Abstract
Blood from cattle with subclinical Mycobacterium avium subsp. paratuberculosis infection was stimulated with M. avium subsp. paratuberculosis antigens, and expression of interleukin-1β (IL-1β), tumor necrosis factor alpha (TNF-α), RANTES, monocyte chemoattractant protein 1 (MCP-1), and IL-8 was measured. Expression of TNF-α, RANTES, and MCP-1 was lower in infected than in uninfected cattle. The reduced response may weaken protective immunity and perpetuate infection.
Information is now accumulating on cellular and cytokine changes associated with Mycobacterium avium subsp. paratuberculosis infection in cattle (8, 17-20), but little or no information is available on chemokines, which are involved in granuloma formation. Chemokines are chemotactic cytokines with small molecular size (8 to 14 kDa) that mediate the recruitment of leukocytes from blood to tissues in maintenance of health or in response to inflammation (4, 11). Four subfamilies of chemokines, namely CC, CXC, C, and CX3C, have been designated based on the presence of a cysteine-containing signature motif at the amino-terminal end (11). The response of chemokines to Mycobacterium tuberculosis infection is induced by inflammatory cytokines among other factors (8, 10). The chemokines monocyte chemoattractant protein 1 (MCP-1 [CCL2]), RANTES (CCL5), and interleukin-8 (IL-8 [CXCL8]) are suggested to play a role in formation of granuloma to M. tuberculosis (6, 9, 18); however, no information is available on their responses to M. avium subsp. paratuberculosis infection. In the present study, we evaluated mRNA expression of the chemokines RANTES, MCP-1, and IL-8 and the proinflammatory cytokines IL-1β and tumor necrosis factor alpha (TNF-α) during M. avium subsp. paratuberculosis infection in cattle.
The experimental animals included five 25-month-old castrated male Holstein calves and five age-matched uninfected control animals. The infected animals were orally inoculated with 20.7 × 108 CFU of M. avium subsp. paratuberculosis at 1 week of age. At the beginning of this study, the animals were positive for M. avium subsp. paratuberculosis infection in the gamma interferon (IFN-γ) enzyme-linked immunosorbent assay (2) but were negative for clinical signs, M. avium subsp. paratuberculosis-specific antibodies, and M. avium subsp. paratuberculosis shedding in feces by PCR and culture. Jugular blood was dispensed at 1 ml per well of a 24-well tissue culture plate and stimulated with different stimulants to a final concentration of 10 μg of lipopolysacharide (LPS) per ml from Escherichia coli (Sigma), 10 μg of M. avium subsp. paratuberculosis lysate per ml, 0.5 μg of M. avium subsp. paratuberculosis PPD per ml or 60 × 106 CFU of live M. avium subsp. paratuberculosis. After 0, 1, 3, 6, 12, and 24 h of incubation in a humidified incubator at 37°C and 5% CO2 in air, plasma was separated from the blood cell fraction and tested for IFN-γ with the BioX ELISA kit (Marche-en-Famenne, Belgium). The blood cell fraction was used for RNA isolation with the Trizol reagent (Life Technologies, Rockville, Md.) according to manufacturer's instructions. Real-time reverse transcription-PCR (RT-PCR) was done with the SYBR Green RT-PCR kit (Qiagen, Tokyo, Japan). Primers were designed from the published bovine genome by using Primer Express version 1.5 software (Applied Biosystems, Foster City, Calif.) and are described in Table 1. The 25-μl one-step RT-PCR mixture consisted of 12.5 μl of master mix, 0.5 μl each of a forward and reverse primer, 0.25 μl of RT mix, 10 μl of RNase-free water, and 1.25 μl of template RNA (containing 500 to 1,500 ng). The real-time cycler conditions included 50°C for 30 min and 95°C for 15 min followed by 40 cycles of 94°C for 15 s, 60°C for 30 s, and 72°C for 30 s. Relative quantities for different cytokines were extrapolated from the standard curve generated from 10-fold dilutions of the reference mRNA sample (6-h LPS-stimulated whole blood) and then normalized for GAPDH expression as previously described (http://www.ambion.com./techlib/tn/85/857.html). Data were analyzed by analysis of variance with repeated measures. P values of < 0.05 were considered significant.
TABLE 1.
Primers used for real-time RT-PCR
| Primer | Sequence (5′ to 3′) | Product size (bp) | GenBank accession no. |
|---|---|---|---|
| IL-1β | |||
| Sense | ATCTTCGAAACGTCCTCCGAC | 187 | M37211 |
| Antisense | CCTCTCCTTGCACAAAGCTCA | ||
| TNF-α | |||
| Sense | CCATCAACAGCCCTCTGGTT | 138 | AF011926 |
| Antisense | CCATGAGGGCATTGGCATAC | ||
| RANTES | |||
| Sense | GCCCTGCTGCTTTGCCTATAT | 190 | BTA7043 |
| Antisense | TCCACCCTAGCTCAACTCCAA | ||
| MCP-1 | |||
| Sense | CCTCCTGTGCCTGCTACTCA | 236 | L32659 |
| Antisense | CTGGACCCATTTCTGCCTGG | ||
| IL-8 | |||
| Sense | CCACACCTTTCTACCCCCAAA | 142 | AF061524 |
| Antisense | CCTTCTGCACCCACTTTTCCT | ||
| GAPDH | |||
| Sense | GGCGTGAACCACGAGAAGTATAA | 118 | U85042 |
| Antisense | CCTCCACGATGCCAAAGTG |
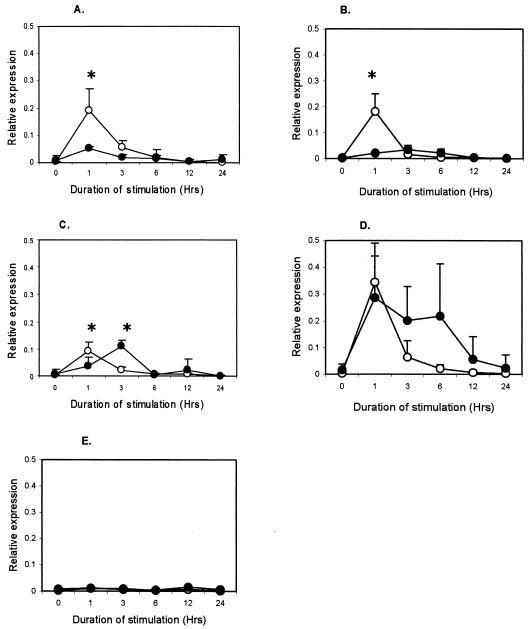
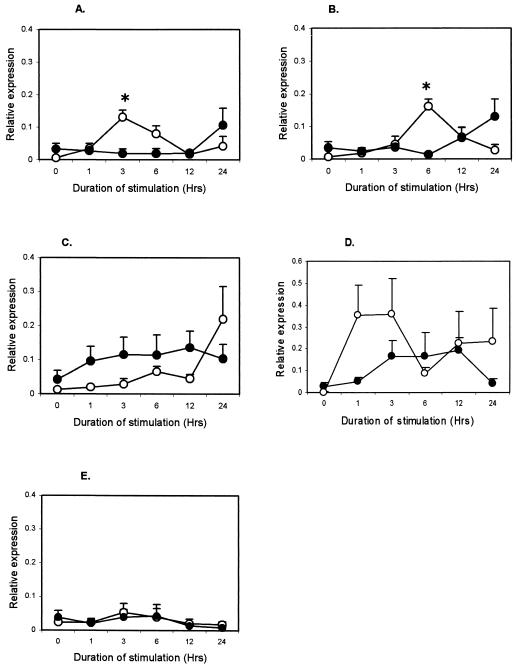
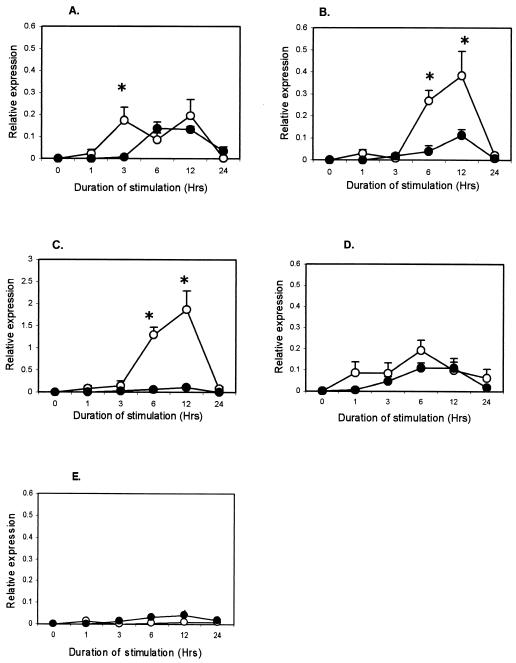
M. avium subsp. paratuberculosis antigens induced IFN-γ production in plasma from infected animals (P < 0.001), but not from uninfected animals (data not shown), confirming the infection status (2, 16). To the contrary, mycobacterial antigens induced lower TNF-α, RANTES, and MCP-1 responses in the infected animals. Responses for IL-1β and IL-8 were not different between groups (data not shown). The TNF-α expression in infected cattle was lower (P < 0.001) at 1 h after purified protein derivative (PPD) (Fig. 1A), M. avium subsp. paratuberculosis lysate (Fig. 1B), and live M. avium subsp. paratuberculosis stimulation (Fig. 1C). Live M. avium subsp. paratuberculosis, however, induced higher (P < 0.001) TNF-α expression in infected animals at 3 h. Expression of RANTES in the infected group was lower (P < 0.01) at 3 h after PPD stimulation (Fig. 2A) and at 6 h after M. avium subsp. paratuberculosis lysate stimulation (P < 0.001) (Fig. 2B), but live M. avium subsp. paratuberculosis did not confer differential expression (Fig. 2C). MCP-1 expression was lower (P < 0.01) in infected animals at 3 h after stimulation with PPD (Fig. 3A) but was much lower (P < 0.001) at 6 and 12 h after stimulation with M. avium subsp. paratuberculosis lysate (Fig. 3B) and live M. avium subsp. paratuberculosis (Fig. 3C). Stimulation with LPS, a non-M. avium subsp. paratuberculosis antigen, failed to induce differential expression between groups (Fig. 1D, 2D, and 3D), and unstimulated samples did not show change in expression (Fig. 1E, 2E, and 3E).
FIG. 1.
Kinetics of TNF-α in whole-blood samples from M. avium subsp. paratuberculosis-infected (•) and uninfected (○) cattle after in vitro stimulation with 0.5 μg of M. avium subsp. paratuberculosis PPD per ml (A), 10 μg of M. avium subsp. paratuberculosis lysate per ml (B), 60 × 106 live M. avium subsp. paratuberculosis CFU (C), 10 μg of E. coli LPS per ml (D), and medium (E). Each point represents the mean ± standard error of five animals. Differences between groups were considered significant when P < 0.05 (*).
FIG. 2.
Kinetics of RANTES in whole-blood samples from M. avium subsp. paratuberculosis-infected (•) and uninfected (○) cattle after in vitro stimulation with 0.5 μg of M. avium subsp. paratuberculosis PPD per ml (A), 10 μg of M. avium subsp. paratuberculosis lysate per ml (B), 60 × 106 live M. avium subsp. paratuberculosis CFU (C), 10 μg of E. coli LPS per ml (D), and medium (E). Each point represents the mean ± standard error of five animals. Differences between groups were considered significant when P < 0.05 (*).
FIG. 3.
Kinetics of MCP-1 in whole-blood samples from M. avium subsp. paratuberculosis-infected (•) and uninfected (○) cattle after in vitro stimulation with 0.5 μg of M. avium subsp. paratuberculosis PPD per ml (A), 10 μg of M. avium subsp. paratuberculosis lysate per ml (B), 60 × 106 live M. avium subsp. paratuberculosis CFU (C), 10 μg of E. coli LPS per ml (D), and medium (E). Each point represents the mean ± standard error of five animals. Differences between groups were considered significant when P < 0.05 (*).
The reduced TNF-α, RANTES, and MCP-1 responses observed may have a negative effect on granuloma formation and function. TNF-α plays an important role in the host defense against mycobacterium infections, including the initiation and formation of granulomas and activation of antimycobacterial killing mechanisms (3, 5, 7, 13). RANTES and MCP-1 contribute to granuloma formation through chemotactic effects on T cells, monocytes, and macrophages (1, 9, 14, 15, 21). Our results concur with observations that fewer if any granulomas are found in cattle during the subclinical stage of M. avium subsp. paratuberculosis infection (3, 5, 22). On the other hand, increased MCP-1 expression during M. avium subsp. paratuberculosis infection in mice was associated with high numbers of granulomas in liver, indicating its direct association with granuloma formation (E. Momotani, M. Miyama, T. L. To, K. Yoshihara, and H. Gotoh, 14th European Immunology Meeting, Poznan, Poland, Abstr. Immunol. Lett., abstr. 490, 2000). Interestingly, the reduced levels of TNF-α, RANTES, and MCP-1 expression occurred after stimulation with M. avium subsp. paratuberculosis antigens rather than with a non-M. avium subsp. paratuberculosis antigen LPS, suggesting the presence of suppressive substances emanating from the specific response. In this regard, it has been suggested that IL-10 secreted by M. avium subsp. paratuberculosis-infected macrophages suppresses TNF-α expression (20). At the same time, induction of RANTES (11) and MCP-1 (12, 13) by mycobacterial antigens is mediated by TNF-α. It is possible therefore that M. avium subsp. paratuberculosis antigens induced IL-10, which in turn suppressed TNF-α production with subsequent reduction in RANTES and MCP-1 expression. Despite an increased cell-mediated immune response during the subclinical stage of M. avium subsp. paratuberculosis infection in cattle, the infection persists in a number of cases, ending with precipitation of the clinical disease (18). The suppression of TNF-α, RANTES, and MCP-1, among other factors, may compromise this response, allowing M. avium subsp. paratuberculosis to escape destruction. Further studies are still needed to directly correlate peripheral chemokine responses with intestinal granuloma formation.
Acknowledgments
This work was supported by the Japan Bio-oriented Technology Research Advancement Institute (BRAIN).
We thank T. Kamio of the Parasitic Disease Section for granting access to some animals used in this study and S. Enoki of the Paratuberculosis and IBD research team for support in preparation of various experiments.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Ajuebor, M., R. Flower, R. Hannon, M. Christie, K. Bowman, A. Varity, and M. Paerretti. 1998. Endogenous monocyte chemoattractant protein-1 recruits monocytes in the zymosan peritonitis model. J. Leukoc. Biol. 63:108-116. [DOI] [PubMed] [Google Scholar]
- 2.Billman-Jacobe, H., M. Carrigan, and F. Cochram. 1992. A comparison of the interferon gamma assay with absorbed ELISA for the diagnosis of Johne's disease in cattle. Aust. Vet. J. 69:25-28. [DOI] [PubMed] [Google Scholar]
- 3.Clarke, C. J. 1997. The pathology and pathogenesis of paratuberculosis in ruminants and other species. J. Comp. Pathol. 116:217-261. [DOI] [PubMed] [Google Scholar]
- 4.Gangur, V., N. P. Birmingham, and S. Thanesvorakul. 2002. Chemokines in health and disease. Vet. Immunol. Immunopathol. 86:127-136. [DOI] [PubMed] [Google Scholar]
- 5.Harris, N. B., and R. G. Barletta. 2001. Mycobacterium avium subsp. paratuberculosis in veterinary medicine. Clin. Microbiol. Rev. 14:489-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeevan, A., T. Yoshimura, G. Foster, and D. N. McMurray. 2002. Effect of Mycobacterium bovis BCG vaccination on interleukin-1β and RANTES mRNA expression in guinea pig cells exposed to attenuated and virulent mycobacteria. Infect. Immun. 70:1245-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kindler, V., A. P. Sappino, G. E. Grau, P. F. Piguet, and P. Vassalli. 1989. The inducing role of tumor necrosis factor in the development of bacterial granulomas during BCG infection. Cell 56:731-739. [DOI] [PubMed] [Google Scholar]
- 8.Lee, H., J. R. Stabel, and M. E. Kerli, Jr. 2001. Cytokine gene expression in ileal tissues of cattle infected with Mycobacterium paratuberculosis. Vet. Immunol. Immunopathol. 82:73-85. [DOI] [PubMed] [Google Scholar]
- 9.Lyons, M. J., T. Yoshimura, and D. N. McMurray. 2002. Mycobacterium bovis BCG vaccination augments interleukin-8 mRNA expression and protein production in guinea pig alveolar macrophages infected with Mycobacterium tuberculosis. Infect. Immun. 70:5471-5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marfaing-Koka, A., O. Devergne, G. Gorgone, A. Portier, T. J. Schall, P. Galanaud, and D. Emilie. 1995. Regulation of the production of the RANTES chemokine by endothelial cells. Synergistic induction by IFN-gamma plus TNF-alpha and inhibition by IL-4 and IL-13. J. Immunol. 154:1870-1878. [PubMed] [Google Scholar]
- 11.Peters, W., and J. D. Ernst. 2003. Mechanisms of cell recruitment in the immune response to Mycobacterium tuberculosis. Microb. Infect. 5:151-158. [DOI] [PubMed] [Google Scholar]
- 12.Qiu, B., K. A. Frait, F. Reich, E. Komuniecki, and S. W. Chensue. 2001. Chemokine expression dynamics in mycobacterial (type-1) and schistosomal (type-2) antigen-elicited pulmonary granuloma formation. Am. J. Pathol. 158:1503-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roach, D. R., A. G. D. Bean, C. Demangel, M. P. France, H. Briscoe, and W. J. Britton. 2002. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J. Immunol. 168:4620-4627. [DOI] [PubMed] [Google Scholar]
- 14.Schall, T. J. 1991. Biology of the RANTES/SIS cytokine family. Cytokine 3:165-183. [DOI] [PubMed] [Google Scholar]
- 15.Schall, T. J., K. Bacon, K. J. Toy, and D. V. Goeddel. 1990. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature 347:669-671. [DOI] [PubMed] [Google Scholar]
- 16.Stabel, J. R. 1996. Production of γ-interferon by peripheral blood mononuclear cells: an important diagnostic tool for detection of preclinical paratuberculosis. J. Vet. Diagn. Investig. 8:345-350. [DOI] [PubMed] [Google Scholar]
- 17.Stabel, J. R. 2000. Cytokine secretion by peripheral blood mononuclear cells from cows infected with Mycobacterium paratuberculosis. Am. J. Vet. Res. 61:754-760. [DOI] [PubMed] [Google Scholar]
- 18.Stabel, J. R. 2000. Transitions in immune responses to Mycobacterium paratuberculosis. Vet. Microbiol. 77:465-473. [DOI] [PubMed] [Google Scholar]
- 19.Sweeney, R. W., D. E. Jones, P. Habecker, and P. Scott. 1998. Interferon-γ and interleukin-4 expression in cows infected with Mycobacterium paratuberculosis. Am. J. Vet. Res. 59:842-847. [PubMed] [Google Scholar]
- 20.Weiss, D. J., O. A. Evanson, A. Moritz, M. Q. Deng, and M. S. Abrahamsen. 2002. Differential responses of bovine macrophages to Mycobacterium avium subsp. paratuberculosis and Mycobacterium avium subsp. avium. Infect. Immun. 70:5556-5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wempe, F., A. Henschen, and K. H. Scheit. 1991. Gene expression and cDNA cloning identified a major basic protein constituent of bovine seminal plasma as bovine monocyte-chemoattractant protein-1 (MCP-1). DNA Cell Biol. 10:671-679. [DOI] [PubMed] [Google Scholar]
- 22.Whittington, R. J., and E. S. G. Sergeant. 2001. Progress towards understanding the spread, detection and control of Mycobacterium avium subsp. paratuberculosis in animal populations. Aust. Vet. J. 79:267-278. [DOI] [PubMed] [Google Scholar]