Abstract
Heterologous priming-boosting vaccination regimens involving priming with plasmid DNA antigen constructs and inoculating (boosting) with the same recombinant antigen expressed in replication-attenuated poxviruses have recently been demonstrated to induce immunity, based on CD4+- and CD8+-T-cell responses, against several diseases in both rodents and primates. We show that similar priming-boosting vaccination strategies using the 85A antigen of Mycobacterium tuberculosis are effective in inducing antigen-specific gamma interferon-secreting CD4+ and CD8+ T cells, detected by a bovine enzyme-linked immunospot assay, in Bos indicus cattle. T-cell responses induced by priming with either plasmid DNA or fowlpox virus 85A constructs were enhanced by boosting with modified vaccinia virus Ankara expressing the same antigen administered intradermally. On the basis of the data, it appears that intradermal priming was more effective than intramuscular delivery of the priming dose for boosting with the modified vaccinia virus Ankara strain in cattle. Using either fowlpox virus or DNA priming, there was a significant bias toward induction of CD4+- rather than CD8+-T-cell responses. These data illustrate the general applicability of priming-boosting vaccination strategies for induction of antigen-specific T-cell responses and suggest that the method may be useful for development of veterinary vaccines.
Infections with Mycobacterium bovis in cattle and wildlife hosts constitute a major economic burden worldwide in terms of loss of production and the cost of disease surveillance (9). Infected cattle often suffer clinical disease, bovine tuberculosis, and pose a considerable public health risk, especially in certain communities in developing countries that consume raw milk, where the incidence of this disease is increasing with the advent of pandemic human immunodeficiency virus (HIV)-induced syndrome (14). Control of M. bovis infections by the test-and-slaughter strategy is largely effective but not widely applied because of its uneconomical nature. Although initial experimental studies in which cattle were vaccinated with M. bovis bacillus Calmette-Guérin (BCG) demonstrated modest efficacy levels (36), subsequent trials have provided evidence for protection against clinical disease (8). However, the adoption of this vaccination regimen as a control measure for bovine tuberculosis has hitherto been complicated by the difficulty associated with differentiating between vaccinated and infected cattle (9, 34). This complication has been addressed in more recent studies indicating the potential of defined antigens to distinguish between these two groups of exposed cattle (34, 35). Studies with BCG in humans and animals have, however, been useful in providing insights into the mechanisms of protection against tuberculosis, which is crucial to the rational design of new improved tuberculosis vaccines (8, 19, 27, 30).
Intense efforts are, therefore, currently focused on developing subunit vaccines capable of inducing robust immunity based on antigen-specific CD4+- and CD8+-T-cell responses. Initial attempts to immunize cattle with recombinant protein or plasmid DNA failed to induce appropriate immune responses (9), which highlights the need to evaluate vaccination regimens that have been shown, in other systems, to induce strong cell-mediated immune responses. Studies using animal models of malaria, tuberculosis, and HIV infection have shown that the immunogenicity of plasmid DNA, influenza virus, or adenovirus in priming T-cell responses to defined antigens can be markedly enhanced by inoculating (boosting) with the modified vaccinia virus Ankara strain (MVA) or fowlpox virus (FP9) expressing the same antigens (1, 7, 12, 17, 21, 24, 28, 29). These experiments also demonstrated that the efficacy of protection to lethal challenge was enhanced upon boosting, and in some cases sterile immunity was achieved (7, 28).
A number of mycobacterial antigens have been studied in murine models and in BCG-vaccinated humans for their potential as targets for T-cell immunity. Secreted extracellular antigens have been found to be prime candidates. The antigen 85 complex, which comprises three distinct but highly conserved proteins (85A, -B, and -C), constitutes 30% of the M. tuberculosis and M. bovis BCG culture filtrate proteins (37). 85A represents a major portion of the complex and has been shown to be a key antigenic target for CD4+- and CD8+-T-cell responses in BCG-vaccinated donors (20, 26, 30, 31). Immunization of mice using 85A plasmid DNA has resulted in significant induction of CD4+- and CD8+-T-cell responses but only partial immunity to challenge (11, 15, 22). Further studies of mice have demonstrated enhancement of T-cell immunogenicity and improved protection to challenge following 85A plasmid DNA priming and protein boosting (32).
The present study seeks to evaluate the utility of a strategy involving priming with plasmid DNA or recombinant FP9 and boosting with recombinant MVA expressing 85A to induce antigen-specific T-cell responses in cattle as a basis for developing a subunit vaccine against bovine tuberculosis. Priming with either of the two agents and boosting with recombinant MVA elicited significant frequencies of peptide-specific gamma interferon (IFN-γ)-secreting T cells in immunized cattle. In general, IFN-γ-secreting T cells were capable of proliferating upon further peptide stimulation. These findings raise the prospect of assessing this vaccination regimen in cattle challenged with virulent M. bovis.
MATERIALS AND METHODS
Plasmid DNA constructs
The 85A coding sequence was PCR amplified from M. tuberculosis genomic DNA using the following primers: 85A upper (5′-AGATCTATGCAGCTTGTTGACAGGGTTCG-3′) and 85A lower (5′-GGATCCACGTTGCAGGTCGGGCTTCATA-3′). This amplified a product encoding amino acids 1 to 323 of 85A. The PCR product was ligated to other sequences to create in-frame fusions of the human tissue plasminogen activator (tPA) leader at the N terminus and a monoclonal antibody recognition tag at the C terminus. The tPA leader sequence was found to increase expression and immunogenicity of 85A (23, 24). The C-terminal tag is recognized by anti-P-K monoclonal antibody (Serotec, Oxford, United Kingdom) and consists of the amino acid sequence IPNPLLGLD. This was included to allow detection of expression of the antigen in different vaccine delivery systems.
The resulting tPA/85A/P-K sequence was ligated into a DNA vaccine vector, pSG2 (24), downstream of the cytomegalovirus promoter to make pSG2-85A. The resulting plasmid was purified by anion-exchange chromatography (QIAGEN GmbH, Hilden, Germany) and diluted in endotoxin-free phosphate-buffered saline (PBS) (Sigma Chemical Co., Poole, Dorset, United Kingdom) for injection.
Construction of recombinant MVA and FP9.
The tPA/85A/P-K sequence was also ligated into the vaccinia virus shuttle vector pSC11 (10). Primary chicken embryo fibroblasts (CEFs) were infected with MVA (Anton Mayr, University of Munich) and transfected with pSC11-85A. Recombinants were identified by expression of beta-galactosidase and purified by repeated plaque picking.
To generate recombinant attenuated FP9, the insert was ligated into the vector FP9-GFP. This shuttle vector allows expression of the insert using the vaccinia P7.5 early-late promoter as in pSC11, and the expression of the marker gene coding for green fluorescent protein (GFP) from the FP9 late promoter fp4b (S. Gilbert, unpublished data). CEFs were infected with FP9 (Michael Skinner, Institute of Animal Health, Compton, United Kingdom) and transfected with FP9-GFP-85A. Recombinants express GFP and were enriched by sorting of infected CEFs using a fluorescence-activated cell sorter (FACSVantage; Becton Dickinson) and then purified by plaque picking. Recombinant MVA and FP9 were produced in primary CEFs, purified through a sucrose cushion by ultracentrifugation, and diluted in PBS for injection.
Experimental cattle.
Female and male Boran (Bos indicus) calves 6 to 8 months old were used in the study. Animals were screened for T-cell reactivity to purified protein derivative (PPD) of M. bovis and M. avium before and during the study. These animals were selected from the International Livestock Research Institute cattle resource and were handled in accordance with the guidelines of the institute's Animal Care and Use Committee.
Experimental design and cattle inoculations.
Four groups of four animals each were subjected to priming-boosting immunizations. Data for groups 1, 2, and 3 are indicated in Tables 1 and 2. Group 4 consisted of four male cattle: animals BV157, BV158, BV160, and BV166. These animals were primed with 2 × 108 PFU of FP-85A given intradermally (i.d.). First and second booster immunizations with 109 PFU of MVA-85A were administered i.d. at weeks 4 and 8, respectively.
TABLE 1.
Experiment 1: regimen of immunization of group 1 by priming with plasmid DNA and boosting with recombinant MVA
| Immunizations received | Animala | DNA (mg) administered at wk 0 | PFU of virus administeredb at wk:
|
||
|---|---|---|---|---|---|
| 4 | 24 | 72 | |||
| pSG2-85A and MVA-85A (test) | BT37 | 2 i.d., 2 i.m. | 5 × 108 i.v. | 109 i.v. | 109 i.v. |
| BT99 | 2 i.d., 2 i.m. | 5 × 108 i.d. | 109 i.d. | 109 i.d. | |
| pSG2 and MVA (control) | BT53 | 2 i.d., 2 i.m. | 5 × 108 i.v. | 109 i.v. | Not done |
| BT47 | 2 i.d., 2 i.m. | 5 × 108 i.d. | 109 i.d. | Not done | |
Animal BT53 was female; the others in this group were male.
i.v., intravenously.
TABLE 2.
Experiment 2: comparison of results of i.d. and i.m. DNA priming followed by MVA boosting
| Group (animala) | pSG2-85A (mg) administered at wk:
|
MVA-85A (PFU) administered at wk:
|
||
|---|---|---|---|---|
| 0 | 4 | 8 | 12 | |
| 2 (BV156, BV159, BV162, BV165) | 4 (i.m.) | 4 (i.m.) | 109 (i.d.) | 109 (i.d.) |
| 3 (BV155, BV161, BV163, BV164) | 4 (i.d.) | 4 (i.d.) | 109 (i.d.) | 109 (i.d.) |
All animals in groups 2 and 3 were male.
Marsupialization of the spleen.
In order to gain easy access to the spleen for repetitive biopsies, the spleens of cattle in group 1 were “marsupialized.” Briefly, the animals were put under general anesthesia and a laparotomy was performed on the left side via the 12th rib. The tail of the spleen was secured in a pouch between the skin and the intercostal muscle anterior to the 13th rib. By introducing a 16-guage needle through the overlying skin and into the spleen, samples of spleen could be aspirated with a syringe containing Alsever's solution. As the tail of the spleen was held snugly in the pouch, postaspiration hemorrhage was minimal.
Preparation of splenocytes and PBMC.
Isolation of peripheral blood mononuclear cells (PBMC) from the venous blood-Alsever's solution mixture and splenocytes teased from spleen needle biopsy specimens was achieved by flotation on Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) as described before (13). Cells were counted before cell depletions were conducted.
Cell depletions.
Splenocytes and PBMC were depleted of T cells bearing the γδ antigen receptor and B lymphocytes (hereinafter referred to as d-SPL and d-PBL, respectively) before use in in vitro assays. In certain instances, CD4+ or CD8+ T cells plus monocytes were purified and used in assays. Cell purifications were achieved by negative selection. Undesirable cell subpopulations were stained with specific monoclonal antibodies, and anti-mouse immunoglobulin G antibody-conjugated ferrous beads (Dynal, Oslo, Norway) were added. The cell-bead mixture was put in a magnet, and unbound cells were collected.
Peptides.
Overlapping peptides spanning the entire length of the 85A protein of M. tuberculosis were purchased from Research Genetics (Huntsville, Ala.). The peptides were 20 residues long and overlapped by 10 amino acids as shown in Table 3. Peptide synthesis was confirmed by high-performance liquid chromatography and mass spectrometry profiles to be on the order of >80% purity.
TABLE 3.
Synthetic peptides derived from M. tuberculosis protein 85A
| Peptide | Sequence |
|---|---|
| p1 | MQLVDRVRGAVTGMSRRLVV |
| p2 | VTGMSRRLVVGAVARLVSGL |
| p3 | GAVARLVSGLVGAVGGTATA |
| p4 | VGAVGGTATAGAFSRPGLPV |
| p5 | GAFSRPGLPVEYLQVPSPSM |
| p6 | EYLQVPSPSMGRDIKVQFQS |
| p7 | GRDIKVQFQSGGANSPALYL |
| p8 | GGANSPALYLLDGLRAQDDF |
| p9 | LDGLRAQDDFSGWDINTPAF |
| p10 | SGWDINTPAFEWYDQSGLSV |
| p11 | EWYDQSGLSVVMPVGGQSSF |
| p12 | VMPVGGQSSFYSDWYQPACR |
| p13 | YSDWYQPACRKAGCQTYKWE |
| p14 | KAGCQTYKWETFLTSELPGW |
| p15 | TFLTSELPGWLQANRHVKPT |
| p16 | LQANRHVKPTGSAVVGLSMA |
| p17 | GSAVVGLSMAASSALTLAIY |
| p18 | ASSALTLAIYHPQQFVYAGA |
| p19 | HPQQFVYAGAMSGLLDPSQA |
| p20 | MSGLLDPSQAMGPTLIGLAM |
| p21 | MGPTLIGLAMGDAGGYKASD |
| p22 | GDAGGYKASDMWGPKEDPAW |
| p23 | MWGPKEDPAWQRNDPLLNVG |
| p24 | QRNDPLLNVGKLIANNTRVW |
| p25 | KLIANNTRVWVYCGNGKPSD |
| p26 | VYCGNGKPSDLGGNNLPAKF |
| p27 | LGGNNLPAKFLEGFVRTSNI |
| p28 | LEGFVRTSNIKFQDAYNAGG |
| p29 | KFQDAYNAGGRHNGVFDFPD |
| p30 | RHNGVFDFPDSGTHSWEYWG |
| p31 | SGTHSWEYWGAQLNAMKPDL |
Quantification of peptide-specific T cells by the ELISPOT assay.
The frequency of peptide-specific IFN-γ-releasing T cells in immunized cattle was determined using a direct ex vivo enzyme-linked immunospot (ELISPOT) assay essentially as described before (25, 31) with modifications. Briefly, 96-well polyvinylidene difluoride-backed plates (MAIP S45; Millipore) were precoated with anti-bovine IFN-γ capture monoclonal antibody 2-2-1 (5 μg/ml; Serotec) overnight at 4°C. Plates were washed twice with Optimem (Gibco BRL, Paisley, United Kingdom)-2% (vol/vol) heat-inactivated fetal calf serum (FCS) (Life Technologies Ltd., Paisley, United Kingdom) and blocked with Optimem-10% FCS for 2 h at 37°C. Peptides at a final concentration of 10 μg/ml in Optimem-2% FCS were added in 50-μl aliquots to the wells. A control well with medium alone and another well with concanavalin A (Sigma Chemical Co.) at a final concentration of 5 μg/ml were included. Responder cells (d-SPL, d-PBL, CD4+, or CD8+ T cells) were added in 50-μl aliquots containing 2.5 × 105 cells per well. A precoated well with no cells added was also included. Plates were kept for 20 h in a humidified incubator at 37°C and 5% CO2. After the cells were shaken off, the plates were washed twice with distilled water and then thrice with PBS 0.05%-Tween 20 (Sigma Chemical Co.), each time with a plate being shaken on a shaker for 20 s before the wash fluid was flicked off. A second rabbit anti-bovine IFN-γ antibody (an in-house reagent used at 1:1,500) was added in 100-μl aliquots, and the plates were incubated for 1 h at room temperature. A further three washes with PBS-Tween 20 were performed without shaking the plates before addition of an anti-rabbit immunoglobulin G-alkaline phosphatase conjugate (clone R696 [100 μl/well; 1:2,000 in PBS-T-bovine serum albumin]; Sigma Chemical Co.) for 1 h at room temperature. Plates were further washed six times before 100 μl of chromogenic alkaline phosphatase substrate (5-bromo-4-chloro-3-indolylphosphate-nitroblue tetrazolium; Sigma Chemical Co.) was added to each well for up to 5 min in the dark to allow spot development. Copious amounts of tap water were added, and the plates were air dried. Spots were initially observed under a dissection microscope (Ernst Leitz Wetzlar GmbH, Germany) and then counted using an ELISPOT Reader (Autoimmun Diagnostika GmbH, Strassberg, Germany).
Proliferation assays.
Cultures of d-SPL or d-PBL were established in triplicate wells containing 200-μl aliquots of 5 × 105 cells per well in 96-well flat-bottom microtiter plates (Costar) in the presence of 10-μg/ml peptide pools. After 5 days of incubation at 37°C and 5% CO2, cultures were pulsed with 100 μCi of [125I]iododeoxyuridine (Amersham, Little Chalfont, United Kingdom) for 8 h before harvesting of the cells on DNA filters using a cell harvester. The amount of radioisotope incorporated into dividing cells was monitored using a gamma counter (ICN Micromedic Systems, Huntsville, Ala.).
Statistical analysis.
Individual ELISPOT values were transformed to logarithms. These were then analyzed using a repeated-measures analysis of variance by immunization group. The means on the logarithmic scale were then detransformed to derive geometric means.
RESULTS
Priming with 85A plasmid DNA and boosting with recombinant MVA elicits peptide-specific IFN-γ-secreting T cells.
To assess whether priming with pSG2-85A and boosting with MVA-85A induces 85A-specific IFN-γ-producing T cells in cattle, two animals (BT37 and BT99) were inoculated with recombinant plasmid DNA and recombinant MVA, and another two cattle (BT47 and BT53) were inoculated with empty plasmid DNA and wild-type MVA as shown in Table 1. ELISPOT assays were performed with d-SPL obtained prior to vaccination; 14 days after DNA immunization; 7, 14, and 21 days and 6 months after the first MVA booster injection; and 7, 14, and 32 days and 16 weeks after the second MVA booster and at 10 days after the third MVA booster. Due to the initial problems of high background in the assays, cells taken before vaccination; 14 days after DNA inoculation; and at 7, 14, and 21 days after the first MVA booster were cryopreserved during further assay optimization. For assays performed at the other time points, freshly isolated cells were utilized without cryopreservation.
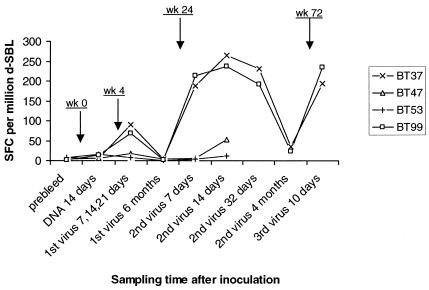
As shown in Fig. 1, peptide-specific T cells detectable in all the animals prior to immunization were at marginal levels (<5 to 15 spot-forming cells (SFC) per 106 cells), and these frequencies were similar to those observed at 14 days after DNA inoculation. By contrast, at days 7, 14, and 21 (cells at these three time points were pooled to obtain sufficient numbers) following the first MVA booster, the frequencies of peptide-specific T cells from 85A-immunized animals increased nearly fivefold to 70 to 90 SFC per 106 cells, while those observed with cells from control animals were either unaltered or showed a slight increase above marginal levels. When tested next at 6 months, peptide-specific cytokine responses in all the animals were either undetectable or marginal. Further assays conducted at days 7, 14, and 32 following a second virus booster revealed levels as high as 220 to 260 SFC/106 cells in the test animals, compared with 10 to 50 SFC/106 cells observed in the control group. However, at 16 weeks after the second virus booster, frequencies of peptide-specific T cells detected in the test animals had declined approximately 8- to 10-fold (corresponding assays were not performed with the control group). The test animals received further boosters with recombinant MVA, and assays performed at 10 days showed a steep rise (190 to 230 SFC/106 cells) in the frequencies of peptide-specific cells.
FIG. 1.
Frequencies of peptide-specific T cells in cattle primed with 85A plasmid DNA and boosted several times with recombinant MVA. BT37 and BT99 were inoculated with pSG2-85A and MVA-85A as the test group, while BT47 and BT53 received empty pSG2 and wild-type MVA to serve as controls. Cells were obtained at the indicated time points following inoculation (at weeks 0, 4, 24, and 72) and utilized in IFN-γ ELISPOT assays. Results are presented as sums of the numbers of cytokine-releasing cells per million d-SPL responding to individual peptides after correcting for responses in medium-only control wells.
Collectively, these data indicate that the induction of peptide-specific T cells in the animals is a consequence of immunization with 85A-expressing plasmid DNA and boosting with recombinant MVA and that priming with 85A DNA is insufficient, on its own, to induce detectable responses. The data also demonstrate that detectable responses last for periods less than 6 months but that the animals can again be vaccinated with the booster to obtain high-level responses, suggesting that memory T cells are maintained in vivo despite the inability to detect them in vitro.
Comparison of peptide-specific responses in spleen and peripheral blood.
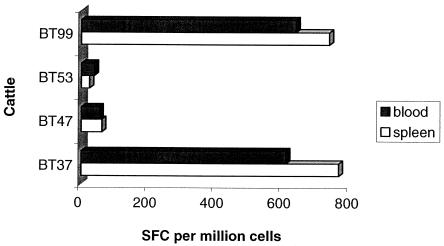
Splenocytes and PBMC were prepared from group 1 cattle to compare the levels of peptide-specific T-cell responses in spleen and peripheral blood. Assays were conducted with cells obtained at 6 months after the first MVA dose and at 7, 14, and 32 days and 4 months after the second MVA dose. The cumulative responses in individual animals are reported in Fig. 2. It is evident from the results that responses observed in both spleen and peripheral blood at the indicated time points following MVA boosters are comparable in magnitude and specificity. Based on these observations, all subsequent assays were performed utilizing PBMC.
FIG. 2.
Reactivity of splenocytes and PBMC to 85A. d-SPL and d-PBL were prepared at different time points following virus boosters and assessed for their capacity to respond to 85A peptides in IFN-γ ELISPOT assays. Data are presented as cumulative sums of cytokine-secreting T cells per million d-SPL or d-PBL.
Comparison of i.d. and i.m. DNA priming following i.d. boosting by recombinant MVA.
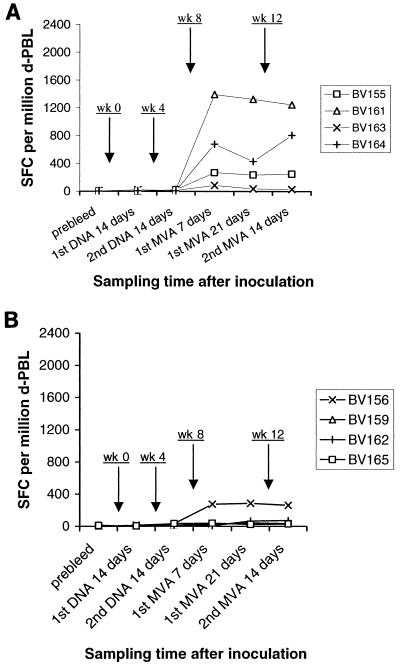
Experiments were performed using two groups of four cattle to determine the efficiency of inducing 85A-specific IFN-γ-secreting T cells by priming with DNA either i.d. or intramuscularly (i.m.) and boosting with recombinant MVA i.d. The animals were immunized as indicated in Table 2. d-PBL were prepared from the animals prior to immunization, 14 days after each DNA prime, 7 and 21 days after the first MVA booster, and 14 days after the second MVA booster and were subjected to ELISPOT assays. As shown in Fig. 3A, frequencies ranging from <5 to 24 SFC/106 cells were observed prior to immunization and after each i.d. DNA prime in all the animals. By contrast, large numbers (86 to 1,395 SFC/106 cells) of responding cells were detected at 7 and 21 days after the first MVA booster, representing increases up to 80-fold. When tested at 14 days following a second MVA booster, similar frequencies of peptide-specific T cells were detected. The magnitude of these responses varied between individual animals; BV161 exhibited high-level responses (1,245 to 1,395 SFC/106 cells), BV155 and BV164 exhibited medium-level responses (238 to 808 SFC/106 cells), and BV163 exhibited low-level responses (27 to 86 SFC/106 cells).
FIG. 3.
(A) Responses following i.d. DNA priming and i.d. recombinant MVA boosting. Cells were obtained from cattle at indicated time points following inoculation (at weeks 0, 4, 8, and 12) and assessed for peptide reactivity in ELISPOT assays as described in Materials and Methods. Results are presented as sums of the numbers of cytokine-releasing cells per million d-PBL responding to individual peptides after correcting in medium-only control wells. (B) Responses following i.m. DNA priming and i.d. recombinant MVA boosting. The assays were performed and results are presented as reported for Fig. 3A.
Corresponding analyses were carried out with cells obtained from cattle immunized by priming with DNA i.m. and boosting with MVA i.d. Results of these experiments are shown in Fig. 3B. Frequencies of <5 to 14 SFC/106 cells were observed prior to immunization and after the first DNA prime, and these responses showed a general, albeit slight, increase to 15 to 36 SFC/106 cells following the second DNA prime. Assays performed 7 and 14 days after the first MVA booster showed either a slight or no increase (10 to 66 SFC/106 cells) in the frequencies of responding cells from 3 animals (BV159, BV162 and BV165), and a markedly enhanced response (275 to 287 SFC/106 cells) in 1 animal (BV156). Similar levels of responses were observed at 14 days after the second MVA booster.
The data from these two groups of cattle (experiment 2), compared to those from experiment 1, indicate that an increase in the amount and frequency of DNA administered using either route of inoculation resulted in a slight or no enhancement of peptide-specific T cells detectable after priming. However, upon boosting with recombinant MVA, three of four cattle primed by DNA i.d. showed medium to high levels of cytokine responses while the fourth animal exhibited a low response. By contrast, of four animals primed by DNA i.m., one animal gave a medium response while the rest had a low response following boosting with recombinant MVA. Geometric means, with ranges in parentheses, were 308 (44 to 1,321) SFC/106 cells and 56 (30 to 274) SFC/106 cells for DNA i.d. and DNA i.m. groups, respectively. Statistical analysis on a logarithmic scale showed that the mean for animals primed i.d. was higher than the mean for animals primed i.m. (P = 0.09).
Recombinant FP9 priming generates moderate but clearly detectable responses that are boosted significantly by recombinant MVA.
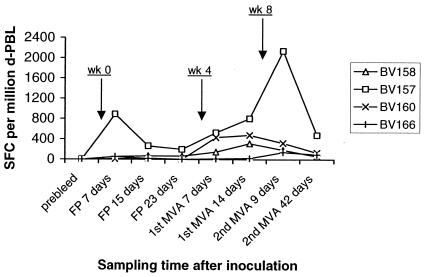
To evaluate the utility of priming with recombinant FP and boosting with MVA expressing 85A to induce antigen-specific T cells, four cattle were immunized as described in experiment 3 above, and ELISPOT assays were conducted at different time points. As shown in Fig. 4, while preimmunization samples yielded <5 SFC/106 cells in all animals, cells obtained at 7, 15, and 23 days following priming with FP9 generated various levels of responses in individual cattle. Animal BV157 exhibited a response of 201 to 890 SFC/106 cells, and BV158 exhibited a response of 57 to 71 SFC/106 cells, while animals BV160 and BV166 exhibited a response of 11 to 57 SFC/106 cells. Assays performed at 7 and 14 days after a first MVA booster yielded various increases in the frequencies of responding cells; animal BV158 showed a three- to fivefold increase, to 150 to 318 SFC/106 cells; animal BV160 showed a 20- to 40-fold increase, to 435 to 480/106 cells; and animals BV157 (530 to 808 SFC/106 cells) and BV166 (13 to 24 SFC/106 cells) did not show an increase in the response. When tested at 9 days after a second MVA booster, responses in BV157 were enhanced by nearly threefold, to 2,142 SFC/106 cells, while those observed in BV158 and BV160 slightly decreased, to 189 to 324 SFC/106 cells. It is notable that the response detected in BV166 had risen by approximately sixfold, to 146 SFC/106 cells. By 42 days after the second MVA booster was administered, there was a general decline in the frequencies of responding cells in all the animals.
FIG. 4.
Responses induced by FP priming and recombinant MVA boosting. ELISPOT assays of d-PBL were performed at the indicated time points following inoculations at weeks 0, 4, and 8. Results are presented as a sum of the SFC per million d-PBL in positive wells.
Together, the data indicate that FP priming induced responses detectable above the preimmunization levels, although considerable variation between animals was exhibited. The other major observation is the general boosting effect by recombinant MVA seen in all the animals. The geometric mean, 205 SFC/106 cells (range, 46 to 815 SFC/106 cells), of responses after the MVA booster is similar to that for the group subjected to DNA priming i.d. but higher than that for the group subjected to DNA priming i.m. (P = 0.09).
T-cell reactivity to individual peptides was disparate in the majority of animals.
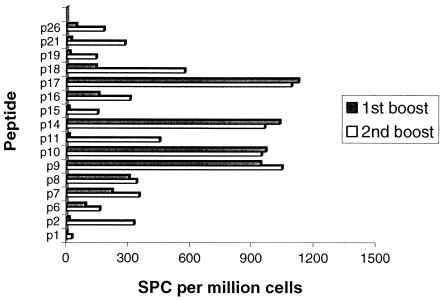
Responses to all regions of 85A were detected in different animals, as shown in Fig. 5, indicating that there appears to be no obvious immunodominant portion of the molecule. Responses to some peptides were detected only after a second booster, exhibiting the phenomenon of “epitope spreading” as described previously (33).
FIG. 5.
T-cell reactivity to individual peptides across animal groups. Responses of CD4 and CD8 T cells detected against individual peptides in all animals were analyzed following the first and second MVA boosters to determine the spread of activity.
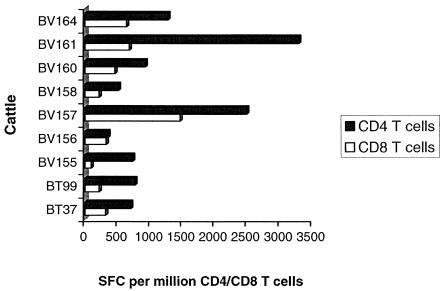
The frequency of peptide-specific IFN-γ-secreting CD4+ T cells induced was higher than that of CD8+ T cells irrespective of the immunization regimen.
Assays were carried out utilizing purified CD4+ or CD8+ T cells in the presence of monocytes and 85A peptides to determine T-cell-subset-restricted responses. Cells from the four groups of cattle reflecting four different immunization regimens were included in the assays. Results of these experiments are shown in Fig. 6. Frequencies of peptide-specific CD8+ T cells ranged between 91 and 1,465 SFC/106 cells, while those of CD4+ T cells ranged from 349 to 3,292 SFC/106 cells. Except for animal BV156, in which the CD4/CD8 T-cell responding ratio was 1:1, all the other animals exhibited a CD8+-T-cell response that was 10 to 50% that of the CD4+ T cells. It is evident from these findings that the majority of animals generated a predominantly CD4+-biased T-cell peptide-specific response regardless of the immunization protocol.
FIG. 6.
CD4+- and CD8+-T-cell responses to 85A peptides. Cells were purified and used in IFN-γ ELISPOT assays as described in Materials and Methods. Results are presented as the sum of responses following recombinant MVA boosters.
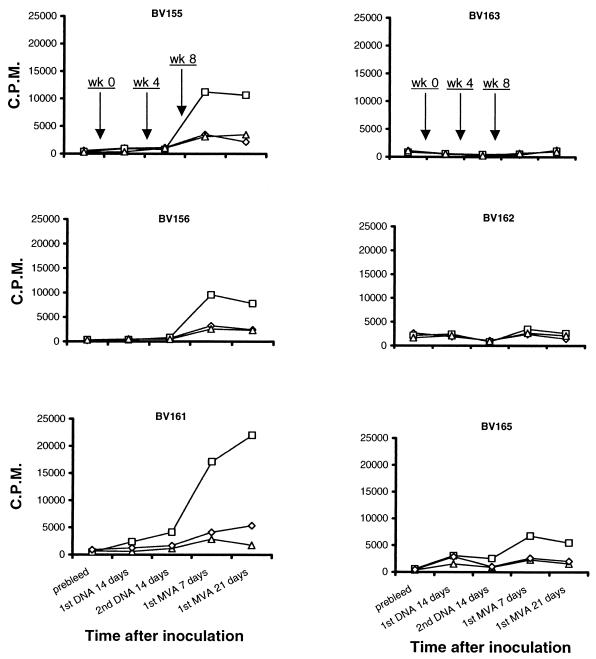
Peptide-specific IFN-γ-secreting T cells are capable of undergoing proliferation in response to further stimulation.
Experiments were carried out to determine whether T cells that produced IFN-γ in response to 85A peptides were capable of peptide-specific lymphoproliferation. Cultures of d-PBL were incubated in the presence of 85A peptides as described in Materials and Methods. Cultures to which no peptide or irrelevant peptides derived from a Theileria parva polymorphic immunodominant molecule were added served as controls. Figure 7 shows data obtained with cells from cattle immunized by the i.d. DNA-i.d. MVA or i.m. DNA-i.d. MVA regimens. Compared with the corresponding ELISPOT data (Fig. 3), it is evident that animals that exhibited an IFN-γ response were capable of mounting a peptide-specific T-cell proliferative activity.
FIG. 7.
Cultures of d-PBL obtained from different cattle at various time points following inoculations at weeks 0, 4, and 8 as indicated were set up to determine peptide-specific T-cell proliferative activity. Cultures incorporated 85A peptides (□), irrelevant peptides (▵), or no peptide (⋄).
T-cell reactivity to PPD of M. bovis and M. avium.
As described in Materials and Methods, d-SPL and d-PBL were evaluated for their capacity to proliferate to PPD derived from M. bovis and M. avium prior to and periodically after immunization. Marginal or no reactivity to PPD was demonstrated (data not shown).
DISCUSSION
An effective vaccine against M. bovis infection in cattle is needed in both developed and developing countries. In the United Kingdom an expensive and extensive test-and-slaughter policy has failed to prevent a sharp increase in cases of bovine tuberculosis, and a recent scientific review has concluded that the best prospect for long-term control of the disease is the development of a vaccine (18). In African countries, such as Tanzania, milk is not normally pasteurized, and in specific foci within pastoralist agro-ecosystems, M. bovis can be present in milk for human consumption, representing a serious public health risk in a country where the rate of HIV is increasing (16). One promising option for control of M. bovis infection in such a setting is an inexpensive, safe, and effective vaccine capable of generating long-term immunity. Vaccination with the BCG attenuated live vaccine can often be ineffective due to prior exposure of livestock or humans to related strains of M. bovis (6). A similar problem should not apply to subunit vaccines based on priming and boosting with defined recombinant antigens.
Heterologous priming-boosting immunization has been used to generate antigen-specific T-cell responses in several animal models of human diseases, and clinical trials are now in progress for immunization againstmalaria, HIV, tuberculosis, and melanoma using different versions of this technology, but in particular using a recombinant attenuated poxvirus to boost a previously primed response to the same antigen. We report here the application of this technology to cattle to immunize against an economically important cattle disease.
Immunization with 85A in both DNA-MVA and FP9-MVA priming-boosting regimens induced high levels of T-cell responses in the majority of immunized animals. Responses could be detected in T cells obtained from both spleen and blood. No response to PPD from either M. bovis or M. avium was detected, indicating that the cattle had not previously been exposed to these microorganisms. Responses were very low or undetectable following DNA immunization whether administered i.m. or i.d., despite using a high dose (4 mg). Allowing for the small sample size, the results suggest that the route of DNA administration had an effect on the responses obtained after MVA boosting; higher responses were obtained after i.d. DNA priming. Skin contains more antigen-presenting cells, including Langerhans cells and dendritic cells (5), than muscle. Although the effector T-cell response (as measured by ELISPOT assay) generated after both i.d. and i.m. DNA priming is low, i.d. priming results in a larger pool of memory cells that are available to be boosted by recombinant MVA. Priming with recombinant FP9 i.d. resulted in similar responses, after boosting, to i.d. DNA and i.d. MVA. Further comparison of the two regimens will be necessary to decide which is the more immunogenic. For practical purposes, the FP9-MVA regimen has an advantage over that with DNA-MVA since the manufacturing processes for both FP9- and MVA-based vaccines are very similar. Production of both vaccines is straightforward, and production plants could be set up in the developing countries where the vaccine is needed, thereby reducing the production costs. Manufacture of DNA vaccines requires a different process and more-specialized reagents and is therefore more expensive, making DNA-MVA immunization a less-economic alternative.
The majority of the antigen-specific T cells were CD4+, although CD8+ cells were also generated. There is strong evidence from animal and human studies that CD4+ T cells are necessary for protective immunity against Mycobacterium infections (3, 4, 19, 27). However, CD8+ T cells can also be detected following infection and may contribute to protection (24).
The T-cell responses measured by ELISPOT declined to low levels within 4 months of the MVA booster. However, responses could be boosted to high levels again by a second administration of the MVA, indicating that a population of memory cells had been generated following the initial priming-boosting immunization. In a study using a combination of antigens delivered together, macaques immunized by DNA-MVA priming and boosting against simian immunodeficiency virus were protected from development of AIDS symptoms when challenged with a highly pathogenic strain 7 months after the boosting immunization (2), demonstrating the longevity of protective responses generated by this type of immunization.
Responses to each of the immunization regimens varied between animals, as expected in an outbred population. Indeed, it is surprising that responses were generated to such a small antigen (323 amino acids) in almost all animals immunized. The aim of this study was to examine the efficacy of heterologous priming-boosting immunization to induce T-cell responses in cattle, but as strong responses were generated in the majority of cattle using a single small antigen, the protective efficacy of this regimen should be tested using 85A alone. Should this prove partially protective it may be possible to increase protective efficacy by the inclusion of a second antigen in the vaccines. None of the vaccines resulted in systemic or local side effects following immunization. DNA, MVA, and FP9 therefore appear to be both safe and capable of inducing T-cell responses in cattle and should therefore be further evaluated as vaccines against M. bovis infection. The heterologous priming-boosting regimen would also be worth testing as a delivery system for antigens for other cattle diseases in which T-cell responses are believed to be important in mediating protection, including theileriosis, contagious bovine pleuropneumonia, and cowdriosis.
Acknowledgments
We are thankful to James Gachanja, Reeves Njamunggeh, and Paul Muiya for excellent assistance with assays and to David Kennedy for spleen surgery. Initial advice on the bovine IFN-γ ELISPOT protocol was provided by Martin Vordermeier, for which he is kindly appreciated. John Rowlands and William Reece are gratefully acknowledged for their expertise in statistical analysis.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Allen, T. M., T. U. Vogel, D. H. Fuller, B. R. Mothe, S. Steffen, J. E. Boyson, T. Shipley, J. Fuller, T. Hanke, A. Sette, J. D. Altman, B. Moss, A. J. McMichael, and D. I. Watkins. 2000. Induction of AIDS virus-specific CTL activity in fresh, unstimulated peripheral blood lymphocytes from rhesus macaques vaccinated with a DNA prime/modified vaccinia virus Ankara boost regimen. J. Immunol. 164:4968-4978. [DOI] [PubMed] [Google Scholar]
- 2.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H.-L. Ma, B. D. Grimm, M. L. Hulsey, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2002. Control of a mucosal challenge and prevention of AIDS by a multi-protein DNA/MVA vaccine. Vaccine 20:1949-1955. [DOI] [PubMed] [Google Scholar]
- 3.Andersen, P., A. B. Andersen, A. L. Sorensen, and S. Nagai. 1995. Recall of long-lived immunity to Mycobacterium tuberculosis infection in mice. J. Immunol. 154:3359-3372. [PubMed] [Google Scholar]
- 4.Barnes, P. F., S. Lu, J. S. Abrams, E. Wang, M. Yamamura, and R. L. Modlin. 1993. Cytokine production at the site of disease in human tuberculosis. Infect. Immun. 61:3482-3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bos, J. D., and M. L. Kapsenberg. 1993. The skin immune system: progress in cutaneous biology. Immunol. Today 14:75-78. [DOI] [PubMed] [Google Scholar]
- 6.Brandt, L., J. F. Cunha, A. W. Olsen, B. Chilima, P. Hirsch, R. Appelberg, and P. Andersen. 2002. Failure of the Mycobacterium bovis BCG vaccine: some species of environmental mycobacteria block multiplication of BCG and induction of protective immunity to tuberculosis. Infect. Immun. 70:672-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruna-Romero, O., G. Gonzalez-Aseguinolaza, J. C. R. Hafalla, M. Tsuji, and R. S. Nussenzweig. 2001. Complete, long-lasting protection against malaria of mice primed and boosted with two distinct viral vectors expressing the same plasmodial antigen. Proc. Natl. Acad. Sci. USA 98:11491-11496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buddle, B. M., G. W. De Lisle, A. Pfeffer, and F. E. Aldwell. 1995. Immunological responses and protection against Mycobacterium bovis in calves vaccinated with a low dose of BCG. Vaccine 13:1123-1130. [DOI] [PubMed] [Google Scholar]
- 9.Buddle, B. M. 2001. Vaccination of cattle against Mycobacterium bovis. Tuberculosis (Edinburgh) 81:125-132. [DOI] [PubMed] [Google Scholar]
- 10.Chakrabarti, S., K. Brechling, et al. 1985. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol. Cell. Biol. 5:3403-3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denis, O., A. Tanghe, K. Palfliet, F. Jurion, T.-P. van der Berg, A. Vanonckelen, J. Ooms, E. Saman, J. B. Ulmer, J. Content, and K. Huygen. 1998. Vaccination with plasmid DNA encoding mycobacterial antigen 85A stimulates a CD4+ and CD8+ T-cell epitopic repertoire broader than that stimulated by Mycobacterium tuberculosis H37Rv infection. Infect. Immun. 66:1527-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilbert, S. C., J. Schneider, M. Plebanski, C. M. Hannan, T. J. Blanchard, G. L. Smith, and A. V. S. Hill. 1999. Ty virus-like particles, DNA vaccines and modified vaccinia virus Ankara; comparisons and combinations. Biol. Chem. 380:299-303. [DOI] [PubMed] [Google Scholar]
- 13.Goddeeris, B. M., and W. I. Morrison. 1998. Techniques for the generation, cloning and characterisation of bovine cytotoxic T cells specific for the protozoan Theileria parva. J. Tiss. Culture Methods 11:101-110. [Google Scholar]
- 14.Grange, J. M. 2001. Mycobacterium bovis infection in human beings. Tuberculosis (Edinburgh) 81:71-77. [DOI] [PubMed] [Google Scholar]
- 15.Huygen, K., J. Content, O. Denis, D. L. Montgomery, A. M. Yawman, R. R. Deck, C. M. DeWitt, I. M. Orme, S. Baldwin, C. D'Souza, A. Drowart, E. Lozes, P. Vandenbussche, J.-P. van Vooren, M. A. Liu, and J. B. Ulmer. 1996. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat. Med. 2:893-898. [DOI] [PubMed] [Google Scholar]
- 16.Kazwala, R. R., C. J. Daborn, L. J. M. Kusiluka, S. F. H. Jiwa, J. M. Sharp, and D. M. Kambarage. 1998. Isolation of Mycobacterium species from raw milk of pastoral cattle of the southern highlands of Tanzania. Trop. Anim. Health Prod. 30:233-239. [DOI] [PubMed] [Google Scholar]
- 17.Kent, S. J., A. Zhao, S. J. Best, J. D. Chandler, D. B. Boyle, and I. A. Ramshaw. 1998. Enhanced T-cell immunogenicity and protective efficacy of a human immunodeficiency virus type 1 vaccine regimen consisting of consecutive priming with DNA and boosting with recombinant fowlpox virus. J. Virol. 72:10180-10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krebs, J. R. 1997. Bovine tuberculosis in cattle and badgers. London Fisheries and Food Publications, London, United Kingdom.
- 19.Ladel, C. H., S. Daugelat, and S. H. E. Kaufmann. 1995. Immune response to Mycobacterium bovis bacille Calmette Guerin infection in major histocompatibility complex class I- and II-deficient knock-out mice: contribution of CD4 and CD8 T cells to acquired resistance. Eur. J. Immunol. 25:377-384. [DOI] [PubMed] [Google Scholar]
- 20.Launois, P., R. DeLeys, M. N. Niang, A. Drowart, M. Adrien, P. Dierckx, J.-L. Cartel, J.-L. Sarthou, J.-P. van Vooren, and K. Huygen. 1994. T-cell-epitope mapping of the major secreted mycobacterial antigen Ag85A in tuberculosis and leprosy. Infect. Immun. 62:3679-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li, S., M. Rodrigues, D. Rodriguez, J. R. Rodriguez, M. Esteban, P. Palese, R. S. Nussenzweig, and F. Zavala. 1993. Priming with recombinant influenza virus followed by administration of recombinant vaccinia virus induces CD8+ T-cell-mediated protective immunity against malaria. Proc. Natl. Acad. Sci. USA 90:5214-5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lozes, E., K. Huygen, J. Content, O. Denis, D. L. Montgomery, A. M. Yawman, P. Vandenbussche, J.-P. van Vooren, A. Drowart, J. B. Ulmer, and M. A. Liu. 1997. Immunogenicity and efficacy of a tuberculosis DNA vaccine encoding the components of the secreted antigen 85 complex. Vaccine 15:830-833. [DOI] [PubMed] [Google Scholar]
- 23.Malin A. S., K. Huygen, J. Content, M. Mackett, L. Brandt, P. Andersen, S. M. Smith, and H. M. Dockrell. 2000. Vaccinia expression of Mycobacterium tuberculosis-secreted proteins: tissue plasminogen activator signal sequence enhances expression and immunogenicity of M. tuberculosis Ag85. Microbes Infect. 2:1677-1685. [DOI] [PubMed] [Google Scholar]
- 24.McShane, H., R. Brookes, S. C. Gilbert, and A. V. S. Hill. 2001. Enhanced immunogenicity of CD4+ T-cell responses and protective efficacy of a DNA-modified vaccinia virus Ankara prime-boost vaccination regimen for murine tuberculosis. Infect. Immun. 69:681-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyahira, Y., K. Murata, D. Rodriguez, J. R. Rodriguez, M. Esteban, M. M. Rodrigues, and F. Zavala. 1995. Quantification of antigen-specific CD8+ T cells using an ELISPOT assay. J. Immunol. Methods 181:45-54. [DOI] [PubMed] [Google Scholar]
- 26.Munk, M. E., J. De Bruyn, H. Gras, and S. H. E. Kaufmann. 1994. The Mycobacterium bovis 32-kilodalton protein antigen induces human cytotoxic T-cell responses. Infect. Immun. 62:726-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ravn, P., H. Boesen, B. K. Pedersen, and P. Andersen. 1997. Human T cell responses induced by vaccination with Mycobacterium bovis bacillus Calmette-Guerin. J. Immunol. 158:1949-1955. [PubMed] [Google Scholar]
- 28.Schneider, J., S. C. Gilbert, T. J. Blanchard, T. Hanke, K. J. Robson, C. M. Hannan, M. Becker, R. Sinden, G. L. Smith, and A. V. S. Hill. 1998. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat. Med. 4:397-402. [DOI] [PubMed] [Google Scholar]
- 29.Schneider, J., S. C. Gilbert, C. M. Hannan, P. Degano, E. Prieur, E. G. Sheu, M. Plebanski, and A. V. S. Hill. 1999. Induction of CD8+ T cells using heterologous prime-boost immunisation strategies. Immunol. Rev. 170:29-38. [DOI] [PubMed] [Google Scholar]
- 30.Smith, S. M., A. S. Malin, P. T. Lukey, S. E. Atkinson, J. Content, K. Huygen, and H. M. Dockrell. 1999. Characterization of human Mycobacterium bovis bacille Calmette-Guérin-reactive CD8+ T cells. Infect. Immun. 67:5223-5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith, S. M., R. Brookes, M. R. Klein, A. S. Malin, P. T. Lukey, A. S. King, G. S. Ogg, A. V. S. Hill, and H. M. Dockrell. 2000. Human CD8+ CTL specific for the mycobacterial major secreted antigen 85A. J. Immunol. 165:7088-7095. [DOI] [PubMed] [Google Scholar]
- 32.Tanghe, A., S. D'Souza, V. Rosseels, O. Denis, T. H. Ottenhoff, W. Dalemans, C. Wheeler, and K. Huygen. 2001. Improved immunogenicity and protective efficacy of a tuberculosis DNA vaccine encoding Ag85 by protein boosting. Infect. Immun. 69:3041-3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vanderlugt, C. L., W. S. Begolka, K. L. Neville, Y. Katz-Levy, L. M. Howard, T. N. Eagar, J. A. Bluestone, and S. D. Miller. 1998. The functional significance of epitope spreading and its regulation by co-stimulatory molecules. Immunol. Rev. 164:63-72. [DOI] [PubMed] [Google Scholar]
- 34.Vordermeier, H. M., P. C. Cockle, A. Whelan, S. Rhodes, N. Palmer, D. Bakker, and R. G. Hewinson. 1999. Development of diagnostic reagents to differentiate between Mycobacterium bovis BCG vaccination and M. bovis infection in cattle. Clin. Diagn. Lab. Immunol. 6:675-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vordermeier, H. M., M. A. Chambers, P. J. Cockle, A. O. Whelan, J. Simmons, and R. G. Hewinson. 2002. Correlation of ESAT-6-specific gamma interferon production with pathology in cattle following Mycobacterium bovis BCG vaccination against experimental bovine tuberculosis. Infect. Immun. 70:3026-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waddington, F. G., and D. C. Ellwood. 1972. An experiment to challenge the resistance to tuberculosis in BCG vaccinated cattle in Malawi. Br. Vet. J. 128:541-552. [DOI] [PubMed] [Google Scholar]
- 37.Wiker, H. G., and M. Harboe. 1992. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiol. Rev. 56:648-661. [DOI] [PMC free article] [PubMed] [Google Scholar]