Abstract
Diabetic complications include infection and cardiovascular disease. Within the immune system, host-pathogen and regulatory host-host interactions operate through binding of oligosaccharides by C-type lectin. A number of C-type lectins recognise oligosaccharides rich in mannose and fucose – sugars with similar structures to glucose. This raises the possibility that high glucose conditions in diabetes affect protein-oligosaccharide interactions via competitive inhibition. Mannose binding lectin, soluble DC-SIGN & DC-SIGNR, and surfactant protein D, were tested for carbohydrate binding in the presence of glucose concentrations typical of diabetes, via surface plasmon resonance and affinity chromatography. Complement activation assays were performed in high glucose. DC-SIGN and DC-SIGNR expression in adipose tissues was examined via immunohistochemistry. High glucose inhibited C-type lectin binding to high-mannose glycoprotein and binding of DC-SIGN to fucosylated ligand (blood group B) was abrogated in high glucose. Complement activation via the lectin pathway was inhibited in high glucose and also in high trehalose - a nonreducing sugar with glucoside stereochemistry. DC-SIGN staining was seen on cells with DC morphology within omental and subcutaneous adipose tissues. We conclude that high glucose disrupts C-type lectin function, potentially illuminating new perspectives on susceptibility to infectious and inflammatory disease in diabetes. Mechanisms involve competitive inhibition of carbohydrate-binding within sets of defined proteins, in contrast to broadly indiscriminate, irreversible glycation of proteins.
Introduction
Defining characteristics of diabetes mellitus (DM) are the elevated concentrations of free glucose in the circulation and body fluids. Lowering of glucose levels alleviates the pathological symptoms of the disease and improves the prognosis of the patients. In addition to perpetuating metabolic disturbances, high glucose directly causes tissue damage via irreversible glycation (1), a process that involves nonenzymatic, covalent attachment of glucose molecules to proteins via an amine-driven nucleophilic attack of the aldehyde group exposed in the glucose open-chain configuration. Damaged proteins subsequently may exhibit impaired function. However, glucose circulates predominantly in its thermodynamically stable pyranoside configuration, and in this cyclic form, it possesses biochemical features common to other carbohydrate molecules in the body such as oligosaccharide constituents and commensal bacterial cell wall substructures. Important components of the innate immune system have evolved to recognize microbial polysaccharides and oligosaccharides rich in mannose and fucose. Carbohydrate-binding proteins of the C-type lectin family play major roles in the execution of immunological responses towards these glycans and their associated pathogens (2). For example, mannose-binding lectin (MBL), binds directly to mannoside/fucoside units on microbial surfaces and drives activation of the complement system, leading to opsonization, neutralization and stimulation of immune responses (3). The complement system can drive both proinflammatory responses such as cell lysis, leucocyte recruitment and vascular permeability, but also anti-inflammatory processes such as apoptotic cell clearance (4-6). Also, recent studies indicate that opsonic MBL influences antigen processing (7). DC-SIGN (CD209) and DC-SIGNR (CD299) are type II transmembrane proteins believed to possess immunological functions such as cell adhesion and pathogen capture (8-12). Structural analyses of mannose binding by MBL, DC-SIGN and DC-SIGNR reveal the critical importance of the equatorial stereochemistry of the hydroxyl groups at the C3 and C4 positions of the pyranoside ring (13, 14). Glucose shares identical stereochemistry at the C3 and C4 hydroxyls with mannose, varying only at the C2 position, wherein the glucoside hydroxyl group assumes an equatorial orientation in contrast to an axial one (Figure 1). Radioligand competition assays of monosaccharide binding to MBL, DC-SIGN and DC-SIGNR have shown similarities between glucose and mannose binding, with typical KI values in the region of 1-3 mM (15, 16). Mannose does not exist in mammals as a free monosaccharide, although it is a constituent of host oligosaccharides. However, glucose circulates typically at concentrations close to 5 mM and this can rise to 25 mM or more in DM, especially in postprandial conditions (17-19). Therefore, we hypothesize that in DM, particularly poorly controlled DM, raised levels of glucose inhibit C-type lectin-mediated immune functions via reversible competitive inhibition, involving noncovalent interactions. Immunological disturbances such as depression of antimicrobial defences, poor wound healing, and dysregulated inflammatory responses represent major clinical complications in DM. Furthermore, a number of C-type lectins, for example MBL, DC-SIGN and DC-SIGNR, exist in compartments of the circulation and vasculature such as plasma, monocytes, platelets and endothelial cells, such that noncovalent functional inhibition of these molecules could contribute to diabetic cardiovascular and renal complications.
Figure 1. Sugar structures and stereochemistry.
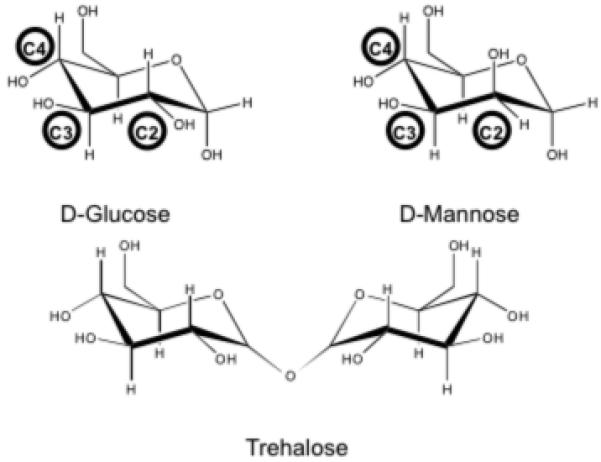
Chemical structures of d-glucose, d-mannose and trehalose, indicating the relative orientation of hydroxyl groups at critical positions within the pyranoside ring.
Materials and Methods
Ethics statement
Human tissues used in this study were obtained with informed written consent from healthy volunteers and approved by the University Hospitals Coventry and Warwickshire Ethics Committee in accordance with principles expressed in the Declaration of Helsinki. Data from human tissue samples were analyzed anonymously.
Proteins and protein purification
Recombinant rat mannose-binding lectin, soluble recombinant human DC-SIGN and DC-SIGNR extracellular domains, and native human surfactant protein D (SP-D) were generated as described previously (16, 20, 21). Purified recombinant HIV gp120 was a gift from Prof K Reid, MRC Immunochemistry Unit, Oxford, UK. All sugars, invertase, and buffer components were purchased from Sigma Chemical Co. (Poole, UK).
Protein-Oligosaccharide Interaction Analysis Via Surface Plasmon Resonance (SPR)
Streptavidin- or Neutravidin-coated sensor chips were treated with biotinylated HIV gp120 or biotinylated yeast invertase, selected as rich high mannose ligands. Sensorgrams were recorded at 25°C using a BIAcore 2000 instrument (flow rate 10 μL/min), or a Biorad ProteOn XPR36 biosensor (flow rate 30 μL/min), with each lectin analyte prepared at 40 μg/ml in 25 mM HEPES, 150 mM NaCl, 5 mM CaCl2, 0.001% Tween-20 supplemented with d-glucose or d-trehalose at concentrations of 5, 10, 15 and 20 mM. No pre-incubation step was carried out and sensorgram data were collected in real time. HEPES-NaCl buffer containing 5 mM EDTA was used to regenerate the sensor chip surface. Sensorgram data were processed using supporting software (BIAevaluation or ProteOn Manager).
Protein-Oligosaccharide Interaction Analysis Via Affinity Chromatography
Glycorex B, a source of immobilized Blood Group B antigen, was used as affinity medium. Columns of 1 ml settled resin were equilibrated in 25 mM HEPES, 150 mM NaCl, 5 mM CaCl2 supplemented with appropriate concentrations of test sugar (5 mM glucose, 20 mM glucose; 5 mM trehalose, 20 mM trehalose). Soluble recombinant DC-SIGN (250 μL of a 2 mg/ml solution) was prepared in sugar-supplemented buffer and applied to the corresponding Glycorex B column. Wash fractions (3 × 1 ml) in corresponding buffer were collected, followed by elution fractions (5 × 0.5 ml) in 25 mM HEPES, 150 mM NaCl, 5 mM EDTA. Aliquots of each fraction (15 μL) were visualized via SDS-PAGE using 4-12% Bis-Tris gels in the NuPAGE system (Invitrogen, Paisley, UK). Positive binding to Group B oligosaccharide was determined through the presence of protein bands in the elution fractions. Optical density at 280 nm was measured for each fraction.
Complement Activation Assays
The Wieslab ELISA-style complement activity assays were used throughout (Eurodiagnostica) following established protocols involving detection of terminal pathway components (22). Assays were carried out measuring classical, alternative and lectin pathway activity within normal human serum in the presence of glucose, mannose, galactose, and fucose at concentrations between 0-100 mM. Additional lectin pathway data were collected using trehalose. Samples were measured in triplicate.
Immunohistochemistry
Formalin-fixed adipose tissue sections, prepared at 3 μm thickness, were stained for DC-SIGN and DC-SIGNR using monoclonal antibodies (R&D Systems) at 800 ng/ml and 2 μg/ml respectively, in conjunction with a diaminobenzidine-immunoperoxidase detection kit (Vector Labs), and counterstaining with Mayers haematoxylin. Negative controls were prepared by the omission of primary antibody, and also the inclusion of soluble recombinant DC-SIGN and DC-SIGNR as competitive inhibitors in the primary antibody solution at 10 μg/ml.
Results
Binding studies showed that high mannose recognition by the C-type lectins MBL, SP-D, DC-SIGNR and DC-SIGN was inhibited by increased concentrations of glucose (Figure 2). The effects of high glucose on all recorded ligand binding events, visualised in real-time via surface plasmon resonance, were instantaneous given that samples were applied to the chip without a prior incubation step and thus insufficient conditions were available for effective glycation to take place. MBL and DC-SIGNR binding was particularly sensitive, with apparent loss of all binding signal in 20 mM glucose. SP-D showed a marked reduction in binding in the presence of 20 mM glucose and although binding of DC-SIGN was observed at 20 mM, it was 33% less than that obtained in 5 mM glucose. We expanded the analysis of DC-SIGN binding using immobilized invertase, a microbial high-mannose glycoprotein with oligosaccharides similar to potential endogenous ligands such as ICAM-3. Here we observed dose-dependent inhibition of glycoprotein recognition in the presence of elevated glucose. Interestingly, the binding signal at 0 mM glucose was substantially greater than that observed with 5 mM glucose, consistent with previous radioligand competition assays (16). Significantly, DC-SIGN binding was affected in a similar fashion using equivalent concentrations of trehalose, a nonreducing sugar incapable of glycation but with glucoside stereochemistry at equivalent C3 and C4 pyranosyl hydroxyls (Figure 1).
Figure 2. C-type lectin-glycoprotein interactions.
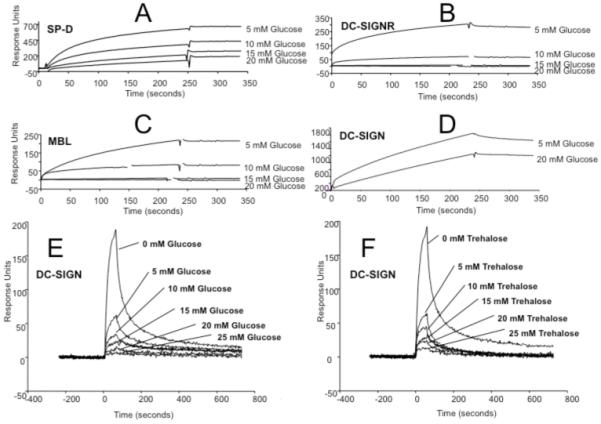
Measurement of binding via surface plasmon resonance using immobilized HIV gp120 (A-D) and immobilized yeast invertase (E and F). Glycoprotein binding by lectins SP-D (A), DC-SIGNR (B), MBL (C) and DC-SIGN (D-F) was recorded in the sugar concentrations indicated.
DC-SIGN has also been shown to bind endogenous fucosylated oligosaccharides such as blood group antigens (23). We examined the effects of high glucose on DC-SIGN binding to blood group B via affinity chromatography. As seen in Figure 3, binding of DC-SIGN to Glycorex B resin was greatly reduced in the presence of 20 mM glucose, as seen by the abundance of protein recovered in the early wash region of the elution profile. In contrast, DC-SIGN bound well to the Glycorex B column in the presence of 5 mM glucose, with the majority of protein eluting in the later, EDTA-containing fractions (Figure 3A and 3B). A similar pattern was observed using 5 mM and 20 mM trehalose (Figure 3C and 3D). As expected, MBL, SP-D, DC-SIGNR, and BSA did not bind to the columns (data not shown).
Figure 3. DC-SIGN-Blood Group B interactions.
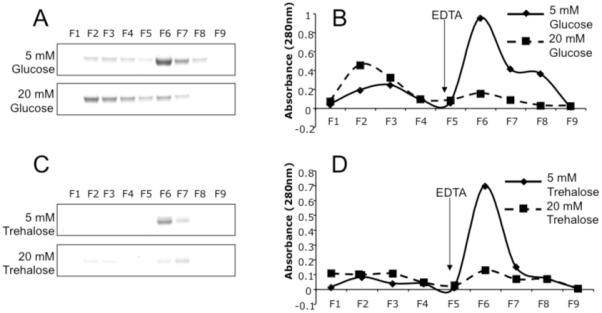
Binding of soluble DC-SIGN to immobilized blood group antigen was carried out in the presence of 5 mM and 20 mM glucose (A and B) and 5 mM and 20 mM trehalose (C and D). The relative appearance of elution profiles are illustrated by gel electrophoresis (A and C) and A280 measurements (B and D) of sequential fractions, with EDTA added to each profile at fraction 5.
Complement activation assays showed that classical and alternative pathway activities were not affected by elevated levels of glucose or other hexoses tested (Figure 4A and 4B). However, activation of the lectin pathway was substantially affected, with complement activity being markedly reduced in the presence of glucose, mannose and fucose (Figure 4C). As expected, mannose (IC50 = 5.8 ± 0.4) and fucose (IC50 = 3.7 ± 0.3) were more effective inhibitors relative to glucose (IC50 = 14.4 ± 0.4), and galactose (IC50 = 20.8 ± 0.7) was less effective, reflecting previous MBL binding studies (15). Analysis of lectin pathway activation in the presence of trehalose (IC50 = 23.8 ± 1.9) showed inhibition at higher concentrations, in a pattern similar to that observed for glucose (Figure 4D).
Figure 4. Complement Activation Assays.
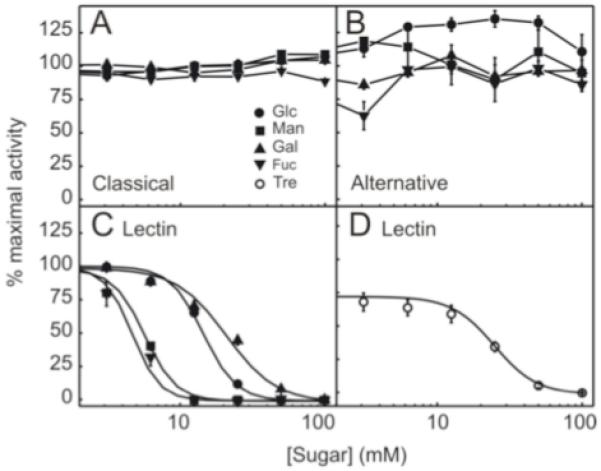
Measurement of classical (A), alternative (B) and lectin (C and D) pathway activation of complement in the presence of monosaccharides: glucose (Glc), mannose (Man), fucose (Fuc), galactose (Gal), and trehalose (Tre).
Interactions between C1q and carbohydrates has been reported (24), and thus it is surprising that monosaccharides did not appear to affect classical pathway activity. However, the classical pathway assay protocol uses microwell plates coated with IgM heavy chain and we hypothesise that as a result, C1q interacts with this target via a mode of binding that operates independently of its putative carbohydrate binding sites.
DC-SIGN and DC-SIGNR are plasma membrane proteins expressed on cells in a range of tissues including liver, lymph node and spleen. We examined the expression of these glucose-sensitive receptors in adipose tissues, anatomically strong candidate sites for fostering intimate communications between immunity and metabolism (25). Immunostaining for DC-SIGN demonstrated scattered, strongly immunopositive cells with dendritic morphology, in both omental (Figure 5A and 5B) and subcutaneous (Figure 5C and 5D) adipose tissues. While occasional dendritic cells appear to be between adipocytes away from blood vessels, the majority appear perivascular in their distribution. No immunostaining of other cell types in adipose tissue with anti-DC-SIGN was observed. Positive staining for DC-SIGNR in adipose tissues was not observed.
Figure 5. Immunohistochemistry.
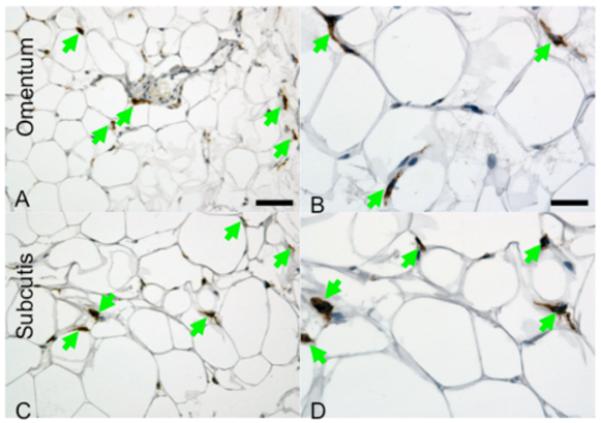
Staining for DC-SIGN using the immunoperoxidase technique demonstrates strongly immunopositive cells (brown, marked with arrows) with dendritic morphology, in keeping with dendritic cells, in both omental (A and B) and subcutaneous (C and D) adipose tissue. The larger, cells with clear, colourless cytoplasm that make up the majority of the tissue in these images represent adipocytes. While occasional dendritic cells appear to be between adipocytes away from blood vessels, the majority appear perivascular in their distribution. There is no immunostaining of other cell types in adipose tissue with anti-DC-SIGN. The scale bar in panel a represents 40 microns and pertains to panels A and C, while the scale bar in panel a represents 20 microns and pertains to panels B and D.
Discussion
Host recognition of carbohydrate structures is essential for maintaining the healthy function of the mammalian immune system. Disruption of selected oligosaccharide-binding functions leads to impairment of immune processes, exemplified in hereditary human MBL deficiencies (26). In a group of mammalian C-type lectins that bind to mannoside structures, we have shown that elevated concentrations of free glucose disrupt protein-oligosaccharide interactions via noncovalent, competitive inhibition. We also observe inhibition of both lectin binding and function in the presence of trehalose, which through its alpha-1,1 glycosidic linkage cannot adopt the open chain conformation necessary for protein glycation. These findings provide novel mechanistic explanations for many pathological complications associated with diabetes, especially cases wherein glucose levels are difficult to control. Gram-negative infections are very common in diabetes, especially Pseudomonas infections. Cell wall structures associated with gram-negative bacteria are often rich in mannose and similar sugars, as are fungal cell walls such as those found in Candida organisms, similarly prevalent in diabetic infections. C-type lectins such as MBL are known to participate in the neutralisation and control of these pathogens (27-29). In addition, MBL is known to interact with apoptotic cells, enhancing their uptake and processing (30). Impaired handling of apoptotic cells by complement proteins such as C1q has been shown to enhance or predispose to chronic inflammatory autoimmune diseases (31, 32), and whilst MBL deficiency may not contribute substantially to the breaking of immunological tolerance, the absence of adequate functional MBL may contribute to poorer resolution of inflammation. Furthermore, epidemiological data describe an association between depressed MBL function, insulin resistance and obesity (33). SP-D is an important component of innate immunity in the lung and it has been suggested that glucose can inhibit SP-D binding in vivo wherein hyperglycaemic mice show greater susceptibility to viral infection (34). Thus, our biochemical data support whole animal models with potential to be explored further.
Activation of innate immune pathways with concomitant infiltration of adipose tissue macrophages leads to adipocyte inflammation, and this has been proposed as a fundamental process linking obesity to T2DM and atherosclerosis (35, 36). Studies have shown that expression levels of pro-inflammatory toll-like receptors on monocytes are elevated in patients with type 1 DM, this being supported by separate studies showing that insulin exerts significant suppressive effects on the expression of toll-like receptors on mononuclear leucocytes (37, 38). DC-SIGN is expressed on dendritic cells and specialised macrophage populations that play vital roles in regulating inflammatory and adaptive immune responses (8). DC-SIGN has been shown to participate in cross-talk with toll-like receptors, showing evidence of immunosuppressive effects (39). Our novel observation of DC-SIGN-positive cells in both omental and subcutaneous compartments suggests that this receptor may participate in adipose tissue function. Disruption of DC-SIGN-oligosaccharide interactions, typically associated with anti-inflammatory signalling and IL-10 production (40), in hyperglycaemia could affect normal adipose DC/macrophage physiology and thus communications between metabolism and immunity/inflammation. DC-SIGN is also expressed on platelets, suggesting that it may play a role in regulating coagulation and angiogenesis (41). The glucose-sensitive binding of DC-SIGN to blood group antigens, extensively expressed on erythrocytes, is particularly interesting within this context. In addition, DC-SIGNR is expressed on specialised endothelia such as those found in lymph node and hepatic sinuses (11) proximal to large T cell repositories, and DC-SIGN has been reported to interact with vascular ICAM-2, suggesting that both of these receptors may be involved in lymphocyte and dendritic cell trafficking (10). These functions may influence systemic inflammatory responses, including those located within adipose tissues.
Novel data indicate a highly dynamic set of signalling characteristics displayed by DC-SIGN wherein mannose ligands invoke different signalling responses and outcomes to fucose ligands (42). This report shows that, mannose and fucose monosaccharides themselves can induce signalling events and therefore it is not inconceivable that glucose itself can, rather paradoxically contribute to selected modes of DC-SIGN signalling. Such contributions to cellular sensing and signalling, in addition to other potential hyperglycaemic and DM-related disturbances, may affect the levels and distribution of DC-SIGN and other lectin expression within the body – something that warrants further investigation.
The reversible and fast-acting effects of glucose on C-type lectins contrasts directly with the slower kinetics of glycation and as such may contribute to a number of transient, postprandial effects of high glucose (43). Our data using trehalose could reflect upon fundamental aspects of animal metabolic control, as trehalose rather than glucose serves as the major nutritional carbohydrate within insect haemolymph. In an insect model of obesity, disease is associated with elevated trehalose, increased lethargy and a specific parasitic infection, all implicitly in the absence of glycation (44). Insect C-type lectins with sugar-binding characteristics similar to those of mammals have been characterised and these proteins could potentially participate within processes regulating immunity, behaviour and metabolism (45). Other mammalian mannose-selective lectins exist, including the mannose receptor (CD206) and pulmonary surfactant protein A. Further elucidation of C-type lectin function, susceptibility to glucose, and participation within metabolic circuits heralds exciting opportunities for discovery within metabolic disease.
Acknowledgments
R.I. is supported by a PhD Studentship from the University of Warwick. D.A.M. and R.W. are Research Councils UK Academic Fellows, and R.W. is further supported by Wellcome Trust Project Grant 077400. D.A.M. wishes to thank the Warwick Institute for Advanced Studies for support and Robert Doms & Carl Davis at University of Pennsylvania for helpful discussions. We also thank Sean James at the University Hospital, Coventry for assistance with histology.
Footnotes
Conflict of Interest
The authors indicate no conflicts of interest.
References
- 1.Ahmed N, Thornalley PJ. Advanced glycation endproducts: what is their relevance to diabetic complications? Diabetes Obes Metab. 2007;9:233. doi: 10.1111/j.1463-1326.2006.00595.x. [DOI] [PubMed] [Google Scholar]
- 2.Weis WI, Taylor ME, Drickamer K. The C-type lectin superfamily in the immune system. Immunol Rev. 1998;163:19. doi: 10.1111/j.1600-065x.1998.tb01185.x. [DOI] [PubMed] [Google Scholar]
- 3.Turner MW. Mannose-binding lectin: the pluripotent molecule of the innate immune system. Immunol Today. 1996;17:532. doi: 10.1016/0167-5699(96)10062-1. [DOI] [PubMed] [Google Scholar]
- 4.Nauta AJ, Raaschou-Jensen N, Roos A, Daha MR, Madsen HO, Borrias-Essers MC, Ryder LP, Koch C, Garred P. Mannose-binding lectin engagement with late apoptotic and necrotic cells. Eur J Immunol. 2003;33:2853. doi: 10.1002/eji.200323888. [DOI] [PubMed] [Google Scholar]
- 5.Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 6.Walport MJ. Complement. Second of two parts. N Engl J Med. 2001;344:1140. doi: 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- 7.Ip WK, Takahashi K, Moore KJ, Stuart LM, Ezekowitz RA. Mannose-binding lectin enhances Toll-like receptors 2 and 6 signaling from the phagosome. J Exp Med. 2008;205:169. doi: 10.1084/jem.20071164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geijtenbeek TB, Torensma R, van Vliet SJ, van Duijnhoven GC, Adema GJ, van Kooyk Y, Figdor CG. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- 9.Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, Middel J, Cornelissen IL, Nottet HS, KewalRamani VN, Littman DR, Figdor CG, van Kooyk Y. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 10.Geijtenbeek TB, Krooshoop DJ, Bleijs DA, van Vliet SJ, van Duijnhoven GC, Grabovsky V, Alon R, Figdor CG, van Kooyk Y. DC-SIGN-ICAM-2 interaction mediates dendritic cell trafficking. Nat Immunol. 2000;1:353. doi: 10.1038/79815. [DOI] [PubMed] [Google Scholar]
- 11.Soilleux EJ, Barten R, Trowsdale J. DC-SIGN; a related gene, DC-SIGNR; and CD23 form a cluster on 19p13. J Immunol. 2000;165:2937. doi: 10.4049/jimmunol.165.6.2937. [DOI] [PubMed] [Google Scholar]
- 12.Pohlmann S, Soilleux EJ, Baribaud F, Leslie GJ, Morris LS, Trowsdale J, Lee B, Coleman N, Doms RW. DC-SIGNR, a DC-SIGN homologue expressed in endothelial cells, binds to human and simian immunodeficiency viruses and activates infection in trans. Proc Natl Acad Sci U S A. 2001;98:2670. doi: 10.1073/pnas.051631398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weis WI, Drickamer K, Hendrickson WA. Structure of a C-type mannose-binding protein complexed with an oligosaccharide. Nature. 1992;360:127. doi: 10.1038/360127a0. [DOI] [PubMed] [Google Scholar]
- 14.Feinberg H, Mitchell DA, Drickamer K, Weis WI. Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science. 2001;294:2163. doi: 10.1126/science.1066371. [DOI] [PubMed] [Google Scholar]
- 15.Lee RT, Ichikawa Y, Fay M, Drickamer K, Shao MC, Lee YC. Ligand-binding characteristics of rat serum-type mannose-binding protein (MBP-A). Homology of binding site architecture with mammalian and chicken hepatic lectins. J Biol Chem. 1991;266:4810. [PubMed] [Google Scholar]
- 16.Mitchell DA, Fadden AJ, Drickamer K. A novel mechanism of carbohydrate recognition by the C-type lectins DC-SIGN and DC-SIGNR. Subunit organization and binding to multivalent ligands. J Biol Chem. 2001;276:28939. doi: 10.1074/jbc.M104565200. [DOI] [PubMed] [Google Scholar]
- 17.UKPDS UK Prospective Diabetes Study 7: response of fasting plasma glucose to diet therapy in newly presenting type II diabetic patients, UKPDS Group. Metabolism. 1990;39:905. [PubMed] [Google Scholar]
- 18.Holman RR, Turner RC. The basal plasma glucose: a simple relevant index of maturity-onset diabetes. Clin Endocrinol (Oxf) 1981;14:279. doi: 10.1111/j.1365-2265.1981.tb00196.x. [DOI] [PubMed] [Google Scholar]
- 19.Turner RC, Holman RR. Lessons from UK prospective diabetes study. Diabetes Res Clin Pract. 1995;28(Suppl):S151. doi: 10.1016/0168-8227(95)01105-m. [DOI] [PubMed] [Google Scholar]
- 20.Wallis R, Drickamer K. Asymmetry adjacent to the collagen-like domain in rat liver mannose-binding protein. Biochem J. 1997;325(Pt 2):391. doi: 10.1042/bj3250391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strong P, Kishore U, Morgan C, Lopez Bernal A, Singh M, Reid KB. A novel method of purifying lung surfactant proteins A and D from the lung lavage of alveolar proteinosis patients and from pooled amniotic fluid. J Immunol Methods. 1998;220:139. doi: 10.1016/s0022-1759(98)00160-4. [DOI] [PubMed] [Google Scholar]
- 22.Seelen MA, Roos A, Wieslander J, Mollnes TE, Sjoholm AG, Wurzner R, Loos M, Tedesco F, Sim RB, Garred P, Alexopoulos E, Turner MW, Daha MR. Functional analysis of the classical, alternative, and MBL pathways of the complement system: standardization and validation of a simple ELISA. J Immunol Methods. 2005;296:187. doi: 10.1016/j.jim.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 23.Guo Y, Feinberg H, Conroy E, Mitchell DA, Alvarez R, Blixt O, Taylor ME, Weis WI, Drickamer K. Structural basis for distinct ligand-binding and targeting properties of the receptors DC-SIGN and DC-SIGNR. Nat Struct Mol Biol. 2004;11:591. doi: 10.1038/nsmb784. [DOI] [PubMed] [Google Scholar]
- 24.Paidassi H, Tacnet-Delorme P, Lunardi T, Arlaud GJ, Thielens NM, Frachet P. The lectin-like activity of human C1q and its implication in DNA and apoptotic cell recognition. FEBS Lett. 2008;582:3111. doi: 10.1016/j.febslet.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Carlow DA, Gold MR, Ziltener HJ. Lymphocytes in the peritoneum home to the omentum and are activated by resident dendritic cells. J Immunol. 2009;183:1155. doi: 10.4049/jimmunol.0900409. [DOI] [PubMed] [Google Scholar]
- 26.Worthley DL, Bardy PG, Mullighan CG. Mannose-binding lectin: biology and clinical implications. Intern Med J. 2005;35:548. doi: 10.1111/j.1445-5994.2005.00908.x. [DOI] [PubMed] [Google Scholar]
- 27.Moller-Kristensen M, Ip WK, Shi L, Gowda LD, Hamblin MR, Thiel S, Jensenius JC, Ezekowitz RA, Takahashi K. Deficiency of mannose-binding lectin greatly increases susceptibility to postburn infection with Pseudomonas aeruginosa. J Immunol. 2006;176:1769. doi: 10.4049/jimmunol.176.3.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Held K, Thiel S, Loos M, Petry F. Increased susceptibility of complement factor B/C2 double knockout mice and mannan-binding lectin knockout mice to systemic infection with Candida albicans. Mol Immunol. 2008;45:3934. doi: 10.1016/j.molimm.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 29.Lillegard JB, Sim RB, Thorkildson P, Gates MA, Kozel TR. Recognition of Candida albicans by mannan-binding lectin in vitro and in vivo. J Infect Dis. 2006;193:1589. doi: 10.1086/503804. [DOI] [PubMed] [Google Scholar]
- 30.Stuart LM, Takahashi K, Shi L, Savill J, Ezekowitz RA. Mannose-binding lectin-deficient mice display defective apoptotic cell clearance but no autoimmune phenotype. J Immunol. 2005;174:3220. doi: 10.4049/jimmunol.174.6.3220. [DOI] [PubMed] [Google Scholar]
- 31.Savill J, Fadok V. Corpse clearance defines the meaning of cell death. Nature. 2000;407:784. doi: 10.1038/35037722. [DOI] [PubMed] [Google Scholar]
- 32.Botto M, Dell’Agnola C, Bygrave AE, Thompson EM, Cook HT, Petry F, Loos M, Pandolfi PP, Walport MJ. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet. 1998;19:56. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 33.Fernandez-Real JM, Straczkowski M, Vendrell J, Soriguer F, Perez Del Pulgar S, Gallart L, Lopez-Bermejo A, Kowalska I, Manco M, Cardona F, Garcia-Gil MM, Mingrone G, Richart C, Ricart W, Zorzano A. Protection from inflammatory disease in insulin resistance: the role of mannan-binding lectin. Diabetologia. 2006;49:2402. doi: 10.1007/s00125-006-0381-6. [DOI] [PubMed] [Google Scholar]
- 34.Reading PC, Allison J, Crouch EC, Anders EM. Increased susceptibility of diabetic mice to influenza virus infection: compromise of collectin-mediated host defense of the lung by glucose? J Virol. 1998;72:6884. doi: 10.1128/jvi.72.8.6884-6887.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeyda M, Farmer D, Todoric J, Aszmann O, Speiser M, Gyori G, Zlabinger GJ, Stulnig TM. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int J Obes (Lond) 2007;31:1420. doi: 10.1038/sj.ijo.0803632. [DOI] [PubMed] [Google Scholar]
- 36.Permana PA, Menge C, Reaven PD. Macrophage-secreted factors induce adipocyte inflammation and insulin resistance. Biochem Biophys Res Commun. 2006;341:507. doi: 10.1016/j.bbrc.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 37.Devaraj S, Dasu MR, Rockwood J, Winter W, Griffen SC, Jialal I. Increased toll-like receptor (TLR) 2 and TLR4 expression in monocytes from patients with type 1 diabetes: further evidence of a proinflammatory state. J Clin Endocrinol Metab. 2008;93:578. doi: 10.1210/jc.2007-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghanim H, Mohanty P, Deopurkar R, Sia CL, Korzeniewski K, Abuaysheh S, Chaudhuri A, Dandona P. Acute modulation of toll-like receptors by insulin. Diabetes Care. 2008;31:1827. doi: 10.2337/dc08-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krutzik SR, Tan B, Li H, Ochoa MT, Liu PT, Sharfstein SE, Graeber TG, Sieling PA, Liu YJ, Rea TH, Bloom BR, Modlin RL. TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nat Med. 2005;11:653. doi: 10.1038/nm1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caparros E, Munoz P, Sierra-Filardi E, Serrano-Gomez D, Puig-Kroger A, Rodriguez-Fernandez JL, Mellado M, Sancho J, Zubiaur M, Corbi AL. DC-SIGN ligation on dendritic cells results in ERK and PI3K activation and modulates cytokine production. Blood. 2006;107:3950. doi: 10.1182/blood-2005-03-1252. [DOI] [PubMed] [Google Scholar]
- 41.Chaipan C, Soilleux EJ, Simpson P, Hofmann H, Gramberg T, Marzi A, Geier M, Stewart EA, Eisemann J, Steinkasserer A, Suzuki-Inoue K, Fuller GL, Pearce AC, Watson SP, Hoxie JA, Baribaud F, Pohlmann S. DC-SIGN and CLEC-2 mediate human immunodeficiency virus type 1 capture by platelets. J Virol. 2006;80:8951. doi: 10.1128/JVI.00136-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gringhuis SI, den Dunnen J, Litjens M, van der Vlist M, Geijtenbeek TB. Carbohydrate-specific signaling through the DC-SIGN signalosome tailors immunity to Mycobacterium tuberculosis, HIV-1 and Helicobacter pylori. Nat Immunol. 2009;10:1081. doi: 10.1038/ni.1778. [DOI] [PubMed] [Google Scholar]
- 43.Ceriello A. Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes. 2005;54:1. doi: 10.2337/diabetes.54.1.1. [DOI] [PubMed] [Google Scholar]
- 44.Schilder RJ, Marden JH. Metabolic syndrome and obesity in an insect. Proc Natl Acad Sci U S A. 2006;103:18805. doi: 10.1073/pnas.0603156103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bouyain S, Silk NJ, Fabini G, Drickamer K. An endogenous Drosophila receptor for glycans bearing alpha 1,3-linked core fucose residues. J Biol Chem. 2002;277:22566. doi: 10.1074/jbc.M202825200. [DOI] [PubMed] [Google Scholar]


