Abstract
Entrapment of mammalian cells in natural or synthetic biomaterials represents an important tool for both basic and applied research in tissue engineering. For instance, the encapsulation procedures allow to physically isolate cells from the surrounding environment, after their transplantation maintaining the normal cellular physiology. The first part of the current paper describes different microencapsulation techniques including bulk emulsion technique, vibrating-nozzle procedure, gas driven mono-jet device protocol and microfluidic based approach. In the second part, the application of a microencapsulation procedure to the embedding of IB3-1 cells is also described. IB3-1 is a bronchial epithelial cell line, derived from a cystic fibrosis (CF) patient. Different experimental parameters of the encapsulation process were analyzed, including frequency and amplitude of vibration, polymer pumping rate and distance between the nozzle and the gelling bath. We have found that the microencapsulation procedure does not alter the viability of the encapsulated IB3-1 cells. The encapsulated IB3-1 cells were characterized in term of protein secretion, analysing the culture medium by Bio-Plex strategy. The analyzed factors include members of the interleukin family (IL-6), chemokines (IL-8 and MCP-1) and growth factors (G-CSF). The experiments demonstrated that most of the analyzed proteins, were secreted both by the free and encapsulated cells, even if in a different extent.
Keyword: Biomaterials, Encapsulation, Alginate, Cystic fibrosis
Introduction
Every organ that can be broken apart into single cells or cell clusters, without disrupting the original function is potentially suitable for generating bioartificial organs that can be applied to restore or improve damaged tissue function (Goldstein 2002). However, it is generally difficult to preserve the functions of cells when they are implanted in environmental conditions, usually very different from their native site.
A key role in preserving the cell functions is played by biomaterials, that are increasingly important for the development of tissue engineering devices. For instance, biomaterials can be used to produce cellular scaffolds suitable for implanting cells into the host or immobilizing them for long-term systemic delivery of biomolecules.
In this respect, it should be considered that only few biomaterials are fully biocompatible towards both the immobilised cells and the host’s tissue, and special care must be taken not only to assess the material’s physical-chemical properties and biocompatibility, but also to select the tissue’s sources, in compliance with physiological competence and safety principles.
Among biomaterials, alginate or other polysaccharides with similar properties, such as pectins, carrageenans, xanthans, chitosans and natural and semi-synthetic celluloses. occupy a prominent position and they has been very often proposed as biomaterial for cell encapsulation/immobilization.
These polysaccharides require very mild conditions for their gelation and they are, biocompatible, cheap and worldwide commercially available.
For instance, alginates are a family of unbranched binary copolymers of 1→4 linked β-D-mannuronic acid (M) and α-L-guluronic acid (G), of widely varying compositions and sequential structures. These differences are very important since they influence alginate characteristics such as gel properties, biocompatibility, stability, mechanical resistance, permeability, biodegradability and swelling behaviour.
Alginate possess the ability to form gels by reaction of divalent cations with G blocks in a selective and cooperative fashion (Strand et al. 2000). For this reason alginate cross-linked with Ca or Ba ions has been used successfully to encapsulate cells and to maintain their function in tissue culture (De Vos et al. 2006). Cells immobilized in alginate gels maintain good viability during long-term culture due to the mild environment of the gel network.
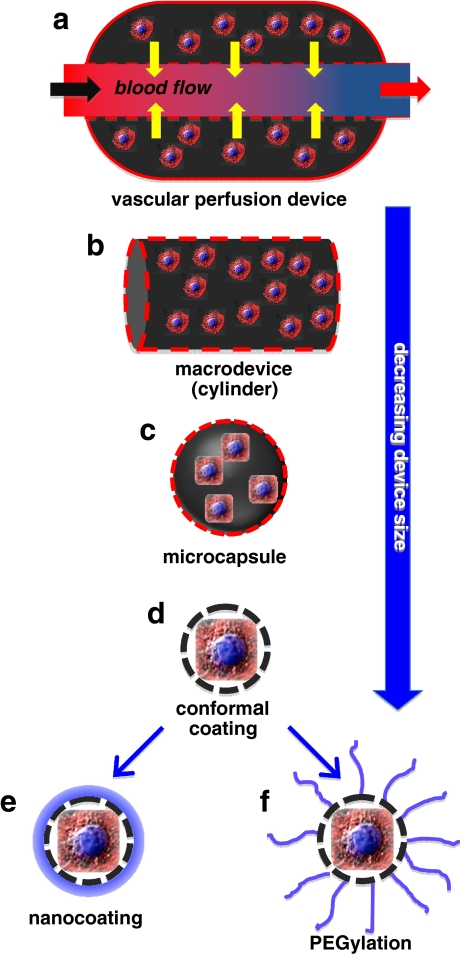
Different prototypes of Bio-Artificial Organs (BAO) have been designed and developed over the past few years and in general, it is still valid the classification that keeps macro- and micro-devices as distinct systems (see Fig. 1).
Fig. 1.
General scheme of biomaterials based scaffolds suitable for in vivo cells transplantation protocols. Vascular chamber containing matrix-embedded cells (a); macrodevice (cylinder) containing alginate encapsulated cells (b); microcapsule containing embeded cells (c); conformal microcapsule (d); nanocoated microcapsule (e) and finally PEGylated microcapsule (f)
An example of the use of macrodevices for cell transplantation is represented by the seeding of cells in a special compartment chamber, directly anastomosed to blood vessels, usually as arterio-vein shunts. In this way, the cells are continuously perfused by blood ultrafiltrate, which seemingly facilitated biochemical exchange. The membrane, at contact with the blood stream, is associated with an appropriate molecular weight cut off (commonly below 100 kD) to avoid that immune cells or antibodies would cross the cell containing chamber.
Among micro-devices, microcapsules represent the most widely known and studied microimmunobarrier system for cell transplantation and numerous cell microencapsulation techniques have been developed over the years.
Figures 2, 3, 4 and 5 report the general schemes of a selected number of encapsulation procedures, including among others: bulk emulsion technique (Fig. 2), vibrating-nozzle procedure (Fig. 3), gas driven mono-jet device protocol (Fig. 4) and microfluidic based approach (Fig. 5). Nevertheless, many others microencapsulation techniques have been also proposed such as spinning disk atomization, coaxial airflow dropping, jetcutter technology and electrostatic droplet generation technology (Herrero et al. 2006).
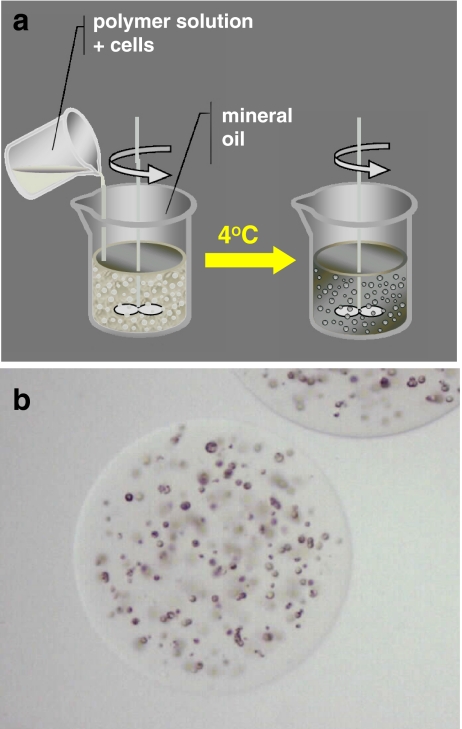
Fig. 2.
Schematic representation of the encapsulation procedure based on bulk emulsion technique and relative optical stereophotomicrographs of an agarose microcapsule
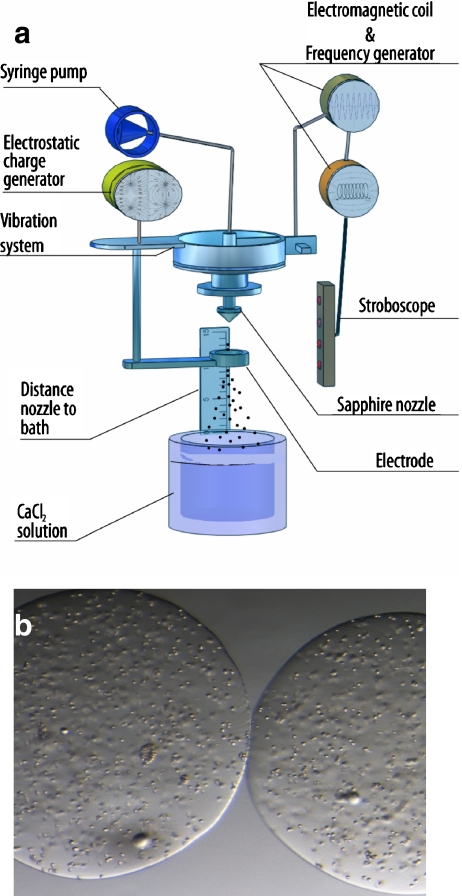
Fig. 3.
Schematic representation of the encapsulation procedure based on vibrating nozzle device and relative optical sterephotomicrographs of alginate based microcapsules
Fig. 4.
Schematic representation of the encapsulation procedure based on gas driven mono-jet device and relative optical stereophotomicrographs of alginate based microcapsules
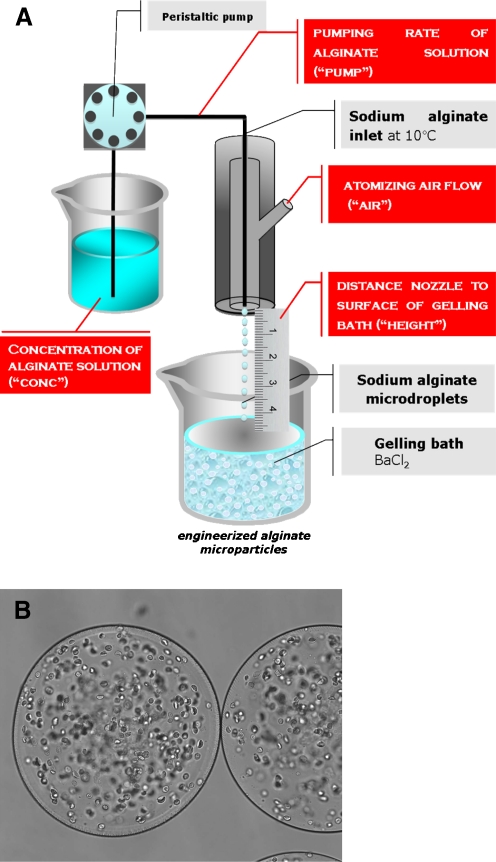
Fig. 5.
Schematic representation of the encapsulation procedure based on microfluidic technique and relative optical stereophotomicrographs of microchip and alginate based microcapsules
The scheme of the preparation procedure reported in Fig. 2, summarizes the encapsulation procedure suitable for the production of agarose based microcapsules. Agarose, like alginate, is a gelling polymer extracted from seaweeds, and it is composed of repeating units of alternating β-D-galactopyranosyl and 3,6-anhydro-α-L-galactopyranosyl. Depending upon temperature conditions, agarose forms thermally reversible gels, with gelling occurring at temperatures that are far below the gel fusion point [23]. For instance, bulk emulsion techniques can be used to produce agarose microcapsules to immunoprotect transplanted cells from the host’s immune attack.
Typically, 0.5–5% (w/v) agarose solutions can be employed to obtain microcapsules, Microparticles are usually manufactured following a general experimental procedure that consists of few easy steps: (a) dissolution of agarose powder in water heated at 80°C, (b) emulsification of the agarose solution into an oil phase at 37°C with a suitable stabilizer, (c) cooling of the emulsion to a temperature below the agarose gelling point, in order to obtain the droplet gelation, (d) isolation of the obtained microparticles after washing several times with ethanol and water to eliminate the oil, by centrifugation (see Fig. 2b).
The obtained agarose microcapsules present no major problems adversely affecting the enveloped cell viability.
The scheme of the preparation procedure reported in Fig. 3, summarizes the encapsulation procedure based on a vibrating-nozzle instrumentation that is suitable for the production of a large range of polysaccharic microcapsules.
The encapsulation device based on a vibrating-nozzle is composed (see Fig. 3a) of a 2-liter glass reaction vessel with stainless steel top and bottom plates. The top plate contains a feed-line connected to a syringe or hydraulic polymer reservoir and a nozzle. A nozzle with an internal diameter of 300 mm was used. The nozzle is connected, via a steel or PTFE membrane, to a vibrating device, which is insulated from the surrounding structures by rubber mounts to avoid the generation of resonance frequencies in the system. The flow of polymer to the nozzle is achieved by a precision syringe.
The morphological and dimensional characteristics of polysaccharidic microcapsules can be varied by changing different experimental parameters such as the nozzle vibrational frequency, the nozzle vibrational amplitude, the polymer pumping rate and the distance between the nozzle and the surface of the gelling bath. This method results in the production of microcapsules with optimal characteristic for cell trasplantation protocols, at least from a morphological point of view, such as, spherical shape, smooth surface, very narrow size distribution, absence of tails and coalescences (see Fig. 3b).
The scheme of the preparation procedure reported in Fig. 4, summarizes the encapsulation procedure based on a gas driven mono-jet device protocol that probably represent the most used technique for the production of polysaccharic microcapsules intended for cell encapsulation.
The encapsulation system is composed by a gas driven mono-jet device connected to a precision peristaltic pump (for the feeding of the different alginate dispersions) and to a gas flask (usually nitrogen) equipped with a flow meter (providing the gas for the atomization of the alginate dispersion). Typically, the cell suspension is continuously mixed by a magnetic stirrer to prevent cell clumping, which would possibly lead to inhomogeneous cell distribution within the microparticles. Also by this technique, different microparticle can be prepared by changing the following experimental parameters: nozzle-to-gelling bath distance, atomizing gas, feeding rate and polymer concentration. The microdroplets generated by the gas driven mono-jet device are transformed into microparticles (see Fig. 4b) by an ionic gelation procedure using a gelling bath generally constituted by a divalent cation solutions.
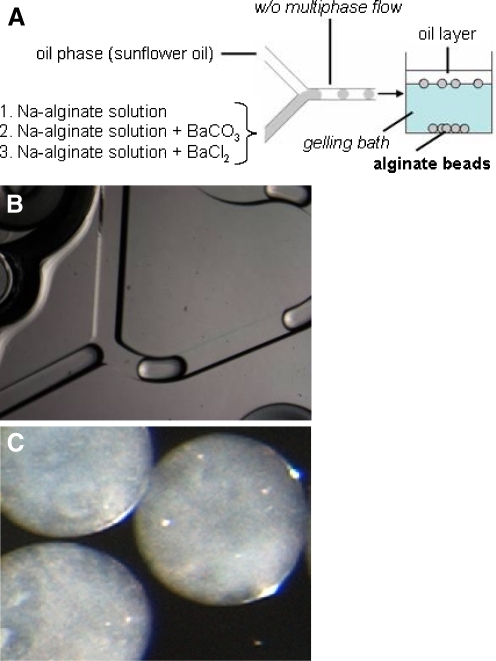
Recently different methods to obtain polysaccharidic microparticles by microfluidic approaches have been developed.
In this respect, in Fig. 5 is reported the general scheme of the use of microfluidic devices for the production of alginate microparticles.
For the injection, into the microfluidic chip, of both dispersed and continuous phases, syringe/syringe pump systems are usually employed, that are connected through silicone tubes. Sodium alginate dispersions are used as aqueous internal phase and injected into a reagent inlet of a squeezing geometry microchannel (Fig. 5b). The second immiscible liquid is injected into the other inlet as continuous phase to form a multiphasic flow (droplets) represented by a w/o emulsion. Finally, the Na-alginate microdroplets were gelled by dripping, the formed w/o emulsion, into a gelling solution, to produce the final consolidated microcapsules (Fig. 5c).
The current paper has two main aims, firstly in order to enlarge the number of different living cells efficiently encapsulated in polysaccharidic based microcapsules, the feasibility to entrap IB3-1 cells into alginate microcapsules, by a gas driven mono-jet device protocol, was analyzed. Secondly, and more importantly, the IB3-1 cells encapsulated into microcapsules were employed, as model system, to study the cell signaling by cytokines through the capsular structure. (Fig. 6)
Fig. 6.
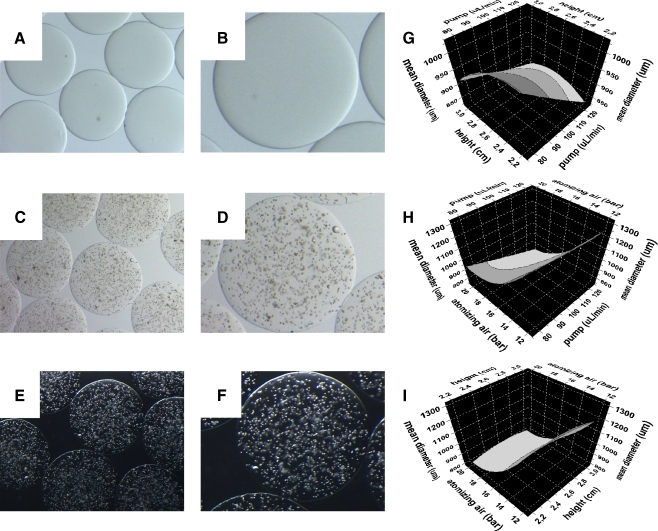
Optical stereophotomicrographs of empty (a, b) and IB3-1 containing alginate microcapsules (c–f) produced by the mono-jet air driven device. Response surface plots, generated by DoE analysis, showing the relationship between the experimental factors height vs pump (g), pump vs air (h) and pump vs height (i) and the response size, representing the mean diameter of the produced microcapsules
In fact, IB3-1 is a bronchial epithelial cell line, derived from a cystic fibrosis (CF) patient with a CFTR genotype of F508del/W1282X, therefore carrying the associated cystic fibrosis mutation (Bezzerri et al. 2008). This cell line can be induced to high expression of pro-inflammatory proteins, following infection with Pseudomonas aeruginosa or by treatment with TNF-α. Twenty-four hours treatment with TNF-α is usually sufficient to induce, in the IB3-1 cells, a deep alteration of mRNA expression and protein secretion profile with a typical increase of IL-6 and IL-8 mRNA and IL-6/IL-8 release (Borgatti et al. 2007; Bezzerri et al. 2008).
Taking into consideration what stated above, it would be of great interest to develop a specific system to possibly study the mechanism of bacterial activation of IB3-1 cell, as well as the effect of the secreted chemokines on target cell populations, in co-colture experiments. For instance, IB3-1 cells could be co-cultured with Pseudomonas aeruginosa or polymorphonuclear cells (PMN), the major phagocytic cell of blood and also with other inflammatory cells such as basophils, eosinophils and T-cells (Lund and Østerud 2004; Terheggen-Lagro et al. 2005).
Summarizing, the paper describes: (a) the encapsulation for IB3-1 cells in alginate microcapsules following a microencapsulation procedure developed in our laboratory that is based on the generation of monodisperse droplets by an air-driven droplet generator for cell encapsulation; (b) the characterization of alginate microcapsules produced, with different experimental parameters, including atomizing air, polymer pumping rate and distance between the nozzle and the gelling bath; (c) the determination of viability of the encapsulated IB3-1 cells and finally (d) the characterization of the encapsulated IB3-1 cells, in term of secretomic profile, analysing the culture medium by Bio-Plex strategy (Rizzo et al. 2009; Borgatti et al. 2008). The analyzed factors include members of the interleukin family, chemokines, growth factors and soluble forms of adhesion molecules.
Materials and methods
Encapsulation of IB3-1 cells
IB3-1 cells were obtained from LGC Promochem. Cells were grown in LHC-8 basal medium (Biofluids, Rockville, MD, USA), supplemented with 5% FBS in the absence of gentamycin. Alginate microcapsules containing IB3-1 cells were prepared using an air-driven droplet generator. Briefly, IB3-1 cells were suspended in a 1.5% (w/v) aqueous solution of highly purified sodium alginate (Inotech, Dottikon, Switzerland) at a concentration of 8–12 × 106 cells/mL. The resulting cell suspension was continuously aspirated by a syringe pump and extruded through the air-driven droplet generator, under sterile conditions. The generated microdroplets were harderned by an ionotropic gelling process into a 1.2% (w/v) barium chloride solution that resulted in the production of barium alginate microcapsules. After 3 min incubation into the gelling bath, the microcapsules were washed twice with saline and placed in LHC-8 basal medium (Biofluids), supplemented with 5% FBS at 37°C in an humidified atmosphere of 5% CO2.
To study the effect and the influence of different experimental parameters on the size and size distribution of alginate microcapsules, a randomized central composite face-centered design (CCF) consisting of 17 runs, was used. The experimental design and the evaluation of the experiments were performed by the PC software MODDE 8.0 (Umetrics AB, Sweden), followed by multiple linear regression (MLR) algorithms. The following experimental parameters (“factors”) were considered: the atomizing air flow (“air”), the alginate pumping rate (“pump”) and the distance between the nozzle of air-driven droplet generator and the surface of the gelling bath (“height”).
Characterization of IB3-1 containing alginate microcapsules
The morphology of barium alginate microcapsules was evaluated by optical microscopy and stereomicroscopy (Nikon microscopes, Tokyo, Japan). Microcapsule size was determined by photomicrograph analyses (Eclipsenet version 1.16.5; Laboratory AU5c Imaging s.r.o. for Nikon B.V.). Samples of 200–400 beads were considered.
After encapsulation, the viability of IB3-1 cells was analyzed by double staining with propidium iodide (PI) and Calcein-AM, following manufacturer’s instructions. For the propidium iodide (PI) and Calcein-AM analysis, cells were visualized under a fluorescence microscope (Nikon, Optiphot-2, Nikon Corporation, Japan). Viable cells were stained in green, while the dead ones were stained in red.
Treatment of IB3-1 with TNF-α
Treatment of monolayers: IB3-1 cells were seeded at the initial concentration of 30,000 cells/cm2 and the cell number/ml determined after 3 days of culture. Cell number/ml was determined after trypsin treatment by using a model ZBI Coulter Counter (Coulter Electronics, Hialeah, FL, USA). Treatment of monolayers with 80 ng/ml TNF-α (PeProTech EC, London, UK) was performed on 70% confluent cells for 24 h. Treatment of encapsulated cells: equal quantity (20 × 106 cells) of free and encapsulated cells (derived from the same flask, previously cultured for 3 days and successively detached by trypsin) were treated with 80 ng/ml TNF-α for 24 h.
Quantification of IL-8 transcripts
Total RNA was isolated (High Pure RNA isolation kit, Roche), retro transcribed (Promega Corporation, Madison, USA) and the resulting cDNA was quantified by relative quantitative real-time PCR. The sequences of the oligonucleotides used for amplification of IL-8 mRNA were: 5′-GTG CAG TTT TGC CAA GGA GT-3′ (forward) and 5′-TTA TGA ATT CTC AGC CCT CTT CAA AAA CTT CTC-3′ (reverse); for GAPDH mRNA: 5′-AAG GTC GGA GTC AAC GGA TTT-3′ (forward); 5′-ACT GTG GTC ATG AGT CCT TCC A-3′ (reverse). For PCR, 0,5/20 μl aliquots of cDNA were used for each Sybr Green real-time PCR reaction to quantify the relative tissue expression of IL-8 transcripts. Each 25 μl of total reaction volume contained 0.5 μl of cDNA, 10 pmol of primers, 1× iQ™ SYBR® Green Supermix (Bio-Rad Laboratories, Hercules, CA). Real-time PCR reactions were performed for a total of 40 cycles (denaturation, 95°C for 10 s; annealing, 68°C for 30 s for IL-8, 65°C for 30 s for IL-6; elongation, 72°C for 60 s) using an iCycler IQ® (Bio-Rad Laboratories, Hercules, CA). The relative proportions of each template amplified were determined based on the threshold cycle (Tc) value for each PCR reaction. The ΔΔCt method was used to compare gene expression data. Each sample was quantified in duplicate from at least two independent experiments. Mean ± S.D. values were determined for each fold difference. Amplification of human GAPDH cDNA served as internal standards (housekeeping gene). Duplicate negative controls (no template cDNA) were also run with every experimental plate to assess specificity and indicate potential contamination.
Production of IL-8
Cytokines in tissue culture supernatants released from the cells under analysis, were measured by Bio-Plex cytokine assay (Bio-Rad Laboratories, Hercules, CA) (Borgatti et al. 2008) as described by the manufacturer. The Bio-Plex cytokine assay is designed for the multiplexed quantitative measurement of multiple cytokines in a single well using as little as 50 μl of sample. In our experiments, the premixed multiplex beads of the Bio-Plex human cytokine 7-plex which included seven cytokines, including IL-8, were incubated with 50 μl of anti-cytokine conjugated beads in 96-well filter plates for 30 min at room temperature with shaking. Plates were then washed by vacuum filtration three times with 100 μl of Bio-Plex wash buffer, 25 μl of diluted detection antibody were added, and plates were incubated for 30 min at room temperature with shaking. After three filter washes, 50 μl of streptavidin-phycoerythrin was added, and the plates were incubated for 10 min at room temperature with shaking. Finally, plates were washed by vacuum filtration three times, beads were suspended in Bio-Plex assay buffer, and samples were analyzed on a Bio-Rad 96-well plate reader using the Bio-Plex Suspension Array System and Bio-Plex Manager software (Bio-Rad Laboratories, Hercules, CA).
Results and discussion
IB3-1 cell encapsulation in alginate microcapsules
IB3-1 cells were embedded into alginate microcapsules by a air-driven droplet generator for cell encapsulation (see scheme in Fig. 4). The encapsulation procedure consisted of a limited number of steps. In order to achieve complete biocompatibility, essential for mammalian cells, the entire procedure was conducted at room temperature, under physiologic pH and tonicity using a pyrogen-free alginate. The hardening of the generated alginate microdroplets was accomplished by an ionic gelation procedure, by barium chloride. The resulting barium alginate microcapsules were elastic and transparent, thus facilitating the microscopic observation of cell morphology and viability, during the in vitro studies, as evident by the brightfield and darkfield stereophotomicrographs shown in Fig. 7.
Fig. 7.
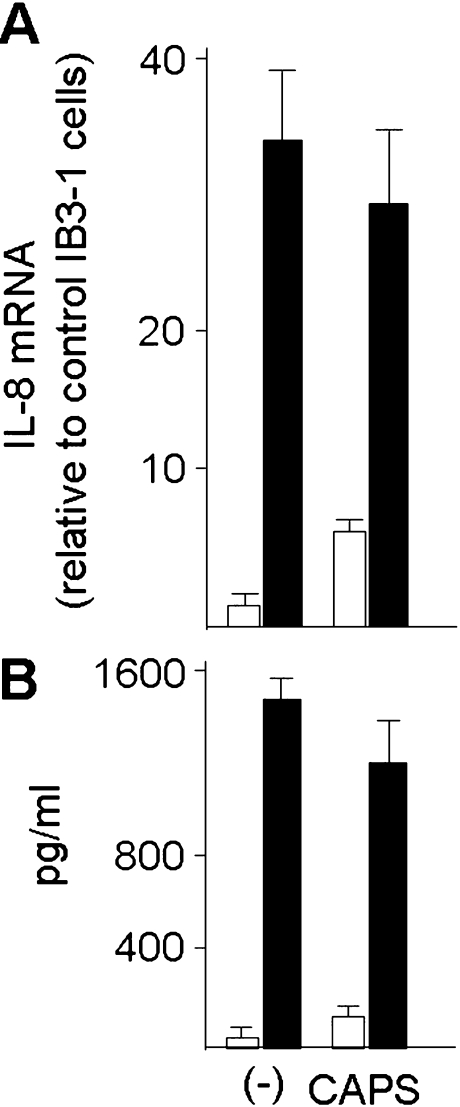
Analysis of the mRNA levels (a) and release (b) of IL-8 by IB3-1 cells. Data are referred to control free cells growing as monolayer (-) and cells encapsulated in alginate microcapsules (CAPS). Both free and encapsulated cells were cultured for 24 h, in the absence (open bars) or in the presence of TNF-α 80 ng/ml) (closed bars). Data represent the average of three independent experiments±SD
For the screening and optimisation of the experimental parameters, a “design of the experiments” (DoE), was performed, after design by MODDE software. The DoE reduces the number of experiments and provides statistical information about the effects of different variables and their possible interactions.
The high and low values of each variable were defined based on preliminary experiments.
The factors/responses and the general results of DoE analysis are reported in Table 1. In addition, Fig. 7g-i, reports the response surface plots of the factors “size”.
Table 1.
Results of the DoE (design of experiments) approach for alginate microcapsules
| Batch # | Atomizing air (Bar) | Factors | Responses | ||
|---|---|---|---|---|---|
| Distance between the nozzle and the gelling bath (cm) | Polymer pumping rate Pump (μm/mL) | Mean diameter (um) | Standard deviation (±um) | ||
| “air” | “height” | “pump” | “size” | “SD” | |
| #1 | 12.0 | 2.2 | 75.0 | 1350.5 | 63.2 |
| #2 | 20.0 | 2.2 | 75.0 | 983.1 | 68.4 |
| #3 | 12.0 | 3.0 | 75.0 | 1234.1 | 389.5 |
| #4 | 20.0 | 3.0 | 75.0 | 917.5 | 60.5 |
| #5 | 12.0 | 2.2 | 125.0 | 1177.5 | 296.5 |
| #6 | 20.0 | 2.2 | 125.0 | 716.7 | 99.2 |
| #7 | 12.0 | 3.0 | 125.0 | 1161.1 | 83.6 |
| #8 | 20.0 | 3.0 | 125.0 | 724.8 | 106.7 |
| #9 | 12.0 | 2.6 | 100.0 | 1293.6 | 47.6 |
| #10 | 20.0 | 2.6 | 100.0 | 835.4 | 322.3 |
| #11 | 16.0 | 2.2 | 100.0 | 970.5 | 54.8 |
| #12 | 16.0 | 3.0 | 100.0 | 851.6 | 36.3 |
| #13 | 16.0 | 2.6 | 75.0 | 920.6 | 47.2 |
| #14 | 16.0 | 2.6 | 125.0 | 974.9 | 58.1 |
| #15a | 16.0 | 2.6 | 100.0 | 920.8 | 43.1 |
| #15b | 16.0 | 2.6 | 100.0 | 920.8 | 43,1 |
| #15c | 16.0 | 2.6 | 100.0 | 920.8 | 43.1 |
The main observation was that a change in “air” value from a low to a high level (12 to 20 bar) results in a sharp decrease of the microcapsule “size”. The “height” and “pump” parameters exert an influence at their high and low levels causing the increase of size.
Increase of accumulation of IL-8 mRNAs and release of IL-8 by encapsulated IB3-1 cells treated with TNF-α
Free and encapsulated IB3-1 cystic fibrosis cells were treated for 24 h in the presence of 80 ng/ml of TNF-α. After this treatment, microcapsules were separated from the culture medium, washed, fused and entrapped cells isolated, washed and finally lysed for RNA isolated. RT-PCR was performed using primers amplifying IL-8 and GAPDH RNA sequences. The results obtained are reported in Fig. 7a, which clearly indicates that encapsulation has only minor effects on IL-8 mRNA accumulation. Consistently, in TNF-α-treated cells (closed bars) the levels of IL-8 mRNA sequences are higher that those usually found in TNF-α untreated IB3-1 cells (open bars).
In order to determine whether the increases of IL-8 mRNA directs synthesis of the respective proteins and their secretion outside the alginate microbeads, Bio-plex analysis performed on the medium was performed. Free and encapsulated IB3-1 cystic fibrosis cells were treated for 24 h in the presence of 80 ng/ml of TNF-α. After this treatment, microcapsules were separated from the culture medium, which was analyzed for content of IL-8. Figure 7b shows that minor differences in the amounts of secreted IL-8 protein levels are present when mediums isolated from cultures of free (-) and encapsulated (CAPS) IB3-1 cells are compared. On the contrary, when release by TNF-α treated cells is compared to that of control IB3-1 cells (compare black to white histograms of Fig. 7b), the levels of IL-8 increase significantly both in free (-) and in encapsulated (CAPS) IB3-1 cells. The TNF-α mediated fold induction of IL-8 release was found similar in free and encapsulated IB3-1 cells (Fig. 7b, right side of the panel). Table 2 reports data obtained studying the release of IL-6, IL-8, G-CSF and MCP-1, suggesting that, despite to a different extent, the induction of the release of all the analyzed proteins occurs after treatment with TNF-a af both free and encapsulated IB3-1.
Table 2.
Fold increase of cytokines and chemokines following treatment of free and encapsulated IB3-1 cells with TNF-aa
| IL-6b | IL-8b | G-CSFb | MCP-1b | |
|---|---|---|---|---|
| Free IB3-1 cells | 9.50 | 25.50 | 10.20 | 14.90 |
| (CAPS) IB3-1 cells | 8.52 | 12.26 | 4.48 | 40.00 |
aFor the experimental conditions, see legends to Fig. 7.
bIL-6, Interleukin 6; IL-8, Interleukin-8, G-CSF, Granulocyte-macrophage colony stimulating factor; MCP-1, Monocyte chemotactic protein-1.
Conclusions
We have encapsulated cystic fibrosis IB3-1 cells in alginate microbreads and found that the microencapsulation procedure does not alter the viability of the encapsulated cells. In order to determine the biotechnological applications of this procedure, we determined whether encapsulated IB3-1 cells could be induced to pro-inflammatory responses after treatment with TNF-alpha. In this experimental set-up, encapsulated and free IB3-1 cells were treated, for 24 h, with TNF-α, thereafter the culture media from both cell populations were collected. As expected, TNF-α induced a sharp increase of IL-8 mRNA content and secretion of IL-8, demonstrating that TNF-α mediated induction of IL-8 occurs also by encapsulated IB3-1 cells. Cystic fibrosis is a common genetic disease caused by mutations of the Cystic Fibrosis Transmembrane conductance Regulator (CFTR) gene, which encodes for a chloride channel expressed in several epithelia (Boucher 2004; Feriotto et al. 2004; Corradini et al. 2004) Defective CFTR causes chronic pathology in lungs, pancreas, liver, reproductive system, being the airway tract disease the most relevant cause of morbidity and mortality in CF (Boucher 2004) Interestingly, In human bronchial epithelial cells, NaCl induction of IL-8 has been described and is dependent from binding sites for NFkappaB, AP-1 and NF-IL6. Moreover, and of great relevance for our study, human bronchial epithelial cells with the DeltaF508/W1282X CFTR mutation, including IB3-1, produce an exaggerated amount of basal and NaCl-induced IL-8 (Chan et al. 2006; Bodas and Vij 2010).
Acknowledegments
Roberto Gambari has recieved grants from AIRC, Fondazione CARIPARO (Cassa di Risparmio di Padova e Rovigo), Cofin-2002, STAMINA Project (University of Ferrara), UE ITHANET Project and Telethon (contract GGP07257). Monica Borgatti has recieved a 2006 Young Investigator Grant from the University of Ferrara, Italy. This research is also supported by The Emilia-Romagna Region and by Associazione Veneta per la Lotta alla Talassemia, Rovigo.
Footnotes
Stefania Mazzitelli and Monica Borgatti contributed equally to this work.
References
- Bezzerri V, Borgatti M, Nicolis E, Lampronti I, Dechecchi MC, Mancini I, Rizzotti P, Gambari R, Cabrini G. Transcription factor oligodeoxynucleotides to NF-kappaB inhibit transcription of IL-8 in bronchial cells. Am J Resp Cell Mol Biol. 2008;39:86–96. doi: 10.1165/rcmb.2007-0176OC. [DOI] [PubMed] [Google Scholar]
- Bodas M, Vij N. The NF-kappaB signaling in cystic fibrosis lung disease: pathophysiology and therapeutic potential. Discov Med. 2010;9:346–56. [PMC free article] [PubMed] [Google Scholar]
- Borgatti M, Bezzerri V, Mancini I, Nicolis E, Dechecchi MC, Lampronti I, Rizzotti P, Cabrini G, Gambari R. Induction of IL-6 gene expression in a CF bronchial epithelial cell line by Pseudomonas aeruginosa is dependent on transcription factors belonging to the Sp1 superfamily. Bioch Bioph Res Commun. 2007;357:977–983. doi: 10.1016/j.bbrc.2007.04.081. [DOI] [PubMed] [Google Scholar]
- Borgatti M, Rizzo R, Canto MB, Fumagalli D, Renzini MM, Fadini R, Stignani M, Baricordi OR, Gambari R. Release of sICAM-1 in oocytes and in vitro fertilized human embryos. PLoS ONE. 2008;3:e3970. doi: 10.1371/journal.pone.0003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher RC. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur Respir J. 2004;23:146–158. doi: 10.1183/09031936.03.00057003. [DOI] [PubMed] [Google Scholar]
- Chan MM, Chmura K, Chan ED. Increased NaCl-induced interleukin-8 production by human bronchial epithelial cells is enhanced by the DeltaF508/W1282X mutation of the cystic fibrosis transmembrane conductance regulator gene. Cytokine. 2006;33:309–16. doi: 10.1016/j.cyto.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Corradini R, Feriotto G, Sforza S, Marchelli R, Gambari R. Enhanced recognition of cystic fibrosis W1282X DNA point mutation by chiral peptide nucleic acid probes by a surface plasmon resonance biosensor. J Mol Recognit. 2004;17:76–84. doi: 10.1002/jmr.646. [DOI] [PubMed] [Google Scholar]
- Vos P, Faas MM, Strand B, Calafiore R. Alginate-based microcapsules for immunoisolation of pancreatic islets. Biomat. 2006;27:5603–5617. doi: 10.1016/j.biomaterials.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Feriotto G, Breveglieri G, Finotti A, Gardenghi S, Gambari R. Real-time multiplex analysis of four beta-thalassemia mutations employing surface plasmon resonance and biosensor technology. Lab Invest. 2004;84:796–803. doi: 10.1038/labinvest.3700106. [DOI] [PubMed] [Google Scholar]
- Goldstein SA. Tissue engineering: functional assessment and clinical outcome. Ann NY Acad Sci. 2002;961:183–192. doi: 10.1111/j.1749-6632.2002.tb03079.x. [DOI] [PubMed] [Google Scholar]
- Herrero EP, Martìn Del Valle EM, Galàn MA. Development of a new technology for the production of microcapsules based in atomization processes. Chem Eng J. 2006;117:137–142. doi: 10.1016/j.cej.2005.12.022. [DOI] [Google Scholar]
- Lund T, Østerud B. The effect of TNF-A, PMA, and LPS on plasma and cell-associated IL-8 in human leukocytes. Thromb Res. 2004;113:75–83. doi: 10.1016/j.thromres.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Rizzo R, Dal Canto MB, Stignani M, Fadini R, Fumagalli D, Renzini MM, Borgatti M, Gambari R, Baricordi OR. Production of sHLA-G molecules by in vitro matured cumulus-oocyte complex. Intern J Mol Med. 2009;24:523–530. doi: 10.3892/ijmm_00000261. [DOI] [PubMed] [Google Scholar]
- Strand BL, Mǿrch YA, Skjåk Bræk G. Alginate as immobilization matrix for cells. Minerva Biotecnol. 2000;12:223–233. [Google Scholar]
- Terheggen-Lagro SW, Rijkers GT, Ent CK. The role of airway epithelium and blood neutrophils in the inflammatory response in cystic fibrosis. J Cyst Fibros. 2005;4:15–23. doi: 10.1016/j.jcf.2005.05.007. [DOI] [PubMed] [Google Scholar]