Abstract
Approximately 30 years ago, researchers reported intracellular bacteria in filarial nematodes. These bacteria are relatives of the arthropod symbiont Wolbachia and occur in many filarial nematodes, including Brugia pahangi and Brugia malayi. Wolbachia bacteria have been implicated in a variety of roles, including filaria development and fecundity and the pathogenesis of lymphatic lesions associated with filarial infections. However, the role of the bacteria in worm biology or filarial disease is still not clear. The present experiments support previous data showing that tetracycline eliminates or reduces Wolbachia bacteria in B. pahangi in vivo. The elimination of Wolbachia was closely linked to a reduction in female fecundity and the viability of both sexes, suggesting that the killing of Wolbachia is detrimental to B. pahangi. The gerbils treated with tetracycline showed reduced levels of interleukin-4 (IL-4) and IL-5 mRNA in renal lymph nodes and spleens compared with the levels in B. pahangi-infected gerbils not treated with tetracycline. However, similar findings were noted in B. pahangi-infected gerbils treated with ivermectin, suggesting that the loss of circulating microfilariae, not the reduction of Wolbachia bacteria, was associated with the altered cytokine profile. Despite the change in T-cell cytokines, there was no difference in the sizes of renal lymph nodes isolated from gerbils in each treatment group. Furthermore, the numbers, sizes, or cellular compositions of granulomas examined in the lymphatics or renal lymph nodes did not differ with treatment. These data suggest that Wolbachia may not play a primary role in the formation of lymphatic lesions in gerbils chronically infected with B. pahangi.
Lymphatic filariasis is caused primarily by the filarial nematodes Wuchereria bancrofti and Brugia malayi and affects more than 120 million people throughout the tropics and subtropics. The disease presents as a broad range of clinical and subclinical symptoms, including fever, acute and chronic inflammation, lymphatic edema, and elephantiasis. While much research has focused on the disease caused by infection with Brugia or Wuchereria, the mechanism(s) underlying the pathogenesis of lymphatic filariasis has not been clearly defined. In both acute and chronic infections, these mechanisms probably involve a diverse range of inflammatory reactions attributable to the parasite, host inflammatory responses, and opportunistic infections (40).
Approximately 30 years ago, several researchers reported intracellular bacteria in filarial nematodes (31, 37, 54). These bacteria have since been identified as relatives of the arthropod symbiont Wolbachia (46). Wolbachia bacteria have now been reported to exist in many filarial nematodes, including B. malayi and Brugia pahangi (3, 30, 49, 55). Interestingly, Wolbachia has recently been proposed to play a role in the induction of the host immune response to filariae (9, 44, 50, 51). Some researchers suggest that lipopolysaccharide (LPS)-like molecules from filarial Wolbachia bacteria may contribute to the pathogenesis associated with lymphatic filariasis (50) and the ocular lesions associated with onchocerciasis (44). However, these conclusions rely on data collected from experiments involving the use of parasite extracts that are inoculated into animal models or are used to stimulate homogenous cell lines. Neither of these situations is necessarily representative of natural parasitic infections. Consequently, in vivo experiments using a suitable animal model may provide more pertinent information regarding the role of Wolbachia in both the biology of the parasite and the immune response of the host.
The B. pahangi-Mongolian gerbil model of human lymphatic filariasis has previously been used to investigate the biology of Brugia spp. and the inflammatory lesions associated with lymphatic filariasis (1, 2, 16, 21, 27, 32, 37, 38). Gerbils develop chronic infections with B. pahangi that result in lymphatic damage through the formation of well-characterized granulomatous lesions (21, 54). These lesions consist primarily of macrophages, lymphocytes, epithelioid cells, and eosinophils (21). Similar lesions also occur in humans (14) and other natural hosts (45). Consequently, quantifying the lymphatic lesions observed in the Brugia-Mongolian gerbil model system can provide relevant information concerning the pathogenesis associated with Brugia infections in humans.
Experiments were conducted to examine the role of Wolbachia in the biology of B. pahangi and the immune response of Mongolian gerbils to B. pahangi infection. To accomplish this, age-matched gerbils infected subcutaneously (s.c.) with B. pahangi were treated in three ways to establish three types of infections. One group of gerbils were infected with B. pahangi and given tetracycline (B. pahangi+TET gerbils). Previous studies have shown that TET will eradicate Wolbachia from B. pahangi in vivo and in vitro (4, 47). In addition, TET treatment has also been shown to interrupt embryogenesis in many filariae (4, 10, 18, 19). B. pahangi+TET gerbils, then, carried B. pahangi infections that were Wolbachia negative and amicrofilaremic. A second group of B. pahangi-infected gerbils were treated with ivermectin (B. pahangi+IVM gerbils). At the dose used, IVM did not affect Wolbachia or microfilaria production in gerbils (8). However, it did kill circulating microfilariae upon release from female B. pahangi worms. B. pahangi+IVM gerbils were Wolbachia positive and amicrofilaremic. A third group of B. pahangi-infected gerbils were left untreated (B. pahangi+0 gerbils). B. pahangi+0 gerbils were Wolbachia positive and microfilaremic. In addition, a fourth group of age-matched gerbils were not infected but were included as controls (0+0 gerbils). Three experiments were conducted, with necropsies carried out at 76, 86, and 100 days postinfection (DPI). All worms were recovered at necropsy, and data were collected on parasite development, fecundity, and the presence of Wolbachia DNA. In addition, information was gathered concerning gerbil renal lymph node (RLN) size and histology, the number, severity, and cellular composition of lymphatic lesions in gerbils, the presence of circulating anti-Wolbachia antibodies in sera, and the localized (RLN) and systemic (spleen) host cytokine response to B. pahangi infection.
MATERIALS AND METHODS
Gerbils and parasites
Mongolian gerbils approximately 8 weeks of age were obtained from Charles River (Wilmington, Mass.) and were maintained on standard rodent chow and water ad libitum. B. pahangi infective third-stage larval (L3) organisms were recovered from infected Aedes aegypti mosquitoes by use of a previously described Baermann technique (27). Aliquots of 100 B. pahangi L3 organisms in 0.5 ml of RPMI medium were injected s.c. Control gerbils were injected s.c. with 0.5-ml aliquots of RPMI medium recovered from the Baermann apparatus.
Experimental design
Gerbils were randomly divided into four groups of 36 each. Three groups were infected with B. pahangi, and one group was left uninfected as controls (the 0+0 gerbils). Of the three groups of infected gerbils, one was treated with TET (B. pahangi+TET gerbils), one was treated with IVM (B. pahangi+IVM gerbils), and one group was left untreated (B. pahangi+0 gerbils). TET was given at a concentration of 2.5 mg/ml in drinking water from 14 DPI to the time of necropsy (7). IVM was given at 28, 42, 56, and 76 DPI by gavage (10 mg/kg of body weight). This dose has been proven effective against circulating microfilariae without being detrimental to adult worms (8). Gerbils were bled throughout the course of infection and at necropsy. Blood was examined for microfilariae, and sera were harvested for the analysis of antibodies against Wolbachia surface protein (WSP) (41). Necropsies and worm recoveries were performed at 76, 86, and 100 DPI. Twelve gerbils from each treatment group were killed at each necropsy. Worms were sexed and enumerated, and female worms were analyzed for fecundity and the presence of Wolbachia. Gerbil spleens and RLNs were collected for cytokine mRNA quantitation. The number and size of lymphatic granulomas were recorded for each gerbil. Selected lesions from spermatic cords and lymphatics were fixed in 10% formalin for histological examination. RLNs were also weighed as an indicator of cellular reactivity.
Worm recoveries and fecundity analysis
Worms were recovered from infected gerbils at necropsy by dissecting out major lymphatics and organs, teasing the tissues apart, and soaking them in phosphate-buffered saline (PBS), as previously described (25). Upon recovery, the worms were sexed and counted.
Blood was collected from all infected gerbils at necropsy. Whole blood (0.5 ml) was mixed with 9 ml of 2% formalin and analyzed for microfilariae by Knott's test. Samples (15 drops of blood from a Pasteur pipette) were counted in duplicate. The mean number of microfilariae (± the standard error [SE]) per 15 drops of blood was calculated for each treatment group.
When possible, two female worms were taken from each gerbil and placed in 2 ml of RPMI medium (with 25 mM HEPES and penicillin-streptomycin). Following 24 h of incubation at 37°C, the media were collected and centrifuged at 2,000 × g for 10 min. The supernatant was discarded, and the pellet, containing all reproductive stages released by the female, was resuspended in 500 μl of RPMI medium. Two aliquots of 20 μl were examined under a microscope, and eggs, developing embryos (DE), and microfilariae were counted. Female worms from 76 and 86 DPI were fixed in 70% ethanol-10% glycerin and microscopically examined. Particular note was taken of the internal structures of the worms and the presence of eggs, DE, and microfilariae within the uteri. Female worms collected at 86 DPI were also measured to identify any effect of TET treatment on worm development. Mean lengths ± SEs were calculated for each treatment group.
PCR analysis of Wolbachia
Two female worms were collected from each gerbil, when available, for PCR analysis for Wolbachia. Genomic DNA (gDNA) was collected from single worms as previously described (12). The gDNA was used as a template for the amplification of the nematode 5S spacer and Wolbachia sp. 16S ribosomal DNA (rDNA) gene by PCR. Primer sequences for the nematode 5S and Wolbachia 16S genes have been previously published for use with Litomosoides sigmodontis (20). Four microliters of gDNA was added to a PCR master mixture and amplified as previously described (12). Plasmids containing cloned sequences for the 5S and 16S genes were used as positive controls with water as a negative control. PCRs were carried out in duplicate, and products were analyzed by agarose gel (1%) electrophoresis.
To increase the sensitivity of the detection of Wolbachia 16S rDNA, the PCR product (15 μl) was also denatured and applied to a nylon membrane (Hybond N; Amersham Pharmacia Biotech, Buckinghamshire, England) as previously described (12). Probe labeling, hybridization, and signal detection were conducted using an ECL direct nucleic acid labeling and detection system as per the manufacturer's instructions (Amersham Pharmacia Biotech). Probes consisted of 100 ng (10 ng/μl) of gel-purified B. pahangi 5S or Wolbachia 16S PCR products. The blot was exposed to film (Hyperfilm ECL; Amersham Pharmacia Biotech) for 30 min, and an automated X-ray developer was used to develop the film. The DNA hybridization experiments were conducted twice.
Cytokine quantitation in spleen and RLN tissues
Gerbil spleen and RLN tissues were collected for the quantitation of interleukin-4 (IL-4) (GenBank accession no. L37779), IL-5 (accession no. L37780), IL-10 (accession no. L37781), gamma interferon (IFN-γ) (accession no. L37782), and hypoxanthine phosphoribosyltransferase (accession no. L37778). RNA was isolated from these tissues and reverse transcribed to cDNA as previously described (11, 49). This cDNA was employed as a template in quantitative PCRs using an ABI PRISM 7700 sequence detection system (TaqMan; Applied Biosystems, Foster City, Calif.).
Gene-specific oligonucleotide primers and probes for IL-4, IL-5, IL-10, IFN-γ, and hypoxanthine phosphoribosyltransferase in the gerbils were generated commercially (GeneLab, Baton Rouge, La.; Baron, Plymouth, Mass.; PE Biosystems). All cDNA and oligonucleotide sequences have been previously described (11, 33, 34, 35). Primers were unlabeled, and probes were labeled with the reporter dye 6-FAM (6-carboxyfluorescein) and the quencher dye TAMRA (6-carboxytetramethylrhodamine).
TaqMan PCRs were carried out using Applied Biosystems Universal PCR Master Mix as per the manufacturer's directions. All PCR analyses were carried out in duplicate. Relative standard curves were constructed using cDNA prepared from spleen cells stimulated with concanavalin A (10 μg/ml) for 24 h (11, 29). Data are presented as the mean fold change for a treatment group compared with the mean value for the control group (±SE).
RLN weight as a measure of cellular reactivity
RLNs were collected from half of the gerbils at each necropsy date and fixed in 10% buffered formalin. At a later time, these RLNs were removed from the formalin, blotted to remove excess liquid, and weighed. Mean RLN weights ± SEs were calculated for each treatment group.
Estimation of lymphatic and RLN lesions
Lymphatic vessels were removed from gerbils at necropsy and examined under a dissecting microscope for the presence of lesions. Lymphatic granulomas (lymphatic thrombi) were identified and classified as small, medium, or large based upon the number of cells present and the degree of lymphatic vessel occlusion. Representative lesions from each treatment group were fixed in 10% formalin. After being weighed, RLNs were embedded in paraffin and stained with hematoxylin and eosin for histologic examination.
The alterations in lymphatic vessels and the composition of inflammatory-cell infiltrate associated with the RLNs and perinodal lymphatics were characterized and recorded. Sections were examined in a single-blind fashion. The severities of inflammation and lymphatic vascular changes were subjectively scored on a scale of 1 (slight) to 3 (severe). Only lesions noted in and around the perinodal lymphatics were examined. In addition, lymphatic changes were noted according to the degree of dilation and villiform proliferation and the amount of inflammation present around lymphatic vessels.
Determination of anti-WSP antibodies
Sera collected weekly from all gerbils were analyzed for the presence of anti-WSP antibodies at 0, 8, 17, 23, 43, 58, and 86 DPI. Recombinant WSP was produced in bacteria, purified by column chromatography(41), and used to coat 96-well plates at 0.5 μg/ml. Plates were blocked with 0.3% PBST (0.1 M PBS plus 0.3% Tween 20) for 1 h at 4°C, and then sera (diluted 1:50 in 0.05% PBST) were applied in duplicate and incubated for 2 h at room temperature. Plates were then incubated with 50 μl of rabbit anti-gerbil immunoglobulin G (1:2,000 in PBST) for 1 h at room temperature, followed by 50 μl of an alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin G antibody (1:1,000) for 1 h at room temperature, with washing in between. The assay was developed by the addition of 0.1% p-nitrophenylphosphate, 3 mM MgCl2, and 10% diethanolamine at a pH of ∼9.8. A serum sample obtained from a gerbil immunized with recombinant WSP was included as a positive control on all plates, and mean optical density (OD) values for all other serum samples on a plate were divided by the OD of this sample to correct for variations between plates.
Statistics.
One-way analysis of variance was carried out to determine if significant differences existed between groups with respect to worm recoveries, worm fecundity in vivo and in vitro, worm development, cytokine mRNA levels in spleen and RLN tissue, RLN weight, and lesion numbers, sizes, and cellular compositions. All analyses were conducted using SigmaStat (SPSS Science, Chicago, Ill.).
RESULTS
TET treatment results in worm death and decreased fecundity
Worm recoveries indicate that TET treatment results in worm death over time (Fig. 1). At 76 DPI, similar numbers of worms were recovered from gerbils regardless of treatment. By 86 DPI, fewer worms were recovered from B. pahangi+TET gerbils than from B. pahangi+IVM and B. pahangi+0 gerbils, although these differences were not significant. By 100 DPI, however, B. pahangi+TET gerbils harbored significantly (P < 0.05) fewer worms than gerbils from the other two groups, suggesting that TET treatment does result in worm death.
FIG. 1.
Mean number of worms recovered from B. pahangi-infected gerbils at necropsy. Gerbils were infected with B. pahangi and treated with TET (Bp+Tet) or IVM (Bp+IVM) or were left untreated as controls (Bp+0). Worms were collected from dissected gerbil tissues and enumerated. Data are presented as mean numbers of worms ± SEs. Differences at 100 DPI were significant.
Female B. pahangi worms collected from B. pahangi+TET gerbils showed a significant (P < 0.05) reduction in fecundity compared with females from B. pahangi+0 gerbils, both in vivo and in vitro. Knott's test conducted at necropsy revealed no microfilariae in B. pahangi+TET gerbils at 72, 82, or 100 DPI (Table 1). Some microfilariae were detected in B. pahangi+IVM gerbils at all time points, although levels were significantly (P < 0.05) lower than in B. pahangi+0 gerbils.
TABLE 1.
Mean B. pahangi microfilaria count in gerbil blood at necropsya
| Treatment group | Microfilaria count (mean ± SE)
|
||
|---|---|---|---|
| 76 DPI | 86 DPI | 100 DPI | |
| B. pahangi+TET | 0 | 0 | 0 |
| B. pahangi+IVM | 2.25 ± 0.56 | 14.83 ± 3.72 | 38.16 ± 24.73 |
| B. pahangi+0 | 7.58 ± 3.93 | 113.25 ± 32.34 | 179.60 ± 49.17 |
Microfilariae were enumerated in 15 drops of blood. Values shown are means for each treatment group ± SEs. Microfilaria counts in B. pahangi+TET and B. pahangi+IVM gerbils were significantly different than those from B. pahangi+0 gerbils at all time points. Counts in B. pahangi+IVM gerbils were significantly different from those in B. pahangi+TET gerbils at 86 and 100 DPI.
Overnight cultures of female B. pahangi worms suggested that TET and IVM affected worms very differently (Fig. 2). Whereas worms from B. pahangi+TET gerbils showed a marked reduction in microfilaria production, females from B. pahangi+IVM gerbils showed levels of microfilaria production similar to levels in females from B. pahangi+0 gerbils (Fig. 2A). These data show that the effect of IVM is directed against the circulating microfilariae and not embryogenesis. At all time points, female B. pahangi worms from B. pahangi+TET gerbils also produced fewer eggs (Fig. 2B) and DE (Fig. 2C) than did females from B. pahangi+0 and B. pahangi+IVM gerbils, suggesting that TET treatment may indeed interrupt embryogenesis in B. pahangi.
FIG. 2.
Measurement of female B. pahangi worm fecundity in vitro. At necropsy, two female B. pahangi adult worms were isolated from each gerbil. Worms were incubated overnight in 2 ml of RPMI medium. Enumeration of microfilariae (A), eggs (B), and DE (C) was carried out once each with two 20-μl aliquots of the culture. Data are presented as the means of these two counts.
Microscopic analysis of female B. pahangi worms at 72 DPI did not show significant differences in the uterine contents, regardless of treatment group (data not shown). All female worms isolated from B. pahangi+0 and B. pahangi+IVM gerbils showed eggs, DE, and microfilariae in utero. Four of the five female B. pahangi worms isolated from B. pahangi+TET gerbils showed all developmental stages, while one worm did not show microfilariae in utero. Further analysis of reproductive stages present in utero at 86 DPI showed that female B. pahangi worms from B. pahangi+0 and B. pahangi+IVM gerbils contained eggs, DE, and microfilariae (Table 2). Female B. pahangi worms recovered from B. pahangi+TET gerbils contained no eggs and significantly fewer microfilariae and DE (P < 0.05). Only one female worm from a B. pahangi+TET gerbil contained microfilariae and DE. This worm was also shown, by PCR and Southern blot, to be Wolbachia positive. Sixteen female worms from B. pahangi+TET gerbils did contain eggs. However, the eggs were empty. These data suggest that the effect of TET on the fecundity of female B. pahangi worms occurred after 62 days of treatment (76 DPI).
TABLE 2.
In utero fecundity in female B. pahangi worms at 86 DPI
| Treatment groupa | No. of worms harboringb:
|
||
|---|---|---|---|
| Eggs | DE | Microfilariae | |
| B. pahangi+TET (23) | 16c | 1d | 1d |
| B. pahangi+IVM (20) | 20 | 20 | 20 |
| B. pahangi+0 (23) | 20 | 18 | 22 |
The numbers in parentheses indicate the numbers of female worms examined.
Mean counts of DE and microfilariae were significantly lower in B. pahangi worms isolated from B. pahangi+TET gerbils than those in female worms from B. pahangi+0 or B. pahangi+IVM gerbils.
Eggs found in these worms were empty.
The same worm contained microfilariae and DE and was also positive for Wolbachia by PCR and Southern blot.
No female worms were microscopically examined at 100 DPI due to a significant decrease in the number of female B. pahangi worms recovered at this time.
TET treatment did not significantly inhibit B. pahangi growth
Measurements of 12 female worms recovered from each treatment group at 86 DPI suggested that worms from B. pahangi+TET gerbils were slightly smaller (mean length, 15.58 ± 3.88 mm) than those recovered from B. pahangi+0 (22.66 ± 3.28 mm) and B. pahangi+IVM (22.41 ± 3.78 mm) gerbils. However, these differences were not statistically significant (P > 0.05).
Wolbachia is eliminated from TET-treated worms prior to death
At all time points, B. pahangi+TET gerbils had fewer Wolbachia-positive female worms than did B. pahangi+IVM and B. pahangi+0 gerbils (Table 3). At 76 and 100 DPI, all worms taken from control and IVM-treated gerbils were positive for Wolbachia, while at 86 DPI, 17 of 22 (70%) and 12 of 17 (77%) female worms were positive from the B. pahangi+0 and B. pahangi+IVM gerbils, respectively. Significantly (P = 0.03) fewer B. pahangi worms recovered from B. pahangi+TET gerbils were positive for Wolbachia at both 76 DPI (0 of 24) and 86 DPI (1 of 16 or 6.2%). While 20% of female B. pahangi worms from B. pahangi+TET gerbils were positive at 100 DPI, it must be noted that only five worms were analyzed at this time due to a marked decrease in the number of worms recovered.
TABLE 3.
Presence of Wolbachia in female B. pahangi worms at necropsy
| Treatment group | No. of worms positive/total no. ata:
|
||
|---|---|---|---|
| 76 DPI | 86 DPI | 100 DPI | |
| B. pahangi+TET | 0/24 | 1/16 | 1/5 |
| B. pahangi+IVM | 23/23 | 17/22 | 23/23 |
| B. pahangi+0 | 24/24 | 12/17 | 24/24 |
Values are the number of worms positive for Wolbachia relative to the number of worms examined. Worms were considered positive following the amplification and Southern hybridization of Wolbachia 16S rDNA from gDNA isolated from individual worms. The number of B. pahangi worms that were positive for Wolbachia was significantly lower in worms recovered from B. pahangi+TET gerbils than in those recovered from B. pahangi+IVM and B. pahangi+0 gerbils at 86 and 100 DPI.
TET- and IVM-treated gerbils show decreased type 2 cytokines
Analysis of IL-4 and IL-5 mRNA revealed that these cytokines were increased in RLN tissues from B. pahangi+0 gerbils at 76 and 100 DPI (Fig. 3). However, levels of IL-4 and IL-5 mRNA were lower in the RLNs of gerbils treated with TET or IVM than in control gerbils. These differences were not significant due to the large variance in mRNA levels within groups. A similar pattern of cytokine expression was observed in spleen cells (data not shown). Levels of IFN-γ in spleen and RLN tissues were similar in both B. pahangi+TET and B. pahangi+0 gerbils at all time points. However, B. pahangi+IVM gerbils showed elevated levels of IFN-γ in RLN at 76 DPI (Fig. 3A).
FIG. 3.
Cytokine mRNA production in RLNs of gerbils at 76 (A) or 100 (B) DPI. Gerbils were infected with B. pahangi and treated with either TET (Bp+Tet) or IVM (Bp+IVM) or were left untreated (Bp+0). IL-4, IL-5, and IFN-γ mRNA levels were measured with the ABI PRISM 9700 sequence detection system. Data are presented as the fold change in cytokine mRNA relative to the values measured in uninfected control (0+0) gerbils ± SEs.
RLN weight does not indicate a difference in immune reactivity between groups
RLNs obtained from B. pahangi-infected gerbils (B. pahangi+TET, B. pahangi+IVM, and B. pahangi+0) were larger than those obtained from control (0+0) gerbils at 76 and 86 DPI, suggesting that infection with Brugia invokes an immune response in the RLNs. By 100 DPI, however, the size of the RLNs from Brugia-infected gerbils decreased and was nearly equal to that of B. pahangi+0 gerbils at 100 DPI. This is indicative of the down regulation of the gerbil immune response observed during chronic Brugia infections (25). At 76 DPI, RLN weights were as follows: for B. pahangi+TET gerbils, 0.1 ± 0.01 g; B. pahangi+IVM, 0.12 ± 0.02 g; B. pahangi+0, 0.18 ± 0.02 g; and 0+0, 0.05 ± 0.007 g. At 86 DPI, the RLN weights were as follows: for B. pahangi+TET gerbils, 0.06 ± 0.02 g; B. pahangi+IVM, 0.06 ± 0.03 g; B. pahangi+0, 0.05 ± 0.03 g; and 0+0, 0.02 ± 0.002 g. At 100 DPI, a similar pattern was observed: for B. pahangi+TET gerbils, 0.08 ± 0.02 g; B. pahangi+IVM, 0.1 ± 0.02 g; B. pahangi+0, 0.09 ± 0.02 g; and 0+0, 0.08 ± 0.007 g. While RLNs from B. pahangi+TET and B. pahangi+IVM gerbils showed different cytokine responses than those from B. pahangi+0 gerbils (see above), there was no significant difference in the mean weights of RLNs from B. pahangi-infected gerbils (B. pahangi+TET, B. pahangi+IVM, and B. pahangi+0) at any time.
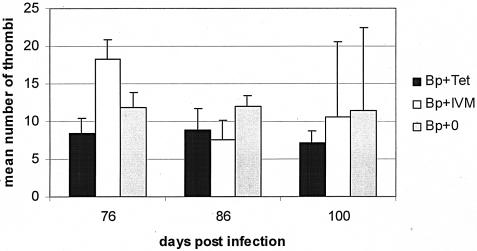
Lymphatic lesion formation is not dependent solely on the presence of Wolbachia bacteria or microfilariae
Enumeration of granulomatous lesions in the lymphatics of B. pahangi-infected gerbils at day 76 DPI revealed an increase in the number of lesions in B. pahangi+IVM gerbils compared with the numbers in B. pahangi+TET and B. pahangi+0 gerbils (Fig. 4). However, this difference was not statistically significant. Similar numbers of lesions were recorded for gerbils from all three groups at both 86 and 100 DPI. Additionally, when lesions were classified by size (small, medium, or large), there were no significant differences between the three treatment groups of gerbils at any time (data not shown).
FIG. 4.
Mean number of lymphatic thrombi measured in B. pahangi-infected gerbils at necropsy. Gerbils were infected with B. pahangi and treated with either TET (Bp+Tet) or IVM (Bp+IVM) or were left untreated (Bp+0). Lesions were enumerated under a dissecting microscope. Data are presented as the mean numbers of lymph thrombi ± SEs.
The lymphatics and RLNs of gerbils from each treatment group were histologically examined, and levels of inflammation were scored. The lymphatics of infected gerbils showed various degrees of vascular dilation, villiform proliferation, and perivascular inflammation consisting primarily of lymphocytes, plasma cells, and macrophages. Lymphatics often contained intraluminal thrombi composed of foamy macrophages mixed with lesser numbers of eosinophils. RLNs showed prominent germinal centers with mild increases in eosinophil numbers within both the follicles and sinuses. Aggregates of large foamy macrophages were present within the subcapsular and medullary sinuses. Histological examination and scoring did not reveal apparent differences in cellular composition or severity of lesions recovered from B. pahangi-infected gerbils, regardless of treatment.
B. pahangi+TET gerbils do not show increased anti-WSP antibody production
Low levels of anti-WSP antibodies were observed in all B. pahangi-infected gerbils prior to 58 DPI (Fig. 5). After this time, the levels of anti-WSP antibodies increased in both B. pahangi+0 and B. pahangi+IVM gerbils. The levels of anti-WSP antibodies produced by B. pahangi+TET gerbils were not significantly greater than the levels in gerbils in other treatment groups at any time. Indeed, at 86 DPI, B. pahangi+TET gerbils produced significantly lower levels of anti-WSP antibodies than did B. pahangi+0 (P = 0.008) and B. pahangi+IVM gerbils (P = 0.004), despite the killing of developmental stages in utero and the death of adult worms around 86 to 100 DPI.
FIG. 5.
Enzyme-linked immunosorbent assay measurement of anti-WSP antibodies in either uninfected (0+0) or B. pahangi-infected (Bp+0, Bp+Tet, and Bp+IVM) gerbils. Values shown are adjusted ODs.
DISCUSSION
Previous reports have shown that treating 14-day-old B. pahangi infections with TET for a limited time has no effect on worm viability in gerbils (7). Similarly, we observed no effect of TET on worm recoveries following treatment from 14 until 76 DPI (62 days of treatment). At 86 and 100 DPI, however, we recovered fewer worms from B. pahangi+TET gerbils than from B. pahangi+0 or B. pahangi+IVM gerbils. These differences were significant at 100 DPI. These data suggest that TET not only may target developing larvae as previously suggested (8, 10, 19, 32) but may also affect adult worm viability if given for a sufficient period of time. Other experiments in our laboratory have confirmed the lethal effect of TET on adult worms. Adult B. pahangi worms transplanted into the peritoneal cavities of gerbils undergoing TET treatment show a reduction in worm numbers within 21 days. This reduction is significant after 35 days (13). In addition, studies with Onchocerca ochengi in cattle have shown adult worm burden reduction and onchocercomata resolution following treatment with oxytetracycline (31). Treatment of Onchocerca volvulus in humans with doxycycline has not shown the same resolution of onchocercomata but does result in increased worm death within nodules (19).
In the present experiments, TET treatment eliminated Wolbachia bacteria from B. pahangi prior to death. At 76 DPI, all worms examined from B. pahangi+TET gerbils were Wolbachia negative. At this time, there was no difference in the mean numbers of worms recovered from each treatment group, suggesting that B. pahangi may not immediately die upon elimination of Wolbachia in vivo. At 86 and 100 DPI, only one out of five worms from B. pahangi+TET gerbils was positive for Wolbachia compared with >70% of worms isolated from B. pahangi+0 and B. pahangi+IVM gerbils, confirming the effect of TET on Wolbachia. However, at 86 and 100 DPI, we also noted a decrease in the mean number of worms recovered from gerbils, indicating that B. pahangi may survive for only a short time following the eradication of Wolbachia. We recently reported a similar relationship between Wolbachia elimination and worm viability following TET treatment of intraperitoneal B. pahangi infections in gerbils. In these intraperitoneal infection experiments, B. pahangi+TET and B. pahangi+0 gerbils had similar numbers of female and male B. pahangi worms following 28 days of treatment. After this time point, however, the mean number of worms recovered from B. pahangi+TET gerbils was lower than that for B. pahangi+0 gerbils. In addition, worm death followed a decrease in the number of worms found to harbor Wolbachia (13). The data collected from these two systems support the theory that B. pahangi may not live for a long period of time following Wolbachia elimination.
Whether the death of B. pahangi is directly due to the loss of Wolbachia is still unknown. It has been suggested that the filarial nematode-Wolbachia relationship is an obligatory mutualistic one (51), with each organism requiring the other to survive. Acanthocheilonema viteae, Onchocerca flexuosa, and Setaria equina have all been reported to be Wolbachia negative (12, 49). Yet, these parasites are able to maintain normal biological function in the absence of Wolbachia. In a similar manner, B. pahangi and other Wolbachia-positive filarial nematodes may not be dependent on Wolbachia for normal biological function. One alternative explanation for parasite death following Wolbachia elimination might be that, upon their death, Wolbachia bacteria release products that are toxic to the nematode. Alternatively, there is also the possibility that TET has as yet undescribed direct effects on nematode viability.
In the present experiments, gerbils treated with TET never developed microfilaremia, and in vitro cultures confirmed that female B. pahangi worms taken from B. pahangi+TET gerbils produced fewer microfilariae than did females taken from B. pahangi+0 gerbils. Similar antimicrofilarial effects have been observed with TET against various filarial nematodes in vivo and in vitro (4, 13, 18, 31, 43). Female B. pahangi worms taken from B. pahangi+TET gerbils did produce a greater number of eggs than microfilariae. However, the eggs that were present did not contain embryos, confirming that TET treatment blocks early embryogenesis. Furthermore, microscopic analysis of B. pahangi+TET female worms revealed mostly empty uteri. Similar results have been observed in vitro and in vivo with TET and doxycycline (4, 17, 19, 20, 43). In contrast, females taken from B. pahangi+IVM gerbils showed levels of in vitro microfilaria production that were similar to those in females taken from B. pahangi+0 gerbils, confirming that the activity of IVM was not based upon the interruption of embryogenesis as it was for TET.
The data described above confirm that we had manipulated B. pahangi infections in three ways. Infections in control (B. pahangi+0) gerbils were microfilaremic and Wolbachia positive. In B. pahangi+IVM gerbils, infections were low in microfilariae but were Wolbachia positive. Finally, in B. pahangi+TET gerbils, B. pahangi infections were amicrofilaremic and Wolbachia negative. Further analyses were conducted to determine if the different infections would induce different host responses in the infected Mongolian gerbils.
While RLN weight did not suggest major differences in cellular responsiveness between B. pahangi-infected gerbils, analysis of RLN cytokine mRNA showed marked differences between infected control (B. pahangi+0) gerbils and treated (B. pahangi+TET or B. pahangi+IVM) gerbils. Specifically, B. pahangi+TET gerbils produced lower levels of IL-4 and IL-5 mRNA than did B. pahangi+0 gerbils. Similarly, low levels of these cytokines were also observed in B. pahangi+IVM gerbils, suggesting that the effect of Th2 suppression may be unrelated to the presence or absence of Wolbachia. Previous studies with mice have shown that the presence of adult B. pahangi worms stimulates host IL-4 production (15). In contrast, our data suggest that adult B. pahangi worms alone are insufficient to maintain high levels of host IL-4 (and IL-5) and that circulating microfilariae may be largely responsible for the induction of these cytokines in gerbils.
Interestingly, we did not observe high levels of IFN-γ mRNA in spleen or RLN tissue, even during the death of adult worms and assumed release of Wolbachia. In many intracellular bacterial infections, tumor necrosis factor alpha (TNF-α) and bacterium-derived products such as LPS are known to activate IFN-γ production by natural killer cells (23). Furthermore, examination of limb lymphedema and hydrocele fluid from humans infected with Wuchereria bancrofti has revealed high levels of IL-1β, IL-6, IL-8, TNF-α, and granulocyte-macrophage colony-stimulating factor (39). These are inflammatory molecules that can be activated in the presence of LPS via CD14 and toll-like receptor 4 (6, 29, 52). The lack of IFN-γ mRNA observed in gerbil RLNs during these experiments suggests that there was little, if any, LPS stimulation during these experiments. In contrast, IFN-γ may not be the most sensitive indicator of LPS-induced inflammation. The measurement of levels of molecules initially induced in the host response to LPS, such as TNF-α, IL-1β, and nitric oxide, may give a better indication of LPS stimulation.
Finally, we aimed to determine if the presence or absence of Wolbachia bacteria had a significant effect on the granulomatous inflammatory response in the lymphatics or RLNs of B. pahangi-infected gerbils. Recently, Wolbachia bacteria have been implicated in the pathogenesis of filarial infections (9, 44, 50, 51). An LPS-like component of soluble O. volvulus and B. malayi extracts can elicit inflammatory molecules from human monocytes and murine macrophages in vitro (9, 50). Soluble extracts of the nematode A. viteae, shown to be Wolbachia free (3, 49, 50), do not induce these proinflammatory molecules, a finding which implicates Wolbachia as the source of LPS. LPS induction of inflammatory molecules is initially high. It is then down regulated through the production of anti-inflammatory cytokines and chemokines (e.g., IL-4, IL-10, transforming growth factor β, prostaglandin-E2) (22). This down regulation, also known as the development of LPS tolerance (42, 55), protects the host from developing an uncontrolled immunological response, resulting in endotoxic shock. However, it may also result in a decreased ability of the host to respond to secondary infections, thereby contributing to the development of chronic pathology in lymphatic filariasis (51). Interestingly, we did not observe a strong effect of Wolbachia on lymphatic lesion formation associated with chronic infection in the Mongolian gerbil model of human lymphatic filariasis. There was no significant difference in the mean numbers of lesions recorded for each treatment group at 76, 86, and 100 DPI. Additionally, lesions collected from gerbils in all treatment groups were of similar sizes and had similar cellular compositions. Not surprisingly, these data suggest that Wolbachia is not the only factor contributing to lesion formation in this animal model.
One point worthy of note was an increase in the mean number of lymphatic lesions observed in B. pahangi+IVM gerbils at 76 DPI, corresponding to an increase in IFN-γ in RLNs. At least two possible stimuli are suggested as being responsible for the observed increase in lymphatic lesion formation. First, the sudden influx of microfilaria antigen may be the cause. Microfilariae are continually produced and released by female B. pahangi worms from ∼60 DPI (2; T. R. Klei, unpublished observations). In B. pahangi+IVM gerbils, however, microfilariae are subsequently killed, providing a continuous source of worm- and bacterium-derived antigenic material to the host. An increased host inflammatory response to these antigens may certainly result in increased lesion formation, as microfilariae have previously been documented to act as a nidus for lymphatic lesions (21). Second, the antigenic source for lymphatic lesion formation may be the large number of eggs produced by female B. pahangi worms from B. pahangi+IVM gerbils at 76 DPI. The cause of this increased egg production is not known, although the killing of circulating microfilariae by IVM may remove a feedback molecule that limits fertility in adult female B. pahangi worms. Regardless of its origin, the increase in egg antigen at 76 DPI in B. pahangi+IVM gerbils may certainly result in increased granuloma formation.
Previously, Wolbachia has been found in blood taken from O. volvulus-infected patients following treatment with diethylcarbamazine (24), suggesting that in some cases, worm death does result in the release of bacteria. In addition, researchers have suggested that the natural attrition of filarial parasites results in the constant release of Wolbachia and therefore Wolbachia-derived products (51). While we did not examine gerbil blood for the presence of Wolbachia organisms, we did analyze gerbil sera for the presence of antibodies to a major WSP (5). There is little information currently available regarding the relationship between filarial infection status and anti-WSP antibody production. Bazzocchi et al. (5) reported the universal production of anti-WSP antibodies in Dirofilaria immitis-infected cats, suggesting that the constant release of Wolbachia organisms through natural worm death was sufficient to induce an antibody response. In contrast, others have reported an increase in antibodies to WSP in two B. malayi-infected rhesus monkeys only at the time of transition from microfilaremic to amicrofilaremic status and during attacks of lymphedema (41).
We observed little production of anti-WSP antibodies in B. pahangi-infected gerbils prior to 58 DPI. After this time, however, the data suggest that B. pahangi-infected gerbils do develop an anti-WSP antibody response, as both B. pahangi+0 and B. pahangi+IVM gerbils showed increasing levels of anti-WSP antibodies compared with those of 0+0 gerbils. In contrast, B. pahangi+TET gerbils did not develop anti-WSP antibodies, suggesting that the early elimination of Wolbachia bacteria from B. pahangi worms, as evidenced by PCR and Southern hybridization analysis of female B. pahangi worms at 76 DPI, may interrupt the development of this response. Indeed, at 85 DPI, B. pahangi+TET gerbils showed significantly lower levels of anti-WSP antibodies than both B. pahangi+0 and B. pahangi+IVM gerbils did. These data suggest that the constant release of Wolbachia organisms due to (i) the killing of developmental stages in utero in B. pahangi+TET gerbils and (ii) the killing of circulating microfilariae following treatment in B. pahangi+IVM gerbils does not result in a strong anti-WSP response. Furthermore, the levels of anti-WSP antibodies did not increase in B. pahangi+TET gerbils around the time of adult worm death (86 to 100 DPI), despite previous suggestions that adult worm death may indeed be linked to anti-WSP antibody production in monkeys (41).
The identification of rickettsia-like bacteria within filarial nematodes has created new questions for human lymphatic filariasis research. Elucidation of the role of Wolbachia in filarial nematode biology and host pathogenesis has become the focus of many recent investigations. However, while Wolbachia has now been reported to exist in many filarial nematodes, its contribution to parasite survival and/or host immune response is still unclear. The data presented here confirm previous reports of worm death following the chemotherapeutic elimination of Wolbachia. However, we have been unable to distinguish between a requirement of B. pahangi for Wolbachia and the toxic effects of bacterial death within the worm. Furthermore, we have shown that the elimination of Wolbachia organisms from B. pahangi in Mongolian gerbils does not significantly alter lymphatic lesion formation, demonstrating that Wolbachia does not play a major role in lymphatic lesion pathogenesis in this model. Indeed, it is likely that various worm-derived products, including worm fragments, soluble products, and embryonic stages, as well as released bacteria and bacterial products (e.g., LPS), are responsible for inducing lymphatic lesions, and the removal of one of these is not sufficient to entirely inhibit granuloma formation.
Acknowledgments
We thank Andrew DeRosa for manuscript revision and Walter Wiles for the fecundity analysis.
This work was supported by NIH grant AI-19199-18.
Editor: J. M. Mansfield
REFERENCES
- 1.Ah, H.-S., T. R. Klei, J. W. McCall, and P. E. Thompson. 1974. Brugia pahangi infections in Mongolian jirds and dogs following the ocular inoculation of infective larvae. J. Parasitol. 60:643-648. [PubMed] [Google Scholar]
- 2.Ash, L. R., and J. M. Riley. 1970. Development of Brugia pahangi in the jird, Meriones unguiculatus, with notes on infections in other rodents. J. Parasitol. 56:962-968. [PubMed] [Google Scholar]
- 3.Bandi, C., T. J. Anderson, C. Genchi, and M. L. Blaxter. 1998. Phylogeny of Wolbachia in filarial nematodes. Proc. R. Soc. Lond. B 265:2407-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bandi, C., J. W. McCall, C. Genchi, S. Corona, L. Venco, and L. Sacchi. 1999. Effects of tetracycline on the filarial worms Brugia pahangi and Dirofilaria immitis and their bacterial endosymbionts Wolbachia. Int. J. Parasitol. 29:357-364. [DOI] [PubMed] [Google Scholar]
- 5.Bazzocchi, C., F. Ceciliani, J. W. McCall, I. Ricci, C. Genchi, and C. Bandi. 2000. Antigenic role of the endosymbionts of filarial nematodes: IgG response against the Wolbachia surface protein in cats infected with Dirofilaria immitis. Proc. R. Soc. Lond. B 267:2511-2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beutler, B. 2000. Endotoxin, toll-like receptor 4, and the afferent limb of innate immunity. Curr. Opin. Microbiol. 3:23-28. [DOI] [PubMed] [Google Scholar]
- 7.Bosshardt, S. C., J. W. McCall, S. U. Coleman, K. L. Jones, T. A. Petit, and T. R. Klei. 1993. Prophylactic activity of tetracycline against Brugia pahangi infection in jirds (Meriones unguiculatus). J. Parasitol. 77:775-777. [PubMed] [Google Scholar]
- 8.Bosshardt, S. C., S. U. Coleman, C. S. McVay, K. L. Jones, and T. R. Klei. 1995. Impact of microfilaremia on maintenance of a hyporesponsive cellular immune response in Brugia-infected gerbils (Meriones unguiculatus). Infect. Immun. 63:940-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brattig, N. W., U. Rathjens, M. Ernst, F. Geisinger, A. Renz, and F. W. Tischendorf. 2000. Lipopolysaccharide-like molecules derived from Wolbachia endobacteria of the filarial Onchocerca volvulus are candidate mediators in the sequence of inflammatory and anti-inflammatory responses of human monocytes. Microbes Infect. 2:1147-1157. [DOI] [PubMed] [Google Scholar]
- 10.Casiraghi, M., J. W. McCall, L. Simoncini, L. H. Kramer, L. Sacchi, C. Genchi, J. H. Werren, and C. Bandi. 2002. Tetracycline treatment and sex-ratio distortion: a role for Wolbachia in the moulting of filarial nematodes? Int. J. Parasitol. 32:1457-1468. [DOI] [PubMed] [Google Scholar]
- 11.Chirgwin, S. R., P. H. Elzer, S. U. Coleman, J. M. Nowling, S. D. Hagius, M. D. Edmonds, and T. R. Klei. 2002. Infection outcome and cytokine gene expression in Brugia pahangi-infected gerbils (Meriones unguiculatus) sensitized with Brucella abortus. Infect. Immun. 70:5938-5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chirgwin, S. R., K. H. Porthouse, J. M. Nowling, and T. R. Klei. 2002. The filarial endosymbiont Wolbachia sp. is absent from Setaria equina. J. Parasitol. 88:1248-1250. [DOI] [PubMed] [Google Scholar]
- 13.Chirgwin, S. R., J. M. Nowling, S. U. Coleman, and T. R. Klei. 2003. Brugia pahangi and Wolbachia: the kinetics of bacteria elimination, worm viability, and host responses following tetracycline treatment. Exp. Parasitol. 103:16-26. [DOI] [PubMed] [Google Scholar]
- 14.Connor, D. H., J. R. Palmiere, and D. W. Gibson. 1986. Pathogenesis of lymphatic filariasis in man. Z. Parasitenkd. 72:13-28. [DOI] [PubMed] [Google Scholar]
- 15.Devaney, E., V. Gillan, I. Wheatley, J. Jenson, R. O'Connor, and P. Balmer. 2002. Interleukin-4 influences the production of microfilariae in a mouse model of Brugia infection. Parasite Immunol. 24:29-37. [DOI] [PubMed] [Google Scholar]
- 16.Farrar, R. G., T. R. Klei, C. S. McVay, and S. U. Coleman. 1991. Qualitative characterization of antibody responses to single and multiple Brugia pahangi infections in jirds. J. Parasitol. 77:718-726. [PubMed] [Google Scholar]
- 17.Genchi, C., L. Sacchi, C. Bandi, and L. Venco. 1998. Preliminary results on the effect of tetracycline on the embryogenesis and symbiotic bacteria (Wolbachia) of Dirofilaria immitis. An update and discussion. Parassitologia 40:247-249. [PubMed] [Google Scholar]
- 18.Hoerauf, A., K. Nissen-Pahle, C. Schmetz, K. Henkle-Duhrsen, M. L. Blaxter, D. W. Buttner, M. Y. Gallin, K. M. Al-Qaoud, R. Lucius, and B. Fleischer. 1999. Tetracycline therapy targets intracellular bacteria in the filarial nematode Litomosoides sigmodontis and results in filarial infertility. J. Clin. Investig. 103:11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoerauf, A., L. Volkmann, C. Hamelmann, O. Adjei, I. B. Autenrieth, B. Fleischer, and D. W. Buttner. 2000. Endosymbiotic bacteria in worms as targets for a novel chemotherapy in filariasis. Lancet 355:1242-1243. [DOI] [PubMed] [Google Scholar]
- 20.Hoerauf, A., L. Volkmann, K. Nissen-Paehle, C. Schmetz, I. Autenrieth, D. W. Buttner, and B. Fleischer. 2000. Targeting of Wolbachia endobacteria in Litomosoides sigmodontis: comparison of tetracyclines with chloramphenicol, macrolides and ciproflaxin. Trop. Med. Int. Health 5:275-279. [PubMed] [Google Scholar]
- 21.Jeffers, G. W., T. R. Klei, F. M. Enright, and W. G. Henk. 1987. The granulomatous inflammatory response in jirds, Meriones unguiculatus, to Brugia pahangi: an ultrastuctural and histochemical comparison of the reaction in the lymphatics and peritoneal cavity. J. Parasitol. 73:1220-1233. [PubMed] [Google Scholar]
- 22.Karima, R., S. Matsumoto, H. Higashi, and K. Matsushima. 1999. The molecular pathogenesis of endotoxic shock and organ failure. Mol. Med. Today 5:123-132. [DOI] [PubMed] [Google Scholar]
- 23.Kaufmann, A. H. E. 1993. Immunity to intracellular bacteria, p. 1251-1256. In W. E. Paul (ed.), Fundamental immunology. Raven Press, New York, N.Y.
- 24.Keiser, P. B., S. M. Reynolds, K. Awadzi, E. A. Ottesen, M. J. Taylor, and T. B. Nutman. 2002. Bacterial endosymbionts of Onchocerca volvulus in the pathogenesis of posttreatment reactions. J. Infect. Dis. 185:805-811. [DOI] [PubMed] [Google Scholar]
- 25.Klei, T. R., F. M. Enright, D. P. Blanchard, and S. A. Uhl. 1982. Effects of presensitization on the development of lymphatic lesions in Brugia pahangi-infected jirds. Am. J. Trop. Med. Hyg. 31:280-291. [DOI] [PubMed] [Google Scholar]
- 26.Klei, T. R., K. C. McDonough, S. U. Coleman, and F. M. Enright. 1987. Induction of lymphatic lesions by Brugia pahangi in jirds with large and small preexisting homologous intraperitoneal infections. J. Parasitol. 73:290-294. [PubMed] [Google Scholar]
- 27.Klei, T. R., C. S. McVay, V. A. Dennis, S. U. Coleman, F. M. Enright, and H. W. Casey. 1990. Brugia pahangi: effects of duration of infection and parasite burden on lymphatic lesion severity, granulomatous hypersensitivity, and immune responses in jirds (Meriones unguiculatus). Exp. Parasitol. 71:393-405. [DOI] [PubMed] [Google Scholar]
- 28.Klei, T. R., C. S. McVay, S. U. Coleman, F. M. Enright, and U. R. Rao. 1991. Adoptive transfer of granulomatous inflammation to Brugia antigens in jirds. J. Parasitol. 83:626-629. [PubMed] [Google Scholar]
- 29.Kopp, E. B., and R. Medzhitov. 1999. The Toll-receptor family and control of innate immunity. Curr. Opin. Immunol. 11:13-18. [DOI] [PubMed] [Google Scholar]
- 30.Kozek, W. J. 1977. Transovarially-transmitted intracellular microorganisms in adult and larval stages of Brugia malayi. J. Parasitol. 63:992-1000. [PubMed] [Google Scholar]
- 31.Langworthy, N. G., A. Renz, U. Mackenstedt, K. Henkle-Duhrsen, M. B. de Bronsvoort, V. N. Tanya, M. J. Donnelly, and A. J. Trees. 2000. Macrofilaricidal activity of tetracycline against the filarial nematode Onchocerca ochengi: elimination of Wolbachia precedes worm death and suggests a dependent relationship. Proc. R. Soc. Lond. B 267:1063-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin, D. S., S. U. Coleman, U. R. Rao, and T. R. Klei. 1995. Absence of protective resistance to homologous challenge infections in jirds with chronic, amicrofilaremic infections of Brugia pahangi. J. Parasitol. 81:643-646. [PubMed] [Google Scholar]
- 33.Mai, Z., G. K. Kousoulas, D. W. Horohov, and T. R. Klei. 1994. Cross-species cloning of gerbil (Meriones unguiculatus) interleukin-2 cDNA and its expression in COS-7 cells. Vet. Immunol. Immunopathol. 40:63-71. [DOI] [PubMed] [Google Scholar]
- 34.Mai, Z. 1996. Experimental lymphatic filariasis in gerbils (Meriones unguiculatus): molecular cloning and expression of gerbil cytokines and measurement of cytokine gene expression during a primary infection of Brugia pahangi. Ph.D. thesis. Louisiana State University, Baton Rouge.
- 35.Mai, Z., D. W. Horohov, and T. R. Klei. 1998. Hypoxanthine phosphoribosyltransferase cDNA in gerbils (Meriones unguiculatus). Lab. Anim. Sci. 48:179-183. [PubMed] [Google Scholar]
- 36.McLaren, D. J., M. J. Worms, B. R. Laurence, and M. G. Simpson. 1975. Micro-organisms in filarial larvae (Nematoda). Trans. R. Soc. Trop. Med. Hyg. 69:509-514. [DOI] [PubMed] [Google Scholar]
- 37.Nasarre, C., S. U. Coleman, U. R. Rao, and T. R. Klei. 1997. Brugia pahangi: differential induction and regulation of jird inflammatory responses by life-cycle stages. Exp. Parasitol. 87:20-29. [DOI] [PubMed] [Google Scholar]
- 38.Nasarre, C., J. L. Krahenbuhl, and T. R. Klei. 1998. Down regulation of macrophage activation in Brugia pahangi-infected jirds (Meriones unguiculatus). Infect. Immun. 66:1063-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olszewski, W. L., S. Jamal, B. Lukomska, G. Manokaran, and I. Grzelak. 1992. Immune proteins in peripheral tissue fluid-lymph in patients with filarial lymphedema of the lower limbs. Lymphology 25:166-171. [PubMed] [Google Scholar]
- 40.Ottesen, E. A. 1992. The Wellcome Trust Lecture. Infection and disease in lymphatic filariasis: an immunological perspective. Parasitology 104:S71-S79. [DOI] [PubMed] [Google Scholar]
- 41.Punkosdy, G. A., V. A. Dennis, B. L. Lasater, G. Tzertzinis, J. M. Foster, and P. J. Lammie. 2002. Detection of serum IgG antibodies for Wolbachia surface protein in rhesus monkeys infected with Brugia malayi. J. Infect. Dis. 184:385-389. [DOI] [PubMed] [Google Scholar]
- 42.Randow, F., U. Sybre, C. Meisel, D. Krausch, H. Zuckermann, C. Platzer, and H. D. Volk. 1995. Mechanism of endotoxin desensitization: involvement of interleukin 10 and transforming growth factor beta. J. Exp. Med. 181:1887-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rao, R. U., H. Moussa, and G. J. Weil. 2002. Brugia malayi: effects of antibacterial agents on larval viability and development in vitro. Exp. Parasitol. 101:77-81. [DOI] [PubMed] [Google Scholar]
- 44.Saint Andre, A., N. M. Blackwell, L. R. Hall, A. Hoerauf, N. W. Brattig, L. Volkmann, M. J. Taylor, L. Ford, A. G. Hise, J. H. Lass, E. Diaconu, and E. Pearlman. 2002. The role of endosymbiotic Wolbachia bacteria in the pathogenesis of river blindness. Science 295:1892-1895. [DOI] [PubMed] [Google Scholar]
- 45.Schacher, J. E., and P. F. Sahyoun. 1967. A chronological study of the histopathology of filarial disease in cats and dogs caused by Brugia pahangi (Buckley and Edison, 1956). Trans. R. Soc. Trop. Med. Hyg. 61:234-243. [DOI] [PubMed] [Google Scholar]
- 46.Sironi, M., C. Bandi, L. Sacchi, B. Di Sacco, G. Damiani, and C. Genchi. 1995. Molecular evidence for a close relative of the arthropod endosymbiont Wolbachia in a filarial worm. Mol. Biochem. Parasitol. 74:223-227. [DOI] [PubMed] [Google Scholar]
- 47.Smith, H. L., and T. V. Rajan. 2000. Tetracycline inhibits development of the infective-stage larvae of filarial nematodes in vitro. Exp. Parasitol. 95:265-270. [DOI] [PubMed] [Google Scholar]
- 48.Swiderski, C. E., T. R. Klei, and D. W. Horohov. 1999. Quantitative measurement of equine cytokine mRNA expression by polymerase chain reaction using target-specific standard curves. J. Immunol. Methods 222:155-169. [DOI] [PubMed] [Google Scholar]
- 49.Taylor, M. J., and A. Hoerauf. 1999. Wolbachia bacteria of filarial nematodes. Parasitol. Today 15:437-442. [DOI] [PubMed] [Google Scholar]
- 50.Taylor, M. J., H. F. Cross, and K. Bilo. 2000. Inflammatory responses induced by the filarial nematode Brugia malayi are mediated by lipopolysaccharide-like activity from endosymbiotic Wolbachia bacteria. J. Exp. Med. 191:1429-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taylor, M. J., H. F. Cross, L. Ford, W. H. Makunde, G. B. K. S. Prasad, and K. Bilo. 2001. Wolbachia bacteria in filarial immunity and disease. Parasite Immunol. 23:401-409. [DOI] [PubMed] [Google Scholar]
- 52.Ulevitch, R. J., and P. S. Tobias. 1999. Recognition of gram-negative bacteria and endotoxin by the innate immune system. Curr. Opin. Immunol. 11:19-22. [DOI] [PubMed] [Google Scholar]
- 53.Vincent, A. L., L. R. Ash, and S. P. Frommes. 1975. The ultrastructure of adult Brugia malayi (Brug, 1927) (Nematoda: Filarioidea). J. Parasitol. 61:499-512. [PubMed] [Google Scholar]
- 54.Vincent, A. L., L. R. Ash, G. E. Rodrick, and W. A. Sodeman, Jr. 1980. The lymphatic pathology of Brugia pahangi in the Mongolian jird. J. Parasitol. 66:613-620. [PubMed] [Google Scholar]
- 55.Ziegler-Heitbrock, H. W. 1995. Molecular mechanism in tolerance to lipopolysaccharide. J. Inflamm. 45:13-26. [PubMed] [Google Scholar]