Abstract
Osteopontin (OPN) is a matricellular protein that binds to a number of cell surface receptors including integrins and CD44. It is expressed in many tissues and secreted into body fluids including blood, milk and urine. OPN plays important physiological roles in bone remodeling, immune response and inflammation. It is also a tumour-associated protein, and elevated OPN levels are associated with tumour formation, progression and metastasis. Research has revealed a promising role for OPN as a cancer biomarker. OPN is subject to alternative splicing, as well as post-translational modifications such as phosphorylation, glycosylation and proteolytic cleavage. Functional differences have been revealed for different isoforms and post-translational modifications. The pattern of isoform expression and post-translational modification is cell-type specific and may influence the potential role of OPN in malignancy and as a cancer biomarker.
Keywords: Osteopontin, Cancer, Metastasis, Matricellular protein, Post-translational modification, Alternative splicing
Introduction
Osteopontin (OPN) is a matricellular protein that is involved in both physiological and pathological processes (Giachelli and Steitz 2000; Kyriakides and Bornstein 2003; Rangaswami et al. 2006; Weber 2001). It is a secreted, integrin-binding glycophosphoprotein, involved in inflammation, wound healing, bone formation and remodelling, as well as atherosclerosis and cancer (Cho et al. 2009; Sodek et al. 2000; Tuck et al. 2007; Weber 2001). A wide variety of cell types express OPN, including osteoclasts, osteoblasts, endothelial cells, vascular smooth muscle cells, epithelial cells (such as kidney, breast, and skin), neural cells (neurons, glial cells and Schwann cells) and activated immune cells (such as T-cells, B-cells, macrophages, natural killer (NK) and Kupffer cells) (Kunii et al. 2009; Rangaswami et al. 2006; Wai and Kuo 2004; Wai and Kuo 2008). OPN is found as both an immobilized extracellular matrix (ECM) molecule in some (eg. mineralized) tissues and as a secreted protein in body fluids such as milk, blood, urine, saliva, seminal fluid and bile (Rangaswami et al. 2006; Wai and Kuo 2004). It signals through different integrin receptors including αVβ1, αVβ3, αVβ5, αVβ6, α4β1, α5β1, α8β1, and α9β1 and CD44 variant receptors, through RGD-dependent and independent mechanisms, leading to a wide variety of effects (Rodrigues et al. 2007; Wai and Kuo 2004).
OPN is heavily post-translationally modified with serine/threonine phosphorylation, glycosylation and tyrosine sulfation, which allows for a monomeric molecular weight ranging from 41 to 75 kDa (Christensen et al. 2008). These modifications can be cell type-specific (Christensen et al. 2007), may depend on other physiological and pathological factors and may impact both OPN structure and function (Anborgh et al. 2009; Kazanecki et al. 2007; Zhang et al. 2007). This review will focus on OPN’s role in cancer and how splice variants and post-translational modifications may influence its effects in cancer cells.
OPN and cancer
OPN has been clinically and functionally associated with cancer for many years (reviewed in (Furger et al. 2001; Rittling and Chambers 2004; Tuck and Chambers 2001; Tuck et al. 2007; Weber 2001)). Clinically, there are numerous studies showing OPN expression in both tumour cells and cells found within the tumour microenvironment (reviewed in (Anborgh et al. 2010)). OPN expression in tumour cells has been shown in a variety of cancer types (Brown et al. 1994; Coppola et al. 2004) including carcinomas of breast (Tuck et al. 1998), prostate (Forootan et al. 2006; Hotte et al. 2002), colon (Agrawal et al. 2002), ovary (Bao et al. 2007), stomach (Imano et al. 2009), liver (Chen et al. 2010; Lin et al. 2010; Ye et al. 2003) and lung (Chambers et al. 1996; Zhang et al. 2001; Zhao et al. 2011), mesotheliomas (Pass et al. 2005), squamous cell carcinomas (Chien et al. 2009), sarcomas (Bramwell et al. 2005; Sulzbacher et al. 2002) and multiple myeloma (Saeki et al. 2003). OPN produced by other cells in the tumour microenvironment, such as macrophages and stromal cells, has been seen in a number of different cancer types as well (Reinholt et al. 1990). For example, in a cohort of lymph node negative breast cancer patients, OPN mRNA and protein were detected in both tumour cells and tumour infiltrating inflammatory cells (Tuck et al. 1998). Tumour cells and macrophages were determined to be OPN-positive in a sample of pulmonary artery sarcomas, where OPN had also been incorporated into the extracellular matrix (ECM) (Gaumann et al. 2001) and Chang et al. (2008) found similar results in cutaneous squamous cell carcinoma, as most cases showed the presence of OPN in both cancer cells and in ECM adjacent to the tumour.
Functionally, OPN alters the behaviour of cancer cells both in vitro and in vivo, in most cases in a manner that promotes malignancy. For example, adding OPN (exogenous/recombinant or transfected) to breast cancer cells in vitro can increase their adhesion and migration abilities, and has also been shown to change breast cancer cells’ gene expression profiles, affecting genes involved in all six hallmarks of cancer (Allan et al. 2006; Cook et al. 2005; Hanahan and Weinberg 2000). In colon cancer cells, both endogenous OPN and exogenous OPN increased motility and invasive abilities of cells in vitro (Irby et al. 2004). Knockdown models emphasize the effects OPN has on cancer cell behaviour in vitro. For example, in human hepatocellular carcinoma cells, siRNA specific for OPN decreased colony formation and invasion (Lin et al. 2010) and in PC-3 prostate cancer cells, OPN specific shRNA decreased proliferation, migration and invasion (Liu et al. 2010). Similarly, in the highly tumourigenic and metastatic breast MDA-MB-435 cell line, OPN knockdown significantly decreased invasion, migration, and colony formation in soft agar (Shevde et al. 2006). Comparable results are seen in breast cancer cell lines when OPN is blocked with either an anti-OPN antibody (Dai et al. 2010) or with RNA aptamers (small structured single stranded antisense RNA) (Mi et al. 2009).
In vivo studies also illustrate the pro-malignant effects of OPN. Breast cancer cells transfected to over-express OPN had increased tumour growth, lymphovascular invasion, lymph node metastases, and earlier occurring lung micrometastases in a xenograft tumour model (Allan et al. 2006). Weakly tumourigenic colon cancer cells showed enhanced tumourigenicity after stable transfection with OPN (Irby et al. 2004). Similarly, human lung cancer cells transfected to overexpress OPN had increased tumour growth and neovascularisation (Cui et al. 2007). As expected, decreasing the expression of OPN has malignancy-inhibiting effects in mouse models. OPN knockdown via shRNA, siRNA or treatment with an anti-OPN antibody decreased tumour take, tumour progression, metastases and expression of OPN-mediated signaling proteins of breast cancer cells (Chakraborty et al. 2008; Dai et al. 2010; Mi et al. 2009; Shevde et al. 2006). Anti-sense oligonucleotide targeting of OPN in hepatocellular cancer decreased lung metastases (Chen et al. 2010) and OPN specific shRNA decreased prostate tumour growth (Liu et al. 2010). Thus, OPN promotes tumour growth and metastases in experimental models in a variety of cancer types.
OPN as a biomarker in cancer
There have been numerous studies determining how OPN levels in tumour tissue and patients’ plasma/serum correlate with prognosis and survival in a variety of cancers (reviewed in (Terpos et al. 2009; Tuck et al. 2007; Wai and Kuo 2008; Weber et al. 2010)). For example, in breast cancer, elevated OPN expression, measured both in plasma and in tumour tissue, has been associated with decreased survival. Tuck et al. (1998) showed that OPN immunopositivity of tumour cells correlated with decreased disease-free and overall survival in a group of lymph node negative breast cancer patients. Similarly, in another study of 333 breast cancer patients (lymph node negative and positive), OPN immunostaining was negatively correlated with survival (Rudland et al. 2002). Singhal et al. (1997) also found significantly higher levels of plasma OPN in a group of women with metastatic breast cancer, compared with a group of healthy women and a group of breast cancer survivors. Furthermore, within the group of metastatic cancer patients, increased OPN levels were correlated with poorer prognosis and decreased survival (Singhal et al. 1997).
OPN has also been shown to be a potentially valuable biomarker in other cancer types. In advanced gastric cancer, OPN expression was the most significant predictor of poor prognosis, as patients with OPN-positive tumours (as determined by IHC) had decreased 5-year survival compared to those with OPN-negative cancer (Zhang et al. 2009). Elevated OPN serum levels were negatively correlated with survival in glioblastoma (Sreekanthreddy et al. 2010) and in soft tissue sarcoma patients (Bache et al. 2010). OPN levels were also associated with increased stage and grade and larger tumour size in soft tissue sarcomas (Bache et al. 2010). In colorectal cancer patients, increased OPN mRNA was significantly correlated with stage, lymph node metastasis and lymphatic or venous invasion, as well as shorter disease-free and overall survival rates (Likui et al. 2010). Thus, OPN levels, whether in tumour tissue or patient plasma, may provide useful prognostic information.
Additionally, OPN levels may be useful to monitor progression of some cancers. When measured throughout breast cancer disease progression, increases in plasma OPN levels over time are strongly correlated with decreased survival (Bramwell et al. 2006). In early stage NSCLC patients, plasma OPN levels decreased after tumour resection surgery compared to pre-surgery values, whereas in patients showing recurrence of NSCLC, OPN levels at the time of recurrence were elevated compared to values immediately post-surgery (Blasberg et al. 2010). Thus, in a number of cancers, OPN may be a good biomarker to monitor disease progression, in addition to providing useful prognostic information.
OPN splice variants/isoforms
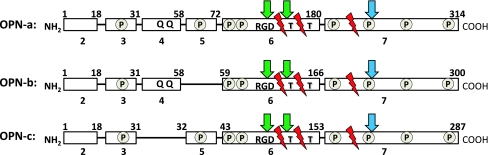
In addition to full length OPN (OPN-a), there are two other known splice variants (OPN-b, OPN-c). Full length OPN pre-mRNA consists of 7 exons (Rodrigues et al. 2007) while OPN-b lacks exon 5 and OPN-c lacks exon 4 (Hijiya et al. 1994; Kiefer et al. 1989; Saitoh et al. 1995; Young et al. 1990) (Fig. 1). Limited information is available about the function of the N-terminal region of OPN where alternative splicing occurs. However, certain inferences can be made on differential effects of the three splice variants, as a result of what has been shown regarding function of the exons they contain. For example, the N-terminal region of OPN contains two conserved amino acid sequence motifs (QLYxxYP and WLxPDP), which promote migration and survival of lymphocytes via activation of MAPK/WEK/AP-1 pathways (Cao et al. 2008; Dai et al. 2009). Sequences corresponding to exon 5, absent in OPN-b, contain one of several clusters of phosphorylated serine/threonine residues (Christensen et al. 2005). Also, two glutamine residues essential for transglutaminase crosslinking are present in exon 4 (Sorensen et al. 1994), thus OPN-a and OPN-b, but not OPN-c, are able to form polymeric OPN complexes which have altered functional properties (Higashikawa et al. 2007; Sorensen et al. 1994), as discussed in greater detail below.
Fig. 1.
Schematic of three OPN splice variants, OPN-a, OPN-b and OPN-c, highlighting important functional domains and the differences between isoforms. Full length OPN pre-mRNA is 7 exons in length, with 6 translated exons. Integrin binding (green arrow) occurs at RGD and cryptic SVVYGLR sequences, while CD44 variant receptor binding (blue arrow) occurs near the C-terminus. Thrombin cleavage (T) and MMP cleavage (red zig zag) sites are present in all three isoforms. Serine/threonine phosphorylated residues occur in clusters (circled P) throughout and are modified based on origin and function of OPN. Transglutaminase cross-linking occurs at glutamine residues (Q Q). OPN-b lacks exon 5 and is therefore missing certain phosphorylated sites, while OPN-c lacks exon 4 and is unable to undergo transglutaminase cross-linking. Clinical and functional differences do exist between OPN isoforms, however, these differences appear to be cancer cell type specific
Little is known about the regulation of the alternative splicing of the OPN message. Venables et al. (2008) observed a shift towards expression of splice variant OPN-b in three cell lines (HeLa, PC-3 and BJT) upon siRNA knockdown of the splicing protein hnRNP K, although secondary effects of this knock-down on the expression of other hnRNP family members could not be excluded (Venables et al. 2008). A change in the splicing pattern of a protein is one of the mechanisms that can alter its function and lead to malignancy-promoting effects (Brinkman 2004). While most clinical and experimental studies regarding OPN have not distinguished between splice variants, a number of recent studies have begun to examine their relative expression, at both mRNA and protein level, as well as to assess what different effects, if any, the OPN splice variants play in cancer and metastasis. In the following section we will review the (sometimes contrasting) findings of these studies.
Expression of OPN isoforms in human tumours
Recently, a number of studies in various cancer types have sought to identify expression patterns of OPN isoforms in an effort to determine if clinical and functional differences exist (Chae et al. 2009; Goparaju et al. 2010; He et al. 2006; Ivanov et al. 2009; Mirza et al. 2008; Patani et al. 2008; Sullivan et al. 2009; Yan et al. 2010). These studies are summarized in Table 1. It appears that OPN isoforms are differentially expressed and may have diverse effects, and this may be cancer type specific. Conflicting evidence exists and much remains unclear regarding which isoform is most clinically relevant, or if all three splice variants are important. For example, in the work of He et al. (2006) and Mirza et al. (2008), using both RT-PCR and immunohistochemistry, OPN-c was detected in breast carcinomas and invasive ductal carcinomas, while normal breast tissue surrounding the tumour or normal breast tissue (obtained by reduction mammoplasty) was devoid of OPN-c (He et al. 2006; Mirza et al. 2008). OPN-a was detected in both tumour tissue and at variable levels in normal tissue (He et al. 2006; Mirza et al. 2008), while OPN-b was expressed at low levels in tumour, tumour-adjacent tissue and normal breast tissue (Mirza et al. 2008). Mirza et al. (2008) also found that the intensity of OPN-c staining increased from tumour grade 1 to grade 3 (Mirza et al. 2008). High OPN-c levels were also associated with tumour grade, poor prognosis, increased recurrence rates and poorer disease-free survival in another breast cancer study (Patani et al. 2008). These studies suggested that OPN-c may be the most clinically relevant isoform, as it was found to be the predominant form expressed in tumour tissue. However, the majority of studies published regarding OPN expression in breast cancer have not differentiated between isoforms and have still identified OPN as a strong clinical prognostic factor (Bramwell et al. 2006; Rudland et al. 2002; Singhal et al. 1997; Tuck et al. 1997; Tuck et al. 1998), and studies examining function of transfected OPN have indicated functional effect of OPN-b as well (Allan et al. 2006; Tuck et al. 1999). Therefore, while OPN-c may indeed provide clinically useful information, further studies must be done to clarify the relative contributions of the three different OPN isoforms in breast cancer.
Table 1.
Summary of clinical studies which have looked at differential splice variant expression/levels
| Cancer Type | Reference | Method | Splice Variantsa | Resultsb |
|---|---|---|---|---|
| Breast | He et al. (2006) | RT-PCR | OPN-a, OPN-c | Normal: OPN-a > OPN-c |
| Tumour: OPN-a ~ OPN-c* | ||||
| Breast | Patani et al. (2008) | RT-PCR | OPN-a,OPN-b, OPN-c | Normal: OPN-a ~ OPN-b > OPN-c |
| Tumour: OPN-a ~ OPN-b > OPN-c | ||||
| Breast | Mirza et al. (2008) | RT-PCR, IHC | OPN-a,OPN-b, OPN-c | Normal: OPN-a > OPN-b > OPN-c |
| Tumour: OPN-c > OPN-a > OPN-b | ||||
| Lung | Goparaju et al (2010) | RT-PCR | OPN-a,OPN-b, OPN-c | Normal: OPN-a ~ OPN-c |
| Tumour: OPN-a > OPN-c | ||||
| Lung | Courter et al (2010) | qRT-PCR | OPN-a, OPN-b | Normal: OPN-a ~ OPN-b |
| Tumour: OPN-a ~ OPN-b | ||||
| Liver | Chae et al (2009) | RT-PCR | OPN-a,OPN-b, OPN-c | Normal: OPN-a ~ OPN-b ~ OPN-c |
| Tumour: OPN-a > OPN-b > OPN-c | ||||
| Glioma | Yan et al (2010) | RT-PCR | OPN-a,OPN-b, OPN-c | Normal: OPN-a ~ OPN-b ~ OPN-c |
| Tumour: OPN-b > OPN-a > OPN-c | ||||
| Head & neck | Courter et al (2010) | qRT-PCR | OPN-a, OPN-b | Normal: OPN-a ~ OPN-b |
| Tumour: OPN-a ~ OPN-b | ||||
| Mesothelioma | Ivanov et al (2009) | RT-PCR | OPN-a,OPN-b, OPN-c | Normal: OPN-a > OPN-b > OPN-c |
| Tumour: OPN-a > OPN-b; no OPN-c | ||||
| Pancreas | Sullivan et al (2009) | RT-PCR, IHC | Total OPN, OPN-c | Normal: Low total and OPN-c |
| Tumour: Total OPN ~ OPN-c | ||||
| Soft tissue sarcoma | Courter et al (2010) | RT-PCR | OPN-a, OPN-b | Tumour: OPN-a ~ OPN-b |
| Colon | Mirza et al. (2008) | qRT-PCR | OPN-a, OPN-c | Normal: OPN-a ~ OPN-c |
| Tumour: OPN-c > OPN-a |
a. Indicates splice variants assessed in each study
b. > greater expression than; ~ equivalent expression
* Bold face indicates tumour tissue OPN values were found to be significantly higher than for normal tissue
Isoform-specific studies have also been done in glioma (Yan et al. 2010), lung (Goparaju et al. 2010), liver (Chae et al. 2009), mesothelioma (Ivanov et al. 2009) and pancreatic cancers (Sullivan et al. 2009) (Table 1). Again, these studies found isoform specific differences. However, which OPN isoform is most clinically relevant depends on cancer type, with OPN-a and OPN-b, not OPN-c, predominating in lung cancer, liver cancer and mesotheliomas. Goparaju et al. (2010) found that OPN-a was most tumour associated in a cohort of non-small cell lung cancer (NSCLC) patients as OPN-a was over-expressed in most tumour samples compared to normal lung tissue, whereas OPN-c was detected in normal lung tissue but not in patients’ tumour tissue (Goparaju et al. 2010). Similar results were reported in another cohort of NSCLC patients, head and neck squamous cell carcinomas (HNSCC) and soft tissue sarcomas (Courter et al. 2010). Studies in hepatocellular carcinoma (HCC) and malignant mesothelioma (MM) also found that tumour tissue predominately expressed OPN-a, compared to control tissue (surrounding non-tumour liver or normal liver samples and normal peritoneal tissue) (Chae et al. 2009; Ivanov et al. 2009). Both of these studies speculate that OPN-a and OPN-b may be associated with poor prognosis. In HCC, the ratio of OPN-a and OPN-b to OPN-c increased as the tumours developed (Chae et al. 2009) and Ivanov et al. (2009) found that OPN-a and OPN-b (but not OPN-c) increased substantially in one patient measured before and after MM recurrence (Ivanov et al. 2009). All three OPN splice variants showed increased expression in high grade gliomas with respect to low grade gliomas and normal brain tissue, with OPN-b being the dominant isoform (Yan et al. 2010).
In contrast, similar to the breast cancer studies discussed above, tumour tissue from patients with invasive pancreatic ductal adenocarcinomas (PDA) revealed high OPN-c mRNA and protein expression in 72% of smokers and 36% of the non smokers compared to benign tumours (premalignant intrapapillary mucinous neoplasms), in which OPN-c mRNA or protein was rarely detected (Sullivan et al. 2009). Similarly, OPN-c was expressed at high levels in a small number of colon carcinoma samples and at moderate levels in tumour-adjacent tissue, while OPN-a was expressed at very low levels in tumour tissue (Mirza et al. 2008). These studies emphasize the variability amongst OPN isoform expression in different types of cancer.
While keeping in mind that most of the above studies on OPN splice variant expression in tumours were based on small numbers of patients, it appears that differential expression of OPN splice variants occurs in tumour versus normal tissues and that in breast cancer this may be associated with tumour grade, malignancy and patient prognosis (Mirza et al. 2008). However, which one of the OPN isoforms is dominantly expressed appears to depend upon the origin of the tumour. In breast, pancreatic and colon cancer, OPN-c and/or OPN-b may be good candidates both as biomarkers and as therapeutic targets. In other types of cancer such as lung cancer, liver cancer, malignant mesothelioma and glioma the role of OPN-c is less clear, as OPN-a and OPN-b seem to be the predominant isoforms expressed by these types of tumours. It is also not known if differential OPN expression at the mRNA level or protein level in tumours is associated with a difference of OPN isoforms present in plasma, as currently available OPN ELISA systems do not distinguish between isoforms.
OPN isoform expression and functional differences in cell lines
Similar heterogeneity exists for OPN splice variant expression in tumour cell lines and for the functional effects of different OPN isoforms on the behavior of these cells. In one study using breast cancer cells, He et al. (2006) reported that recombinant OPN-a, whether bacterially expressed or over-expressed and secreted from non-invasive breast cancer MFC-7 cells, is more prone to form aggregates in the presence of calcium, and is more active in supporting cell adhesion compared to OPN-c (He et al. 2006). In contrast, OPN-c was found to be soluble and to strongly support anchorage-independent growth of these cells (He et al. 2006). Very little OPN-b was secreted, as it was degraded by the proteasome when over-expressed in HEK293 cells or MCF-7 cells (Mirza et al. 2008). In contrast, several other studies have shown that in OPN-b over-expressing breast cancer cells (21T mammary epithelial cell series and MDA-MB-468 cells transfected to over-express OPN-b), OPN-b was both secreted into media and increased cell adhesion, migration, invasion and metastasis (Allan et al. 2006; Cook et al. 2005; Schulze et al. 2008; Tuck et al. 1999). Also, the malignant breast cancer cell line MDA-MB-231 was reported to express each of the 3 OPN splice variants, as were a number of other invasive cancer cell lines of breast and other origins (He et al. 2006). On the other hand, there is evidence that non-invasive breast epithelial cell lines express either no OPN, or low levels of OPN-a mRNA, without any OPN-b or OPN-c mRNA (He et al. 2006). Therefore, OPN processing and utilization differs depending on cell type, and as suggested from the above, differences in isoform function may exist even when of the same tissue origin (i.e. breast).
Conflicting evidence also exists regarding OPN isoform function in lung cancer. OPN-a was the dominant splice variant found in lung cancer cell lines that endogenously express OPN (A549, H460, H157, H1299, and Calu-3), with less OPN-b expressed and no endogenous OPN-c expression in all cell lines tested (Blasberg et al. 2009). Blasberg et al. (2009) suggested that OPN-a and OPN-b promote angiogenesis in lung cancer, as both isoforms stimulate tubule formation in bovine capillary endothelial cells and increase vascular endothelial growth factor (VEGF) expression, while OPN-c may actually inhibit angiogenesis (Blasberg et al. 2009). In addition, over-expression of OPN-a, and to a lesser extent OPN-b but not OPN-c, resulted in increased cellular proliferation, migration, invasion and anchorage independent growth of NSCLC cells, including the cell lines H358, A549 and H460 (Goparaju et al. 2010). However, Zhao et al. (2011) reported contrasting results for the NSCLC cell line A549, as overexpression of OPN-c, but not OPN-a or OPN-b, resulted in increased invasion (Zhao et al. 2011).
A number of studies have also explored OPN splice variants in other tumour types, including liver cancer (Chae et al. 2009; Takafuji et al. 2007), mesothelioma (Ivanov et al. 2009) and glioma (Yan et al. 2010). In HCC cell lines, OPN splice variant expression correlated with tumourigenecity, where OPN-a and OPN-b were mainly expressed by more migratory and invasive cells (SK-Hep1 and HepG2) and OPN-c was the dominant form expressed in non-malignant cells (Hep3B, PLC/PRF/5 and CHANG) (Chae et al. 2009). Functionally, OPN-a and OPN-b induced cell migration of non-malignant cells (Hep3B) and activated metastasis associated signaling pathways (urokinase plasminogen activator and MAPK) while OPN-c reduced migration in malignant cells (Chae et al. 2009). Surprisingly, OPN-c was more effective in stimulating anchorage-independent growth of the Hep3B cells (Chae et al. 2009). This unexpected malignancy-promoting effect of OPN-c in HCC was supported by Takafuji et al. (2007) who determined that over-expression of OPN-c in Hep3B cells increased cellular invasion, potentially due to the formation of a 5 kDa OPN fragment (aa167-210), generated by cleavage of OPN-c by MMP-9, and mediated by CD44 (Takafuji et al. 2007). In mesothelioma cell lines, functional effects differ between OPN isoforms, as OPN-a, but not OPN-b or OPN-c, stimulated cell proliferation and cell migration, OPN-a and OPN-b, but not OPN-c, stimulated cell invasion, and all three isoforms stimulated anchorage independent growth (Ivanov et al. 2009). In glioma cells, it was OPN-a and OPN-c, not OPN-b, that were able to promote invasiveness, by inducing the expression of proteins such as uPA, MMP-2 and MMP-9 (Yan et al. 2010). These studies again emphasize that OPN splice variants do in fact appear to have differing expression and functional effects, however, it is highly dependent on cell type, and much remains to be learned about which functional roles may be variant-specific.
Post-translational modifications of OPN
Post-translational modifications help to regulate OPN’s function in physiological and pathological processes. OPN can be highly phosphorylated and glycosylated, may contain a sulfated tyrosine residue, be subject to proteolytic cleavage and can be cross-linked by transglutaminase 2 (Fig. 1).
Phosphorylation of OPN occurs primarily by intracellular Golgi kinase and casein kinase II (Christensen et al. 2005; Lasa et al. 1997; Weber 2001). Most phosphorylated residues in OPN appear in groups of 2–5, with stretches of unphosphoryated residues in between the groups (Christensen et al. 2005; Christensen et al. 2007). Phosphorylation patterns have been described for OPN isolated from human and bovine milk (Christensen et al. 2005; Sorensen et al. 1995), human urine (Christensen et al. 2008), rat bone (Keykhosravani et al. 2005) and chicken osteoblasts (Salih et al. 1997), as well as from Ras-transformed mouse fibroblasts and differentiated mouse osteoblasts (Christensen et al. 2007). In most of these cases, OPN phosphorylation status was found to be heterogeneous and complex (Zhang et al. 2007). Only in OPN from bovine milk were all 28 phosphorylation sites completely occupied by phosphate groups (Christensen et al. 2005). OPN isolated from human milk can be phosphorylated at 36 different serine/threonine residues, although the average number of phosphate groups per OPN molecule was found to be 32 (Christensen et al. 2005). In human urinary OPN, 30 potential phosphorylation sites have been found, of which on average only 8 are actually phosphorylated (Christensen et al. 2008). Chicken osteoblast OPN contained an average of 6 phosphate groups per molecule (Salih et al. 1997). In OPN expressed by murine ras-transformed fibroblasts, 16 potential phosphorylation sites were found, however, on average only 4 phosphate groups per OPN molecule were identified. OPN from a non-transformed parental mouse fibroblast line, as well as OPN from a differentiated mouse osteoclast cell line, appeared to be highly phosphorylated (Christensen et al. 2007; Kazanecki et al. 2007).
Functionally, phosphorylation has been found to increase or decrease adhesion and/or migration, depending on the cell type. For example, it has been shown that native human milk OPN must be highly phosphorylated in order to stimulate the migration of human choriocarcinoma cells (Al-Shami et al. 2005). In contrast, dephosphorylation of bovine milk or rat bone OPN increased osteoclast migration at the expense of adhesion (Ek-Rylander et al. 1994; Ek-Rylander and Andersson 2010). In both murine and human breast cancer cells, unphosphorylated human recombinant OPN increased adhesion, migration, and invasion (Christensen et al. 2007; Tuck et al. 1999; Tuck et al. 2000; Tuck et al. 2003; Xuan et al. 1994) and OPN with low phosphorylation status promoted adhesion of human tumour (MDA-MB-435 breast cancer) cells to a greater extent than a highly phosphorylated form of OPN (Christensen et al. 2007). Although in general, for most “normal” fibroblasts, osteoclasts and osteoblasts, higher level phosphorylation of OPN appears to be associated with increased adhesion and decreased migration (Christensen et al. 2007), most tumour cells appear to express hypophosphorylated OPN and there is conflicting literature on how the degree of phosphorylation affects their behavior. Indeed, it is possible that total protein phosphorylation is not as important biologically as phosphorylation at specific sites. Now that there is knowledge of the positions of the various phosphorylations in OPN, studies will be possible to more clearly determine the effect of site specific phosphorylation.
Native OPN isolated from human milk contains five O-glycosylated threonine residues, located in one region in the N-terminal half of the molecule (Christensen et al. 2005), that appear to be fully occupied by oligosaccharides. Variation exists in the type of glycan structures which may consist of different combinations of N-acetylhexosamine, hexose, and sialic acid residues (Christensen et al. 2007; Christensen et al. 2008; Keykhosravani et al. 2005). Corresponding regions in OPN from other sources likewise contain O-glycosylated threonines and serines. In a murine breast cancer model, OPN has been identified as one of the proteins bearing the Sialyl-Thomsen-nouvelle antigen (Julien et al. 2009). This glycan is expressed in about 30% of human breast cancers and is associated with decreased survival and lack of response to chemotherapy (Cazet et al. 2010). In keeping with the importance of OPN glycosylation to function, it has been found that a generalized reduction of sialylation may prevent OPN from binding to cell surface receptors (Shanmugam et al. 1997). N-glycosylation, on the other hand, is a post-translational modification that has so far only been reported for OPN isolated from human bone (Masuda et al. 2000).
Sulfation of tyrosine residues is a modification that is found in many proteins that are membrane bound or secreted into the extracellular matrix and is thought to play an important role in controlling protein-protein interactions (Stone et al., 2009). In OPN, tyrosine sulfation has been linked to tissue mineralization (Nagata et al. 1989), and has been found in OPN isolated from rat bone (Keykhosravani et al. 2005), mouse osteoblasts (Ecarot-Charrier et al. 1989) and also from human urine (Christensen et al. 2008), but not in human milk OPN (Christensen et al. 2005). To our knowledge, there is at present no literature regarding the role of OPN sulfation on malignant properties of cancer cells.
Proteolytic processing
Proteolytic processing by both thrombin and MMPs is another important post-translational modification which helps to regulate and alter OPN’s function. OPN contains two highly conserved thrombin cleavage sites (Fig. 1), one is six amino acids from the RGD domain at Arg169-Ser170, and the second one is within the RGD domain, at Arg160-Gly161 (Senger et al. 1989; Smith et al. 1996). Thrombin cleavage results in two major fragments, an N-terminal fragment and a C-terminal fragment which differ in function from each other and full length OPN (Schulze et al. 2008; Smith et al. 1996; Takafuji et al. 2007; Takahashi et al. 1998). Thrombin cleavage has been shown to enhance OPN-mediated cell attachment and spreading, possibly because OPN fragments provide increased accessibility to the RGD binding site to cell surface receptors (Senger et al. 1994). In addition, a cryptic binding site, SVVYGLR, becomes accessible after thrombin cleavage of OPN, allowing α9β1 integrin receptors to bind to N-terminal OPN (Yokosaki et al. 1999). Neutrophils, smooth muscle cells and some epithelial cells express α9β1 integrin, and activation of α9β1 integrin leads to cell migration and proliferation (Yokosaki et al. 1999). Thus, thrombin cleavage of OPN can help to regulate migration of certain cell types to desired areas (Grassinger et al. 2009; Yokosaki et al. 1999), a useful function in inflammation and tissue remodeling, and at times a pathologic function in cancer. The C-terminal fragment of thrombin cleaved OPN is able to bind CD44 variant receptors, leading to activation of downstream signaling pathways to promote cell survival, cell migration and cell adhesion (Kazanecki et al. 2007; Wai and Kuo 2004) and is able to activate Akt1/2 and MMP-2, upon binding to cyclophilin C, via interaction with the CD147 glycoprotein surface receptor (Mi et al. 2007). It also contains a calcium binding domain and two heparin binding domains (Tuck et al. 2007). Inhibition of thrombin in OPN-expressing breast cancer cells results in decreased cell growth, colony formation, adhesion and migration in vitro and decreased primary tumour growth and lymphatic metastasis in vivo (Schulze et al. 2008). Therefore, thrombin cleavage is an important regulator of OPN function in certain tissues and situations and may represent a therapeutic target.
OPN is also a substrate for cleavage by matrix metalloproteases (MMPs), including MMP-2, -3, -7 and −9 (Agnihotri et al. 2001; Dean and Overall 2007; Takafuji et al. 2007). MMP-3 and −7 cleave OPN between G166-L167, leading to N-terminal fragments containing the RGD site (Agnihotri et al. 2001). Similar to thrombin cleaved OPN, MMP cleaved OPN also stimulates increased adhesion and migratory abilities in a variety of cell types (Agnihotri et al. 2001). MMP-9 cleaves OPN into 5 fragments, predominately at residues G166 and D210 (Takafuji et al. 2007). A 5 kDa fragment released after MMP-9 cleavage was found to induce cell invasion in HEK-293, SMMC-7721 and Hep3B cells through the CD44 receptor (Takafuji et al. 2007). In addition, OPN is able to induce MMP-2 and −9 expression in cancer cells (Liu et al. 2010; Rangaswami et al. 2004), thus increasing cells’ invasion abilities and increasing its own proteolytic processing. Therefore, MMP cleavage also appears to activate function of OPN, such that blocking MMP activity may be a potential way to target and decrease OPN-mediated cell aggressiveness.
Transglutaminase cross-linking
Osteopontin is also a substrate of tissue transglutaminase (TG2) (Kaartinen et al. 2002; Prince et al. 1991). TG2 is a widely distributed intra- and extracellular calcium dependent enzyme which leads to polymerization by cross-linking of its substrate proteins (Higashikawa et al. 2007; Kaartinen et al. 2002; Kaartinen et al. 2007). OPN contains two highly conserved transglutaminase-reactive glutamines, Gln-50 and Gln-52 (Sorensen et al. 1994), with which TG2 can form isopeptide cross-links between OPN monomers or fragments and between OPN and ECM components (Higashikawa et al. 2007). These glutamines are found in exon-4, present in OPN-a and OPN-b but not in OPN-c (Sorensen et al. 1994). In at least some instances, polymeric OPN has increased integrin-mediated cell adhesion, spreading and migration stimulating abilities, in addition to an altered conformation which enhances its collagen binding properties (Higashikawa et al. 2007). It has been speculated that OPN polymerization leads to concentration of integrin binding sites, which may enhance OPN-integrin signaling in addition to potentially exposing new integrin binding sites due to the altered conformation of OPN polymers (Higashikawa et al. 2007). TG2 cross-links are resistant to proteolytic cleavage and increase the stability of protein aggregates (Collighan and Griffin 2009), enhancing their potential functional effects. In breast cancer, TG2 expression is often increased in lymph node metastases compared to primary tumours (Mehta et al. 2004) and is thought to play an important role in the development of the metastatic phenotype (Mangala et al. 2007). For example, in MDA-MB-231 breast cancer cells, TG2 associates with integrin receptors and promotes motility, invasion and survival, compared to cells with minimal TG2 levels (Mangala et al. 2007). Also, siRNA inhibition of TG2 in these cells decreased their invasive abilities and survival in serum-free conditions (Mangala et al. 2007). While there are no clinical studies which specifically examine the relationship between OPN and TG2 in cancer, both have been independently identified as increased in metastatic breast cancer (Bramwell et al. 2006; Mehta et al. 2004; Singhal et al. 1997), and experimental evidence suggests that TG2 crosslinking of OPN promotes aggressive cancer cell behaviour (Higashikawa et al. 2007). The possibility that OPN polymers formed due to TG2 cross-linking may have different and/or enhanced functional effects in cancer cells is thus a subject worthy of further, more direct investigation.
Conclusion
OPN is a multifunctional protein that plays an important role in cancer progression and metastasis. It shows promise as a useful prognostic biomarker in a variety of cancer types, as well as a potential therapeutic target. More research is required regarding the expression of its three isoforms, OPN-a, OPN-b and OPN-c in different benign and malignant situations, and their functional consequences. From what we have learned so far, expression of a specific OPN isoform may have different, and sometimes opposite, effects in cancer cells of different origins. Also required is a better understanding of how post-translational modifications affect OPN function and which modifications are most common and important in cancer. OPN regulation is complex, much like the many functions of this protein, and improved understanding will better allow us to appropriately utilize OPN as a biomarker and as a possible therapeutic target.
Acknowledgements
This work is supported by an award from the Lloyd Carr-Harris Foundation. JCM is supported by a Canadian Graduate Award from the Canadian Institutes of Health Research and a Studentship from the Translational Breast Cancer Research Unit at the London Regional Cancer Program. AFC is a Canada Research Chair in Oncology, supported by the Canada Research Chairs Program.
Abbreviations
- ECM
Extracellular matrix
- ELISA
Enzyme-linked immunosorbent assay
- HCC
Hepatocellular carcinoma
- HNSCC
Head and neck squamous cell carcinoma
- MM
Malignant mesothelioma
- MMP
Matrix metalloproteinase
- mRNA
Messenger ribonucleic acid
- NSCLC
Non-small cell lung cancer
- OPN
Osteopontin
- PDA
Pancreatic ductal adenocarcinoma
- Q
Glutamine
- qRT-PCR
Quantitative real time polymerase chain reaction
- RT-PCR
Real time polymerase chain reaction
- RGD
Arginine-Glycine-Aspartic acid
- siRNA
Small interfering ribonucleic acid
- shRNA
Short hairpin ribonucleic acid
- SVVYGLR
Serine-Valine-Valine-Tyrosine-Glycine-Leucine-Arginine
- TG2
Tissue transglutaminase
- uPA
Urokinase plasminogen activator
References
- Agnihotri R, Crawford HC, Haro H, Matrisian LM, Havrda MC, Liaw L. Osteopontin, a novel substrate for matrix metalloproteinase-3 (stromelysin-1) and matrix metalloproteinase-7 (matrilysin) J Biol Chem. 2001;276(30):28261–28267. doi: 10.1074/jbc.M103608200. [DOI] [PubMed] [Google Scholar]
- Agrawal D, Chen T, Irby R, Quackenbush J, Chambers AF, Szabo M, Cantor A, Coppola D, Yeatman TJ. Osteopontin identified as lead marker of colon cancer progression, using pooled sample expression profiling. J Natl Cancer Inst. 2002;94(7):513–521. doi: 10.1093/jnci/94.7.513. [DOI] [PubMed] [Google Scholar]
- Allan AL, George R, Vantyghem SA, Lee MW, Hodgson NC, Engel CJ, Holliday RL, Girvan DP, Scott LA, Postenka CO, Al-Katib W, Stitt LW, Uede T, Chambers AF, Tuck AB. Role of the integrin-binding protein osteopontin in lymphatic metastasis of breast cancer. Am J Pathol. 2006;169(1):233–246. doi: 10.2353/ajpath.2006.051152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shami R, Sorensen ES, Ek-Rylander B, Andersson G, Carson DD, Farach-Carson MC. Phosphorylated osteopontin promotes migration of human choriocarcinoma cells via a p70 S6 kinase-dependent pathway. J Cell Biochem. 2005;94(6):1218–1233. doi: 10.1002/jcb.20379. [DOI] [PubMed] [Google Scholar]
- Anborgh PH, Wilson SM, Tuck AB, Winquist E, Schmidt N, Hart R, Kon S, Maeda M, Uede T, Stitt LW, Chambers AF. New dual monoclonal ELISA for measuring plasma osteopontin as a biomarker associated with survival in prostate cancer: clinical validation and comparison of multiple ELISAs. Clin Chem. 2009;55(5):895–903. doi: 10.1373/clinchem.2008.117465. [DOI] [PubMed] [Google Scholar]
- Anborgh PH, Mutrie JC, Tuck AB, Chambers AF. Role of the metastasis-promoting protein osteopontin in the tumor microenvironment. J Cell Mol Med. 2010;14(8):2037–2044. doi: 10.1111/j.1582-4934.2010.01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bache M, Kappler M, Wichmann H, Rot S, Hahnel A, Greither T, Said HM, Kotzsch M, Wurl P, Taubert H, Vordermark D. Elevated tumor and serum levels of the hypoxia-associated protein osteopontin are associated with prognosis for soft tissue sarcoma patients. BMC Cancer. 2010;10:132. doi: 10.1186/1471-2407-10-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao LH, Sakaguchi H, Fujimoto J, Tamaya T. Osteopontin in metastatic lesions as a prognostic marker in ovarian cancers. J Biomed Sci. 2007;14(3):373–381. doi: 10.1007/s11373-006-9143-1. [DOI] [PubMed] [Google Scholar]
- Blasberg JD, Goparaju CM, Pass HI, Donington JS. Lung cancer osteopontin isoforms exhibit angiogenic functional heterogeneity. J Thorac Cardiovasc Surg. 2009;139(6):1587–1593. doi: 10.1016/j.jtcvs.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasberg JD, Pass HI, Goparaju CM, Flores RM, Lee S, Donington JS. Reduction of elevated plasma osteopontin levels with resection of non-small-cell lung cancer. J Clin Oncol. 2010;28(6):936–941. doi: 10.1200/JCO.2009.25.5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramwell VH, Tuck AB, Wilson SM, Stitt LW, Cherian AK, Rorke SC, Al-Katib W, Postenka CO, Chambers AF. Expression of osteopontin and HGF/met in adult soft tissue tumors. Cancer Biol Ther. 2005;4(12):1336–1341. doi: 10.4161/cbt.4.12.2166. [DOI] [PubMed] [Google Scholar]
- Bramwell VH, Doig GS, Tuck AB, Wilson SM, Tonkin KS, Tomiak A, Perera F, Vandenberg TA, Chambers AF. Serial plasma osteopontin levels have prognostic value in metastatic breast cancer. Clin Cancer Res. 2006;12(11 Pt 1):3337–3343. doi: 10.1158/1078-0432.CCR-05-2354. [DOI] [PubMed] [Google Scholar]
- Brinkman BM. Splice variants as cancer biomarkers. Clin Biochem. 2004;37(7):584–594. doi: 10.1016/j.clinbiochem.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Brown LF, Papadopoulos-Sergiou A, Berse B, Manseau EJ, Tognazzi K, Perruzzi CA, Dvorak HF, Senger DR. Osteopontin expression and distribution in human carcinomas. Am J Pathol. 1994;145(3):610–623. [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Dai J, Fan K, Wang H, Ji G, Li B, Zhang D, Hou S, Qian W, Zhao J, Wang H, Guo Y. A novel functional motif of osteopontin for human lymphocyte migration and survival. Mol Immunol. 2008;45(14):3683–3692. doi: 10.1016/j.molimm.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Cazet A, Julien S, Bobowski M, Burchell J, Delannoy P. Tumour-associated carbohydrate antigens in breast cancer. Breast Cancer Res. 2010;12(3):204. doi: 10.1186/bcr2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae S, Jun HO, Lee EG, Yang SJ, Lee DC, Jung JK, Park KC, Yeom YI, Kim KW. Osteopontin splice variants differentially modulate the migratory activity of hepatocellular carcinoma cell lines. Int J Oncol. 2009;35(6):1409–1416. doi: 10.3892/ijo_00000458. [DOI] [PubMed] [Google Scholar]
- Chakraborty G, Jain S, Patil TV, Kundu GC. Down-regulation of osteopontin attenuates breast tumour progression in vivo. J Cell Mol Med. 2008;12(6A):2305–2318. doi: 10.1111/j.1582-4934.2008.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers AF, Wilson SM, Kerkvliet N, O’Malley FP, Harris JF, Casson AG. Osteopontin expression in lung cancer. Lung Cancer. 1996;15(3):311–323. doi: 10.1016/0169-5002(95)00595-1. [DOI] [PubMed] [Google Scholar]
- Chang PL, Harkins L, Hsieh YH, Hicks P, Sappayatosok K, Yodsanga S, Swasdison S, Chambers AF, Elmets CA, Ho KJ. Osteopontin expression in normal skin and non-melanoma skin tumors. J Histochem Cytochem. 2008;56(1):57–66. doi: 10.1369/jhc.7A7325.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RX, Xia YH, Xue TC, Zhang H, Ye SL (2011) Down-regulation of osteopontin inhibits metastasis of hepatocellular carcinoma cells via a mechanism involving MMP-2 and uPA. Oncol Rep 25(3):803-808 [DOI] [PubMed]
- Chien CY, Su CY, Chuang HC, Fang FM, Huang HY, Chen CH, Chen CM, Huang CC. Comprehensive study on the prognostic role of osteopontin expression in oral squamous cell carcinoma. Oral Oncol. 2009;45(9):798–802. doi: 10.1016/j.oraloncology.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Cho HJ, Cho HJ, Kim HS. Osteopontin: A multifunctional protein at the crossroads of inflammation, atherosclerosis, and vascular calcification. Curr Atheroscler Rep. 2009;11(3):206–213. doi: 10.1007/s11883-009-0032-8. [DOI] [PubMed] [Google Scholar]
- Christensen B, Nielsen MS, Haselmann KF, Petersen TE, Sorensen ES. Post-translationally modified residues of native human osteopontin are located in clusters: Identification of 36 phosphorylation and five O-glycosylation sites and their biological implications. Biochem J. 2005;390(Pt 1):285–292. doi: 10.1042/BJ20050341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen B, Kazanecki CC, Petersen TE, Rittling SR, Denhardt DT, Sorensen ES. Cell type-specific post-translational modifications of mouse osteopontin are associated with different adhesive properties. J Biol Chem. 2007;282(27):19463–19472. doi: 10.1074/jbc.M703055200. [DOI] [PubMed] [Google Scholar]
- Christensen B, Petersen TE, Sorensen ES. Post-translational modification and proteolytic processing of urinary osteopontin. Biochem J. 2008;411(1):53–61. doi: 10.1042/BJ20071021. [DOI] [PubMed] [Google Scholar]
- Collighan RJ, Griffin M. Transglutaminase 2 cross-linking of matrix proteins: Biological significance and medical applications. Amino Acids. 2009;36(4):659–670. doi: 10.1007/s00726-008-0190-y. [DOI] [PubMed] [Google Scholar]
- Cook AC, Tuck AB, McCarthy S, Turner JG, Irby RB, Bloom GC, Yeatman TJ, Chambers AF. Osteopontin induces multiple changes in gene expression that reflect the six “hallmarks of cancer” in a model of breast cancer progression. Mol Carcinog. 2005;43(4):225–236. doi: 10.1002/mc.20105. [DOI] [PubMed] [Google Scholar]
- Coppola D, Szabo M, Boulware D, Muraca P, Alsarraj M, Chambers AF, Yeatman TJ. Correlation of osteopontin protein expression and pathological stage across a wide variety of tumor histologies. Clin Cancer Res. 2004;10(1 Pt 1):184–190. doi: 10.1158/1078-0432.ccr-1405-2. [DOI] [PubMed] [Google Scholar]
- Courter D, Cao H, Kwok S, Kong C, Banh A, Kuo P, Bouley DM, Vice C, Brustugun OT, Denko NC, Koong AC, Giaccia A, Le QT. The RGD domain of human osteopontin promotes tumor growth and metastasis through activation of survival pathways. PLoS One. 2010;5(3):e9633. doi: 10.1371/journal.pone.0009633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui R, Takahashi F, Ohashi R, Gu T, Yoshioka M, Nishio K, Ohe Y, Tominaga S, Takagi Y, Sasaki S, Fukuchi Y, Takahashi K. Abrogation of the interaction between osteopontin and alphavbeta3 integrin reduces tumor growth of human lung cancer cells in mice. Lung Cancer. 2007;57(3):302–310. doi: 10.1016/j.lungcan.2007.03.019. [DOI] [PubMed] [Google Scholar]
- Dai J, Cao Z, Kang Y, Fan K, Ji G, Yang H, Wang H, Gao J, Wang H, Guo Y. A functional motif QLYxxYP is essential for osteopontin induced T lymphocyte activation and migration. Biochem Biophys Res Commun. 2009;380(3):715–720. doi: 10.1016/j.bbrc.2009.01.157. [DOI] [PubMed] [Google Scholar]
- Dai J, Li B, Shi J, Peng L, Zhang D, Qian W, Hou S, Zhao L, Gao J, Cao Z, Zhao J, Wang H, Guo Y. A humanized anti-osteopontin antibody inhibits breast cancer growth and metastasis in vivo. Cancer Immunol Immunother. 2010;59(3):355–366. doi: 10.1007/s00262-009-0754-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean RA, Overall CM. Proteomics discovery of metalloproteinase substrates in the cellular context by iTRAQ labeling reveals a diverse MMP-2 substrate degradome. Mol Cell Proteomics. 2007;6(4):611–623. doi: 10.1074/mcp.M600341-MCP200. [DOI] [PubMed] [Google Scholar]
- Ecarot-Charrier B, Bouchard F, Delloye C. Bone sialoprotein II synthesized by cultured osteoblasts contains tyrosine sulfate. J Biol Chem. 1989;264(33):20049–20053. [PubMed] [Google Scholar]
- Ek-Rylander B, Andersson G. Osteoclast migration on phosphorylated osteopontin is regulated by endogenous tartrate-resistant acid phosphatase. Exp Cell Res. 2010;316(3):443–451. doi: 10.1016/j.yexcr.2009.10.019. [DOI] [PubMed] [Google Scholar]
- Ek-Rylander B, Flores M, Wendel M, Heinegard D, Andersson G. Dephosphorylation of osteopontin and bone sialoprotein by osteoclastic tartrate-resistant acid phosphatase. modulation of osteoclast adhesion in vitro. J Biol Chem. 1994;269(21):14853–14856. [PubMed] [Google Scholar]
- Forootan SS, Foster CS, Aachi VR, Adamson J, Smith PH, Lin K, Ke Y. Prognostic significance of osteopontin expression in human prostate cancer. Int J Cancer. 2006;118(9):2255–2261. doi: 10.1002/ijc.21619. [DOI] [PubMed] [Google Scholar]
- Furger KA, Menon RK, Tuck AB, Bramwell VH, Chambers AF. The functional and clinical roles of osteopontin in cancer and metastasis. Curr Mol Med. 2001;1(5):621–632. doi: 10.2174/1566524013363339. [DOI] [PubMed] [Google Scholar]
- Gaumann A, Petrow P, Mentzel T, Mayer E, Dahm M, Otto M, Kirkpatrick CJ, Kriegsmann J. Osteopontin expression in primary sarcomas of the pulmonary artery. Virchows Arch. 2001;439(5):668–674. doi: 10.1007/s004280100452. [DOI] [PubMed] [Google Scholar]
- Giachelli CM, Steitz S. Osteopontin: a versatile regulator of inflammation and biomineralization. Matrix Biol. 2000;19(7):615–622. doi: 10.1016/s0945-053x(00)00108-6. [DOI] [PubMed] [Google Scholar]
- Goparaju CM, Pass HI, Blasberg JD, Hirsch N, Donington JS. Functional heterogeneity of osteopontin isoforms in non-small cell lung cancer. J Thorac Oncol. 2010;5(10):1516–1523. doi: 10.1097/JTO.0b013e3181eba6bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassinger J, Haylock DN, Storan MJ, Haines GO, Williams B, Whitty GA, Vinson AR, Be CL, Li S, Sorensen ES, Tam PP, Denhardt DT, Sheppard D, Choong PF, Nilsson SK. Thrombin-cleaved osteopontin regulates hemopoietic stem and progenitor cell functions through interactions with alpha9beta1 and alpha4beta1 integrins. Blood. 2009;114(1):49–59. doi: 10.1182/blood-2009-01-197988. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- He B, Mirza M, Weber GF. An osteopontin splice variant induces anchorage independence in human breast cancer cells. Oncogene. 2006;25(15):2192–2202. doi: 10.1038/sj.onc.1209248. [DOI] [PubMed] [Google Scholar]
- Higashikawa F, Eboshida A, Yokosaki Y. Enhanced biological activity of polymeric osteopontin. FEBS Lett. 2007;581(14):2697–2701. doi: 10.1016/j.febslet.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Hijiya N, Setoguchi M, Matsuura K, Higuchi Y, Akizuki S, Yamamoto S. Cloning and characterization of the human osteopontin gene and its promoter. Biochem J. 1994;303(Pt 1):255–262. doi: 10.1042/bj3030255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotte SJ, Winquist EW, Stitt L, Wilson SM, Chambers AF. Plasma osteopontin: associations with survival and metastasis to bone in men with hormone-refractory prostate carcinoma. Cancer. 2002;95(3):506–512. doi: 10.1002/cncr.10709. [DOI] [PubMed] [Google Scholar]
- Imano M, Satou T, Itoh T, Sakai K, Ishimaru E, Yasuda A, Peng YF, Shinkai M, Akai F, Yasuda T, Imamoto H, Okuno K, Ito H, Shiozaki H, Ohyanagi H. Immunohistochemical expression of osteopontin in gastric cancer. J Gastrointest Surg. 2009;13(9):1577–1582. doi: 10.1007/s11605-009-0955-y. [DOI] [PubMed] [Google Scholar]
- Irby RB, McCarthy SM, Yeatman TJ. Osteopontin regulates multiple functions contributing to human colon cancer development and progression. Clin Exp Metastasis. 2004;21(6):515–523. doi: 10.1007/s10585-004-2873-4. [DOI] [PubMed] [Google Scholar]
- Ivanov SV, Ivanova AV, Goparaju CM, Chen Y, Beck A, Pass HI. Tumorigenic properties of alternative osteopontin isoforms in mesothelioma. Biochem Biophys Res Commun. 2009;382(3):514–518. doi: 10.1016/j.bbrc.2009.03.042. [DOI] [PubMed] [Google Scholar]
- Julien S, Picco G, Sewell R, Vercoutter-Edouart AS, Tarp M, Miles D, Clausen H, Taylor-Papadimitriou J, Burchell JM. Sialyl-tn vaccine induces antibody-mediated tumour protection in a relevant murine model. Br J Cancer. 2009;100(11):1746–1754. doi: 10.1038/sj.bjc.6605083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaartinen MT, El-Maadawy S, Rasanen NH, McKee MD. Tissue transglutaminase and its substrates in bone. J Bone Miner Res. 2002;17(12):2161–2173. doi: 10.1359/jbmr.2002.17.12.2161. [DOI] [PubMed] [Google Scholar]
- Kaartinen MT, Murshed M, Karsenty G, McKee MD. Osteopontin upregulation and polymerization by transglutaminase 2 in calcified arteries of matrix gla protein-deficient mice. J Histochem Cytochem. 2007;55(4):375–386. doi: 10.1369/jhc.6A7087.2006. [DOI] [PubMed] [Google Scholar]
- Kazanecki CC, Uzwiak DJ, Denhardt DT. Control of osteopontin signaling and function by post-translational phosphorylation and protein folding. J Cell Biochem. 2007;102(4):912–924. doi: 10.1002/jcb.21558. [DOI] [PubMed] [Google Scholar]
- Keykhosravani M, Doherty-Kirby A, Zhang C, Brewer D, Goldberg HA, Hunter GK, Lajoie G. Comprehensive identification of post-translational modifications of rat bone osteopontin by mass spectrometry. Biochemistry. 2005;44(18):6990–7003. doi: 10.1021/bi050109p. [DOI] [PubMed] [Google Scholar]
- Kiefer MC, Bauer DM, Barr PJ. The cDNA and derived amino acid sequence for human osteopontin. Nucleic Acids Res. 1989;17(8):3306. doi: 10.1093/nar/17.8.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunii Y, Niwa S, Hagiwara Y, Maeda M, Seitoh T, Suzuki T. The immunohistochemical expression profile of osteopontin in normal human tissues using two site-specific antibodies reveals a wide distribution of positive cells and extensive expression in the central and peripheral nervous systems. Med Mol Morphol. 2009;42(3):155–161. doi: 10.1007/s00795-009-0459-6. [DOI] [PubMed] [Google Scholar]
- Kyriakides TR, Bornstein P. Matricellular proteins as modulators of wound healing and the foreign body response. Thromb Haemost. 2003;90(6):986–992. doi: 10.1160/TH03-06-0399. [DOI] [PubMed] [Google Scholar]
- Lasa M, Chang PL, Prince CW, Pinna LA. Phosphorylation of osteopontin by golgi apparatus casein kinase. Biochem Biophys Res Commun. 1997;240(3):602–605. doi: 10.1006/bbrc.1997.7702. [DOI] [PubMed] [Google Scholar]
- Likui W, Hong W, Shuwen Z. Clinical significance of the upregulated osteopontin mRNA expression in human colorectal cancer. J Gastrointest Surg. 2010;14(1):74–81. doi: 10.1007/s11605-009-1035-z. [DOI] [PubMed] [Google Scholar]
- Lin F, Li Y, Cao J, Fan S, Wen J, Zhu G, Du H, Liang Y (2010) Overexpression of osteopontin in hepatocellular carcinoma and its relationships with metastasis, invasion of tumor cells. Mol Biol Rep [DOI] [PubMed]
- Liu H, Chen A, Guo F, Yuan L. A short-hairpin RNA targeting osteopontin downregulates MMP-2 and MMP-9 expressions in prostate cancer PC-3 cells. Cancer Lett. 2010;295(1):27–37. doi: 10.1016/j.canlet.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Mangala LS, Fok JY, Zorrilla-Calancha IR, Verma A, Mehta K. Tissue transglutaminase expression promotes cell attachment, invasion and survival in breast cancer cells. Oncogene. 2007;26(17):2459–2470. doi: 10.1038/sj.onc.1210035. [DOI] [PubMed] [Google Scholar]
- Masuda K, Takahashi N, Tsukamoto Y, Honma H, Kohri K. N-glycan structures of an osteopontin from human bone. Biochem Biophys Res Commun. 2000;268(3):814–817. doi: 10.1006/bbrc.2000.2224. [DOI] [PubMed] [Google Scholar]
- Mehta K, Fok J, Miller FR, Koul D, Sahin AA. Prognostic significance of tissue transglutaminase in drug resistant and metastatic breast cancer. Clin Cancer Res. 2004;10(23):8068–8076. doi: 10.1158/1078-0432.CCR-04-1107. [DOI] [PubMed] [Google Scholar]
- Mi Z, Oliver T, Guo H, Gao C, Kuo PC. Thrombin-cleaved COOH(−) terminal osteopontin peptide binds with cyclophilin C to CD147 in murine breast cancer cells. Cancer Res. 2007;67(9):4088–4097. doi: 10.1158/0008-5472.CAN-06-4066. [DOI] [PubMed] [Google Scholar]
- Mi Z, Guo H, Russell MB, Liu Y, Sullenger BA, Kuo PC. RNA aptamer blockade of osteopontin inhibits growth and metastasis of MDA-MB231 breast cancer cells. Mol Ther. 2009;17(1):153–161. doi: 10.1038/mt.2008.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza M, Shaughnessy E, Hurley JK, Vanpatten KA, Pestano GA, He B, Weber GF. Osteopontin-c is a selective marker of breast cancer. Int J Cancer. 2008;122(4):889–897. doi: 10.1002/ijc.23204. [DOI] [PubMed] [Google Scholar]
- Nagata T, Todescan R, Goldberg HA, Zhang Q, Sodek J. Sulphation of secreted phosphoprotein I (SPPI, osteopontin) is associated with mineralized tissue formation. Biochem Biophys Res Commun. 1989;165(1):234–240. doi: 10.1016/0006-291x(89)91059-0. [DOI] [PubMed] [Google Scholar]
- Pass HI, Lott D, Lonardo F, Harbut M, Liu Z, Tang N, Carbone M, Webb C, Wali A. Asbestos exposure, pleural mesothelioma, and serum osteopontin levels. N Engl J Med. 2005;353(15):1564–1573. doi: 10.1056/NEJMoa051185. [DOI] [PubMed] [Google Scholar]
- Patani N, Jouhra F, Jiang W, Mokbel K. Osteopontin expression profiles predict pathological and clinical outcome in breast cancer. Anticancer Res. 2008;28(6B):4105–4110. [PubMed] [Google Scholar]
- Prince CW, Dickie D, Krumdieck CL. Osteopontin, a substrate for transglutaminase and factor XIII activity. Biochem Biophys Res Commun. 1991;177(3):1205–1210. doi: 10.1016/0006-291x(91)90669-x. [DOI] [PubMed] [Google Scholar]
- Rangaswami H, Bulbule A, Kundu GC. Nuclear factor-inducing kinase plays a crucial role in osteopontin-induced MAPK/IkappaBalpha kinase-dependent nuclear factor kappaB-mediated promatrix metalloproteinase-9 activation. J Biol Chem. 2004;279(37):38921–38935. doi: 10.1074/jbc.M404674200. [DOI] [PubMed] [Google Scholar]
- Rangaswami H, Bulbule A, Kundu GC. Osteopontin: role in cell signaling and cancer progression. Trends Cell Biol. 2006;16(2):79–87. doi: 10.1016/j.tcb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Reinholt FP, Hultenby K, Oldberg A, Heinegard D. Osteopontin–a possible anchor of osteoclasts to bone. Proc Natl Acad Sci U S A. 1990;87(12):4473–4475. doi: 10.1073/pnas.87.12.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittling SR, Chambers AF. Role of osteopontin in tumour progression. Br J Cancer. 2004;90(10):1877–1881. doi: 10.1038/sj.bjc.6601839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues LR, Teixeira JA, Schmitt FL, Paulsson M, Lindmark-Mansson H. The role of osteopontin in tumor progression and metastasis in breast cancer. Cancer Epidemiol Biomarkers Prev. 2007;16(6):1087–1097. doi: 10.1158/1055-9965.EPI-06-1008. [DOI] [PubMed] [Google Scholar]
- Rudland PS, Platt-Higgins A, El-Tanani M, Silva RS, Barraclough R, Winstanley JH, Howitt R, West CR. Prognostic significance of the metastasis-associated protein osteopontin in human breast cancer. Cancer Res. 2002;62(12):3417–3427. [PubMed] [Google Scholar]
- Saeki Y, Mima T, Ishii T, Ogata A, Kobayashi H, Ohshima S, Ishida T, Tabunoki Y, Kitayama H, Mizuki M, Katada Y, Asaoku H, Kitano M, Nishimoto N, Yoshizaki K, Maeda M, Kon S, Kinoshita N, Uede T, Kawase I. Enhanced production of osteopontin in multiple myeloma: clinical and pathogenic implications. Br J Haematol. 2003;123(2):263–270. doi: 10.1046/j.1365-2141.2003.04589.x. [DOI] [PubMed] [Google Scholar]
- Saitoh Y, Kuratsu J, Takeshima H, Yamamoto S, Ushio Y. Expression of osteopontin in human glioma. its correlation with the malignancy. Lab Invest. 1995;72(1):55–63. [PubMed] [Google Scholar]
- Salih E, Ashkar S, Gerstenfeld LC, Glimcher MJ. Identification of the phosphorylated sites of metabolically 32P-labeled osteopontin from cultured chicken osteoblasts. J Biol Chem. 1997;272(21):13966–13973. doi: 10.1074/jbc.272.21.13966. [DOI] [PubMed] [Google Scholar]
- Schulze EB, Hedley BD, Goodale D, Postenka CO, Al-Katib W, Tuck AB, Chambers AF, Allan AL. The thrombin inhibitor argatroban reduces breast cancer malignancy and metastasis via osteopontin-dependent and osteopontin-independent mechanisms. Breast Cancer Res Treat. 2008;112(2):243–254. doi: 10.1007/s10549-007-9865-4. [DOI] [PubMed] [Google Scholar]
- Senger DR, Perruzzi CA, Papadopoulos A. Elevated expression of secreted phosphoprotein I (osteopontin, 2ar) as a consequence of neoplastic transformation. Anticancer Res. 1989;9(5):1291–1299. [PubMed] [Google Scholar]
- Senger DR, Perruzzi CA, Papadopoulos-Sergiou A, Water L. Adhesive properties of osteopontin: regulation by a naturally occurring thrombin-cleavage in close proximity to the GRGDS cell-binding domain. Mol Biol Cell. 1994;5(5):565–574. doi: 10.1091/mbc.5.5.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugam V, Chackalaparampil I, Kundu GC, Mukherjee AB, Mukherjee BB. Altered sialylation of osteopontin prevents its receptor-mediated binding on the surface of oncogenically transformed tsB77 cells. Biochemistry. 1997;36(19):5729–5738. doi: 10.1021/bi961687w. [DOI] [PubMed] [Google Scholar]
- Shevde LA, Samant RS, Paik JC, Metge BJ, Chambers AF, Casey G, Frost AR, Welch DR. Osteopontin knockdown suppresses tumorigenicity of human metastatic breast carcinoma, MDA-MB-435. Clin Exp Metastasis. 2006;23(2):123–133. doi: 10.1007/s10585-006-9013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal H, Bautista DS, Tonkin KS, O’Malley FP, Tuck AB, Chambers AF, Harris JF. Elevated plasma osteopontin in metastatic breast cancer associated with increased tumor burden and decreased survival. Clin Cancer Res. 1997;3(4):605–611. [PubMed] [Google Scholar]
- Smith LL, Cheung HK, Ling LE, Chen J, Sheppard D, Pytela R, Giachelli CM. Osteopontin N-terminal domain contains a cryptic adhesive sequence recognized by alpha9beta1 integrin. J Biol Chem. 1996;271(45):28485–28491. [PubMed] [Google Scholar]
- Sodek J, Ganss B, McKee MD. Osteopontin. Crit Rev Oral Biol Med. 2000;11(3):279–303. doi: 10.1177/10454411000110030101. [DOI] [PubMed] [Google Scholar]
- Sorensen ES, Rasmussen LK, Moller L, Jensen PH, Hojrup P, Petersen TE. Localization of transglutaminase-reactive glutamine residues in bovine osteopontin. Biochem J. 1994;304(Pt 1):13–16. doi: 10.1042/bj3040013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen ES, Hojrup P, Petersen TE. Posttranslational modifications of bovine osteopontin: identification of twenty-eight phosphorylation and three O-glycosylation sites. Protein Sci. 1995;4(10):2040–2049. doi: 10.1002/pro.5560041009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreekanthreddy P, Srinivasan H, Kumar DM, Nijaguna MB, Sridevi S, Vrinda M, Arivazhagan A, Balasubramaniam A, Hegde AS, Chandramouli BA, Santosh V, Rao MR, Kondaiah P, Somasundaram K. Identification of potential serum biomarkers of glioblastoma: Serum osteopontin levels correlate with poor prognosis. Cancer Epidemiol Biomarkers Prev. 2010;19(6):1409–1422. doi: 10.1158/1055-9965.EPI-09-1077. [DOI] [PubMed] [Google Scholar]
- Stone MJ, Chuang S, Hou X, Shoham M, Zhu JZ. Tyrosine sulfation: an increasingly recognised post-translational modification of secreted proteins. New Biotechnol. 2009;25(5):299–317. doi: 10.1016/j.nbt.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Sullivan J, Blair L, Alnajar A, Aziz T, Ng CY, Chipitsyna G, Gong Q, Witkiewicz A, Weber GF, Denhardt DT, Yeo CJ, Arafat HA. Expression of a prometastatic splice variant of osteopontin, OPNC, in human pancreatic ductal adenocarcinoma. Surgery. 2009;146(2):232–240. doi: 10.1016/j.surg.2009.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzbacher I, Birner P, Trieb K, Lang S, Chott A. Expression of osteopontin and vascular endothelial growth factor in benign and malignant bone tumors. Virchows Arch. 2002;441(4):345–349. doi: 10.1007/s00428-002-0671-4. [DOI] [PubMed] [Google Scholar]
- Takafuji V, Forgues M, Unsworth E, Goldsmith P, Wang XW. An osteopontin fragment is essential for tumor cell invasion in hepatocellular carcinoma. Oncogene. 2007;26(44):6361–6371. doi: 10.1038/sj.onc.1210463. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Takahashi F, Tanabe KK, Takahashi H, Fukuchi Y. The carboxyl-terminal fragment of osteopontin suppresses arginine-glycine-asparatic acid-dependent cell adhesion. Biochem Mol Biol Int. 1998;46(6):1081–1092. doi: 10.1080/15216549800204632. [DOI] [PubMed] [Google Scholar]
- Terpos E, Kiagia M, Karapanagiotou EM, Charpidou A, Dilana KD, Nasothimiou E, Harrington KJ, Polyzos A, Syrigos KN. The clinical significance of serum markers of bone turnover in NSCLC patients: surveillance, management and prognostic implications. Anticancer Res. 2009;29(5):1651–1657. [PubMed] [Google Scholar]
- Tuck AB, Chambers AF. The role of osteopontin in breast cancer: clinical and experimental studies. J Mammary Gland Biol Neoplasia. 2001;6(4):419–429. doi: 10.1023/a:1014734930781. [DOI] [PubMed] [Google Scholar]
- Tuck AB, O'Malley FP, Singhal H, Tonkin KS, Harris JF, Bautista D, Chambers AF. Osteopontin and p53 expression are associated with tumor progression in a case of synchronous, bilateral, invasive mammary carcinomas. Arch Pathol Lab Med. 1997;121(6):578–584. [PubMed] [Google Scholar]
- Tuck AB, O'Malley FP, Singhal H, Harris JF, Tonkin KS, Kerkvliet N, Saad Z, Doig GS, Chambers AF. Osteopontin expression in a group of lymph node negative breast cancer patients. Int J Cancer. 1998;79(5):502–508. doi: 10.1002/(sici)1097-0215(19981023)79:5<502::aid-ijc10>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Tuck AB, Arsenault DM, O’Malley FP, Hota C, Ling MC, Wilson SM, Chambers AF. Osteopontin induces increased invasiveness and plasminogen activator expression of human mammary epithelial cells. Oncogene. 1999;18(29):4237–4246. doi: 10.1038/sj.onc.1202799. [DOI] [PubMed] [Google Scholar]
- Tuck AB, Elliott BE, Hota C, Tremblay E, Chambers AF. Osteopontin-induced, integrin-dependent migration of human mammary epithelial cells involves activation of the hepatocyte growth factor receptor (met) J Cell Biochem. 2000;78(3):465–475. doi: 10.1002/1097-4644(20000901)78:3<465::aid-jcb11>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Tuck AB, Hota C, Wilson SM, Chambers AF. Osteopontin-induced migration of human mammary epithelial cells involves activation of EGF receptor and multiple signal transduction pathways. Oncogene. 2003;22(8):1198–1205. doi: 10.1038/sj.onc.1206209. [DOI] [PubMed] [Google Scholar]
- Tuck AB, Chambers AF, Allan AL. Osteopontin overexpression in breast cancer: knowledge gained and possible implications for clinical management. J Cell Biochem. 2007;102(4):859–868. doi: 10.1002/jcb.21520. [DOI] [PubMed] [Google Scholar]
- Venables JP, Koh CS, Froehlich U, Lapointe E, Couture S, Inkel L, Bramard A, Paquet ER, Watier V, Durand M, Lucier JF, Gervais-Bird J, Tremblay K, Prinos P, Klinck R, Elela SA, Chabot B. Multiple and specific mRNA processing targets for the major human hnRNP proteins. Mol Cell Biol. 2008;28(19):6033–6043. doi: 10.1128/MCB.00726-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wai PY, Kuo PC. The role of osteopontin in tumor metastasis. J Surg Res. 2004;121(2):228–241. doi: 10.1016/j.jss.2004.03.028. [DOI] [PubMed] [Google Scholar]
- Wai PY, Kuo PC. Osteopontin: Regulation in tumor metastasis. Cancer Metastasis Rev. 2008;27(1):103–118. doi: 10.1007/s10555-007-9104-9. [DOI] [PubMed] [Google Scholar]
- Weber GF. The metastasis gene osteopontin: a candidate target for cancer therapy. Biochim Biophys Acta. 2001;1552(2):61–85. doi: 10.1016/s0304-419x(01)00037-3. [DOI] [PubMed] [Google Scholar]
- Weber GF, Lett GS, Haubein NC. Osteopontin is a marker for cancer aggressiveness and patient survival. Br J Cancer. 2010;103(6):861–869. doi: 10.1038/sj.bjc.6605834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan JW, Hota C, Chambers AF. Recombinant GST-human osteopontin fusion protein is functional in RGD-dependent cell adhesion. J Cell Biochem. 1994;54(2):247–255. doi: 10.1002/jcb.240540213. [DOI] [PubMed] [Google Scholar]
- Yan W, Qian C, Zhao P, Zhang J, Shi L, Qian J, Liu N, Fu Z, Kang C, Pu P, You Y. Expression pattern of osteopontin splice variants and its functions on cell apoptosis and invasion in glioma cells. Neuro Oncol. 2010;12(8):765–775. doi: 10.1093/neuonc/noq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye QH, Qin LX, Forgues M, He P, Kim JW, Peng AC, Simon R, Li Y, Robles AI, Chen Y, Ma ZC, Wu ZQ, Ye SL, Liu YK, Tang ZY, Wang XW. Predicting hepatitis B virus-positive metastatic hepatocellular carcinomas using gene expression profiling and supervised machine learning. Nat Med. 2003;9(4):416–423. doi: 10.1038/nm843. [DOI] [PubMed] [Google Scholar]
- Yokosaki Y, Matsuura N, Sasaki T, Murakami I, Schneider H, Higashiyama S, Saitoh Y, Yamakido M, Taooka Y, Sheppard D. The integrin alpha(9)beta(1) binds to a novel recognition sequence (SVVYGLR) in the thrombin-cleaved amino-terminal fragment of osteopontin. J Biol Chem. 1999;274(51):36328–36334. doi: 10.1074/jbc.274.51.36328. [DOI] [PubMed] [Google Scholar]
- Young MF, Kerr JM, Termine JD, Wewer UM, Wang MG, McBride OW, Fisher LW. cDNA cloning, mRNA distribution and heterogeneity, chromosomal location, and RFLP analysis of human osteopontin (OPN) Genomics. 1990;7(4):491–502. doi: 10.1016/0888-7543(90)90191-v. [DOI] [PubMed] [Google Scholar]
- Zhang J, Takahashi K, Takahashi F, Shimizu K, Ohshita F, Kameda Y, Maeda K, Nishio K, Fukuchi Y. Differential osteopontin expression in lung cancer. Cancer Lett. 2001;171(2):215–222. doi: 10.1016/s0304-3835(01)00607-3. [DOI] [PubMed] [Google Scholar]
- Zhang H, Hunter GK, Goldberg HA, Lajoie GA, Yeung KK. An integrated procedure of selective injection, sample stacking and fractionation of phosphopeptides for MALDI MS analysis. Anal Chim Acta. 2007;581(2):268–280. doi: 10.1016/j.aca.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Zhang X, Tsukamoto T, Mizoshita T, Ban H, Suzuki H, Toyoda T, Tatematsu M. Expression of osteopontin and CDX2: indications of phenotypes and prognosis in advanced gastric cancer. Oncol Rep. 2009;21(3):609–613. [PubMed] [Google Scholar]
- Zhao B, Sun T, Meng F, Qu A, Li C, Shen H, Jin Y, Li W (2011) Osteopontin as a potential biomarker of proliferation and invasiveness for lung cancer. J Cancer Res Clin Oncol [DOI] [PMC free article] [PubMed]