Abstract
Breast carcinoma is the most common cancer of women. Bones are often involved with breast carcinoma metastases with the resulting morbidity and reduced quality of life. Breast cancer cells arriving at bone tissues mount supportive microenvironment by recruiting and modulating the activity of several host tissue cell types including the specialized bone cells osteoblasts and osteoclasts. Pathologically activated osteoclasts produce osteolytic lesions associated with bone pain, pathological fractures, cord compression and other complications of metastatic breast carcinoma at bone. Over the last decade there has been enormous growth of knowledge in the field of osteoclasts biology both in the physiological state and in the tumor microenvironment. This knowledge allowed the development and implementation of several targeted therapeutics that expanded the armamentarium of the oncologists dealing with the metastases-associated osteolytic disease. While the interactions of cancer cells with resident bone cells at the established metastatic gross lesions are well-studied, the preclinical events that underlie the progression of disseminated tumor cells into micrometastases and then into clinically-overt macrometastases are just starting to be uncovered. In this review, we discuss the established information and the most recent discoveries in the pathogenesis of osteolytic metastases of breast cancer, as well as the corresponding investigational drugs that have been introduced into clinical development.
Keywords: Bone metastases, Breast cancer, Osteoclast, Osteoblast, Metastatic niche, Drug development
Breast cancer is the most common cancer of women. Globally, it accounted for 1.15 million incident cases in 2002, while 2.7 million new cases are projected for 2030 (Ferlay et al. 2010). Screening programs and the introduction of adjuvant therapies affected a decline of about 24% in death related to breast cancer in several developed countries (Berry et al. 2005). However, metastatic cases are still universally incurable.
Bone is the most common organ affected by distant relapse of breast cancer. Around 70% of metastatic patients will have lesions at bone (Hess et al. 2006). The skeleton and the lungs bear the major burden of the disease at autopsy studies (Cho and Choi 1980; Cifuentes and Pickren 1979; Lee 1983; Weiss 1992). Even though, breast carcinoma with metastases confined to bone is generally regarded as an indolent disease, bone secondaries usually present with bone pain and may be complicated with skeletal related events (SREs) such as pathological fractures, cord compression, bone marrow infiltration, or hypercalcemia of malignancy (Chiedozi 1988; Leone et al. 1988; Sherry et al. 1986a, b; Solomayer et al. 2000).
Breast carcinoma cells reach bones through blood spread. Invading metastatic cells landing at bone tissues induce a complex host reaction that alters the local microenvironment in favor of the growth and expansion of the secondary tumor deposits. Understanding the molecular interactions between the invading tumor cells and resident host cells at the metastatic sites allowed the invention of several biological therapies, which show promising results in the clinic. Other therapies are in pre-clinical development. In this review, we discuss the recent discoveries in the biology of tumor-host interactions at the metastatic bone sites and the status of the most promising investigational treatment strategies.
Predictors of occurrence of bone metastases
Investigators have been looking for the risk factors that can predict relapse at bone sites. Breast cancers are divided into distinctive molecular patterns according to their transcriptional profile. Relatively good-prognosis cancers show a gene expression reminiscent of a luminal or normal-like breast pattern, including positive expression of estrogen receptor (ER). More aggressive cancers usually demonstrate a basal-like phenotype or one that is characterized by the expression of human epidermal growth factor receptor 2 (HER2) (Perou et al. 2000; Sorlie et al. 2001; Sorlie et al. 2003). Smid et al. (Smid et al. 2008) found that around two-thirds of bone relapses occurred in luminal (ER+) cases while only 7% belonged to basal tumors. This incidence however roughly corresponds to the general prevalence of these tumor subtypes.
The concept of molecular classification of cancer according to gene signatures was introduced to increase the accuracy of traditional clinico-pathological prognostic markers of breast cancer, aiming at tailoring adjuvant therapy to personalized patient risk of relapse. The scientists at the Netherland Cancer Institute used microarray technology to identify 70-gene expression signature that correlated with early relapse in young breast cancer patients (van ’t Veer et al. 2002). This signature was later validated in a cohort of 295 consecutive patients (van de Vijver et al. 2002). A 67-gene signature was also described that predicted early metastases in node-negative cases (Foekens et al. 2006; Wang et al. 2005). Kang et al. used gene expression profiling to identify 50-genes associated specifically with bone metastases in a model of human breast cancer cell line xenografts. The signature genes included the chemokine receptor CXCR4, the metalloproteinase MMP-1, the cytokines CTGF and IL-11 as collaborative genes that mediate the bone metastatic program (Kang et al. 2003). The same group validated the bone metastases signature in a cohort of clinical specimens with available follow-up data (Minn et al. 2005). Although this 50-gene bone signature did not distinguish tumors that relapsed in bone when the analysis was performed on all specimens available (Minn et al. 2005), it distinguished between tumors relapsed to bone and those relapsed to lungs. The importance of some genes, such as CTGF and IL-11 was confirmed, although some genes identified in the animal model, such as MMP-1, lost their significance in the clinical samples (Minn et al. 2005). Smid et al. (Smid et al. 2006) analyzed samples from 107 node-negative breast cancer patients who experienced relapse. They identified a 31-gene list that predicted relapse to bone with 100% sensitivity, 50% specificity and 79.3% positive predictive value. Their signature agreed with Kang et al. gene set in highlighting the up-regulation of fibroblast growth factor pathway as a predictor of bone relapse. Later, Klein et al. (Klein et al. 2009) confirmed the correlation of bone relapse with cysteine-rich intestinal protein 1 (CRIP1) gene up-regulation reported by Smid et al. Although the data from these studies highlighted certain mechanistic pathways that operate to induce the development of breast cancer metastases at osseous sites, there is relatively little concordance between gene signatures reported by different groups to predict bone relapse in clinical cases. Thus, the long-sought signature that would predict bone relapse with enough accuracy to justify adjuvant bone-targeted treatment is yet to be identified.
The pathogenesis of bone metastases
Normal bone structure depends on the fine balance between two active cellular elements of bone tissues—the bone-forming osteoblasts and the bone-resorbing osteoclasts. Osteolytic metastases depend entirely on the activation of osteoclasts; the sole bone-resorbing cells. Osteoclasts are formed by fusion of precursors of myeloid origin (Vaananen and Laitala-Leinonen 2008). Hematopoietic stem cells are diverted to myeloid lineage under the effect of the transcription factor PU.1 and PU.1-/- mice lack both osteoclasts and macrophages (Tondravi et al. 1997). During osteoclastogenesis, three main signaling axes have been identified through: i) Macrophage Colony Stimulating Factor (MCSF), ii) Receptor Activator of Nuclear Factor κB Ligand and iii) Immunoreceptor Tyrosine-based Activation Motifs (ITAMs).
MCSF induces commitment of myeloid cells to osteoclastic lineage. Binding of MCSF to its cognate receptor c-fms on the surface of hematopoietic cells leads to the up-regulation of the transcription factor c-fos responsible for diversion of myeloid cells to osteoclastic rather than macrophage phenotype. Mice lacking MCSF (op/op) (Kodama et al. 1991; Wiktor-Jedrzejczak et al. 1990), c-fms (Dai et al. 2002) or c-fos (Wang et al. 1992) are devoid of osteoclasts and are osteopetrotic.
Committed osteoclast precursors express the Receptor Activator of Nuclear Factor κB (RANK) on their surface that allows them to respond to its respective ligand. RANK ligand (RANKL) is expressed on the surfaces of osteoblasts, which also secret Osteoprotegerin (OPG), the decoy receptor that sequesters RANKL and blocks its action. RANKL is the master osteoclastogenic mediator, which controls formation and function of mature osteoclasts (Blair and Athanasou 2004; Boyce and Xing 2008; Knowles and Athanasou 2009; Matsuo and Irie 2008; Wada et al. 2006). Binding of RANKL to its receptor on the surface of osteoclasts recruits the TNF Receptor Associated Factor 6 (TRAF6). Phosphorylation of TRAF6 leads to the activation and ultimately nuclear translocation of the p50 or p52 subunits of the transcription factor NFκB, responsible for fusion of pre-osteoclasts to form mature osteoclasts. RANKL signaling also induces the expression of the Nuclear Factor of Activated T cells c1 (NFATc1), the master transcription factor ultimately responsible for the osteoclastic phenotype (Negishi-Koga and Takayanagi 2009). The deficiency of either RANKL (Kong et al. 1999) or its receptor RANK (Dougall et al. 1999) in mice leads to severe osteopetrosis due to the absence of osteoclasts. Similar phenotype is observed in mice lacking TRAF6 (Naito et al. 1999), both p50 and p52 of NFκB (Iotsova et al. 1997), or NFATc1 (Aliprantis et al. 2008).
Further induction of NFATc1 is promoted by the phosphorylation of the ITAMs within the receptor-associated adaptor molecules DNAX-activating protein (DAP)12 and Fc receptor common gamma subunit (FcRγ). Activation by ITAM leads to amplification of NFATc1 expression, and induces its phospholipase Cγ (PLCγ)/calcium/calcineurin-dependent nuclear translocation, both of which are essential for osteoclast formation (Koga et al. 2004; Mocsai et al. 2004). Thus, signaling events essential for induction of osteoclast formation include MCSF/c-fms signal which up-regulates the transcription factor c-fos, the RANKL/RANK signal which up-regulates the transcription factors NFκB and NFATc1 and the immunoreceptor signal down-stream of yet unknown ligand, which induces calcium signaling and activates the transcription factor NFATc1.
Induction of osteoclast resorptive function also depends on specific signaling pathways. Mature osteoclasts express αvβ3 integrins that bind the fibronectin in the organic bone matrix (Nakamura et al. 2007). Integrin signaling induces c-Src-mediated changes in cytoskeletal organization that lead to formation of active actin ring and a tight sealing zone between osteoclast cytoplasmic membrane and the matrix surface (Horne et al. 2005; Miyazaki et al. 2006). Sealing zone is a pre-requisite for effective secretion of the resorptive mediators such as proton ions (H+), cathepsin K and MMP-9 that dissolve the bone matrix (Lakkakorpi and Vaananen 1996; Mulari et al. 2003; Nakamura et al. 2003; Ory et al. 2008; Saltel et al. 2008; Takahashi et al. 2007; Vaananen et al. 1988; Vaananen et al. 2000; Zaidi et al. 1993). Mice lacking β3 integrins or c-Src are osteopetrotic due to the presence of dysfunctional osteoclasts that fail to resorp bone (McHugh et al. 2000; Soriano et al. 1991). Metastatic cancer cells landing at bony sites exploit osteoclasts by inducing one or more of these pathways to elicit the pathological osteoclast formation and activity and excessive bone resorption.
The journey from breast to bone
The metastatic process is classically viewed as a sequential multi-step program (Hanahan and Weinberg 2000). Tumor cells at the primary site acquire the properties allowing them to invade the surrounding stroma and vasculature and gain access to the blood stream (i), to survive in the circulation (ii), to home at specific secondary site(s), and survive at these secondary site (iii) which may or may not entail a variable period of dormancy state (iv) and to establish secondary growth at the metastatic sites (v). Steps from (i) to (iv) constitute the pre-clinical stages of the metastatic process, while the last of these five steps is associated with clinically overt metastatic disease (Fig. 1).
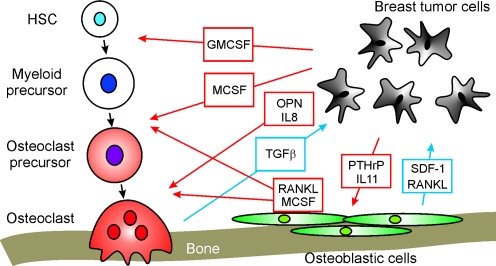
Fig. 1.
Schematics demonstrating the interactions between invading tumor cells and bone cells during breast cancer metastasis to bone. Red arrows indicate the effects of tumor cells on bone cells; blue arrows depict the reciprocal effects of bone cells on tumor cells
The pre-clinical stages of the metastatic process
Invasion at the primary site: Migrating cancer cells execute a complex program known as Epithelial-to-Mesenchymal Transition (EMT). This program entails down-regulation of the homophilic adhesion molecule E-cadherin allowing cancer cells to loss the cohesive properties characteristic of epithelium. EMT also confers cancer cells the motile phenotype necessary for these cells to invade the surrounding stroma and blood vessels (Creighton et al. 2010; Hugo et al. 2007; Tomaskovic-Crook et al. 2009; Trimboli et al. 2008; Willipinski-Stapelfeldt et al. 2005). Associated with these changes is the secretion of various proteolytic enzymes that help opening the path of cancer cells through the tissues (McMahon and Kwaan 2008). Loss of cohesiveness, acquisition of motility and proteolytic enzyme secretion pave the way for cancer cells to escape from primary site to the blood stream.
Survival in the blood stream: The majority of cancer cells liberated to the circulation fail to induce secondary deposits and probably succumb to apoptosis. The surviving minority however find their way to their preferable soil at specific secondary organs (Croker and Allan 2008).
Homing: Arrival of cancer cells to secondary organs is far from being a random process. Different types of cancers, tumors from different patients suffering from the same cancer and even different sub-clones of the same tumor have specific proclivity to home and thrive at specific organ(s). Homing of a particular cancer cell to its preferable secondary organ is facilitated by the directional movement of cancer cells towards a gradient of a soluble mediator (chemotaxis) and/or the active binding to a surface-bound molecule (haptotaxis). Two chemo-attractant interactions are particularly relevant to guiding breast cancer cells (BCCs) to home specifically at bone. One of these interactions is the migration of breast cancer cells expressing RANK (Mori et al. 2009; Santini et al. 2010) towards bone rich in its ligand (RANKL). Jones et al. have identified RANKL as an important factor that directed the migration of RANK-bearing carcinoma and melanoma cells (Jones et al. 2006). Similarly, prostate cancer cells have been shown to exhibit a chemotactic response to RANKL (Armstrong et al. 2008). The other molecule established to guide breast cancer cells expressing chemokine receptor CXCR4 (Hassan et al. 2008, 2010, 2009; Muller et al. 2001) to bone is Stromal-Derived Factor 1 (SDF-1) also known as CXCL12. RANKL and SDF-1 are highly expressed on the surface of osteoblasts residing at the highly vascular endosteal surface of the bone metaphyses (which were also demonstrated to be the first landing site for circulating BCCs (Phadke et al. 2006)), suggesting an important role of osteoblasts in guiding the BCCs to the bone marrow. In keeping, CXCR4 expression in early breast cancer correlated with the risk of relapse in bone (Andre et al. 2009). In metastatic patients, CXCR4 was higher in bony metastatic lesions compared to visceral ones (Cabioglu et al. 2009). Hassan et al. reported that high serum levels of SDF-1, which dump the chemotactic gradient guiding CXCR4-bearing cells to secondary sites, was associated with decreased risk of distant metastases in breast cancer patients (Hassan et al. 2008).
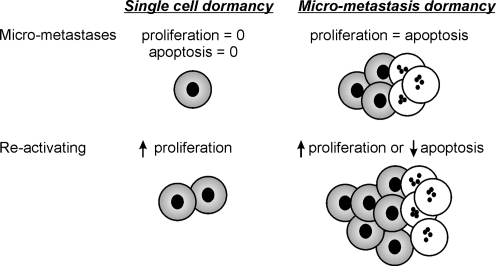
- Dormancy: Prolonged survival of the cancer cells at the secondary sites prior to appearance of clinically detectable metastases is a common phenomenon in breast cancer. Secondary tumors particularly in bone appear after a variable period of disease-free interval that may be as long as several years or even decades. Dormancy state is not well understood since it contrasts with the notion of inevitable exponential growth of cancer. Two models have been proposed to explain the mechanisms that allow BCCs to remain dormant at the metastatic sites. These two mechanisms are the micro-metastatic dormancy (a) and the single-cell dormancy (b) models (Fig. 2).
- In the micro-metastasis model, a microscopic tumor remains short of attaining a clinically detectable size through a maintained balance of its proliferation and apoptosis rates. Escape of such microscopic tumors into progressive growth may be induced by an angiogenic, immunologic, hormonal or other microenvironmental switches. Multiple evidences point to the cell-to-matrix signaling as a putative proliferation switch for BCCs microscopic lesions at bony secondary sites. Binding of α5β1 integrin to its extracellular ligands (fibronectin and to a lesser extent to collagen-1 fragment) induces cell proliferation and cell motility through induction of ERK and FAK pathways respectively (Aguirre-Ghiso et al. 2001; Barkan et al. 2010). Fibroblastic growth factor-2 (FGF-2) signaling on the other hand keeps the cell in a quiescent immotile non-proliferative state (Barrios and Wieder 2009; Korah et al. 2004; Najmi et al. 2005) characterized by the dominance of AKT over ERK and Rho-C over Rho-A (Barrios and Wieder 2009; Chatterjee and van Golen 2010; Danen et al. 2002).
- In the single-cell dormancy model, single scattered cells at the future metastatic organ linger into a prolonged period of cell cycle arrest and remain viable through tonic induction of anti-apoptotic survival signals. This model of arrested apoptosis contrasts with the micro-metastatic model where both proliferation and apoptosis are active. In bone, BCCs may gain a survival advantage through blocking of the receptors for TNF-Related Apoptosis Inducing Ligand (TRAIL). Two survival mechanisms that inhibit TRAIL signaling have been described and may be of relevance to the microenvironment at the bony tissues. TRAIL receptors in BCCs can be blocked by OPG (Fisher et al. 2006; Holen et al. 2005; Rachner et al. 2009; Schubert et al. 2008). Neville-Webb et al. demonstrated that bone marrow stromal cells isolated from breast cancer patients secret enough OPG to inhibit BCCs apoptosis in vitro (Neville-Webbe et al. 2004). More recently, another survival pathway was identified that counteracts the apoptotic TRAIL signaling and is mediated through stimulation of Src; a tyrosin-specific kinase involved in breast cancer progression and metastasis (Zhang et al. 2009).
Fig. 2.
Different models of breast cancer dormancy: In the single-cell model (left), cells detached from indolent breast carcinomas lodge at the sites of future metastases and remain quiescent in a state of prolonged survival. In the micro-metastasis model (right), cells detached from aggressive carcinomas lodge at the sites of future metastases and form microscopic tumor foci that have a balanced state of proliferation and apoptosis
The apparent dichotomy of the two models of dormancy raises the question of which model represents breast cancer dormancy at bone. While there is no definite answer to this question at the present time, we suggest that the answer lies in the well-known heterogeneity of the disease. Indolent breast cancers may fit into the dormant single-cell model while the more aggressive cases fit into the micro-metastatic model. In fact, recent studies corroborate this hypothesis. Zhang et al. reported that up-regulation of Src signaling in breast cancer specimens correlated with hormone-receptor (ER+) status and specifically with late-onset bone relapse (Zhang et al. 2009). Src was shown to induce prolonged survival of a slowly growing breast cancer cell line in the host bone marrow and induced the survival of these cells in bone metastases by counteracting TRAIL signaling. The authors also demonstrated the involvement of SDF-1/CXCR4 in mediating BCC survival, which depended on Src and Akt signaling (Zhang et al. 2009). These data linked Src-mediated anti-apoptotic mechanisms to survival of single BCCs in bone marrow and to late onset bony metastases.
Another indication of the distinctive dormancy mechanisms of indolent versus aggressive breast cancer comes from studies of cell lines in vitro. Barkan et al. (Barkan et al. 2008) compared the growth characteristics of the ER+ breast cancer cell line MCF7 with MDA-MB-231 cell line, which represent the aggressive basal type of the disease. In three-dimensional culture, MDA-MB-231 proliferated readily while MCF7 remained in a state of cell-cycle arrest, potentially linking the dormancy state associated with arrested proliferation with the less aggressive disease phenotype.
Cancer metastases and the stem cell concept
Since bone metastases are classically associated with late onset relapse and indolent metastatic disease, it is convenient to address the dormant single-cell model and the related concept of cancer stem cells in some detail. The concept of a migrating cancer cell that reaches the bone marrow, remain dormant for prolonged period of time and then resurges as a secondary tumor has always been appreciated by scientists and was later directly demonstrated with the description of circulating tumor cells (CTC) and disseminating tumor cells (DTC) that were retrieved from the blood and bone marrow respectively of apparently disease-free breast cancer survivors (Bidard et al. 2008; Slade and Coombes 2007; Tjensvoll et al. 2010). DTCs have been shown beyond doubt to correlate with the risk of subsequent tumor relapse (Bidard et al. 2008; Slade and Coombes 2007; Tjensvoll et al. 2010). Investigators tried to explain how DTC survive in the bone marrow, remains viable and later induce the formation of gross metastases. The fact that human bone marrow contains the niche for the adult hematopoietic stem cells (HSC) inspired the theory of tumor dormancy maintained by a cancer stem cell (CSC) population.
Normal adult stem cells are pluri-potent undifferentiated cells that constitute a reservoir for generation of mature complex tissues at the time of physiological needs. It was proposed that cancers originate from Cancer Stem Cells (CSCs), while metastases arise from Metastases Cancer Stem Cells (MCSCs) lodged at the future metastatic sites. Al-Hajj et al. (Al-Hajj et al. 2003) described a fraction of BCCs characterized by CD44+/CD24−/low expression that exhibited an augmented capacity of forming invasive tumors from heterogeneous cell population. In contrast to the rest of the tumor population, as few as 200 cells of this fraction formed palpable masses when transplanted in SCID mice. CD44+/CD24−/low cells were also found to represent the majority of DTCs in the bone marrow of breast cancer patients (Balic et al. 2006), and CD44+/CD24−/low cells content of the primary tumor was shown to correlate with subsequent development of osseous metastases (Abraham et al. 2005). It is plausible to assume that dormant BCCs at the bone marrow may reside in specific cellular niches that may help them to maintain their quiescent survival. It is also convenient to assume that such a CSC niche may have some resemblance to the better-characterized HSC niche at the bone marrow. There are at least two distinct niches that harbor HSC at the bone marrow. One niche is formed by osteoblasts (Calvi et al. 2003) and/or mesenchymal stem cells (MSCs) (Mendez-Ferrer et al. 2010) at the endosteal surface of bone and generally keeps HSCs at quiescent state. This niche retains HSCs in part through CXCR4/SDF-1 mediated adhesion between HSCs and osteoblasts/MSCs (Mishima et al. 2010; Van Overstraeten-Schlogel et al. 2006). The other niche is formed mainly by pericytes around blood vessels (Kiel and Morrison 2008) and generally favors expansion and mobilization of HSCs at the time of need. It is possible that BCCs exploit a parallel niche system to colonize the bone marrow. Cancer cells may remain dormant in an osteoblastic/MSCs niche and later cascade into the circulation through interactions with blood vessels. Indeed, there are indications to support this claim. Our group demonstrated in vitro that breast cancer cells produce soluble mediators that facilitate the selective attachment of the breast cancer cells to immature osteoblasts and identified γ-secretase-dependent signaling as a critical mediator of this process (Fong et al. 2010). Phadke et al. followed GFP-tagged human breast cancer cell line after intra-cardiac injection in athymic mice, and demonstrated that cancer cells lodged at the endosteal surface in close proximity to osteoblasts and remained as single-cell foci for 72 h post-injection (Phadke et al. 2006). This study provided direct evidence supporting the presence of a bone marrow metastatic niche for mammary carcinoma that is supported by endosteal osteoblasts. As mentioned above, BCCs often express RANK and CXCR4 on their surfaces. These two receptors target the directional migration of BCCs to bone where their respective ligands are highly expressed on the surface of osteoblasts. Having adhered to their osteoblastic niches, BCCs may gain survival advantage through Src mediated signaling down-stream of CXCR4 (Zhang et al. 2009). Mature osteoblasts also secrete increased amounts of OPG which can inhibit TRAIL receptors to maintain survival of BCCs (Neville-Webbe et al. 2004). Thus, osteoblasts at the endosteal side of the bone marrow are rational constituent of the niche for BCCs in the single-cell dormancy pattern.
The role of osteoclasts in driving the osteolysis associated with clinically overt bony metastases has been confirmed beyond doubt (Akhtari et al. 2008; Bendre et al. 2003a, b; Berenson et al. 2006; Casimiro et al. 2009; Chirgwin and Guise 2000; Clines and Guise 2005; Gatien et al. 2010; Guise et al. 2006; Kakonen and Mundy 2003; Kozlow and Guise 2005; Lipton 2006; Siclari et al. 2006). Less evident is the role of osteoclasts in the pre-clinical stages of bone metastases. The current paradigm of HSC niche suggests that osteoblasts maintain HSC at bone marrow while osteoclasts favor mobilization of vascular progenitors towards blood stream (Aicher et al. 2008; Brouard et al. 2010; Cho et al. 2010; Kollet et al. 2007, 2006). Do osteoclasts help mobilize dormant cancer cells and initiate cancer spread? Currently this question has no definite answer. On one hand, there are evidences that osteoclasts can suppress proliferation of leukemia cells at least in vitro (Yokota et al. 2010). On the other hand, prostate cancer cells have been shown to preferentially metastasize to sites of bone turnover containing active osteoclasts (Schneider et al. 2005), which suggests that osteoclasts may contribute to cancer cell homing.
Taken together, an emerging understanding of the pathogenesis of the pre-clinical stages of breast cancer metastases at bone includes the following events. Cancer cells are directed to migrate to bony sites by a chemotactic gradient of RANKL and/or SDF-1. Homing BCCs lodge in an osteoblastic niche where they gain survival advantage probably mediated through Src inhibition of TRAIL apoptotic signaling. Remains to be solved is the nature of the stimuli that later switch-on the macroscopic tumor growth and whether osteoclastic activation may be involved in this event.
The clinically overt metastatic stage
Osteoclasts have a pivotal role in the pathology of the clinically overt osseous lesions of breast carcinoma. The clinical appearance of osseous metastases is associated with increased proliferation of cancer cells, which succeed to mount a supportive microenvironmental reaction. BCCs induce intense osteoclastic response which perpetuates the growth of cancer cells at the involved bone tissues through resorption of bone matrix and the release of growth factors stored in that matrix. This reaction potentially derives more cancer growth at the metastatic site in what came to be known the “vicious cycle of bone metastases” and has been exhaustively reviewed in the literature(Akhtari et al. 2008; Bendre et al. 2003a, b; Berenson et al. 2006; Casimiro et al. 2009; Chirgwin and Guise 2000; Clezardin and Teti 2007; Clines and Guise 2005, 2008; Gatien et al. 2010; Guise et al. 2006; Kakonen and Mundy 2003; Kozlow and Guise 2005; Lipton 2006; Mundy 1997; Reddi et al. 2003; Siclari et al. 2006; Wang et al. 2006). BCCs induce increased osteoclasts formation, survival and function through direct and indirect mechanisms. Cancer cells derived mediators can act directly on osteoclasts or can act on osteoblasts to modulate the expression pattern of osteoclast regulatory molecules.
Direct osteoclastogenic effects of BCCs
Breast cancer cells of different clones were found to produce molecules capable of supporting and inducing osteoclastogenesis. Interleukin-8 (IL-8) was identified as one such molecule produced by bone metastatic subclone of breast carcinoma cells MDA-MB-231 (Bendre et al. 2005). IL-8 was shown to directly induce osteoclastogenesis (Bendre et al. 2003a, b), and its expression was found to correlate with bone metastases in vivo (Bendre et al. 2002). Breast cancer cells can also secret MCSF that directly promotes osteoclasts formation (Mancino et al. 2001) and survival (Gallet et al. 2006, 2004; Hussein et al. 2010). Mancino et al. generated osteoclasts from the bone marrow derived cell line UAMS-33 by supplying soluble RANKL and MCSF. Osteoclasts also developed in the same system when supplied with RANKL and MDA-MB-231 cells as a source of MCSF (Mancino et al. 2001). Gallet et al. showed that MDA-MB-231-derived MCSF is also responsible for inhibition of apoptosis of mature osteoclasts (Gallet et al. 2006, 2004). We recently confirmed the anti-apoptotic effect induced by MDA-MB-231 on mature osteoclasts and we have identified MCFS as a partial mediator of these effects and the pro-apoptotic protein BIM as an osteoclastic target for down-regulation by breast cancer-derived factors (Hussein et al. 2010). These findings suggest that BIM-targeted therapies such as the proteasome inhibitor Bortezomib, which is known to inhibit human osteoclastogenesis (von Metzler et al. 2007), may be interesting candidates for further investigation in the setting of tumor-associated osteolytic disease. Granulocyte Macrophage Colony Stimulating Factor (GM-CSF) has also been shown to induce osteoclasts formation and promote cancer-mediated osteolysis in animal models of metastatic breast cancer (Dai et al. 2010; Park et al. 2007). Our group has also found that during osteoclastogenesis, osteoclast precursors acquire the ability to strongly respond to factors produced by highly proliferating breast cancer cells (Guo et al. 2008; Tiedemann et al. 2009). We have found that breast cancer-derived factors can substitute for RANKL in inducing intracellular calcium signaling and nuclear translocation of NFATc1, resulting in robust osteoclastogenesis in RANKL-primed osteoclast precursors in vitro (Guo et al. 2008). In addition, breast cancer-derived factors induced unique signaling profile in differentiating pre-osteoclasts and mature osteoclasts, which was distinct from normal RANKL-induced differentiation and survival signals (Hussein et al. 2010; Tiedemann et al. 2009), suggesting the involvement of pathological mediators of osteoclastogenesis.
Role of osteoblasts-mediated osteoclastogenesis in BCC establishment in bone
Physiologically, osteoclastogenesis is regulated by osteoblasts, producing both pro- and anti-resorptive mediators. This property of osteoblasts has been used by the invading cancer cells to their advantage. Parathyroid hormone related protein (PTHrP) (Guise et al. 1996; Kitazawa and Kitazawa 2002; Yin et al. 1999), IL-8 (Bendre et al. 2003a, b; Kinder et al. 2008a, b; Singh et al. 2006), IL-6 and Monocyte Chemoattractant Protein (MCP-1) (Chen et al. 2009; Kinder et al. 2008a, b) are the BCCs-derived mediators that act on osteoblasts to induce the expression of the osteoclastogenic mediators such as RANKL (Berenson et al. 2006; Fong et al. 2010; Kozlow and Guise 2005) and Prostaglandin E2 (PGE2) (Chen et al. 2009; Li et al. 2008; Morgan et al. 2004). The resulting increased osteoclasts population at the tumor-bone interface secret proteolytic enzymes such as Cathepsin K (Guo et al. 2008; Le Gall et al. 2007) and Cathepsin G (Wilson et al. 2008, 2009) that resorb the bone matrix and release stored growth factors such as IGF and TGF-β. The latter induces further PTHrP release from BCCs (Chirgwin and Guise 2000; Kozlow and Guise 2005; Yin et al. 1999) setting on the vicious cycle of bone metastases.
PTHrP is one of the most extensively studied tumor-derived mediators that increase RANKL expression by osteoblasts. In one study of metastatic breast cancer cases, PTHrP was detected by immunohistochemistry in 92% of bony metastatic lesions, but in 17% of metastases to other organs (Powell et al. 1991). However, a prospective study of 526 breast cancer patients demonstrated that PTHrP expression at the primary tumor site correlated with better survival and even with fewer incidences of bone metastases, indicating that PTHrP at the primary tumor may delay tumor invasion (Henderson et al. 2006). The authors also examined 19 bone metastases specimens and their corresponding primary tumor lesions. Of the seven PTHrP-negative primary tumors, six cases developed PTHrP-positive bony metastases (Henderson et al. 2006), suggesting that the tumor expression of PTHrP is driven by the local milieu at bone tissue. TGFβ, which is abundant in bone matrix, augments the expression of PTHrP in breast cancer cells (Yin et al. 1999). The metastatic breast cancer cell line MDA-MB-231 grows readily and forms extensive osteolytic metastases when inoculated in nude mice. Treatment of mice with PTHrP blocking antibodies prior to MDA-MB-231 inoculation reduced both osteoclasts number and tumor area compared to controls (Guise et al. 1996). The ability of MDA-MB-231 to form tumor growth was also significantly reduced in TGFβ−/− mice relative to wild type animals (Mourskaia et al. 2009), which further underscore the importance of the host microenvironment in deriving bony metastases.
In addition to its increased ability to induce osteoclastogenesis, bone metastatic breast cancer cells have enhanced ability of secreting Matrix metalloproteases such as MMP1 (Kang et al. 2003) and MMP3 (Rose et al. 2007). Rose et al. demonstrated the importance of osteoactivin, the murine counterpart of Glycogen Nonmetastatic Melanoma Protein B (GPNMB) in inducing MMP3 expression by cancer cells at bony metastatic sites. In their model, forced expression of osteoactivin in the murine mammary carcinoma 4T1 cells enhanced their ability to form osteolytic metastases (Rose et al. 2007). They also reported the prognostic importance of GPNMB as an independent predictor of relapse in breast cancer patients (Rose et al. 2010).
Targeted therapy of bone metastases
Currently, the most promising results of targeted therapy for osseous lesions of MBC came from trials involving drugs that inhibit either osteoclasts function or formation (Bisphosphonates, RANKL inhibitor and Src inhibitors). The published and ongoing trials of targeted therapy of osseous metastases of breast cancer have been recently reviewed (Onishi et al. 2010; Rose and Siegel 2010). In the following paragraphs, we will briefly discuss the current status of the investigational targeted therapies that gain rationale from the above discussion of the disease pathogenesis (Fig. 3).
Bisphosphonates
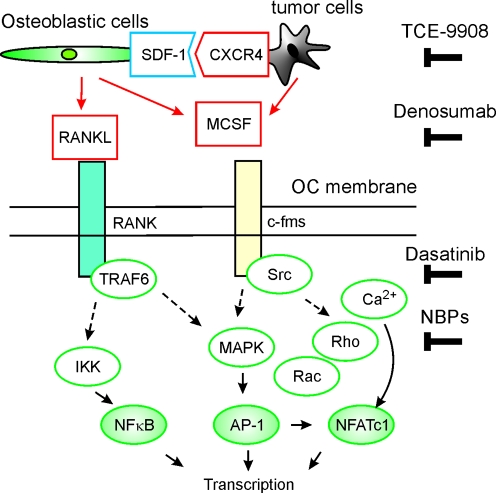
Fig. 3.
Schematics of the main therapeutic targets explored for treatment of breast cancer metastases to bone. TCE 9908 is a CXCR4 inhibitor, Denosumab is a fully humanized monoclonal antibody against RANKL, Dasatinib is an inhibitor of multiple tyrosine kinases including the Src family, NBPs are nitrogen-containing bisphosphonates. The main intracellular signalling molecules in osteoclasts are represented by white ovals while transcription factors are represented by green ovals
Bisphosphonates bind the inorganic matrix of the bone with high affinity due to their structure of two phosphate groups linked to a carbon atom. Systemically administered bisphosphonates are mainly deposited in the bone or excreted by the kidney. Osteoclast activity solubilizes bisphosphonates, which then become internalized by osteoclasts. The first generation bisphosphonates, such as clodronate, induce osteoclast apoptosis, while nitrogen-containing bisphosphonates (NBPs) inhibit farnysyl transferase, which is essential for the post-translational modification of Ras, Rac and Rho small GTPases leading to inhibition of osteoclast activity (Russell 2007). Although non-nitrogenous bisphosphonates showed efficacy in the treatment of breast cancer (Diel et al. 2008), there has been a trend towards using NBPs for this disease.
Zoledronic acid (ZA) is the most potent NBP approved at present. In clinical trials, ZA reduced the incidence of SREs (Carteni et al. 2006; Facchini et al. 2007; Kohno et al. 2005; Lipton et al. 2007a, b; Rosen et al. 2003; Rosen et al. 2004) and improved survival of metastatic breast cancer patients with osseous lesions. Survival benefit of ZA in the metastatic setting was associated with its anti-resorptive effect (Lipton et al. 2007a, b). In the adjuvant setting, ZA decreased bone loss related to cancer therapy (Brufsky et al. 2008, 2007, 2009; Bundred et al. 2008; Gnant et al. 2008, 2009, 2007; Hershman et al. 2008, 2010) and improved the disease-free survival in premenopausal women under hormonal therapy (Gnant et al. 2009). Other pharmacological actions have been attributed to ZA that might contribute to its therapeutic benefit, including direct action on cancer cells (Denoyelle et al. 2003; Hiraga et al. 2004; Ottewell et al. 2008), anti-angiogenic action (Santini et al. 2007) and immunomodulatory action (Dieli et al. 2007; Kondo et al. 2008; Meraviglia et al. 2010). ZA treatment was shown to decrease DTCs population in early breast cancer patients, indicating its potential role in combating tumor dormancy and late relapse (Aft et al. 2010; Rack et al. 2010). The main side effects of ZA are renal toxicity, hypocalcemia (Zuradelli et al. 2009) and osteonecrosis of the jaw (Fehm et al. 2009; Kyrgidis et al. 2008).
Although bisphosphonates demonstrate promising results in cancer setting, their potency seems to be reduced relative to what is observed in osteoporosis (Levine 2006) or Paget’s disease of bone (Josse et al. 2007). In this regard, we recently reported that the effect of NBP on osteoclasts was strongly affected by the presence of breast cancer cells. Soluble factors released from breast cancer cells prevented alendronate or pamidronate from inducing caspase-3 mediated apoptosis of mature osteoclasts. We identified the pro-apoptotic protein BIM as a target of down-regulation by cancer cells-derived mediators that was associated with osteoclasts resistance to spontaneous and bisphosphonate-induced apoptosis (Hussein et al. 2010).
Denosumab
Denosumab is a fully humanized monoclonal antibody against RANKL. Denosumab demonstrated high efficacy in NBPs-naïve (Body et al. 2006; Lipton et al. 2007a, b, 2008) and NBPs-resistant (Fizazi et al. 2009) patients. Fizazi et al. described the results of a randomized trial from participating institutions recruiting patients with solid tumors (lung excluded) or melanoma who had experienced persistent elevated markers of bone resorption despite 8 weeks or more of IV treatment with NBPs. Normalization of resorption markers was achieved in 71% and 29% of the patients in the Denosumab arms and NBPs arm respectively. More patients in the NBPs arm developed first on-study SRE and adverse events were similar across study arms (Fizazi et al. 2009). Denosumab is a viable option for NBPs-resistant patients with bone lesions, however long-term toxicity data remain to be characterized.
Src inhibitors
Src is a logical target for bone metastases prevention and treatment, since it is involved in tumor growth, osteoclasts function and resistance to endocrine therapy (Araujo and Logothetis 2009; Araujo and Logothetis 2010; Hiscox et al. 2010; Saad and Lipton 2010), as well as tumor cells survival and dormancy in bone marrow (Zhang et al. 2009). Dasatinib is the most widely used Src inhibitor in the clinic. It was shown to inhibit proliferation, migration and invasion of basal-like BCCs in vitro (Finn et al. 2007; Nautiyal et al. 2009; Pichot et al. 2009), osteoclasts formation and function (Araujo et al. 2009) and to enhance osteoblasts differentiation (Id Boufker et al. 2010; Lee et al. 2010) that may decrease RANKL/OPG ratio and decrease the osteoclastogenic derive. Clinical trials of Src inhibitors in metastatic breast cancer are under way (Araujo and Logothetis 2009, 2010; Onishi et al. 2010; Saad and Lipton 2010).
CXCR4 antagonists
In view of the strong evidences for CXCR4 involvement in tumor metastases, targeting CXCR4 remains a viable option for both adjuvant and palliative treatment settings. CTCE-9908, a peptide analogue of SDF-1 that competitively inhibits CXCR4, was demonstrated to decrease primary and metastatic tumor burden in mouse models of breast cancer (Hassan et al. 2010; Huang et al. 2009; Richert et al. 2009). The drug has been in Phase II clinical trial and publication of the results is awaited (Hotte et al. 2008).
Blocking the main players of the “vicious cycle”
The classic vicious cycle concept implies that bone metastases are driven by a positive feedback loop initiated by BCCs expressing PTHrP, which induce expression of RANKL by osteoblasts, thus stimulating osteoclastic resoprition and release of TGFβ from bone, which further stimulates tumor growth. Prospective studies however correlated PTHrP expression in primary tumor with better survival and even less bone metastases (Henderson et al. 2006). The explanation of this controversy is not known but the authors demonstrated that BCCs may change their PTHrP expression on arrival to bone. At the moment, we are not aware of an active trial of PTHrP antagonists in bony MBC. Similarly, the multi-facet effects of TGF-β in cancer progression delayed the clinical application of targeting strategies in breast cancer (Mourskaia et al. 2007; Rose and Siegel 2010; Yang and Moses 2008). In fact, abrogation of TGF-β response signaling was associated with poor outcome in several independent human data sets (Bierie et al. 2009).
Other targeted therapies
Cathepsin K inhibitors had a therapeutic benefit in animal models of metastatic breast cancer (Le Gall et al. 2007) but development in clinical phases was halted by the toxicity profile. Similarly, matrix metalloproteinase inhibitors were almost arrested in clinical trials due to dose-limiting toxicities (Pavlaki and Zucker 2003). The mechanistic role of connective tissue growth factor (CTGF) in osteolytic metastases of breast carcinoma was demonstrated in xenograft models (Kang et al. 2003; Shimo et al. 2006). Neutralizing antibody to CTGF decreased tumor associated osteolysis in a mouse model of bone metastases of human breast carcinoma (Shimo et al. 2006). Glembatumumab is a fully humanized monoclonal antibody against the extracellular domain of GPNMB that has shown antitumor activity in phase I/II clinical trials (Naumovski and Junutula 2010).
Closing remarks
Since the discovery of RANKL as a master osteoclastogenic cytokine, the vicious cycle associated with bone metastases has been well-characterized. The potent osteoclast inhibitors ZA and Denosumab proved to be of valuable benefit in the treatment of osteolytic disease of breast cancer bone metastases. Nevertheless, these two drugs are expensive to a prohibitive degree in many societies. In addition, there is a need for second line therapies for the intolerant cases and for the non responders. While the vicious cycle is clearly portrayed, targeting the main molecular players often did not translate to the clinic because of the low therapeutic index in case of cathepsin K and MMPs inhibitors or because of their bidirectional role in the pathogenesis of the disease as in case of TGF-β and PTHrP. Therefore, continuous research into the pathogenesis of clinical stages of metastatic breast cancer is important to identify new therapeutic targets. Such studies, may lead to the development of new treatments, or may provide strong rational for the use of established drugs in the bone metastatic setting. Importantly, the pre-clinical stages of bone metastases are much less understood compared to active metastases. Recent studies of the mechanisms involved in the journey of metastatic cells from the primary site to overt metastases renewed interests in molecular targets such as Src and CXCR4 and revived the hope in finding a curative treatment for breast cancer metastases.
Acknowledgments
We apologize to the colleagues whose work was not cited in this review for reasons of space and knowledge limitation. We appreciate the elaborate discussion with our colleagues, with Dr. P.M. Siegel, Dr. M. Murshed and Dr. M. Basik from McGill University. O.H. is supported by the Merit Doctoral Research Scholarship from the Government of Quebec, by Lloyd Carr-Harris Fellowship and by McGill University. S.V.K. holds a Canada Research Chair in Osteoclast Biology.
Abbreviations
- BCC
Breast Carcinoma Cells
- BIM
Bcl-2-interacting mediator of cell death
- CSC
Cancer Stem Cells
- DTC
Disseminated Tumor Cells
- EMT
Epithelial-Mesenchymal-Transition
- ER
Estrogen Receptors
- GPNMB
Glycoprotein Non-Metastatic B
- HER2
Human Epidermal growth factor Receptor 2
- HSC
Hematopoietic Stem Cells
- ITAM
Immunoreceptor Tyrosine-based Activation Motifs
- MBCC
Metastatic Breast Carcinoma Cells
- MCSF
Macrophage Colony Stimulating Factor
- MSC
Mesenchymal Stem Cells
- mTOR
mammalian Target Of Rapamycin
- NBP
Nitrogen-containing Bisphosphonates
- NFAT
Nuclear Factor of Activated T cell
- OPG
Osteoprotegerin
- PTHrP
Parathyroid Hormone related Protein
- RANKL
Receptor Activator of Nuclear factor κB Ligand
- SRE
Skeletal-Related Events
- SRS
Src Responsive Signature
- TRAIL
TNF-Related Apoptosis Inducing Ligand
- ZA
Zoledronic acid
References
- Abraham BK, Fritz P, et al. Prevalence of CD44+/CD24-/low cells in breast cancer may not be associated with clinical outcome but may favor distant metastasis. Clin Cancer Res. 2005;11(3):1154–1159. [PubMed] [Google Scholar]
- Aft R, Naughton M, et al. Effect of zoledronic acid on disseminated tumour cells in women with locally advanced breast cancer: an open label, randomised, phase 2 trial. Lancet Oncol. 2010;11(5):421–428. doi: 10.1016/S1470-2045(10)70054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre-Ghiso JA, Liu D, et al. Urokinase receptor and fibronectin regulate the ERK(MAPK) to p38(MAPK) activity ratios that determine carcinoma cell proliferation or dormancy in vivo. Mol Biol Cell. 2001;12(4):863–879. doi: 10.1091/mbc.12.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aicher A, Kollet O, et al. The Wnt antagonist Dickkopf-1 mobilizes vasculogenic progenitor cells via activation of the bone marrow endosteal stem cell niche. Circ Res. 2008;103(8):796–803. doi: 10.1161/CIRCRESAHA.107.172718. [DOI] [PubMed] [Google Scholar]
- Akhtari M, Mansuri J, et al. Biology of breast cancer bone metastasis. Cancer Biol Ther. 2008;7(1):3–9. doi: 10.4161/cbt.7.1.5163. [DOI] [PubMed] [Google Scholar]
- Al-Hajj M, Wicha MS, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100(7):3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliprantis AO, Ueki Y, et al. NFATc1 in mice represses osteoprotegerin during osteoclastogenesis and dissociates systemic osteopenia from inflammation in cherubism. J Clin Invest. 2008;118(11):3775–3789. doi: 10.1172/JCI35711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre F, Xia W, et al. CXCR4 expression in early breast cancer and risk of distant recurrence. Oncologist. 2009;14(12):1182–1188. doi: 10.1634/theoncologist.2009-0161. [DOI] [PubMed] [Google Scholar]
- Araujo J, Logothetis C. Targeting Src signaling in metastatic bone disease. Int J Cancer. 2009;124(1):1–6. doi: 10.1002/ijc.23998. [DOI] [PubMed] [Google Scholar]
- Araujo J, Logothetis C. Dasatinib: a potent SRC inhibitor in clinical development for the treatment of solid tumors. Cancer Treat Rev. 2010;36(6):492–500. doi: 10.1016/j.ctrv.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo JC, Poblenz A, et al. Dasatinib inhibits both osteoclast activation and prostate cancer PC-3-cell-induced osteoclast formation. Cancer Biol Ther. 2009;8(22):2153–2159. doi: 10.4161/cbt.8.22.9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong AP, Miller RE, et al. RANKL acts directly on RANK-expressing prostate tumor cells and mediates migration and expression of tumor metastasis genes. Prostate. 2008;68(1):92–104. doi: 10.1002/pros.20678. [DOI] [PubMed] [Google Scholar]
- Balic M, Lin H, et al. Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clin Cancer Res. 2006;12(19):5615–5621. doi: 10.1158/1078-0432.CCR-06-0169. [DOI] [PubMed] [Google Scholar]
- Barkan D, Kleinman H, et al. Inhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeleton. Cancer Res. 2008;68(15):6241–6250. doi: 10.1158/0008-5472.CAN-07-6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan D, El Touny LH, et al. Metastatic growth from dormant cells induced by a col-I-enriched fibrotic environment. Cancer Res. 2010;70(14):5706–5716. doi: 10.1158/0008-5472.CAN-09-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrios J, Wieder R. Dual FGF-2 and intergrin alpha5beta1 signaling mediate GRAF-induced RhoA inactivation in a model of breast cancer dormancy. Cancer Microenviron. 2009;2(1):33–47. doi: 10.1007/s12307-009-0019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendre MS, Gaddy-Kurten D, et al. Expression of interleukin 8 and not parathyroid hormone-related protein by human breast cancer cells correlates with bone metastasis in vivo. Cancer Res. 2002;62(19):5571–5579. [PubMed] [Google Scholar]
- Bendre M, Gaddy D, et al (2003a) Breast cancer metastasis to bone: it is not all about PTHrP. Clin Orthop Relat Res (415 Suppl):S39–S45 [DOI] [PubMed]
- Bendre MS, Montague DC, et al. Interleukin-8 stimulation of osteoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone disease. Bone. 2003;33(1):28–37. doi: 10.1016/s8756-3282(03)00086-3. [DOI] [PubMed] [Google Scholar]
- Bendre MS, Margulies AG, et al. Tumor-derived interleukin-8 stimulates osteolysis independent of the receptor activator of nuclear factor-kappaB ligand pathway. Cancer Res. 2005;65(23):11001–11009. doi: 10.1158/0008-5472.CAN-05-2630. [DOI] [PubMed] [Google Scholar]
- Berenson JR, Rajdev L, et al. Pathophysiology of bone metastases. Cancer Biol Ther. 2006;5(9):1078–1081. doi: 10.4161/cbt.5.9.3306. [DOI] [PubMed] [Google Scholar]
- Berry DA, Cronin KA, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353(17):1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- Bidard FC, Vincent-Salomon A, et al. Disseminated tumor cells of breast cancer patients: a strong prognostic factor for distant and local relapse. Clin Cancer Res. 2008;14(11):3306–3311. doi: 10.1158/1078-0432.CCR-07-4749. [DOI] [PubMed] [Google Scholar]
- Bierie B, Chung CH, et al. Abrogation of TGF-beta signaling enhances chemokine production and correlates with prognosis in human breast cancer. J Clin Invest. 2009;119(6):1571–1582. doi: 10.1172/JCI37480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair HC, Athanasou NA. Recent advances in osteoclast biology and pathological bone resorption. Histol Histopathol. 2004;19(1):189–199. doi: 10.14670/HH-19.189. [DOI] [PubMed] [Google Scholar]
- Body JJ, Facon T, et al. A study of the biological receptor activator of nuclear factor-kappaB ligand inhibitor, denosumab, in patients with multiple myeloma or bone metastases from breast cancer. Clin Cancer Res. 2006;12(4):1221–1228. doi: 10.1158/1078-0432.CCR-05-1933. [DOI] [PubMed] [Google Scholar]
- Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008;473(2):139–146. doi: 10.1016/j.abb.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouard N, Driessen R, et al. G-CSF increases mesenchymal precursor cell numbers in the bone marrow via an indirect mechanism involving osteoclast-mediated bone resorption. Stem Cell Res. 2010;5(1):65–75. doi: 10.1016/j.scr.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Brufsky A, Harker WG, et al. Zoledronic acid inhibits adjuvant letrozole-induced bone loss in postmenopausal women with early breast cancer. J Clin Oncol. 2007;25(7):829–836. doi: 10.1200/JCO.2005.05.3744. [DOI] [PubMed] [Google Scholar]
- Brufsky A, Bundred N, et al. Integrated analysis of zoledronic acid for prevention of aromatase inhibitor-associated bone loss in postmenopausal women with early breast cancer receiving adjuvant letrozole. Oncologist. 2008;13(5):503–514. doi: 10.1634/theoncologist.2007-0206. [DOI] [PubMed] [Google Scholar]
- Brufsky AM, Bosserman LD, et al. Zoledronic acid effectively prevents aromatase inhibitor-associated bone loss in postmenopausal women with early breast cancer receiving adjuvant letrozole: Z-FAST study 36-month follow-up results. Clin Breast Cancer. 2009;9(2):77–85. doi: 10.3816/CBC.2009.n.015. [DOI] [PubMed] [Google Scholar]
- Bundred NJ, Campbell ID, et al. Effective inhibition of aromatase inhibitor-associated bone loss by zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: ZO-FAST Study results. Cancer. 2008;112(5):1001–1010. doi: 10.1002/cncr.23259. [DOI] [PubMed] [Google Scholar]
- Cabioglu N, Sahin AA, et al. Chemokine receptors in advanced breast cancer: differential expression in metastatic disease sites with diagnostic and therapeutic implications. Ann Oncol. 2009;20(6):1013–1019. doi: 10.1093/annonc/mdn740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- Carteni G, Bordonaro R, et al. Efficacy and safety of zoledronic acid in patients with breast cancer metastatic to bone: a multicenter clinical trial. Oncologist. 2006;11(7):841–848. doi: 10.1634/theoncologist.11-7-841. [DOI] [PubMed] [Google Scholar]
- Casimiro S, Guise TA, et al. The critical role of the bone microenvironment in cancer metastases. Mol Cell Endocrinol. 2009;310(1–2):71–81. doi: 10.1016/j.mce.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Chatterjee M, van Golen KL (2010) Farnesyl transferase inhibitor treatment of breast cancer cells leads to altered RhoA and RhoC GTPase activity and induces a dormant phenotype. Int J Cancer [DOI] [PubMed]
- Chen YC, Sosnoski DM, et al. Selenium modifies the osteoblast inflammatory stress response to bone metastatic breast cancer. Carcinogenesis. 2009;30(11):1941–1948. doi: 10.1093/carcin/bgp227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiedozi LC. Prognostic significance of exclusive skeletal metastases in stage IV primary carcinoma of the breast. Surg Gynecol Obstet. 1988;167(4):303–306. [PubMed] [Google Scholar]
- Chirgwin JM, Guise TA. Molecular mechanisms of tumor-bone interactions in osteolytic metastases. Crit Rev Eukaryot Gene Expr. 2000;10(2):159–178. [PubMed] [Google Scholar]
- Cho SY, Choi HY. Causes of death and metastatic patterns in patients with mammary cancer. Ten-year autopsy study. Am J Clin Pathol. 1980;73(2):232–234. doi: 10.1093/ajcp/73.2.232. [DOI] [PubMed] [Google Scholar]
- Cho KA, Joo SY, et al. Osteoclast activation by receptor activator of NF-kappaB ligand enhances the mobilization of hematopoietic progenitor cells from the bone marrow in acute injury. Int J Mol Med. 2010;26(4):557–563. doi: 10.3892/ijmm_00000499. [DOI] [PubMed] [Google Scholar]
- Cifuentes N, Pickren JW. Metastases from carcinoma of mammary gland: an autopsy study. J Surg Oncol. 1979;11(3):193–205. doi: 10.1002/jso.2930110303. [DOI] [PubMed] [Google Scholar]
- Clezardin P, Teti A. Bone metastasis: pathogenesis and therapeutic implications. Clin Exp Metastasis. 2007;24(8):599–608. doi: 10.1007/s10585-007-9112-8. [DOI] [PubMed] [Google Scholar]
- Clines GA, Guise TA. Hypercalcaemia of malignancy and basic research on mechanisms responsible for osteolytic and osteoblastic metastasis to bone. Endocr Relat Cancer. 2005;12(3):549–583. doi: 10.1677/erc.1.00543. [DOI] [PubMed] [Google Scholar]
- Clines GA, Guise TA. Molecular mechanisms and treatment of bone metastasis. Expert Rev Mol Med. 2008;10:e7. doi: 10.1017/S1462399408000616. [DOI] [PubMed] [Google Scholar]
- Creighton CJ, Chang JC, et al. Epithelial-mesenchymal transition (EMT) in tumor-initiating cells and its clinical implications in breast cancer. J Mammary Gland Biol Neoplasia. 2010;15(2):253–260. doi: 10.1007/s10911-010-9173-1. [DOI] [PubMed] [Google Scholar]
- Croker AK, Allan AL. Cancer stem cells: implications for the progression and treatment of metastatic disease. J Cell Mol Med. 2008;12(2):374–390. doi: 10.1111/j.1582-4934.2007.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai XM, Ryan GR, et al. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood. 2002;99(1):111–120. doi: 10.1182/blood.v99.1.111. [DOI] [PubMed] [Google Scholar]
- Dai J, Lu Y, et al. Reversal of chemotherapy-induced leukopenia using granulocyte macrophage colony-stimulating factor promotes bone metastasis that can be blocked with osteoclast inhibitors. Cancer Res. 2010;70(12):5014–5023. doi: 10.1158/0008-5472.CAN-10-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danen EH, Sonneveld P, et al. The fibronectin-binding integrins alpha5beta1 and alphavbeta3 differentially modulate RhoA-GTP loading, organization of cell matrix adhesions, and fibronectin fibrillogenesis. J Cell Biol. 2002;159(6):1071–1086. doi: 10.1083/jcb.200205014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denoyelle C, Hong L, et al. New insights into the actions of bisphosphonate zoledronic acid in breast cancer cells by dual RhoA-dependent and -independent effects. Br J Cancer. 2003;88(10):1631–1640. doi: 10.1038/sj.bjc.6600925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diel IJ, Jaschke A, et al. Adjuvant oral clodronate improves the overall survival of primary breast cancer patients with micrometastases to the bone marrow: a long-term follow-up. Ann Oncol. 2008;19(12):2007–2011. doi: 10.1093/annonc/mdn429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieli F, Vermijlen D, et al. Targeting human {gamma}delta} T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 2007;67(15):7450–7457. doi: 10.1158/0008-5472.CAN-07-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougall WC, Glaccum M, et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999;13(18):2412–2424. doi: 10.1101/gad.13.18.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facchini G, Caraglia M, et al. The clinical response on bone metastasis from breast and lung cancer during treatment with zoledronic acid is inversely correlated to skeletal related events (SRE) J Exp Clin Cancer Res. 2007;26(3):307–312. [PubMed] [Google Scholar]
- Fehm T, Beck V, et al. Bisphosphonate-induced osteonecrosis of the jaw (ONJ): Incidence and risk factors in patients with breast cancer and gynecological malignancies. Gynecol Oncol. 2009;112(3):605–609. doi: 10.1016/j.ygyno.2008.11.029. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Héry C, et al (2010) Global burden of breast cancer. Breast Cancer Epidemiology. C. Li, Springer New York, 1–19
- Finn RS, Dering J, et al. Dasatinib, an orally active small molecule inhibitor of both the src and abl kinases, selectively inhibits growth of basal-type/“triple-negative” breast cancer cell lines growing in vitro. Breast Cancer Res Treat. 2007;105(3):319–326. doi: 10.1007/s10549-006-9463-x. [DOI] [PubMed] [Google Scholar]
- Fisher JL, Thomas-Mudge RJ, et al. Osteoprotegerin overexpression by breast cancer cells enhances orthotopic and osseous tumor growth and contrasts with that delivered therapeutically. Cancer Res. 2006;66(7):3620–3628. doi: 10.1158/0008-5472.CAN-05-3119. [DOI] [PubMed] [Google Scholar]
- Fizazi K, Lipton A, et al. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol. 2009;27(10):1564–1571. doi: 10.1200/JCO.2008.19.2146. [DOI] [PubMed] [Google Scholar]
- Foekens JA, Atkins D, et al. Multicenter validation of a gene expression-based prognostic signature in lymph node-negative primary breast cancer. J Clin Oncol. 2006;24(11):1665–1671. doi: 10.1200/JCO.2005.03.9115. [DOI] [PubMed] [Google Scholar]
- Fong JE, Nihouannen D, et al. Tumor-supportive and osteoclastogenic changes induced by breast cancer-derived factors are reversed by inhibition of {gamma}-secretase. J Biol Chem. 2010;285(41):31427–31434. doi: 10.1074/jbc.M110.114496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallet M, Sevenet N, et al. Breast cancer cell line MDA-MB 231 exerts a potent and direct anti-apoptotic effect on mature osteoclasts. Biochem Biophys Res Commun. 2004;319(2):690–696. doi: 10.1016/j.bbrc.2004.05.033. [DOI] [PubMed] [Google Scholar]
- Gallet M, Mentaverri R, et al. Ability of breast cancer cell lines to stimulate bone resorbing activity of mature osteoclasts correlates with an anti-apoptotic effect mediated by macrophage colony stimulating factor. Apoptosis. 2006;11(11):1909–1921. doi: 10.1007/s10495-006-9507-z. [DOI] [PubMed] [Google Scholar]
- Gatien M, Benjamin O, et al. Therapeutic approach of primary bone tumours by bisphosphonates. Curr Pharm Des. 2010;16(27):2981–2987. doi: 10.2174/138161210793563554. [DOI] [PubMed] [Google Scholar]
- Gnant MF, Mlineritsch B, et al. Zoledronic acid prevents cancer treatment-induced bone loss in premenopausal women receiving adjuvant endocrine therapy for hormone-responsive breast cancer: a report from the Austrian Breast and Colorectal Cancer Study Group. J Clin Oncol. 2007;25(7):820–828. doi: 10.1200/JCO.2005.02.7102. [DOI] [PubMed] [Google Scholar]
- Gnant M, Mlineritsch B, et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 5-year follow-up of the ABCSG-12 bone-mineral density substudy. Lancet Oncol. 2008;9(9):840–849. doi: 10.1016/S1470-2045(08)70204-3. [DOI] [PubMed] [Google Scholar]
- Gnant M, Mlineritsch B, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360(7):679–691. doi: 10.1056/NEJMoa0806285. [DOI] [PubMed] [Google Scholar]
- Guise TA, Yin JJ, et al. Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J Clin Invest. 1996;98(7):1544–1549. doi: 10.1172/JCI118947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guise TA, Mohammad KS, et al. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin Cancer Res. 2006;12(20 Pt 2):6213s–6216s. doi: 10.1158/1078-0432.CCR-06-1007. [DOI] [PubMed] [Google Scholar]
- Guo Y, Tiedemann K, et al. Osteoclast precursors acquire sensitivity to breast cancer derived factors early in differentiation. Bone. 2008;43(2):386–393. doi: 10.1016/j.bone.2008.03.026. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hassan S, Baccarelli A, et al. Plasma stromal cell-derived factor-1: host derived marker predictive of distant metastasis in breast cancer. Clin Cancer Res. 2008;14(2):446–454. doi: 10.1158/1078-0432.CCR-07-1189. [DOI] [PubMed] [Google Scholar]
- Hassan S, Ferrario C, et al. The influence of tumor-host interactions in the stromal cell-derived factor-1/CXCR4 ligand/receptor axis in determining metastatic risk in breast cancer. Am J Pathol. 2009;175(1):66–73. doi: 10.2353/ajpath.2009.080948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan S, Buchanan M, et al (2010) CXCR4 peptide antagonist inhibits primary breast tumor growth, metastasis and enhances the efficacy of anti-VEGF treatment or docetaxel in a transgenic mouse model. Int J Cancer [DOI] [PubMed]
- Henderson MA, Danks JA, et al. Parathyroid hormone-related protein localization in breast cancers predict improved prognosis. Cancer Res. 2006;66(4):2250–2256. doi: 10.1158/0008-5472.CAN-05-2814. [DOI] [PubMed] [Google Scholar]
- Hershman DL, McMahon DJ, et al. Zoledronic acid prevents bone loss in premenopausal women undergoing adjuvant chemotherapy for early-stage breast cancer. J Clin Oncol. 2008;26(29):4739–4745. doi: 10.1200/JCO.2008.16.4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershman DL, McMahon DJ, et al. Prevention of bone loss by zoledronic acid in premenopausal women undergoing adjuvant chemotherapy persist up to one year following discontinuing treatment. J Clin Endocrinol Metab. 2010;95(2):559–566. doi: 10.1210/jc.2009-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess KR, Varadhachary GR, et al. Metastatic patterns in adenocarcinoma. Cancer. 2006;106(7):1624–1633. doi: 10.1002/cncr.21778. [DOI] [PubMed] [Google Scholar]
- Hiraga T, Williams PJ, et al. Zoledronic acid inhibits visceral metastases in the 4T1/luc mouse breast cancer model. Clin Cancer Res. 2004;10(13):4559–4567. doi: 10.1158/1078-0432.CCR-03-0325. [DOI] [PubMed] [Google Scholar]
- Hiscox S, Barrett-Lee P, et al. Combining Src inhibitors and aromatase inhibitors: a novel strategy for overcoming endocrine resistance and bone loss. Eur J Cancer. 2010;46(12):2187–2195. doi: 10.1016/j.ejca.2010.04.012. [DOI] [PubMed] [Google Scholar]
- Holen I, Cross SS, et al. Osteoprotegerin (OPG) expression by breast cancer cells in vitro and breast tumours in vivo—a role in tumour cell survival? Breast Cancer Res Treat. 2005;92(3):207–215. doi: 10.1007/s10549-005-2419-8. [DOI] [PubMed] [Google Scholar]
- Horne WC, Sanjay A, et al. The role(s) of Src kinase and Cbl proteins in the regulation of osteoclast differentiation and function. Immunol Rev. 2005;208:106–125. doi: 10.1111/j.0105-2896.2005.00335.x. [DOI] [PubMed] [Google Scholar]
- Hotte SJ, Hirte HW, et al. 405 POSTER Final results of a Phase I/II study of CTCE-9908, a novel anticancer agent that inhibits CXCR4, in patients with advanced solid cancers. Eur J Cancer Suppl. 2008;6(12):127–127. [Google Scholar]
- Huang EH, Singh B, et al. A CXCR4 antagonist CTCE-9908 inhibits primary tumor growth and metastasis of breast cancer. J Surg Res. 2009;155(2):231–236. doi: 10.1016/j.jss.2008.06.044. [DOI] [PubMed] [Google Scholar]
- Hugo H, Ackland ML, et al. Epithelial–mesenchymal and mesenchymal–epithelial transitions in carcinoma progression. J Cell Physiol. 2007;213(2):374–383. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- Hussein O, Tiedemann K, et al (2010) Breast cancer cells inhibit spontaneous and bisphosphonate-induced osteoclast apoptosis. Bone 48:202–211 [DOI] [PubMed]
- Id Boufker H, Lagneaux L, et al. The Src inhibitor dasatinib accelerates the differentiation of human bone marrow-derived mesenchymal stromal cells into osteoblasts. BMC Cancer. 2010;10:298. doi: 10.1186/1471-2407-10-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iotsova V, Caamano J, et al. Osteopetrosis in mice lacking NF-kappaB1 and NF-kappaB2. Nat Med. 1997;3(11):1285–1289. doi: 10.1038/nm1197-1285. [DOI] [PubMed] [Google Scholar]
- Jones DH, Nakashima T, et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature. 2006;440(7084):692–696. doi: 10.1038/nature04524. [DOI] [PubMed] [Google Scholar]
- Josse RG, Hanley DA, et al. Diagnosis and treatment of Paget’s disease of bone. Clin Invest Med. 2007;30(5):E210–E223. doi: 10.25011/cim.v30i5.2897. [DOI] [PubMed] [Google Scholar]
- Kakonen SM, Mundy GR. Mechanisms of osteolytic bone metastases in breast carcinoma. Cancer. 2003;97(3 Suppl):834–839. doi: 10.1002/cncr.11132. [DOI] [PubMed] [Google Scholar]
- Kang Y, Siegel PM, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3(6):537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol. 2008;8(4):290–301. doi: 10.1038/nri2279. [DOI] [PubMed] [Google Scholar]
- Kinder M, Chislock E, et al. Metastatic breast cancer induces an osteoblast inflammatory response. Exp Cell Res. 2008;314(1):173–183. doi: 10.1016/j.yexcr.2007.09.021. [DOI] [PubMed] [Google Scholar]
- Kinder M, Chislock E, et al. Metastatic breast cancer induces an osteoblast inflammatory response. Exp Cell Res. 2008;314(1):173–183. doi: 10.1016/j.yexcr.2007.09.021. [DOI] [PubMed] [Google Scholar]
- Kitazawa S, Kitazawa R. RANK ligand is a prerequisite for cancer-associated osteolytic lesions. J Pathol. 2002;198(2):228–236. doi: 10.1002/path.1199. [DOI] [PubMed] [Google Scholar]
- Klein A, Olendrowitz C, et al. Identification of brain- and bone-specific breast cancer metastasis genes. Cancer Lett. 2009;276(2):212–220. doi: 10.1016/j.canlet.2008.11.017. [DOI] [PubMed] [Google Scholar]
- Knowles HJ, Athanasou NA. Canonical and non-canonical pathways of osteoclast formation. Histol Histopathol. 2009;24(3):337–346. doi: 10.14670/HH-24.337. [DOI] [PubMed] [Google Scholar]
- Kodama H, Nose M, et al. Essential role of macrophage colony-stimulating factor in the osteoclast differentiation supported by stromal cells. J Exp Med. 1991;173(5):1291–1294. doi: 10.1084/jem.173.5.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga T, Inui M, et al. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature. 2004;428(6984):758–763. doi: 10.1038/nature02444. [DOI] [PubMed] [Google Scholar]
- Kohno N, Aogi K, et al. Zoledronic acid significantly reduces skeletal complications compared with placebo in Japanese women with bone metastases from breast cancer: a randomized, placebo-controlled trial. J Clin Oncol. 2005;23(15):3314–3321. doi: 10.1200/JCO.2005.05.116. [DOI] [PubMed] [Google Scholar]
- Kollet O, Dar A, et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006;12(6):657–664. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- Kollet O, Dar A, et al. The multiple roles of osteoclasts in host defense: bone remodeling and hematopoietic stem cell mobilization. Annu Rev Immunol. 2007;25:51–69. doi: 10.1146/annurev.immunol.25.022106.141631. [DOI] [PubMed] [Google Scholar]
- Kondo M, Sakuta K, et al. Zoledronate facilitates large-scale ex vivo expansion of functional gammadelta T cells from cancer patients for use in adoptive immunotherapy. Cytotherapy. 2008;10(8):842–856. doi: 10.1080/14653240802419328. [DOI] [PubMed] [Google Scholar]
- Kong YY, Yoshida H, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397(6717):315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- Korah R, Choi L, et al. Expression of FGF-2 alters focal adhesion dynamics in migration-restricted MDA-MB-231 breast cancer cells. Breast Cancer Res Treat. 2004;88(1):17–28. doi: 10.1007/s10459-004-6006-2. [DOI] [PubMed] [Google Scholar]
- Kozlow W, Guise TA. Breast cancer metastasis to bone: mechanisms of osteolysis and implications for therapy. J Mammary Gland Biol Neoplasia. 2005;10(2):169–180. doi: 10.1007/s10911-005-5399-8. [DOI] [PubMed] [Google Scholar]
- Kyrgidis A, Vahtsevanos K, et al. Bisphosphonate-related osteonecrosis of the jaws: a case-control study of risk factors in breast cancer patients. J Clin Oncol. 2008;26(28):4634–4638. doi: 10.1200/JCO.2008.16.2768. [DOI] [PubMed] [Google Scholar]
- Lakkakorpi PT, Vaananen HK. Cytoskeletal changes in osteoclasts during the resorption cycle. Microsc Res Tech. 1996;33(2):171–181. doi: 10.1002/(SICI)1097-0029(19960201)33:2<171::AID-JEMT7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Gall C, Bellahcene A, et al. A cathepsin K inhibitor reduces breast cancer induced osteolysis and skeletal tumor burden. Cancer Res. 2007;67(20):9894–9902. doi: 10.1158/0008-5472.CAN-06-3940. [DOI] [PubMed] [Google Scholar]
- Lee YT. Breast carcinoma: pattern of metastasis at autopsy. J Surg Oncol. 1983;23(3):175–180. doi: 10.1002/jso.2930230311. [DOI] [PubMed] [Google Scholar]
- Lee YC, Huang CF, et al. Src family kinase/abl inhibitor dasatinib suppresses proliferation and enhances differentiation of osteoblasts. Oncogene. 2010;29(22):3196–3207. doi: 10.1038/onc.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone BA, Romero A, et al. Stage IV breast cancer: clinical course and survival of patients with osseous versus extraosseous metastases at initial diagnosis. The GOCS (Grupo Oncologico Cooperativo del Sur) experience. Am J Clin Oncol. 1988;11(6):618–622. [PubMed] [Google Scholar]
- Levine JP. Pharmacologic and nonpharmacologic management of osteoporosis. Clin Cornerstone. 2006;8(1):40–53. doi: 10.1016/s1098-3597(06)80064-5. [DOI] [PubMed] [Google Scholar]
- Li Z, Schem C, et al. Increased COX2 expression enhances tumor-induced osteoclastic lesions in breast cancer bone metastasis. Clin Exp Metastasis. 2008;25(4):389–400. doi: 10.1007/s10585-007-9117-3. [DOI] [PubMed] [Google Scholar]
- Lipton A. Future treatment of bone metastases. Clin Cancer Res. 2006;12(20 Pt 2):6305s–6308s. doi: 10.1158/1078-0432.CCR-06-1157. [DOI] [PubMed] [Google Scholar]
- Lipton A, Cook RJ, et al. Zoledronic acid and survival in breast cancer patients with bone metastases and elevated markers of osteoclast activity. Oncologist. 2007;12(9):1035–1043. doi: 10.1634/theoncologist.12-9-1035. [DOI] [PubMed] [Google Scholar]
- Lipton A, Steger GG, et al. Randomized active-controlled phase II study of denosumab efficacy and safety in patients with breast cancer-related bone metastases. J Clin Oncol. 2007;25(28):4431–4437. doi: 10.1200/JCO.2007.11.8604. [DOI] [PubMed] [Google Scholar]
- Lipton A, Steger GG, et al. Extended efficacy and safety of denosumab in breast cancer patients with bone metastases not receiving prior bisphosphonate therapy. Clin Cancer Res. 2008;14(20):6690–6696. doi: 10.1158/1078-0432.CCR-07-5234. [DOI] [PubMed] [Google Scholar]
- Mancino AT, Klimberg VS, et al. Breast cancer increases osteoclastogenesis by secreting M-CSF and upregulating RANKL in stromal cells. J Surg Res. 2001;100(1):18–24. doi: 10.1006/jsre.2001.6204. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Irie N. Osteoclast-osteoblast communication. Arch Biochem Biophys. 2008;473(2):201–209. doi: 10.1016/j.abb.2008.03.027. [DOI] [PubMed] [Google Scholar]
- McHugh KP, Hodivala-Dilke K, et al. Mice lacking beta3 integrins are osteosclerotic because of dysfunctional osteoclasts. J Clin Invest. 2000;105(4):433–440. doi: 10.1172/JCI8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon B, Kwaan HC. The plasminogen activator system and cancer. Pathophysiol Haemost Thromb. 2008;36(3–4):184–194. doi: 10.1159/000175156. [DOI] [PubMed] [Google Scholar]
- Mendez-Ferrer S, Michurina TV, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meraviglia S, Eberl M, et al. In vivo manipulation of Vgamma9Vdelta2 T cells with zoledronate and low-dose interleukin-2 for immunotherapy of advanced breast cancer patients. Clin Exp Immunol. 2010;161(2):290–297. doi: 10.1111/j.1365-2249.2010.04167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minn AJ, Kang Y, et al. Distinct organ-specific metastatic potential of individual breast cancer cells and primary tumors. J Clin Invest. 2005;115(1):44–55. doi: 10.1172/JCI22320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima S, Nagai A, et al. Effective ex vivo expansion of hematopoietic stem cells using osteoblast-differentiated mesenchymal stem cells is CXCL12 dependent. Eur J Haematol. 2010;84(6):538–546. doi: 10.1111/j.1600-0609.2010.01419.x. [DOI] [PubMed] [Google Scholar]
- Miyazaki T, Tanaka S, et al. The role of c-Src kinase in the regulation of osteoclast function. Mod Rheumatol. 2006;16(2):68–74. doi: 10.1007/s10165-006-0460-z. [DOI] [PubMed] [Google Scholar]
- Mocsai A, Humphrey MB, et al. The immunomodulatory adapter proteins DAP12 and Fc receptor gamma-chain (FcRgamma) regulate development of functional osteoclasts through the Syk tyrosine kinase. Proc Natl Acad Sci USA. 2004;101(16):6158–6163. doi: 10.1073/pnas.0401602101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan H, Tumber A, et al. Breast cancer cells induce osteoclast formation by stimulating host IL-11 production and downregulating granulocyte/macrophage colony-stimulating factor. Int J Cancer. 2004;109(5):653–660. doi: 10.1002/ijc.20056. [DOI] [PubMed] [Google Scholar]
- Mori K, Ando K, et al. Receptor activator of nuclear factor-kappa B ligand (RANKL) stimulates bone-associated tumors through functional RANK expressed on bone-associated cancer cells? Histol Histopathol. 2009;24(2):235–242. doi: 10.14670/HH-24.235. [DOI] [PubMed] [Google Scholar]
- Mourskaia AA, Northey JJ, et al. Targeting aberrant TGF-beta signaling in pre-clinical models of cancer. Anticancer Agents Med Chem. 2007;7(5):504–514. doi: 10.2174/187152007781668689. [DOI] [PubMed] [Google Scholar]
- Mourskaia AA, Dong Z, et al. Transforming growth factor-beta1 is the predominant isoform required for breast cancer cell outgrowth in bone. Oncogene. 2009;28(7):1005–1015. doi: 10.1038/onc.2008.454. [DOI] [PubMed] [Google Scholar]
- Mulari M, Vaaraniemi J, et al. Intracellular membrane trafficking in bone resorbing osteoclasts. Microsc Res Tech. 2003;61(6):496–503. doi: 10.1002/jemt.10371. [DOI] [PubMed] [Google Scholar]
- Muller A, Homey B, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410(6824):50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- Mundy GR. Mechanisms of bone metastasis. Cancer. 1997;80(8 Suppl):1546–1556. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1546::aid-cncr4>3.3.co;2-r. [DOI] [PubMed] [Google Scholar]
- Naito A, Azuma S, et al. Severe osteopetrosis, defective interleukin-1 signalling and lymph node organogenesis in TRAF6-deficient mice. Genes Cells. 1999;4(6):353–362. doi: 10.1046/j.1365-2443.1999.00265.x. [DOI] [PubMed] [Google Scholar]
- Najmi S, Korah R, et al. Flavopiridol blocks integrin-mediated survival in dormant breast cancer cells. Clin Cancer Res. 2005;11(5):2038–2046. doi: 10.1158/1078-0432.CCR-04-1083. [DOI] [PubMed] [Google Scholar]
- Nakamura I, Rodan GA, et al. Regulatory mechanism of osteoclast activation. J Electron Microsc (Tokyo) 2003;52(6):527–533. doi: 10.1093/jmicro/52.6.527. [DOI] [PubMed] [Google Scholar]
- Nakamura I, Duong le T, et al. Involvement of alpha(v)beta3 integrins in osteoclast function. J Bone Miner Metab. 2007;25(6):337–344. doi: 10.1007/s00774-007-0773-9. [DOI] [PubMed] [Google Scholar]
- Naumovski L, Junutula JR. Glembatumumab vedotin, a conjugate of an anti-glycoprotein non-metastatic melanoma protein B mAb and monomethyl auristatin E for the treatment of melanoma and breast cancer. Curr Opin Mol Ther. 2010;12(2):248–257. [PubMed] [Google Scholar]
- Nautiyal J, Majumder P, et al. Src inhibitor dasatinib inhibits growth of breast cancer cells by modulating EGFR signaling. Cancer Lett. 2009;283(2):143–151. doi: 10.1016/j.canlet.2009.03.035. [DOI] [PubMed] [Google Scholar]
- Negishi-Koga T, Takayanagi H. Ca2+-NFATc1 signaling is an essential axis of osteoclast differentiation. Immunol Rev. 2009;231(1):241–256. doi: 10.1111/j.1600-065X.2009.00821.x. [DOI] [PubMed] [Google Scholar]
- Neville-Webbe HL, Cross NA, et al. Osteoprotegerin (OPG) produced by bone marrow stromal cells protects breast cancer cells from TRAIL-induced apoptosis. Breast Cancer Res Treat. 2004;86(3):269–279. doi: 10.1023/b:brea.0000036900.48763.b3. [DOI] [PubMed] [Google Scholar]
- Onishi T, Hayashi N, et al (2010) Future directions of bone-targeted therapy for metastatic breast cancer. Nat Rev Clin Oncol [DOI] [PubMed]
- Ory S, Brazier H, et al. Rho GTPases in osteoclasts: orchestrators of podosome arrangement. Eur J Cell Biol. 2008;87(8–9):469–477. doi: 10.1016/j.ejcb.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Ottewell PD, Monkkonen H, et al. Antitumor effects of doxorubicin followed by zoledronic acid in a mouse model of breast cancer. J Natl Cancer Inst. 2008;100(16):1167–1178. doi: 10.1093/jnci/djn240. [DOI] [PubMed] [Google Scholar]
- Park BK, Zhang H, et al. NF-kappaB in breast cancer cells promotes osteolytic bone metastasis by inducing osteoclastogenesis via GM-CSF. Nat Med. 2007;13(1):62–69. doi: 10.1038/nm1519. [DOI] [PubMed] [Google Scholar]
- Pavlaki M, Zucker S. Matrix metalloproteinase inhibitors (MMPIs): the beginning of phase I or the termination of phase III clinical trials. Cancer Metastasis Rev. 2003;22(2–3):177–203. doi: 10.1023/a:1023047431869. [DOI] [PubMed] [Google Scholar]
- Perou CM, Sorlie T, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- Phadke PA, Mercer RR, et al. Kinetics of metastatic breast cancer cell trafficking in bone. Clin Cancer Res. 2006;12(5):1431–1440. doi: 10.1158/1078-0432.CCR-05-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichot CS, Hartig SM, et al. Dasatinib synergizes with doxorubicin to block growth, migration, and invasion of breast cancer cells. Br J Cancer. 2009;101(1):38–47. doi: 10.1038/sj.bjc.6605101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell GJ, Southby J, et al. Localization of parathyroid hormone-related protein in breast cancer metastases: increased incidence in bone compared with other sites. Cancer Res. 1991;51(11):3059–3061. [PubMed] [Google Scholar]
- Rachner TD, Benad P, et al. Osteoprotegerin production by breast cancer cells is suppressed by dexamethasone and confers resistance against TRAIL-induced apoptosis. J Cell Biochem. 2009;108(1):106–116. doi: 10.1002/jcb.22232. [DOI] [PubMed] [Google Scholar]
- Rack B, Juckstock J, et al. Effect of zoledronate on persisting isolated tumour cells in patients with early breast cancer. Anticancer Res. 2010;30(5):1807–1813. [PubMed] [Google Scholar]
- Reddi AH, Roodman D, et al. Mechanisms of tumor metastasis to the bone: challenges and opportunities. J Bone Miner Res. 2003;18(2):190–194. doi: 10.1359/jbmr.2003.18.2.190. [DOI] [PubMed] [Google Scholar]
- Richert MM, Vaidya KS, et al. Inhibition of CXCR4 by CTCE-9908 inhibits breast cancer metastasis to lung and bone. Oncol Rep. 2009;21(3):761–767. [PubMed] [Google Scholar]
- Rose AA, Pepin F, et al. Osteoactivin promotes breast cancer metastasis to bone. Mol Cancer Res. 2007;5(10):1001–1014. doi: 10.1158/1541-7786.MCR-07-0119. [DOI] [PubMed] [Google Scholar]
- Rose AA, Siegel PM. Emerging therapeutic targets in breast cancer bone metastasis. Future Oncol. 2010;6(1):55–74. doi: 10.2217/fon.09.138. [DOI] [PubMed] [Google Scholar]
- Rose AA, Grosset AA, et al. Glycoprotein nonmetastatic B is an independent prognostic indicator of recurrence and a novel therapeutic target in breast cancer. Clin Cancer Res. 2010;16(7):2147–2156. doi: 10.1158/1078-0432.CCR-09-1611. [DOI] [PubMed] [Google Scholar]
- Rosen LS, Gordon D, et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer. 2003;98(8):1735–1744. doi: 10.1002/cncr.11701. [DOI] [PubMed] [Google Scholar]
- Rosen LS, Gordon DH, et al. Zoledronic acid is superior to pamidronate for the treatment of bone metastases in breast carcinoma patients with at least one osteolytic lesion. Cancer. 2004;100(1):36–43. doi: 10.1002/cncr.11892. [DOI] [PubMed] [Google Scholar]
- Russell RG. Bisphosphonates: mode of action and pharmacology. Pediatrics. 2007;119(Suppl 2):S150–S162. doi: 10.1542/peds.2006-2023H. [DOI] [PubMed] [Google Scholar]
- Saad F, Lipton A. SRC kinase inhibition: targeting bone metastases and tumor growth in prostate and breast cancer. Cancer Treat Rev. 2010;36(2):177–184. doi: 10.1016/j.ctrv.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Saltel F, Chabadel A, et al. Actin cytoskeletal organisation in osteoclasts: a model to decipher transmigration and matrix degradation. Eur J Cell Biol. 2008;87(8–9):459–468. doi: 10.1016/j.ejcb.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Santini D, Vincenzi B, et al. Repeated intermittent low-dose therapy with zoledronic acid induces an early, sustained, and long-lasting decrease of peripheral vascular endothelial growth factor levels in cancer patients. Clin Cancer Res. 2007;13(15 Pt 1):4482–4486. doi: 10.1158/1078-0432.CCR-07-0551. [DOI] [PubMed] [Google Scholar]
- Santini D, Perrone G, et al (2010) Expression pattern of receptor activator of NFkappaB (RANK) in a series of primary solid tumors and related bone metastases. J Cell Physiol [DOI] [PubMed]
- Schneider A, Kalikin LM, et al. Bone turnover mediates preferential localization of prostate cancer in the skeleton. Endocrinology. 2005;146(4):1727–1736. doi: 10.1210/en.2004-1211. [DOI] [PubMed] [Google Scholar]
- Schubert A, Schulz H, et al. Expression of osteoprotegerin and receptor activator of nuclear factor-kappaB ligand (RANKL) in HCC70 breast cancer cells and effects of treatment with gonadotropin-releasing hormone on RANKL expression. Gynecol Endocrinol. 2008;24(6):331–338. doi: 10.1080/09513590802095845. [DOI] [PubMed] [Google Scholar]
- Sherry MM, Greco FA, et al. Breast cancer with skeletal metastases at initial diagnosis. Distinctive clinical characteristics and favorable prognosis. Cancer. 1986;58(1):178–182. doi: 10.1002/1097-0142(19860701)58:1<178::aid-cncr2820580130>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Sherry MM, Greco FA, et al. Metastatic breast cancer confined to the skeletal system. An indolent disease. Am J Med. 1986;81(3):381–386. doi: 10.1016/0002-9343(86)90286-x. [DOI] [PubMed] [Google Scholar]
- Shimo T, Kubota S, et al. Pathogenic role of connective tissue growth factor (CTGF/CCN2) in osteolytic metastasis of breast cancer. J Bone Miner Res. 2006;21(7):1045–1059. doi: 10.1359/jbmr.060416. [DOI] [PubMed] [Google Scholar]
- Siclari VA, Guise TA, et al. Molecular interactions between breast cancer cells and the bone microenvironment drive skeletal metastases. Cancer Metastasis Rev. 2006;25(4):621–633. doi: 10.1007/s10555-006-9023-1. [DOI] [PubMed] [Google Scholar]
- Singh B, Berry JA, et al. Involvement of IL-8 in COX-2-mediated bone metastases from breast cancer. J Surg Res. 2006;134(1):44–51. doi: 10.1016/j.jss.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Slade MJ, Coombes RC. The clinical significance of disseminated tumor cells in breast cancer. Nat Clin Pract Oncol. 2007;4(1):30–41. doi: 10.1038/ncponc0685. [DOI] [PubMed] [Google Scholar]
- Smid M, Wang Y, et al. Genes associated with breast cancer metastatic to bone. J Clin Oncol. 2006;24(15):2261–2267. doi: 10.1200/JCO.2005.03.8802. [DOI] [PubMed] [Google Scholar]
- Smid M, Wang Y, et al. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 2008;68(9):3108–3114. doi: 10.1158/0008-5472.CAN-07-5644. [DOI] [PubMed] [Google Scholar]
- Solomayer EF, Diel IJ, et al. Metastatic breast cancer: clinical course, prognosis and therapy related to the first site of metastasis. Breast Cancer Res Treat. 2000;59(3):271–278. doi: 10.1023/a:1006308619659. [DOI] [PubMed] [Google Scholar]
- Soriano P, Montgomery C, et al. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991;64(4):693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- Sorlie T, Perou CM, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98(19):10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorlie T, Tibshirani R, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA. 2003;100(14):8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Ejiri S, et al. Regulation of osteoclast polarization. Odontology. 2007;95(1):1–9. doi: 10.1007/s10266-007-0071-y. [DOI] [PubMed] [Google Scholar]
- Tiedemann K, Hussein O, et al. Breast cancer-derived factors stimulate osteoclastogenesis through the Ca2+/protein kinase C and transforming growth factor-beta/MAPK signaling pathways. J Biol Chem. 2009;284(48):33662–33670. doi: 10.1074/jbc.M109.010785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjensvoll K, Oltedal S, et al. Disseminated tumor cells in bone marrow assessed by TWIST1, cytokeratin 19, and mammaglobin A mRNA predict clinical outcome in operable breast cancer patients. Clin Breast Cancer. 2010;10(5):378–384. doi: 10.3816/CBC.2010.n.050. [DOI] [PubMed] [Google Scholar]
- Tomaskovic-Crook E, Thompson EW, et al. Epithelial to mesenchymal transition and breast cancer. Breast Cancer Res. 2009;11(6):213. doi: 10.1186/bcr2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tondravi MM, McKercher SR, et al. Osteopetrosis in mice lacking haematopoietic transcription factor PU.1. Nature. 1997;386(6620):81–84. doi: 10.1038/386081a0. [DOI] [PubMed] [Google Scholar]
- Trimboli AJ, Fukino K, et al. Direct evidence for epithelial-mesenchymal transitions in breast cancer. Cancer Res. 2008;68(3):937–945. doi: 10.1158/0008-5472.CAN-07-2148. [DOI] [PubMed] [Google Scholar]
- Vaananen HK, Hentunen T, et al. Mechanism of osteoclast mediated bone resorption. Ann Chir Gynaecol. 1988;77(5–6):193–196. [PubMed] [Google Scholar]
- Vaananen HK, Laitala-Leinonen T. Osteoclast lineage and function. Arch Biochem Biophys. 2008;473(2):132–138. doi: 10.1016/j.abb.2008.03.037. [DOI] [PubMed] [Google Scholar]
- Vaananen HK, Zhao H, et al. The cell biology of osteoclast function. J Cell Sci. 2000;113(Pt 3):377–381. doi: 10.1242/jcs.113.3.377. [DOI] [PubMed] [Google Scholar]
- Veer LJ, Dai H, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415(6871):530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- Vijver MJ, He YD, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347(25):1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- Overstraeten-Schlogel N, Beguin Y, et al. Role of stromal-derived factor-1 in the hematopoietic-supporting activity of human mesenchymal stem cells. Eur J Haematol. 2006;76(6):488–493. doi: 10.1111/j.1600-0609.2006.00633.x. [DOI] [PubMed] [Google Scholar]
- Metzler I, Krebbel H, et al. Bortezomib inhibits human osteoclastogenesis. Leukemia. 2007;21(9):2025–2034. doi: 10.1038/sj.leu.2404806. [DOI] [PubMed] [Google Scholar]
- Wada T, Nakashima T, et al. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med. 2006;12(1):17–25. doi: 10.1016/j.molmed.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Wang Y, Klijn JG, et al. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet. 2005;365(9460):671–679. doi: 10.1016/S0140-6736(05)17947-1. [DOI] [PubMed] [Google Scholar]
- Wang J, Loberg R, et al. The pivotal role of CXCL12 (SDF-1)/CXCR4 axis in bone metastasis. Cancer Metastasis Rev. 2006;25(4):573–587. doi: 10.1007/s10555-006-9019-x. [DOI] [PubMed] [Google Scholar]
- Wang ZQ, Ovitt C, et al. Bone and haematopoietic defects in mice lacking c-fos. Nature. 1992;360(6406):741–745. doi: 10.1038/360741a0. [DOI] [PubMed] [Google Scholar]
- Weiss L. Comments on hematogenous metastatic patterns in humans as revealed by autopsy. Clin Exp Metastasis. 1992;10(3):191–199. doi: 10.1007/BF00132751. [DOI] [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W, Bartocci A, et al. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci USA. 1990;87(12):4828–4832. doi: 10.1073/pnas.87.12.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willipinski-Stapelfeldt B, Riethdorf S, et al. Changes in cytoskeletal protein composition indicative of an epithelial-mesenchymal transition in human micrometastatic and primary breast carcinoma cells. Clin Cancer Res. 2005;11(22):8006–8014. doi: 10.1158/1078-0432.CCR-05-0632. [DOI] [PubMed] [Google Scholar]
- Wilson TJ, Nannuru KC, et al. Cathepsin G enhances mammary tumor-induced osteolysis by generating soluble receptor activator of nuclear factor-kappaB ligand. Cancer Res. 2008;68(14):5803–5811. doi: 10.1158/0008-5472.CAN-07-5889. [DOI] [PubMed] [Google Scholar]
- Wilson TJ, Nannuru KC, et al. Cathepsin G recruits osteoclast precursors via proteolytic activation of protease-activated receptor-1. Cancer Res. 2009;69(7):3188–3195. doi: 10.1158/0008-5472.CAN-08-1956. [DOI] [PubMed] [Google Scholar]
- Yang L, Moses HL. Transforming growth factor beta: tumor suppressor or promoter? Are host immune cells the answer? Cancer Res. 2008;68(22):9107–9111. doi: 10.1158/0008-5472.CAN-08-2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin JJ, Selander K, et al. TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Invest. 1999;103(2):197–206. doi: 10.1172/JCI3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota A, Kimura S, et al. Osteoclasts are involved in the maintenance of dormant leukemic cells. Leuk Res. 2010;34(6):793–799. doi: 10.1016/j.leukres.2009.08.034. [DOI] [PubMed] [Google Scholar]
- Zaidi M, Pazianas M, et al. Osteoclast function and its control. Exp Physiol. 1993;78(6):721–739. doi: 10.1113/expphysiol.1993.sp003721. [DOI] [PubMed] [Google Scholar]
- Zhang XH, Wang Q, et al. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell. 2009;16(1):67–78. doi: 10.1016/j.ccr.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuradelli M, Masci G, et al. High incidence of hypocalcemia and serum creatinine increase in patients with bone metastases treated with zoledronic acid. Oncologist. 2009;14(5):548–556. doi: 10.1634/theoncologist.2008-0227. [DOI] [PubMed] [Google Scholar]