Abstract
Protection against Plasmodium falciparum can be induced by vaccination in animal models with merozoite surface protein 1 (MSP1), which makes this protein an attractive vaccine candidate for malaria. In an attempt to produce a product that is easily scaleable and inexpensive, we expressed the C-terminal 42 kDa of MSP1 (MSP142) in Escherichia coli, refolded the protein to its native form from insoluble inclusion bodies, and tested its ability to elicit antibodies with in vitro and in vivo activities. Biochemical, biophysical, and immunological characterization confirmed that refolded E. coli MSP142 was homogeneous and highly immunogenic. In a formulation suitable for human use, rabbit antibodies were raised against refolded E. coli MSP142 and tested in vitro in a P. falciparum growth invasion assay. The antibodies inhibited the growth of parasites expressing either homologous or heterologous forms of P. falciparum MSP142. However, the inhibitory activity was primarily a consequence of antibodies directed against the C- terminal 19 kDa of MSP1 (MSP119). Vaccination of nonhuman primates with E. coli MSP142 in Freund's adjuvant protected six of seven Aotus monkeys from virulent infection with P. falciparum. The protection correlated with antibody-dependent mechanisms. Thus, this new construct, E. coli MSP142, is a viable candidate for human vaccine trials.
The malarial parasite remains a scourge on human civilization, and in recent years the incidence of malaria has been increasing (5, 25).Vaccination against Plasmodium falciparum has the potential to reduce severe malaria-associated morbidity and mortality in areas with the most intense transmission, and it may do so without necessarily preventing blood stage infection (20, 24). Most blood stage vaccine research has been focused on antigens that are expressed on the surface of merozoites (11). Merozoites are released from rupturing red blood cells (RBCs) and quickly invade other RBCs. Merozoite surface protein-specific antibodies, therefore, have only a brief period of time in which they can be active (26). The most widely studied merozoite surface protein is merozoite surface protein 1 (MSP1) (15). This molecule is polymorphic and has a complex folding pattern (8, 21).
P. falciparum MSP1 is a large (∼200-kDa) protein. MSP1 is processed into a complex of polypeptides on the merozoite surface, including an 82-kDa N-terminal polypeptide and 30- and 38-kDa central regions, as well as the 42-kDa C-terminal region (MSP142) (1). At the time of RBC invasion, MSP142 is further processed by proteolytic cleavage into a 33-kDa fragment (MSP133), which is shed from the parasite with the rest of the MSP1 complex, and a C-terminal 19-kDa fragment (MSP119). Only the C-terminal MSP119 fragment remains on the merozoite surface and is carried into parasitized RBCs (2). This so-called secondary processing of MSP1 is completed during the successful invasion of a RBC, suggesting that it is a necessary step (3, 7).
The MSP119 and MSP142 regions of P. falciparum MSP1 are leading malaria vaccine candidates (15). Studies with rodent malaria and challenge studies with P. falciparum in primates have indicated that vaccines based on MSP119 and MSP142 confer protection against malaria (6, 9, 12, 13, 29, 30). Recently, O'Donnell et al. (22) convincingly demonstrated not only that most sera from two high-transmission areas in Papua New Guinea were able to inhibit parasite invasion in vitro but also that the inhibitory activity was primarily directed against MSP1. By constructing a chimeric transfected P. falciparum line, D-10 (D10-PcMEGF), which expressed an antigenically distinct MSP119 domain from the distantly related rodent species Plasmodium chabaudi, these authors showed that MSP119-specific antibodies comprised a large component of the total invasion-inhibiting response of sera from many P. falciparum-immune adults in Papua New Guinea (22). There are two implications of these results that can be used for malaria vaccine development. First, antibodies specific to MSP119 may play a major role in reducing parasite multiplication rates during natural immunity. And second, although in animal models protection elicited with vaccines based on MSP1 requires high antibody titers, the lower levels of antibody obtainable in natural infections have an effect on in vitro parasite growth.
As a part of a strategy for malaria vaccine development based on recombinant MSP1, the following different expression systems for MSP1 production have been evaluated: Saccharomyces cerevisiae (18, 19), Pichia pastoris (4), baculovirus-infected insect cells (29), and milk from transgenic mice (30). Recombinant MSP142 produced in baculovirus-infected insect cells (6, 29) and transgenic milk (30) elicits protective responses in an in vivo model system but has yet to be scaled up for human clinical trials. The purpose of the present study was to examine Escherichia coli expression for the production of MSP142. The E. coli protein expression system, which was the first commercialized system for recombinant protein production, is cost-effective and very efficient for nonglycosylated proteins, such as MSP142. MSP1 is a nonglycosylated protein in its native form, and glycosylation blocks the efficacy of MSP142 produced in transgenic milk (30). Here, we describe methods to produce recombinant MSP142 in its correctly folded conformation, to examine the ability of antibodies raised against recombinant MSP142 to block erythrocyte invasion by P. falciparum in vitro, and to examine the in vivo efficacy of MSP142 in Aotus nancymai monkeys against a lethal challenge with P. falciparum.
MATERIALS AND METHODS
Expression and fermentation conditions for E. coli MSP142
The amino acid sequence of MSP142 FVO (MSP142 of the Vietnam-Oak Knoll or FVO strain; GenBank accession no. L20092)was used to construct a synthetic gene. The coding sequence of the gene was optimized for expression in E. coli by normalizing its AT content on the basis of previously published values for E. coli codon bias. This construct, corresponding to amino acids A-1 to S-355, was generated by PCR techniques and was subcloned into a pCR-blunt vector from Invitrogen. The MSP142 FVO gene was then inserted downstream of the T7 promoter by using an NdeI and XhoI site in the E. coli expression vector pET24d+ (Novagen Inc., Madison, Wis.) to obtain plasmid pPfMSP142FVOPET. The transcribed sequence of pPfMSP142FVOPET contains an additional LEHHHHHH at the C terminus. E. coli BL21(DE3) cells (Novagen) were transformed with pPfMSP142FVOPET and used for expression of recombinant E. coli MSP142 FVO protein. Fermentation was performed in a 1.9-liter culture by using defined medium containing (per liter) 13.3 g of KH2PO4, 4.0 g of NH4HPO4, 1.7 g of citric acid monohydrate, 1.2 g of MgSO4 · 7H2O, 4.5 mg of thiamine-HCl, 25 g of dextrose, 35 mg of kanamycin, and 1 ml of PTM4 trace salts. NH4OH was the nitrogen source, and glucose was the carbon source. Fermentation was carried out at 37°C, and once the optical density at 550 nm reached 35, the culture was induced by adding isopropyl-1-thio-β-galactopyranoside (IPTG) to a final concentration of 1 mM. Induction was continued for 3 h before harvesting by centrifugation and cell pellet storage at −80°C.
Refolding and purification of E. coli MSP142
A portion of the frozen cell pellet was resuspended in 10 volumes of lysis buffer (10 mM Tris-HCl [pH 8.0], 10 mM EDTA, 100 mM NaCl, 5 mM dithiothreitol) and lysed at 19,000 lb/in2 by using a microfluidizer (Microfluidics Corporation, Newton, Mass.). The resulting lysate was mixed with an equal volume of freshly prepared urea wash buffer (10 mM Tris-HCl [pH 8.0], 5 mM EDTA, 2 M urea, 1% Triton X-100) and stirred for 1 h at 4°C. The lysate was centrifuged for 30 min at 10,000 × g, and recombinant E. coli MSP142 was detected in the pellet formed by inclusion bodies. The inclusion body pellet was resuspended in solubilization buffer (10 mM Tris-HCl [pH 8.0], 8 M guanidine-HCl, 100 mM NaCl, 10 mM β-mercaptoethanol) and stirred with a magnetic stirrer for 2 h at room temperature. The guanidine-solubilized material was clarified by centrifugation at 20,000 × g for 30 min at 4°C. The denatured supernatant was then refolded by 33-fold rapid dilution in a redox refolding buffer (50 mM Tris-HCl [pH 7.4], 1 mM EDTA, 50 mM NaCl, 0.5 M arginine, 1 M urea, 25 mM cysteine, 1 mM cystamine). The refolding solution was incubated for 24 h at 4°C with continuous stirring and then dialyzed for 36 h against 50 mM Tris-HCl (pH 8.0)-750 mM urea. The dialyzed solution was clarified by centrifugation and applied to a Q-Sepharose Hi Trap column (Amersham Pharmacia) equilibrated with binding buffer containing 50 mM Tris-HCl (pH 8.0), 250 mM urea, and 12 mM 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS). After sample application, the column was washed with 15 column volumes of binding buffer, and then the E. coli MSP142 was eluted with a linear gradient to 100% elution buffer (50 mM Tris-HCl [pH 8.0], 1 M NaCl, 12 mM CHAPS). Fractions containing E. coli MSP142 were pooled and loaded onto an Ni-nitrilotriacetic acid (NTA) Superflow (Qiagen) column preequilibrated in 2× phosphate-buffered saline (PBS). The Ni-NTA column was washed with 5 column volumes of 2× PBS, and protein was eluted from the column by using 1× PBS containing 250 mM imidazole. Final purification of the refolded E. coli MSP142 eluted from the Ni-NTA was carried out by using a Superdex 75 column (Amersham Pharmacia) with PBS.
Analysis of refolded E. coli MSP142
Reverse-phase high-performance liquid chromatography analysis of refolded E. coli MSP142 was carried out with a Dynamax 300Å C4 column (Varian Inc., Walnut Creek, Calif.). The gradient used for elution was developed from buffer A (0.1% trifluoroacetic acid in water) and buffer B (0.1% trifluoroacetic acid in 90% acetonitrile-10% water). The column was initially equilibrated with 90% buffer A and 10% buffer B and reached a composition of 10% buffer A and 90% buffer B in 75 min. N-terminal amino acid sequencing was performed by the Biological Resources Branch, National Institute of Allergy and Infectious Diseases. Protein concentrations were determined with a bicinchoninic acid (BCA) protein assay kit (Pierce, Rockford, Ill.). Electrophoresis and immunoblotting of sodium dodecyl sulfate (SDS) gels were performed by using standard methods, except that 2.5% bovine serum albumin was used to block the binding sites on nitrocellulose after protein transfer. All washes were performed in 1× PBS containing 0.02% Tween 20 and 0.2% Triton X-100. For development of the blot, the nitrocellulose was treated with a 1:1,000 dilution of five individual conformation-specific monoclonal antibodies raised against baculovirus MSP142 FVO (13). The primary antibody was detected with goat anti-mouse alkaline phosphatase-conjugated secondary antibody (Kirkegard and Perry, Gaithersburg, Md.). Detection was performed by using a 5-bromo-4-chloro-3-indolylphosphate-nitroblue tetrazolium colorimetric kit (Kirkegaard and Perry).
Preparation of rabbit antisera against E. coli MSP142
Rabbit antisera against recombinant refolded E. coli MSP142 were raised by immunization with 50 μg of the antigen emulsified in 100 μl of Montanide ISA 720 (SEPPIC, Fairfield, N.J.). The primary immunization was intramuscular (zero time), and subsequent immunizations were subcutaneous (days 21 and 42). Sera were collected on days 42 and 63. Each rabbit serum was heat inactivated at 56°C for 20 min and then sterilized by filtration through a 0.22-μm-pore-size filter.
Parasite culture and GIA
The following P. falciparum culture-adapted clones were used for growth inhibition assays (GIAs): P. falciparum FVO (27), D10-PfM3′ (Pf-D10), and chimeric parasite D10-PcMEGF (Pf-D10Pc) (22). The chimeric parasite Pf-D10Pc is identical to the parental Pf-D10 clone except that the Pf-D10 MSP119 domain is replaced by the domain from P. chabaudi. Prior to use, each clone was cultured by using standard techniques but was preadapted to grow in 25% normal rabbit serum without a loss of viability. The GIA employed is a reproducible method developed in our laboratory (17). Percentages of inhibition were determined by using the following formula: 100 − {[(A650 of immune sample − A650 of RBC alone)/(A650 of preimmune control − A650 of RBC alone)] × 100}.
Vaccination and challenge infection of malaria-naive Aotus monkeys
Fourteen monkeys were randomly assigned to two groups containing seven monkeys each. One vaccine group received E. coli MSP142, and the control group received an unrelated recombinant Plasmodium vivax antigen, Pvs25H, as a negative control.
The monkeys received three vaccinations consisting of 100 μg of E. coli MSP142 or Pvs25H emulsified in 400 μl of the adjuvant 3 weeks apart, as described previously (29). The initial vaccination preparations were emulsified with complete Freund's adjuvant (Sigma, St. Louis, Mo.), and the subsequent two vaccination preparations were emulsified with Montanide ISA 51. Sera were collected from the vaccinated monkeys on day 15 after the third vaccination, and then the monkeys were challenged by intravenous injection of 5 × 104 infected RBCs from a donor monkey infected with the highly virulent P. falciparum FVO strain.Parasitemia was monitored daily by examining Giemsa-stained thin films until treatment. The monkeys were treated when the parasitemia reached >4.5% or the hematocrit fell below 25%. All monkeys not treated previously were treated on day 28 after challenge. The treatment consisted of mefloquine administered in a single dose of 25 mg/kg of body weight by intubation.
Measurement of antibody responses
Enzyme-linked immunosorbent assays (ELISAs) and indirect immunofluorescence assays were performed as previously described (21). Serum dilutions that gave an absorbance that was 0.5 U above the background value were designated the endpoint of the ELISA titer.
Statistical methods
Aotus monkeys that control their parasitemia (i.e., the parasitemia remains less than 4.5%) either self-cure or suffer from anemia and require treatment. At this point it is impossible to say what would have occurred to a monkey's parasite burden; the monkey may have self-cured, continued to control the parasitemia, or lost control and suffered from a virulent infection. Thus, the primary endpoint data included only data collected until the first monkey was treated for anemia rather than parasitemia. At that time, all monkeys were ranked in the following order. Monkeys that were treated for parasitemia prior to the day of data collection were ranked first, in order of the first day of treatment and then cumulative parasitemia (the sum of a monkey's daily parasite burden). Then the monkeys that required treatment for low hematocrit (thus triggering the endpoint) were ranked in the same fashion. Finally, monkeys that did not require treatment until that point were ranked in order of their cumulative parasitemias (29). A nonparametric, unpaired Mann-Whitney U test was then performed to compare the test group to the control group. Secondary statistical comparisons were also made. Nonlinear Spearman's regression analysis was performed to correlate antibody responses to protection from challenge. Unpaired Mann-Whitney U tests were also used to compare data between the vaccine groups (e.g., days until patent).
RESULTS
Expression, in vitro refolding, and purification of E. coli MSP142
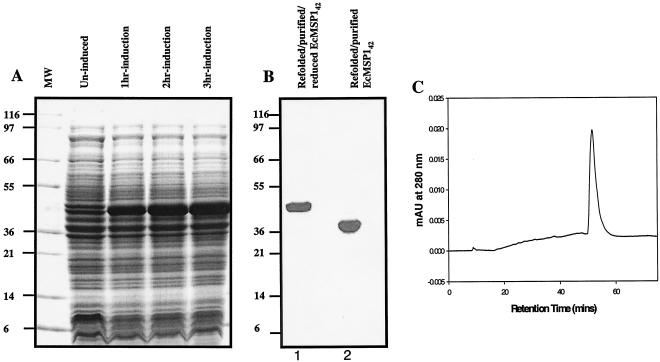
Fermentation of E. coli BL21(DE3) cells containing the pPfMSP142FVOPET plasmid in defined medium showed that there was time-dependent accumulation of a ∼42-kDa protein when the preparation was induced with IPTG (Fig. 1A). Based on an SDS-polyacrylamide gel electrophoresis (PAGE) analysis and light microscopy, expression of recombinant E. coli MSP142 was associated with the insoluble fraction of the cell lysate in the form of inclusion bodies. The recombinant E. coli MSP142 was isolated from the inclusion bodies by solubilization in denaturant and reductant buffer, followed by rapid dilution in refolding buffer. Refolded E. coli MSP142 was further purified by using three stringent chromatography purification processes involving three different chemistries. This purification yielded a homogeneous product. As determined by SDS-PAGE, the refolded E. coli MSP142 had an observed molecular mass under nonreducing conditions of ∼39 kDa, and the molecular mass under reducing conditions was ∼42 kDa (Fig. 1B). Densitometry scanning of the Coomassie blue-stained gel indicated that the purity of the refolded and purified E. coli MSP142 was more than 94%.
FIG. 1.
Expression and analysis of refolded and purified recombinant E. coli MSP142. (A) Expression of recombinant E. coli MSP142 in E. coli. Cell pellets were solubilized in 50 mM Tris (pH 8.0) containing 8 M urea and 10 mM dithiothreitol. Each solubilized cell pellet was diluted in 2× SDS-PAGE sample buffer. Solubilized samples were electrophoresed under reducing conditions on an SDS-4 to 20% PAGE gel. There was time-dependent accumulation of a band at 42 kDa after induction. Lane MW contained molecular weight standards. (B) Coomassie blue stain analysis on an SDS-4 to 20% PAGE gel under nonreducing conditions (lane 2) and reducing conditions (dithiothreitol treatment followed by alkylation with iodoacetamide) (lane 1). The shift in mobility upon reduction indicates the presence of disulfide linkages. EcMSP142, E. coli MSP142. (C) Reverse-phase high-performance liquid chromatography profile of refolded and purified E. coli MSP142. Refolded E. coli MSP142 eluted as a single sharp peak, indicating that it contained a single, homogeneous conformer population. mAU, milliabsorbance unit.
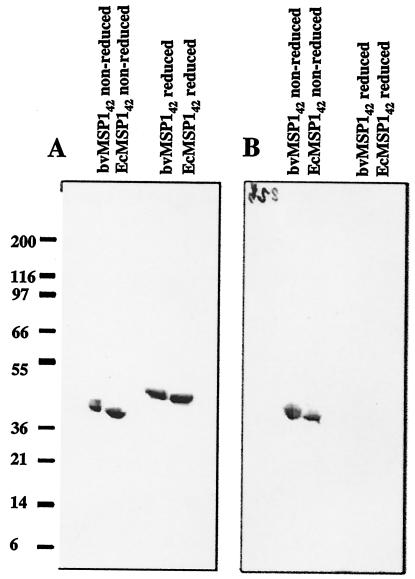
The homogeneity of the refolded and purified E. coli MSP142 was analyzed by reverse-phase high-performance liquid chromatography, which can separate different conformers of a protein based on the change in surface hydrophobicity. Refolded E. coli MSP142 eluted in a single peak during reverse-phase chromatography on a C4 column (Fig. 1C), suggesting that purified E. coli MSP142 is homogeneous. N-terminal sequencing of the refolded E. coli MSP142 yielded the expected sequence (AVTPSVIDNILSKIE) with successful cleavage of the bacterial N-formyl methionine. The mobilities of reduced and nonreduced samples of naturally refolded and purified recombinant baculovirus MSP142 and refolded E. coli MSP142 on SDS-PAGE gels were compared (Fig. 2A). The shifts in mobility after reduction appeared to be similar, although baculovirus MSP142 migrated more slowly than refolded E. coli MSP142under both nonreduced and reduced conditions. This slower migration resulted from the glycosylation of the baculovirus MSP142 (29). Immunological characterization was performed by using conformation-specific monoclonal antibodies and a positive control (baculovirus MSP142). All five conformation-specific monoclonal antibodies reacted with nonreduced E. coli MSP142 but not with reduced E. coli MSP142. An example of the reactivity pattern is shown in Fig. 2B. Monoclonal antibody bv223 reacted with nonreduced refolded E. coli MSP142 and baculovirus MSP142 identically and did not react with the reduced samples.
FIG. 2.
Comparative analysis of refolded and purified recombinant E. coli MSP142 (EcMSP142) with naturally refolded and purified baculovirus MSP142 (bvMSP142). The positions of molecular mass markers (in kilodaltons) are indicated on the left. (A) Coomassie blue stain analysis of SDS-4 to 20% PAGE gel. (B) Western blot developed by using the anti-baculovirus MSP142 FVO 223 monoclonal antibody.
Elicitation of an immune response known to occur in natural infections
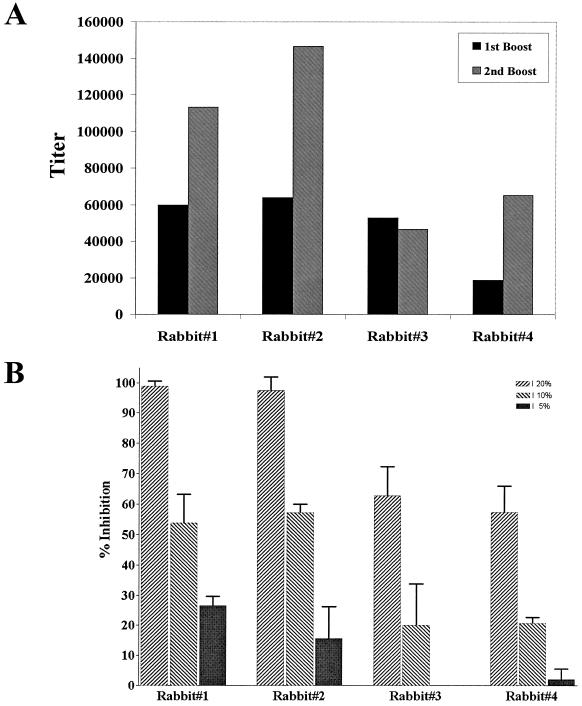
A vaccine formulation suitable for human use (10) was prepared by using the E. coli MSP142 protein (50 μg of protein emulsified in 100 μl of Montanide ISA 720). This formulation was used to immunize rabbits, and the immune sera were tested for inhibition of the growth of P. falciparum parasites in vitro. Immune sera from all four rabbits had high titers (Fig. 3A) and effectively blocked parasite invasion by the P. falciparum FVO laboratory clone (homologous to the immunogen) in a concentration-dependent manner (Fig. 3B). These data suggest that at whole-blood concentrations (effectively 100% serum), parasite growth inhibition would have been total.
FIG. 3.
Antibody responses of rabbits to immunization with E. coli MSP142. (A) Magnitude of the antibody response to E. coli MSP142 immunization as determined by ELISA. (B) Evaluation of the concentration-dependent growth-inhibitory response of E. coli MSP142-immunized rabbit sera to the homologous parasite line (P. falciparum FVO). The error bars indicate the standard deviations observed for nine samples from three independent experiments.
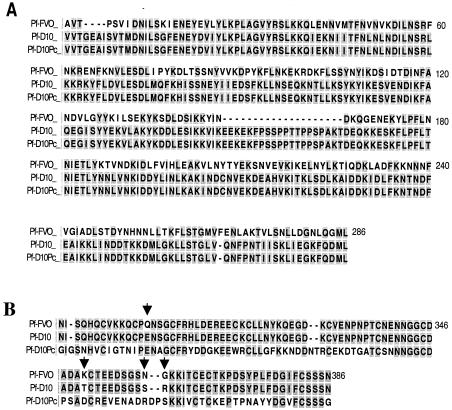
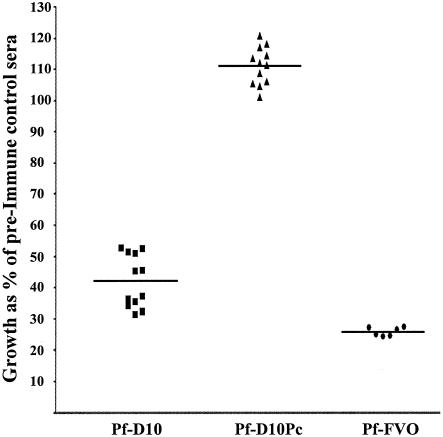
To examine heterologous parasite growth inhibition, Pf-D10 was used in the GIA. Figure 4 shows alignments of the sequences of the P. falciparum FVO and Pf-D10 MSP1 proteins for both the MSP133 (Fig. 4A) and MSP119 (Fig. 4B) domains. The proteins have extensive differences in MSP133 and have four amino acid substitutions in MSP119. Growth inhibition results that are representative of the data obtained with the pooled immune rabbit sera (four rabbits immunized with E. coli MSP142) are shown in Fig. 5. Pooled immune rabbit sera at a concentration of 20% inhibited the growth of the heterologous parasite Pf-D10 significantly (58% inhibition). However, this level of inhibition was significantly lower than the level of inhibition of the homologous parasite P. falciparum FVO (P = 0.033, as determined by a paired Student's t test at a serum concentration of 20%).
FIG. 4.
Alignment of the sequences of MSP-142 from the three Plasmodium parasites used in the GIA, including P. falciparum FVO (Pf-FVO) (GenBank/EMBL/DDBJ accession no. L20092), the Pf-D10 cloned line (GenBank/EMBL/DDBJ accession no. AAA29653), and the P. chabaudi adami (GenBank/EMBL/DDBJ accession no. AF149303)-P. falciparum MSP119 chimera created by O'Donnell et al. (Pf-D10Pc) (22). (A) MSP133 alignment. P. falciparum FVO has the Wellecome type sequence, and Pf-D10 and Pf-D10Pc have the MAD 20 type of sequence. (B) MSP119 alignment. The arrows indicate the four amino acid differences between P. falciparum FVO and Pf-D10.
FIG. 5.
Inhibition of growth invasion of heterologous P. falciparum parasite lines Pf-D10 and Pf-D10Pc determined by using immune sera raised against E. coli MSP142 FVO. A GIA in which two different Plasmodium strains expressing divergent MSP119 domains were compared revealed the important role of MSP119-specific antibodies. The data show the growth of each parasite strain as a percentage of the growth of the same parasite strain in preimmune control sera (20% immune sera pooled from all four rabbits immunized with refolded E. coli MSP142). The data for each group represents 12 different samples from three independent experiments. The horizontal lines indicate the means. Groups were compared by using an unpaired t test to determine statistical significance. Pf-FVO, P. falciparum FVO.
To investigate whether the significant inhibition of the Pf-D10 parasite was due to growth-inhibitory antibodies directed against the C-terminal MSP119 or the N-terminal MSP133 domain of MSP142, the GIA was repeated with the parasite line Pf-D10Pc. Pf-D10Pc is identical to the parental clone, Pf-D10, in the MSP133 region, but the MSP119 domain is replaced by the domain from P. chabaudi. (Fig. 4). No inhibitory effect on Pf-D10Pc parasite growth was observed (Fig. 5).
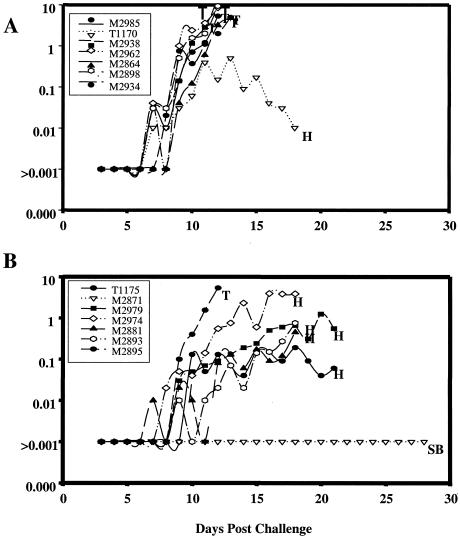
E. coli MSP142 was used to vaccinate A. nancymai monkeys (n = 7), which were examined in conjunction with control animals (n = 7) that received the irrelevant negative control antigen Pvs25H. Fifteen days after the third vaccination all monkeys were challenged with 5 × 104 P. falciparum FVO parasites. The primary technique used to measure efficacy was to rank animals in order of treatment for parasitemia and cumulative parasitemia at the time that the first monkey was treated for anemia, day 18 (see Materials and Methods). Refolded E. coli MSP142 was found to be efficacious, and the cumulative parasitemias were significantly less than those of the control animals (Fig. 6) (P < 0.01). By day 18, one of the seven animals in the E. coli MSP142 group had been treated for parasitemia of >4.5%, compared with six of the seven animals in the control vaccine group. On day 18, four animals were treated for anemia; three of these animals were in the E. coli MSP142 group, and the other animal was in the Pvs25H group.
FIG. 6.
Course of daily parasitemia in individual monkeys in the Aotus vaccine trial. Monkeys were challenged on day 0 with 5 × 104 P . falciparum FVO-parasitized erythrocytes 15 days after the third vaccination. (A) Pvs25-vaccinated group. (B) E. coli MSP142-vaccinated group. Also indicated are the treatment times for uncontrolled parasitemia of >4.5% (T), hematocrit of <20% (H), and subpatent (SB).
Other secondary markers of protection confirmed these results (Table 1). E. coli MSP142-immunized monkeys were significantly different from control animals when the numbers of days to patency, the numbers of days to treatment, the numbers of days to peak parasitemia, and the actual peak parasitemia values were compared (P = 0.04, P = 0.02, P = 0.008, and P = 0.03, respectively, as determined by the Mann-Whitney U test). Overall, six of seven control group animals required treatment for parasitemia of >4.5%. One of the seven E. coli MSP142-immunized animals required treatment for parasitemia, one animal self-cured, and five animals controlled the parasitemia but required treatment for a decrease in hematocrit to <25%. ELISA titers (against the reference antigen baculovirus MSP142) for the vaccinated monkeys are shown in Table 1. Overall, the antibody titers correlated with protection. There was a significant correlation between the antibody titers to E. coli MSP142 and the primary outcome of protection (P = 0.0004; Spearman rank r = −0.79).
TABLE 1.
Course of infection in A. nancymai monkeys challenged with P. falciparum parasites
| Vaccine group | Monkey | ELISA titera | Days to patency | Days to treatmentb | Parasitemia at treatment (%) | Days to peak parasitemia | Peak parasitemia (%) | Outcomec |
|---|---|---|---|---|---|---|---|---|
| Pvs25H | 2864 | — | 9 | 13 | 4.75 | 13 | 4.75 | Virulent |
| 2898 | — | 7 | 12 | 9.35 | 12 | 9.35 | Virulent | |
| 2934 | — | 8 | 13 | 4.95 | 13 | 4.95 | Virulent | |
| 2938 | — | 7 | 12 | 14.30 | 12 | 14.30 | Virulent | |
| 2962 | — | 7 | 12 | 8.50 | 12 | 8.50 | Virulent | |
| 2985 | — | 7 | 12 | 5.30 | 12 | 5.30 | Virulent | |
| T1170 | — | 7 | 18 | 0.01 | 13 | 0.50 | Anemic | |
| Meand | — | 7.4 | 13.1 | 6.70 | 12.4 | 6.80 | ||
| E. coli MSP142 | 2871 | 223,000 | Never patent | 28 | 0 | Never patent | 0 | Self-curede |
| 2881 | 85,025 | 7 | 18 | 0.45 | 18 | 0.45 | Anemic | |
| 2893 | 107,733 | 9 | 18 | 0.75 | 18 | 0.75 | Anemic | |
| 2895 | 48,988 | 9 | 12 | 5.35 | 12 | 5.35 | Virulent | |
| 2974 | 38,863 | 8 | 18 | 3.85 | 16 | 3.90 | Anemic | |
| 2979 | 125,133 | 9 | 21 | 0.55 | 20 | 1.25 | Anemic | |
| T1175 | 95,750 | 10 | 21 | 0.06 | 18 | 0.19 | Anemic | |
| Meand | 89,664.3 | 8.6f | 19.4 | 1.60 | 18.3g | 1.70 |
The values are the reciprocals of the serum dilutions giving an optical density of 0.5 against baculovirus MSP142. —, not measured.
If not already treated, all monkeys were treated on day 28.
Course of infection: virulent, a sharply rising, uncontrolled parasitemia requiring treatment (parasitemia, >4.5%); self-cured, parasites cleared by the animal without intervention; anemic, monkey required treatment for anemia (hematocrit, <25%).
Arithmetic means for each column, except for the ELISA column, in which the values are geometric means.
Monkey 2871 was smear negative during the 28-day course of the challenge.
Monkey 2871 was subpatent, so the arithmetic mean for the days to patency in the E. coli MSP142 group was calculated without data for monkey 2871.
Monkey 2871 was subpatent, so the arithmetic mean of the days to peak parasitemia in the E. coli MSP142 group was calculated without data for monkey 2871.
DISCUSSION
We describe a method used to produce from an E. coli expression system a recombinant E. coli MSP142 that was purified from inclusion bodies and refolded by oxidative rapid dilution. A variety of biochemical, biophysical, and immunological assays were used to demonstrate that the refolded E. coli MSP142 is homogeneous and immunogenic. First, refolded E. coli MSP142 eluted in a single peak during reverse-phase chromatography on a C4 column, suggesting that it is composed of a homogeneous population of conformers. Second, E. coli MSP142 exhibited a reduction-sensitive shift in mobility during SDS-PAGE, suggesting that disulfide bonds were formed. Third, recombinant MSP142 has been expressed previously in its functional form as a secreted protein in insect cells by using baculovirus vectors (29). The reactivity of E. coli MSP142 with conformation-specific monoclonal antibodies is similar to the reactivity of the naturally refolded baculovirus MSP142. Recombinant proteins expressed as secreted proteins in eukaryotic cells are commonly glycosylated. This may be relevant for immunogenicity of vaccine candidate antigens, as previous studies demonstrated that a glycosylated form of MSP142 expressed in transgenic mouse milk did not induce a protective response against malaria (30), while the glycosylated form of MSP142 produced in baculovirus was protective. These differences may be due to the type of glycosylation and the extent of glycosylation on molecules produced in the two different expression systems (30). E. coli is thus well suited for the production of nonglycosylated malarial parasite antigens as vaccine candidates (23, 28), and in this report we provide the first description of methods developed for refolding and purification of functional E. coli MSP142 produced in a bacterial expression system.
Immunization of rabbits with E. coli MSP142 elicits antibodies with significant concentration-dependent in vitro growth-inhibiting activity for the homologous parasite line P. falciparum FVO. The inhibition varies for different rabbit sera and correlates with the ELISA titers in individual rabbit sera against the E. coli MSP142.
The data suggest that the target of the antibodies raised against E. coli MSP142 that block invasion of human erythrocytes is MSP119. This was shown by comparing the inhibition data for two identical parasites that differ only in the replacement of MSP119 of P. falciparum by MSP119 of P. chabaudi (Pf-D10 and Pf-D10Pc) (Fig. 4). Invasion by the P. chabaudi chimera (Pf-D10Pc) was not inhibited, whereas the level of inhibition for the parasite with the P. falciparum sequence (Pf-D10) was around 58% (Fig. 5).
Polymorphisms at four amino acid positions in MSP119 were identified when the FVO and 3D7 sequences were compared (21). These differences may have been selected to minimize cross-protection. We found in the present study that sera raised against the P. falciparum FVO sequence (E. coli MSP142) inhibited invasion of RBCs by the Pf-D10 parasite, which differs from FVO at these four amino acid residues. Similar results were described previously (16); antibodies against parasites that differed at these four amino acids could cross-inhibit, although, as in our study, the inhibition appeared to be less than the inhibition observed with the homologous strain. In another study, however, in which a competitive ELISA with heterologous MSP119 domains was used, some rabbit sera exhibited no cross-reaction with the heterologous recombinant protein (29). Thus, despite the cross-inhibition in rabbits in the two studies, the lack of cross-reactions in other rabbits may indicate that both types of sequences are required in a vaccine.
Immunization with E. coli MSP142 elicits high-titer antibodies in Aotus monkeys and leads to significant protection against a lethal P. falciparum in vivo challenge. A strong correlation between protection and antibody titers was observed, and the variation in protection between animals can be accounted for by variation in the antibody titers (P = 0.0004; Spearman rank r = −0.79). These findings are in accordance with recent data from rodent malaria models, in which protection is also antibody dependent (31, 32). In three previous independent studies of baculovirus MSP142-immunized Aotus monkeys, a consistent pattern of protection was observed with six of seven animals (29), five of seven animals (14), and five of seven animals (30). In the control groups all four animals (29), five of seven animals (14), and six of seven animals (30) developed virulent infections. In the present study E. coli MSP142 protected six of seven monkeys, while six of seven monkeys in the control group developed virulent infections and required treatment for parasitemia. Therefore, we concluded that the E. coli MSP142 efficacy in the protection study was indistinguishable from that previously seen with baculovirus MSP142. In conclusion, immunization with E. coli MSP142 induces protective efficacy, and at least one of the probable effector mechanisms of that efficacy (as determined by an antibody-dependent growth inhibition assay) is a mechanism thought to be instrumental in natural immunity.
As a part of an overall strategy for malaria vaccine development based on recombinant MSP1, the following five different expression systems have been evaluated for MSP1 production: S. cerevisiae (18, 19), P. pastoris (4), baculovirus-infected insect cells (29), transgenic milk (30), and E. coli (this study). We concluded that E. coli is the optimal expression system. Both of the yeast systems failed to make full-length MSP142, and the efficacy of the smaller MSP119 fragment was less consistent (29). Although the efficacies of baculovirus-expressed MSP142, transgenic MSP142, and E. coli MSP142 appear to be indistinguishable, the yields of E. coli MSP142 are superior to those of baculovirus MSP142 (∼30 mg/liter, compared to ∼8 mg/liter), and the E. coli MSP142 development time frame is greatly reduced. As an example of the latter finding, we have been able to successfully manufacture cGMP grade material for human clinical trials of not just the FVO allele of E. coli MSP142 discussed here but also the alternate 3D7 allele (S. Singh and A. Stowers, unpublished data). Thus, the new construct, E. coli MSP142, is a viable candidate for human vaccine trials.
Acknowledgments
We thank Brenden S. Crabb for making the Pf-D10 and Pf-D10Pc parasite lines available and Richard Shrimp, Jr., Lanling Zou, Michael Whitmore, Olga Murtova, Lynn Lambert, Josh Reece, and Brian Keegan for their excellent technical assistance. We were advised during the production, refolding, and scale-up of E. coli MSP142 by the staff at AMGEN, Thousand Oaks, Calif. (especially Tom Boon and Jane Talvenheimo).
This study was supported in part by The Malaria Vaccine Initiative of the Bill & Melinda Gates Foundation.
Editor: J. M. Mansfield
REFERENCES
- 1.Blackman, M. J., I. T. Ling, S. C. Nicholls, and A. A. Holder. 1991. Proteolytic processing of the Plasmodium falciparum merozoite surface protein-1 produces a membrane-bound fragment containing two epidermal growth factor-like domains. Mol. Biochem. Parasitol. 49:29-33. [DOI] [PubMed] [Google Scholar]
- 2.Blackman, M. J., H. G. Heidrich, S. Donachie, J. S. McBride, and A. A. Holder. 1990. A single fragment of a malaria merozoite surface protein remains on the parasite during red cell invasion and is the target of invasion-inhibiting antibodies. J. Exp. Med. 172:379-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blackman, M. J., T. J. Scott-Finnigan, S. Shai, and A. A. Holder. 1994. Antibodies inhibit the protease-mediated processing of a malaria merozoite surface protein. J. Exp. Med. 180:389-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brady, C. P., R. L. Shimp, A. P. Miles, M. Whitmore, and A. W. Stowers. 2001. High-level production and purification of P30P2MSP1(19), an important vaccine antigen for malaria, expressed in the methylotropic yeast Pichia pastoris. Protein Expr. Purif. 23:468-475. [DOI] [PubMed] [Google Scholar]
- 5.Bremen, J. G. 2001. The ears of the hippopotamus: manifestations, determinants, and estimates of the malaria burden. Am. J. Trop. Med. Hyg. 64:1-11. [DOI] [PubMed] [Google Scholar]
- 6.Chang, S. P., S. E. Case, W. L. Gosnell, A. Hashimoto, K. J. Kramer, L. Q. Tam, C. Q. Hashiro, C. M. Nikaido, H. L. Gibson, C. T. Lee-Ng, P. J. Barr, B. T. Yokota, and G. S. Hut. 1996. A recombinant baculovirus 42-kilodalton C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 protects Aotus monkeys against malaria. Infect. Immun. 64:253-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chappel, J. A., and A. A. Holder. 1993. Monoclonal antibodies that inhibit Plasmodium falciparum invasion in vitro recognise the first growth factor-like domain of merozoite surface protein-1. Mol. Biochem. Parasitol. 60:303-311. [DOI] [PubMed] [Google Scholar]
- 8.Chitarra, V., I. Holm, G. A. Bentley, S. Petres, and S. Longacre. 1999. The crystal structure of C-terminal merozoite surface protein 1 at 1.8 A resolution, a highly protective malaria vaccine candidate. Mol. Cell 3:457-464. [DOI] [PubMed] [Google Scholar]
- 9.Daly, T. M., and C. A. Long. 1995. Humoral response to a carboxyl-terminal region of the merozoite surface protein-1 plays a predominant role in controlling blood-stage infection in rodent malaria. J. Immunol. 155:236-243. [PubMed] [Google Scholar]
- 10.Genton, B., I. Betuela, I. Felger, F. Al-Yaman, R. F. Anders, A. Saul, L. Rare, M. Baisor, K. Lorry, G. V. Brown, D. Pye, D. O. Irving, T. A. Smith, H. P. Beck, and M. P. Alpers. 2002. A recombinant blood-stage malaria vaccine reduces Plasmodium falciparum density and exerts selective pressure on parasite populations in a phase 1-2b trial in Papua New Guinea. J. Infect. Dis. 185:820-827. [DOI] [PubMed] [Google Scholar]
- 11.Good, M. F., D. C. Kaslow, and L. H. Miller. 1998. Pathways and strategies for developing a malaria blood-stage vaccine. Annu. Rev. Immunol. 16:57-87. [DOI] [PubMed] [Google Scholar]
- 12.Hirunpetcharat, C., J. H. Tian, D. C. Kaslow, N. van Rooijen, S. Kumar, J. A. Berzofsky, L. H. Miller, and M. F. Good. 1997. Complete protective immunity induced in mice by immunization with the 19-kilodalton carboxyl-terminal fragment of the merozoite surface protein-1 (MSP1[19]) of Plasmodium yoelii expressed in Saccharomyces cerevisiae: correlation of protection with antigen-specific antibody titer, but not with effector CD4+ T cells. J. Immunol. 159:3400-3411. [PubMed] [Google Scholar]
- 13.Hirunpetcharat, C., P. Vukovic, X. Q. Liu, D. C. Kaslow, L. H. Miller, and M. F. Good. 1999. Absolute requirement for an active immune response involving B cells and Th cells in immunity to Plasmodium yoelii passively acquired with antibodies to the 19-kDa carboxyl-terminal fragment of merozoite surface protein-1. J. Immunol. 162:7309-7314. [PubMed] [Google Scholar]
- 14.Hisaeda, H., A. Saul, J. J. Reece, M. C. Kennedy, C. A. Long, L. H. Miller, and A. W. Stowers. 2002. Merozoite surface protein 3 and protection against malaria in Aotus nancymai monkeys. J. Infect. Dis. 185:657-664. [DOI] [PubMed] [Google Scholar]
- 15.Holder, A. A., J. A. Guevara Patino, C. Uthaipibull, S. E. Syed, I. T. Ling, T. Scott-Finnigan, and M. J. Blackman. 1999. Merozoite surface protein 1, immune evasion, and vaccines against asexual blood stage malaria. Parassitologia 41:409-414. [PubMed] [Google Scholar]
- 16.Hui, G. S., C. Hashiro, C. Nikaido, S. E. Case, A. Hashimoto, H. Gibson, P. J. Barr, and S. P. Chang. 1993. Immunological cross-reactivity of the C-terminal 42-kilodalton fragment of Plasmodium falciparum merozoite surface protein 1 expressed in baculovirus. Infect. Immun. 61:3403-3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kennedy, M. C., J. Wang, Y. Zhang, A. P. Miles, F. Chitsaz, A. Saul, C. A. Long, L. H. Miller, and A. W. Stowers. 2002. In vitro studies with recombinant Plasmodium falciparum apical membrane antigen 1 (AMA1): production and activity of an AMA1 vaccine and generation of a multiallelic response. Infect. Immun. 70:6948-6960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar, S., A. Yadava, D. B. Keister, J. H. Tian, M. Ohl, K. A. Perdue-Greenfield, L. H. Miller, and D. C. Kaslow. 1995. Immunogenicity and in vivo efficacy of recombinant Plasmodium falciparum merozoite surface protein-1 in Aotus monkeys. Mol. Med. 1:325-332. [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar, S., W. Collins, A. Egan, A. Yadava, O. Garraud, M. J. Blackman, J. A. Guevara Patino, C. Diggs, and D. C. Kaslow. 2000. Immunogenicity and efficacy in aotus monkeys of four recombinant Plasmodium falciparum vaccines in multiple adjuvant formulations based on the 19-kilodalton C terminus of merozoite surface protein 1. Infect. Immun. 68:2215-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller, L. H., and S. L. Hoffman. 1998. Research toward vaccines against malaria. Nat. Med. 4:520-524. [DOI] [PubMed] [Google Scholar]
- 21.Miller, L. H., T. Roberts, M. Shahabuddin, and T. F. McCutchan. 1993. Analysis of sequence diversity in the Plasmodium falciparum merozoite surface protein-1 (MSP-1). Mol. Biochem. Parasitol. 59:1-14. [DOI] [PubMed] [Google Scholar]
- 22.O'Donnell, R. A., T. F. de Koning-Ward, R. A. Burt, M. Bockarie, J. C. Reeder, A. F. Cowman, and B. S. Crabb. 2001. Antibodies against merozoite surface protein (MSP)-1(19) are a major component of the invasion-inhibitory response in individuals immune to malaria. J. Exp. Med. 193:1403-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pandey, K. C., S. Singh, P. Pattnaik, C. R. Pillai, U. Pillai, A. Lynn, S. K. Jain, and C. E. Chitnis. 2002. Bacterially expressed and refolded receptor binding domain of Plasmodium falciparum EBA-175 elicits invasion inhibitory antibodies. Mol. Biochem. Parasitol. 123:23-33. [DOI] [PubMed] [Google Scholar]
- 24.Richie, T. L., and A. Saul. 2002. Progress and challenges for malaria vaccines. Nature 415:694-701. [DOI] [PubMed] [Google Scholar]
- 25.Sachs, J., and P. Malaney. 2002. The economic and social burden of malaria. Nature 415:680-685. [DOI] [PubMed] [Google Scholar]
- 26.Saul, A. 1987. Kinetic constraints on the development of a malaria vaccine. Parasite Immunol. 9:1-9. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt, L. H. 1972. The course of P. falciparum (Vietnam Oak Knoll strain) in Aotus trivigatus. Trans. R. Soc. Trop. Med. Hyg. 66:521. [DOI] [PubMed] [Google Scholar]
- 28.Singh, S., K. Pandey, R. Chattopadhayay, S. S. Yazdani, A. Lynn, A. Bharadwaj, A. Ranjan, and C. Chitnis. 2001. Biochemical, biophysical, and functional characterization of bacterially expressed and refolded receptor binding domain of Plasmodium vivax duffy-binding protein. J. Biol. Chem. 276:17111-17116. [DOI] [PubMed] [Google Scholar]
- 29.Stowers, A. W., V. Cioce, R. L. Shimp, M. Lawson, G. Hui, O. Muratova, D. C. Kaslow, R. Robinson, C. A. Long, and L. H. Miller. 2001. Efficacy of two alternate vaccines based on Plasmodium falciparum merozoite surface protein 1 in an Aotus challenge trial. Infect. Immun. 69:1536-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stowers, A. W., L. H. Chen Lh, Y. Zhang, M. C. Kennedy, L. Zou, L. Lambert, T. J. Rice, D. C. Kaslow, A. Saul, C. A. Long, H. Meade, and L. H. Miller. 2002. A recombinant vaccine expressed in the milk of transgenic mice protects Aotus monkeys from a lethal challenge with Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 99:339-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wipasa, J., H. Xu, M. Makobongo, M. Gatton, A. Stowers, and M. F. Good. 2002. Nature and specificity of the required protective immune response that develops postchallenge in mice vaccinated with the 19-kilodalton fragment of Plasmodium yoelii merozoite surface protein 1. Infect. Immun. 70:6013-6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang, W., X. Q. Liu, H. Xu, and M. F. Good. 2002. Polyspecific malaria antibodies present at the time of infection inhibit the development of immunity to malaria but antibodies specific for the malaria merozoite surface protein, MSP1, facilitate immunity. Parasite Immunol. 24:233-241. [DOI] [PubMed] [Google Scholar]