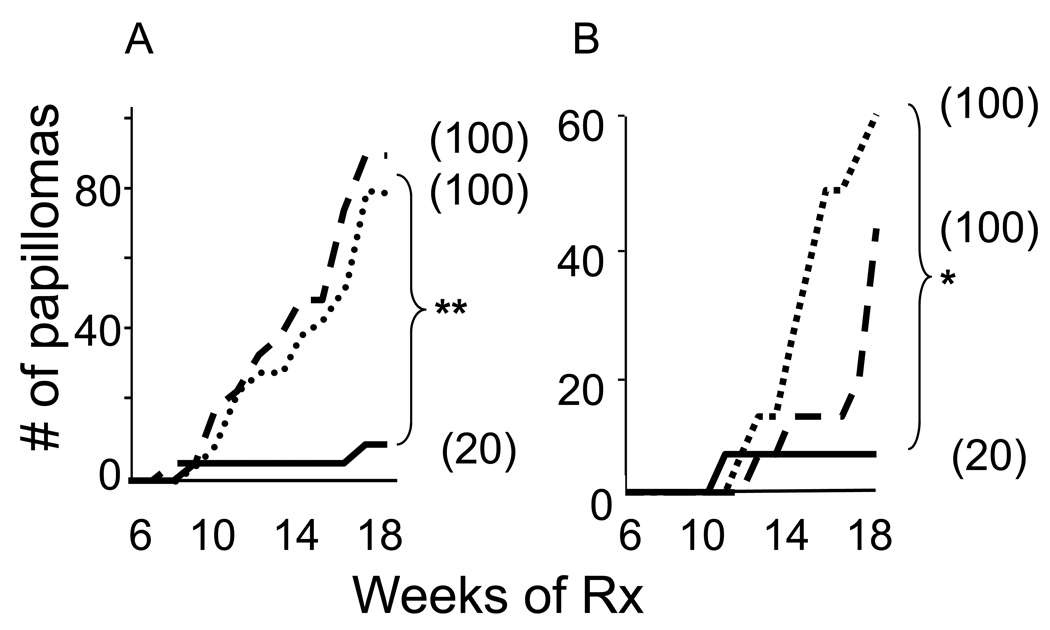
Papillomas were elicited by painting the skin of 7 wk old CD1 mice once with DMBA and then weekly for 4 months with TPA. Papillomas first arose after .10 wk and increased in frequency and size thereafter (Fig 2A).
Figure 2. Effect of immunomodulatory ODN on DMBA/TPA induced papillomas.
CD-1 mice (N = 10/group) were treated at 7 wk of age with 50 ug of DMBA followed by weekly topical applications of 2.5 ug of TPA for 4 months. A) The effect of co-administering 50 ug of control (dashed line) or suppressive (solid line) ODN vs TPA alone (dotted line) on the cumulative number of papillomas is shown. B) The effect of co-administering 50 ug (solid line), 5 ug (dashed line) or 1 ug (dotted line) of suppressive ODN with TPA is shown. In both panels, the percent of mice/group that developed papillomas is shown in parenthesis.
**; p < .01, *; p < .05.
To determine whether the reduction in inflammation found after Sup ODN treatment was associated with decreased papilloma development, ODN were co-administered topically with TPA. Both the number of mice that developed papillomas and the number of papillomas/animal were significantly reduced when Sup ODN was co-administered with TPA (p <.01, Fig 2A). The same effect was observed when 300 ug of Sup OD was administered by i.p. injection within 1 hr of TPA treatment (Supplemental Fig 1). In contrast, control ODN had no impact on papilloma formation (Fig 2A).
Dose-response studies were performed to verify the observation that Sup ODN protected mice from papilloma development. As seen in Fig 2B, the highest dose of ODN administered (50 ug) significantly reduced the number of papillomas when compared to the lowest dose (1 ug, p = 0.027), while the intermediate dose (5 ug) had a detectable but more modest impact on papilloma frequency.
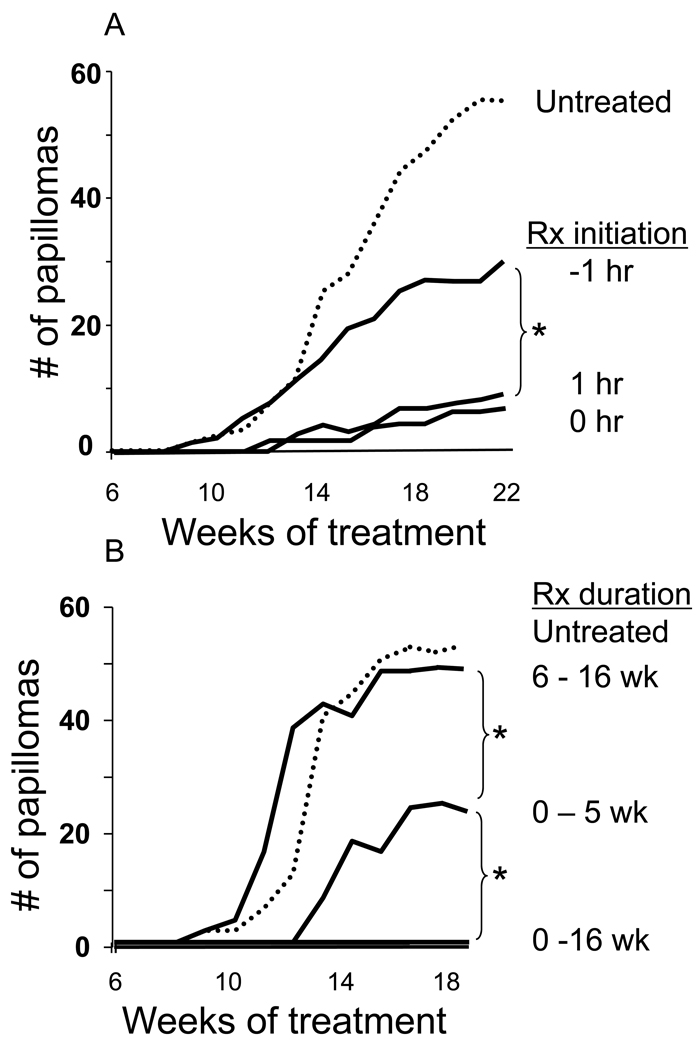
Additional parameters were examined to clarify the conditions under which Sup ODN inhibited papilloma development. Local administration of Sup ODN was highly effective when delivered with or after TPA administration (p <.01, Fig 3A). The efficacy of Sup ODN was significantly reduced when administered prior to TPA, consistent with previous findings showing that Sup ODN have their greatest effect on activated cells (13). The initiation and duration of therapy also impacted efficacy. Uninterrupted treatment with Sup ODN throughout the period of TPA administration was most effective, reducing papilloma incidence by 90 – 100% (Fig 3). When Sup ODN delivery was discontinued after 5 wk, the onset of papilloma formation was significantly delayed (p < .01) but then began to rise. In contrast, no benefit was observed when the effects of TPA were unopposed by Sup ODN therapy for 6 wk (Fig 3B)
Figure 3. The timing and duration of Sup ODN administration effects papilloma development.
CD-1 mice were treated with DMBA and TPA (dotted line) as described in Fig 2. A) 50 ug of Sup ODN was administered before, after, or simultaneous with TPA. B) Mice were treated with 50 ug of Sup ODN for 0–5 wk, 6–16 wk or 0–16 wk. Data show the cumulative number of papillomas in 10 mice/group.
*; p < .05.
Effect of suppressive ODN on markers of inflammation and carcinogenesis
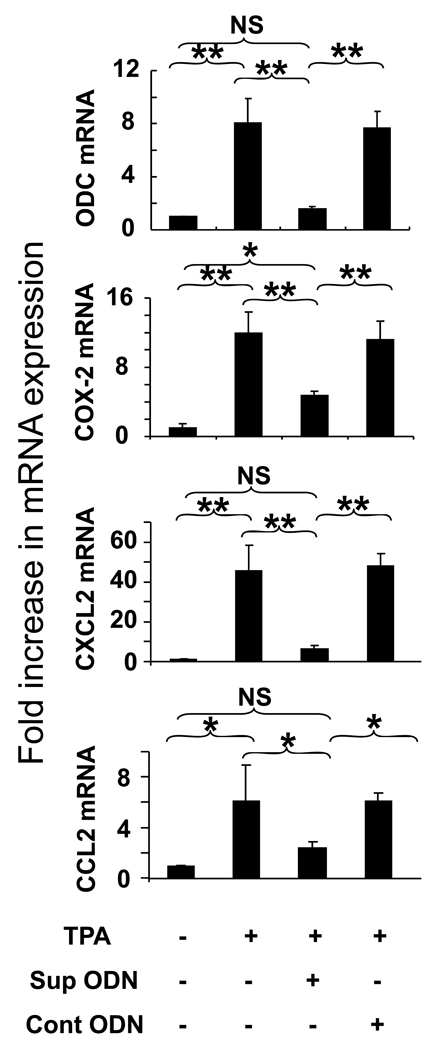
TPA reportedly triggers the up-regulation of mRNA encoding proteins that promote tumor formation (such as ornithine decarboxylase (ODC) and prostaglandin endoperoxide synthase 2 (COX-2) (14, 15) as well as chemokines that promote inflammation (such as CXCL2 and CCL2) (16). Consistent with previous findings, the level of mRNA encoding ODC, COX-2, CXCL2 and CCL2 all rose by >6-fold (p < .05) at sites treated with TPA (Fig 4). The effect of Sup ODN on this TPA dependent change in mRNA expression was monitored by QT-PCR. As seen in Fig 4, Sup ODN significantly reduced the expression of mediators associated with inflammation and carcinogenesis. This activity was sequence specific, as control ODN had no effect.
Figure 4. Effect of Sup ODN on TPA-induced mRNA up-regulation.
Full thickness skin biopsies were taken 8 hr after the topical administration of 2.5 ug of TPA ± 50 ug of ODN. QT-PCR was performed on mRNA isolated from 4 independent samples/treatment group. mRNA levels of the inflammatory mediators ornithine decarboxylase (ODC) and prostaglandin-endoperoxide synthase 2 (COX-2) mRNA and the inflammatory chemokines CXCL2 and CCL2 were monitored. Data show the relative changes in mRNA levels as determined by RT-PCR after correction for the housekeeping gene GAPDH. Results reflect the mean ± SE from 3 independent experiments involving 4 independently studied tissue sections/group/experiment.
**; p < .01, *; p < .05, NS; no significant difference.
Discussion
There is considerable evidence that inflammation can contribute to the initiation and/or progression of cancer. Animal models show that mutagenized cells are better able to survive, proliferate and metastasize when placed in an inflammatory environment (2) while epidemiologic studies show that the risk of developing cancer is significantly increased by chronic infection and/or inflammation (1). Mechanistically, inflammation has been shown to enhance angiogenesis, reduce tumor specific immunity and limit the activity of chemotherapy and radiation therapy (17, 18).
Carcinogenesis in humans is a multi-stage process in which cells exposed to mutagens are subsequently stimulated to grow (and avoid physiologic control mechanisms) through interactions with their environment (9). A murine model that recapitulates important features of this multi-stage process utilizes DMBA to induce mutations in the Ras oncogene followed by TPA to promote inflammation and thus drive papilloma formation (9). This work examines the ability of immunosuppressive oligonucleotides to block TPA-induced inflammation and interrupt the carcinogenic process (manifest by a significant reduction in papilloma development). The Sup ODN used in this work contain 4 repeats of the suppressive TTAGGG motif present in mammalian telomeres (3). Previous studies showed that Sup ODN with 2 – 4 such motifs could prevent or delay both autoimmune and inflammatory diseases including lupus, arthritis, EAE, toxic shock, and silicosis (3–8). Those effects were linked to the ability of Sup ODN to inhibit the ongoing activation of immune cells and concomitantly reduce the production of pro-inflammatory cytokines and chemokines (13, 19).
Delivering Sup ODN throughout the period of TPA administration provided significant protection against papilloma development (p <.01, Figs 2,3). ODN treatment was effective when delivered with, or after, TPA-induced inflammation, consistent with earlier studies showing that Sup ODN activity was maximal in the face of ongoing inflammation (13). This protection was dose dependent and sequence specific, as no protection was conferred by control ODN lacking the TTAGGG motif (Figs 1,2,4). Optimal protection required that Sup ODN be administered throughout the 16 wk period of TPA exposure (Fig 3B). Discontinuing Sup ODN therapy after 5 wk significantly delayed the onset but did not prevent the eventual development of papillomas (Fig 3). Protection was not observed when treatment with Sup ODN was delayed until after TPA had been administered for 6 wk. These outcomes are consistent with those observed in studies of murine lupus: early and continuous treatment with Sup ODN was most effective, starting and then discontinuing therapy slowed but did not prevent disease, while initiating therapy late had little effect on disease progression (4). Although the current study does not document a causative link between inflammation and tumor development, the results are consistent with a model in which repeated TPA administration maintains an inflammatory milieu that drives papilloma formation. Of interest, both processes were blocked by continuous Sup ODN treatment. In contrast, Sup ODN therapy was ineffective if delivered with DMBA (data not shown), suggesting that Sup ODN has no impact of tumor initiation.
To better understand the mechanism underlying these effects, the impact of Sup ODN on TPA-induced chemokine production was examined. Mice treated with TPA develop an inflammatory response characterized by skin edema, epithelial hyperplasia, and leukocyte infiltration (20). The pro-inflammatory cytokines CXCL2 AND CCL2 help mediate these events and are linked to TPA-induced neutrophilic infiltration and papilloma development (21–23). Co-administering Sup ODN with TPA significantly (p < .05) reduced the expression of mRNA encoding these proteins, an effect both dose dependent and sequence specific (Fig 4 and data not shown).
If TPA administration is extended beyond 20 weeks, a fraction of the papillomas develop into squamous cell carcinomas (SCC) (9). This process is preceded by a significant increase in the expression of ODC and COX-2 (10, 24). ODC helps regulate epithelial cell proliferation and provides an excellent marker of neoplastic transformation and tumor growth (10, 25, 26). COX-2 is also associated with the appearance of skin tumors (11, 27) and the over-expression of COX-2 correlates with enhanced tumor invasiveness, increased angiogenesis, and anti-apoptotic cellular responses via the production of PGE2 (11, 27, 28). While the conversion of papillomas to SCC was not monitored in the current study, it is intriguing to note that Sup ODN significantly reduced the level of ODC and COX-2 mRNA in TPA treated skin (p < .01, Fig 4).
Epidemiologic studies indicate that prolonged use of anti-inflammatory agents (such as aspirin and COX-2 inhibitors) may reduce the risk of colon, lung, esophageal and stomach cancer (17, 29, 30). Murine models confirm that chronic administration of certain anti-inflammatory agents can decrease the frequency of chemically induced colon, bladder, lung and skin cancer (31–37). This study is the first to demonstrate that a novel class of oligonucleotides designed to selectively inhibit immune activation also prevent the development of inflammation-dependent cancer. These Sup ODN are patterned after and recapitulate the anti-inflammatory activity of normal human telomeric DNA, and thus are safe when administered repeatedly to mice and non-human primates (reviewed in (38)). Ongoing studies are directed towards determining the impact of Sup ODN in additional models of inflammation-induced cancer and clarify whether there is a causative link between their effect on inflammation and carcinogenesis.
Supplementary Material
CD-1 mice were treated at 7 wk of age with 50 ug of DMBA followed by weekly topical applications of 2.5 ug of TPA for 4 months (dotted line). Suppressive ODN was administered weekly either locally (50 ug, solid line) or systemically (300 ug via i.p. injection, dashed line) at the time of TPA administration. Data show the cumulative number of papillomas in 10 mice/group.
**; p < .01.
Abbreviations
- DMBA
7,12-dimethylbenzanthracene
- ODN
oligodeoxynucleotide
- Sup
suppressive
- TPA
tetradecanoyl phorbol acetate
Footnotes
Conflict of interest: Dr. Klinman and members of his lab hold or have applied for patents concerning the activity of Suppressive ODN, including their use in preventing tumorigenesis. The rights to all such patents have been transferred to the US government.
Reference List
- 1.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 2.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gursel I, Gursel M, Yamada H, Ishii KJ, Takeshita F, Klinman DM. Repetitive elements in mammalian telomeres suppress bacterial DNA-induced immune activation. J Immunol. 2003;171:1393–1400. doi: 10.4049/jimmunol.171.3.1393. [DOI] [PubMed] [Google Scholar]
- 4.Dong I, Ito S, Ishii K, Klinman D. Suppressive Oligodeoxynucleotides Delay the Onset of Glomerulonephritis and Prolong the Survival of Lupus-prone NZB/W Mice. Arthritis Rheum. 2004;52:651–658. doi: 10.1002/art.20810. [DOI] [PubMed] [Google Scholar]
- 5.Zeuner RA, Ishii KJ, Lizak MJ, Gursel I, Yamada H, Klinman DM, Verthelyi D. Reduction of GpG-induced arthritis by suppressive oligodeoxynucleotides. Arthritis Rheum. 2002;46:2219–2224. doi: 10.1002/art.10423. [DOI] [PubMed] [Google Scholar]
- 6.Zeuner RA, Verthelyi D, Gursel M, Ishii KJ, Klinman DM. Influence of stimulatory and suppressive DNA motifs on host susceptibility to inflammatory arthritis. Arthritis Rheum. 2003;48:1701–1707. doi: 10.1002/art.11035. [DOI] [PubMed] [Google Scholar]
- 7.Shirota H, Gursel M, Klinman DM. Suppressive oligodeoxynucleotides inhibit Th1 differentiation by blocking IFN-gamma- and IL-12-mediated signaling. J.Immunol. 2004;173:5002–5007. doi: 10.4049/jimmunol.173.8.5002. [DOI] [PubMed] [Google Scholar]
- 8.Shirota H, Gursel I, Gursel M, Klinman DM. Suppressive oligodeoxynucleotides protect mice from lethal endotoxic shock. J Immunol. 2005;174:4579–4583. doi: 10.4049/jimmunol.174.8.4579. [DOI] [PubMed] [Google Scholar]
- 9.Abel EL, Angel JM, Kiguchi K, DiGiovanni J. Multi-stage chemical carcinogenesis in mouse skin: fundamentals and applications. Nat. Protoc. 2009;4:1350–1362. doi: 10.1038/nprot.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Auvinen M. Cell transformation, invasion, and angiogenesis: a regulatory role for ornithine decarboxylase and polyamines? J Natl.Cancer Inst. 1997;89:533–537. doi: 10.1093/jnci/89.8.533. [DOI] [PubMed] [Google Scholar]
- 11.Prescott SM, Fitzpatrick FA. Cyclooxygenase-2 and carcinogenesis. Biochim.Biophys.Acta. 2000;1470:M69–M78. doi: 10.1016/s0304-419x(00)00006-8. [DOI] [PubMed] [Google Scholar]
- 12.Katiyar SK, Rupp CO, Korman NJ, Agarwal R, Mukhtar H. Inhibition of 12-O-tetradecanoylphorbol-13-acetate and other skin tumor-promoter-caused induction of epidermal interleukin-1 alpha mRNA and protein expression in SENCAR mice by green tea polyphenols. J Invest Dermatol. 1995;105:394–398. doi: 10.1111/1523-1747.ep12321030. [DOI] [PubMed] [Google Scholar]
- 13.Sato T, Shimosato T, Alvord WG, Klinman DM. Suppressive oligodeoxynucleotides inhibit silica-induced pulmonary inflammation. J.Immunol. 2008;180:7648–7654. doi: 10.4049/jimmunol.180.11.7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scholz K, Furstenberger G, Muller-Decker K, Marks F. Differential expression of prostaglandin-H synthase isoenzymes in normal and activated keratinocytes in vivo and in vitro. Biochem.J. 1995;309(Pt 1):263–269. doi: 10.1042/bj3090263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Brien TG, Simsiman RC, Boutwell RK. Induction of the polyamine-biosynthetic enzymes in mouse epidermis by tumor-promoting agents. Cancer Res. 1975;35:1662–1670. [PubMed] [Google Scholar]
- 16.Wang HQ, Smart RC. Overexpression of protein kinase C-alpha in the epidermis of transgenic mice results in striking alterations in phorbol ester-induced inflammation and COX-2, MIP-2 and TNF-alpha expression but not tumor promotion. J Cell Sci. 1999;112(Pt 20):3497–3506. doi: 10.1242/jcs.112.20.3497. [DOI] [PubMed] [Google Scholar]
- 17.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 18.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, Yang H, Amigorena S, Ryffel B, Barrat FJ, Saftig P, Levi F, Lidereau R, Nogues C, Mira JP, Chompret A, Joulin V, Clavel-Chapelon F, Bourhis J, Andre F, Delaloge S, Tursz T, Kroemer G, Zitvogel L. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 19.Klinman DM, xie H, Ivins BE. CpG oligonucleotides improve the protective immune response induced by the licensed anthrax vaccine. Ann.N.Y.Acad.Sci. 2006;1082:137–150. doi: 10.1196/annals.1348.030. [DOI] [PubMed] [Google Scholar]
- 20.Lewis JG, Adams DO. Early inflammatory changes in the skin of SENCAR and C57BL/6 mice following exposure to 12-O-tetradecanoylphorbol- 13-acetate. Carcinogenesis. 1987;8:889–898. doi: 10.1093/carcin/8.7.889. [DOI] [PubMed] [Google Scholar]
- 21.Cataisson C, Ohman R, Patel G, Pearson A, Tsien M, Jay S, Wright L, Hennings H, Yuspa SH. Inducible cutaneous inflammation reveals a protumorigenic role for keratinocyte CXCR2 in skin carcinogenesis. Cancer Res. 2009;69:319–328. doi: 10.1158/0008-5472.CAN-08-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore RJ, Owens DM, Stamp G, Arnott C, Burke F, East N, Holdsworth H, Turner L, Rollins B, Pasparakis M, Kollias G, Balkwill F. Mice deficient in tumor necrosis factor-alpha are resistant to skin carcinogenesis. Nat.Med. 1999;5:828–831. doi: 10.1038/10552. [DOI] [PubMed] [Google Scholar]
- 23.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat.Rev.Immunol. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 24.Afaq F, Saleem M, Krueger CG, Reed JD, Mukhtar H. Anthocyanin- and hydrolyzable tannin-rich pomegranate fruit extract modulates MAPK and NF-kappaB pathways and inhibits skin tumorigenesis in CD-1 mice. Int.J Cancer. 2005;113:423–433. doi: 10.1002/ijc.20587. [DOI] [PubMed] [Google Scholar]
- 25.Thomas T, Thomas TJ. Polyamine metabolism and cancer. J Cell Mol.Med. 2003;7:113–126. doi: 10.1111/j.1582-4934.2003.tb00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohan RR, Challa A, Gupta S, Bostwick DG, Ahmad N, Agarwal R, Marengo SR, Amini SB, Paras F, MacLennan GT, Resnick MI, Mukhtar H. Overexpression of ornithine decarboxylase in prostate cancer and prostatic fluid in humans. Clin.Cancer Res. 1999;5:143–147. [PubMed] [Google Scholar]
- 27.Herschman HR. Regulation of prostaglandin synthase-1 and prostaglandin synthase-2. Cancer Metastasis Rev. 1994;13:241–256. doi: 10.1007/BF00666095. [DOI] [PubMed] [Google Scholar]
- 28.Hirschowitz E, Hidalgo G, Doherty D. Induction of cyclo-oxygenase-2 in non-small cell lung cancer cells by infection with DeltaE1, DeltaE3 recombinant adenovirus vectors. Gene Ther. 2002;9:81–84. doi: 10.1038/sj.gt.3301621. [DOI] [PubMed] [Google Scholar]
- 29.Baron JA, Sandler RS. Nonsteroidal anti-inflammatory drugs and cancer prevention. Annu.Rev.Med. 2000;51:511–523. doi: 10.1146/annurev.med.51.1.511. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Rodriguez LA, Huerta-Alvarez C. Reduced risk of colorectal cancer among long-term users of aspirin and nonaspirin nonsteroidal anti-inflammatory drugs. Epidemiology. 2001;12:88–93. doi: 10.1097/00001648-200101000-00015. [DOI] [PubMed] [Google Scholar]
- 31.Kawamori T, Rao CV, Seibert K, Reddy BS. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer Res. 1998;58:409–412. [PubMed] [Google Scholar]
- 32.Grubbs CJ, Lubet RA, Koki AT, Leahy KM, Masferrer JL, Steele VE, Kelloff GJ, Hill DL, Seibert K. Celecoxib inhibits N-butyl-N-(4-hydroxybutyl)-nitrosamine-induced urinary bladder cancers in male B6D2F1 mice and female Fischer-344 rats. Cancer Res. 2000;60:5599–5602. [PubMed] [Google Scholar]
- 33.Rioux N, Castonguay A. Prevention of NNK-induced lung tumorigenesis in A/J mice by acetylsalicylic acid and NS-398. Cancer Res. 1998;58:5354–5360. [PubMed] [Google Scholar]
- 34.Muller-Decker K, Kopp-Schneider A, Marks F, Seibert K, Furstenberger G. Localization of prostaglandin H synthase isoenzymes in murine epidermal tumors: suppression of skin tumor promotion by inhibition of prostaglandin H synthase-2. Mol.Carcinog. 1998;23:36–44. doi: 10.1002/(sici)1098-2744(199809)23:1<36::aid-mc5>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 35.Fischer SM, Lo HH, Gordon GB, Seibert K, Kelloff G, Lubet RA, Conti CJ. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, and indomethacin against ultraviolet light-induced skin carcinogenesis. Mol.Carcinog. 1999;25:231–240. [PubMed] [Google Scholar]
- 36.Pentland AP, Schoggins JW, Scott GA, Khan KN, Han R. Reduction of UV-induced skin tumors in hairless mice by selective COX-2 inhibition. Carcinogenesis. 1999;20:1939–1944. doi: 10.1093/carcin/20.10.1939. [DOI] [PubMed] [Google Scholar]
- 37.Furstenberger G, Gross M, Marks F. Eicosanoids and multistage carcinogenesis in NMRI mouse skin: role of prostaglandins E and F in conversion (first stage of tumor promotion) and promotion (second stage of tumor promotion) Carcinogenesis. 1989;10:91–96. doi: 10.1093/carcin/10.1.91. [DOI] [PubMed] [Google Scholar]
- 38.Klinman DM, Gursel I, Klaschik S, Dong L, Currie D, Shirota H. Therapeutic potential of oligonucleotides expressing immunosuppressive TTAGGG motifs. Ann.N.Y.Acad.Sci. 2005;1058:87–95. doi: 10.1196/annals.1359.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CD-1 mice were treated at 7 wk of age with 50 ug of DMBA followed by weekly topical applications of 2.5 ug of TPA for 4 months (dotted line). Suppressive ODN was administered weekly either locally (50 ug, solid line) or systemically (300 ug via i.p. injection, dashed line) at the time of TPA administration. Data show the cumulative number of papillomas in 10 mice/group.
**; p < .01.