G&H What are the causes of sinusoidal obstruction syndrome (SOS) in humans?
LD In North America and Western Europe, SOS, or hepatic veno-occlusive disease, is triggered most often by the administration of myeloablative, high-dose chemotherapy for what was once called bone-marrow transplantation and is now referred to as stem-cell transplantation. SOS is also seen sporadically in patients who receive other forms of chemotherapy.
In non-Western nations, SOS is more commonly seen as a complication of ingestion of plant alkaloids called pyrrolizidine alkaloids. Plants containing pyrrolizidine alkaloids sometimes contaminate inadequately winnowed wheat. SOS can reach epidemic proportions when wheat is contaminated. SOS is also seen sporadically in locales, including South Africa, where people make teas from these plants.
G&H Can you describe what is known of the pathophysiology of SOS?
LD From observation in humans, we know that SOS is a microcirculatory disorder. It is a primary circulatory disorder because circulatory manifestations precede the parenchymal dysfunction. However, in a large autopsy series of these patients, 30% had normal veins and venules, showing that venous involvement occurs but is not essential, which points to a root cause at the hepatic microcirculatory level.
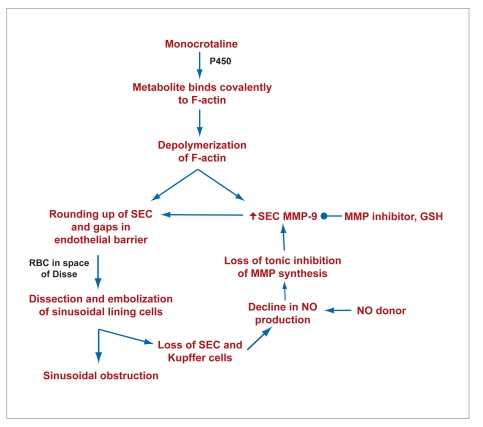
The best characterization of the disease has been performed in the experimental animal model with monocrotaline, one of the pyrrolizine alkaloids. Monocrotaline is P450-activated in sinusoidal endothelial cells. One of the targets of the electrophilic metabolite of monocrotaline is F-actin, a major component of the cytoskeleton. Binding of the activated monocrotaline to F-actin leads to its depolymerization in the sinusoidal endothelial cell. Depolymerization of the F-actin cytoskeleton increases synthesis and activation of matrix metalloproteinase-9 (MMP-9). MMP-9 is an ectoenzyme that, when exocytosed, digests the extracellular matrix tetherings of the sinusoidal endothelial cell to the space of Disse. The combination of depolymerization of F-actin plus digestion of the extracellular matrix in the space of Disse leads to rounding up of the sinusoidal endothelial cells. This rounding up of the endothelial cells creates gaps in the endothelial barrier of the hepatic microcirculation. Red blood cells penetrate through the gaps. Because the rounded up sinusoidal endothelial cells block the sinusoid, the space of Disse becomes the pathway of least resistance for blood. In turn, blood starts flowing in the space of Disse and dissects off the sinusoidal lining cells. The sinusoidal lining cells embolize downstream and obstruct the sinusoid. This obstruction of the hepatic microcirculation leads to ischemic damage with centrilobular hemorrhagic necrosis.
With the loss of sinusoidal endothelial cells and Kupffer cells, the main liver cell sources of nitric oxide (NO), NO declines. Normally NO tonically inhibits synthesis of MMP-9, so that the loss of NO further augments the increase in MMP-9 (Figure 1). This process then causes a vicious cycle of further loss of sinusoidal cells, decreasing NO levels, and further increases in MMP-9.
Interventions that preserve NO levels or inhibitors of MMP-9 protect the integrity of the sinusoidal lining and completely prevent the injury. The sinusoidal origin of the disease is confirmed by the ability to prevent the centrilobular necrosis and subsequent fibrosis by protecting sinusoidal endothelial cells.
G&H What are the signs and symptoms of SOS?
LD In patients who have received myeloablative chemoirradiation, SOS should be considered in cases of right upper quadrant pain of liver origin, weight gain, and/or jaundice. Depending on the extent of the injury, patients can develop severe liver dysfunction, liver failure or, ultimately, multi-organ failure. Diagnosis is frequently made based on these clinical signs and symptoms, but only after other diseases have been ruled out. This patient population is at risk for several complications that could mimic SOS. The differential diagnosis for jaundice or weight gain in this population includes sepsis, acute graft-versus-host disease, viral or fungal infections of the liver, side effects of medications, and weight gain from fluid resuscitation. A combination of these disorders can be difficult to distinguish from SOS. If other causes cannot be ruled out, a transvenous liver biopsy will confirm diagnosis. If a patient has more than one disease process including SOS, it can be difficult to determine the major contributor to the symptoms.
G&H Are there genetic or other factors that make some patients more susceptible to SOS than others?
LD The only directly implicated genetic factor would be related to how patients metabolize cyclophosphamide, which is one of the components used in myeloablative chemotherapy. Other risk factors include a previous history of SOS, hepatitis C, recent treatment with gemtuzumab ozogamicin, or an underlying fibrotic liver disease. A cirrhotic patient who receives chemotherapy will not survive a stem cell transplant, myeloablative or nonmyeloablative.
G&H What are the therapeutic options for patients with confirmed SOS?
LD There is no specific therapy for SOS at this point. Supportive care is the only option and the approach depends on the severity of disease. Patients with mild disease will get better without therapy. These patients need to be monitored, and myeloablative therapies need to be avoided in the future. Patients with moderate disease require analgesics to manage significant pain, as well as management of ascites with diuretics or paracentesis. Patients with severe cases of SOS have a 95% chance of mortality. In patients with severe SOS and multi-organ failure, hemodialysis and mechanical ventilation can be attempted but will most likely not affect outcomes.
G&H Are patients with SOS ever considered as candidates for liver transplantation?
LD Patients with SOS can potentially benefit from liver transplantation, but are considered candidates only if their underlying condition was a benign disorder or has a likely favorable outcome.
G&H What future research should be considered in managing patients with SOS?
LD Future therapy of SOS should focus on prophylactic measures. NO therapies should be examined in this regard as should MMP inhibitors, both of which work very effectively in the animal model. However, these therapies have not yet been tried in humans. The concern with MMP inhibitors is that they conceivably might affect engraftment of a stem-cell transplant, which would preclude their use. If MMP inhibitors can be shown experimentally to not interfere with stem-cell engraftment, this would be an exciting approach to prophylaxis.
The drug defibrotide has been studied in uncontrolled trials and has shown some benefit. Randomized, controlled trials will be needed to determine whether it truly has therapeutic benefit. In addition, it would be interesting to determine whether defibrotide prophylaxis can prevent SOS.
G&H Are there challenges to accruing patients for trials of SOS therapies?
LD We have moved more and more to nonmyeloablative regimens in order to avoid SOS, and as a result, the incidence and pool of patients for study is shrinking. However, this does not mean the problem is solved. The shift to nonmyeloablative therapy has lessened the rate of SOS, but it seems to have increased the rates of late mortality. If we could find an effective prophylactic therapy for SOS, clinicians might be more inclined to administer more toxic myeloablative regimens again and might end up achieving more benefit and more success with stem cell transplantation.
Figure 1.
Microcirculatory pathophysiology of sinusoidal obstruction syndrome.
- GSH
- growth-stimulating hormone
- MMP
- matrix metalloproteinase
- NO
- nitric oxide
- RBC
- red blood cells
- SEC
- sinusoidal endothelial cells.
Modified from DeLeve, LD. American Association for the Study of Liver Diseases, 2007 Postgraduate Course.
Suggested Reading
- McKoy JM, Angelotta C, Bennett CL, Tallman MS, Wadleigh M, et al. Gemtuzumab ozogamicin-associated sinusoidal obstructive syndrome (SOS): an overview from the research on adverse drug events and reports (RADAR) project. Leuk Res. 2007;31:599–604. doi: 10.1016/j.leukres.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Cordonnier C, Maury S, Esperou H, Pautas C, Beaune J, et al. Do minitransplants have minicosts? A cost comparison between myeloablative and nonmyeloablative allogeneic stem cell transplant in patients with acute myeloid leukemia. Bone Marrow Transplant. 2005;36:649–654. doi: 10.1038/sj.bmt.1705109. [DOI] [PubMed] [Google Scholar]
- Hogan WJ, Maris M, Storer B, Sandmaier BM, Maloney DG, et al. Hepatic injury after nonmyeloablative conditioning followed by allogeneic hematopoietic cell transplantation: a study of 193 patients. Blood. 2004;103:78–84. doi: 10.1182/blood-2003-04-1311. [DOI] [PubMed] [Google Scholar]
- DeLeve LD, Wang X, Tsai J, Kanel G, Strasberg S, Tokes ZA. Sinusoidal obstruction syndrome (venoocclusive disease) in the rat is prevented by matrix metalloproteinase inhibition. Gastroenterology. 2003;125:882–890. doi: 10.1016/s0016-5085(03)01056-4. [DOI] [PubMed] [Google Scholar]
- DeLeve LD, Wang X, Kanel GC, et al. Decreased hepatic nitric oxide production contributes to the development of rat sinusoidal obstruction syndrome. Hepatology. 2003;38:900–908. doi: 10.1053/jhep.2003.50383. [DOI] [PubMed] [Google Scholar]
- DeLeve LD, Schulman HM, McDonald GB. Toxic injury to hepatic sinusoids: sinusoidal obstruction syndrome (veno-occlusive disease) Semin Liver Dis. 2002;22:27–42. doi: 10.1055/s-2002-23204. [DOI] [PubMed] [Google Scholar]
- DeLeve LD, McCuskey RS, Wang X, Hu L, McCuskey MK, et al. Characterization of a reproducible rat model of hepatic veno-occlusive diseases. Hepatology. 1999;29:1779–1791. doi: 10.1002/hep.510290615. [DOI] [PubMed] [Google Scholar]