Abstract
Pneumococcal capsular polysaccharide (PS) vaccines induce type-specific immunoglobulin M (IgM), IgG, and IgA. Type-specific IgG to the PS is sufficient to confer protection against the homologous serotype of the pneumococcus, but the efficacies of type-specific IgM and IgA are less well understood. We examined the in vitro activities and efficacies in mice of two human monoclonal antibodies (MAbs) to type 8 PS, NAD (IgA) and D11 (IgM). MAb-mediated opsonophagocytic killing was evaluated after coculture of type 8 pneumococci with human polymorphonuclear cells (PMNs), type-specific or control MAbs, and human complement sources. The effects of the MAbs on PMN interleukin-8 (IL-8) and IL-6 secretion were determined in supernatants from cocultures containing pneumococci and PMNs by enzyme-linked immunosorbent assay. MAb efficacy was determined in an intratracheal model of type 8 infection in mice with classical complement pathway deficiency. Both MAbs were protective in 100% of infected mice. Neither MAb promoted a significant amount of killing of type 8 pneumococci compared to its isotype control MAb. Both type-specific MAbs mediated complement-dependent modulation of PMN IL-8 secretion, with increased secretion at effector/target (E:T) ratios of 500:1 and 50:1 and reduced secretion at 1:5. Trypan blue staining revealed that PMNs cocultured with D11 were less viable at an E:T ratio of 1:5 than PMNs cocultured with the control MAb. PMN IL-6 secretion was increased by both type-specific and control MAbs. These results suggest that certain type-specific IgM and IgAs might contribute to host defense by modulation of the inflammatory response to pneumococci.
Vaccination with pneumococcal capsular polysaccharide (PS)-based vaccines elicits type-specific immunoglobulin M (IgM) and IgA in addition to IgG. Opsonic type-specific IgG to the homologous serotype is known to be sufficient to confer protection in the immune host (34). Type-specific IgM and IgA have also been shown to mediate protection against pneumococci in different models (12, 20, 40, 42, 56). However, mechanisms of IgM and IgA efficacy are less well understood. The use of serum has hampered the examination of mechanisms of IgM and IgA efficacy, since heterogeneous nature makes it difficult to evaluate the activity of individual antibodies (9). The development of human type-specific monoclonal antibodies (MAbs) to PS has made it possible to investigate mechanisms of antibody action with defined antibody reagents. Studies with human IgM MAbs to types 3 and 8 PS have shown that MAbs with a certain specificity can be highly protective in normal mice and mice deficient in either the classical or alternative complement pathway (12, 40, 56). Similar studies have not been performed with type-specific IgA MAbs. Type-specific IgA has been shown to promote phagocytosis of pneumococci by polymorphonuclear cells (PMNs) and enhance the opsonic efficacy of cytokine-stimulated effector cells (21, 35). However, IgA has also been proposed to be antiinflammatory based on its poor complement-activating properties and ability to block surface binding of IgG (22, 39).
The clinical signs and symptoms of pneumococcal disease have been attributed to the host inflammatory response (8, 24, 28). Elderly adults, a population at increased risk for development of and the morbidity of pneumococcal disease, have been shown to manifest a prolonged inflammatory response to pneumococcal infection (8). Recent studies have shown that naturally occurring and specific antibodies can reduce the inflammatory response to a number of pathogens (reviewed in reference 10). Along the same lines, certain antibiotics can reduce the inflammatory response to experimental pneumococcal infection (41, 53), but the influence of type-specific antibodies on the inflammatory response to pneumococci has not been investigated. In this study, we sought to determine the biological activity of type-specific human MAbs to type 8 PS and their effect on the release of proinflammatory mediators from human PMNs cultured with type 8 pneumococci.
MATERIALS AND METHODS
Bacteria and type 8 PS.
Type 8 Streptococcus pneumoniae and purified type 8 PS were obtained from the American Type Culture Collection (ATCC 6308; Rockville, Md.). Type 8 PS contains a pentasaccharide repeat unit and has an approximate molecular mass of 140 kDa (23). For in vitro and in vivo experiments, pneumococci were grown in tryptic soy broth (TSB; Difco, Sparks, Md.) to mid-log phase at 37°C in 5% CO2, frozen in TSB in 10% glycerol, and stored at −80°C until used as described previously (56). For in vivo studies, pneumococci were rapidly thawed just prior to use, placed on ice, and diluted to the desired concentration with TSB. For cytokine studies, pneumococci were washed with phosphate-buffered saline (PBS) and heat killed at 65°C for 1 h before use as described elsewhere (56). For epitope mapping and complement deposition studies, type 8 PS was used. For enzyme-linked immunosorbent assays (ELISAs), plates were coated with 10 μg of type 8 PS/ml as described previously (56).
Antibodies.
The isolation and efficacy of the type 8 PS-reactive human lymphoblastoid cell lines (heretofore referred to as MAbs) D11 and NAD from human PS vaccinees have been described previously (43, 56). D11 is an IgM kappa derived from a VH3 gene segment (56). NAD is an IgA kappa, the sequence of which is reported herein. The MAbs were purified by affinity chromatography using anti-human IgM or protein A-coated agarose beads (Sigma-Aldrich, St. Louis, Mo.). A human myeloma IgM and IgA (catalog numbers 401108 and 400109, respectively; Calbiochem, San Diego, Calif.) were used as negative control MAbs.
Nucleotide sequence analysis.
The nucleotide sequence of NAD was determined by sequencing cDNA amplified from RNA by PCR as described previously (12, 40, 56). Briefly, cDNA of the heavy (VH) and light (VL) chains were generated by reverse transcription of RNA with constant heavy and light chain region primers. VH and VL PCR products were gel purified and cloned into the PCR plasmid 1000 of the TA cloning system (Invitrogen, San Diego, Calif.) according to the manufacturer's instructions. Plasmid DNA was isolated by the Maxi plasmid protocol (Qiagen, Inc., Chatsworth, Calif.), and DNA sequencing was performed by the Cancer Center DNA Synthesis Facility (Albert Einstein College of Medicine, Bronx, N.Y.). Variable region sequences were compared to the database of human immunoglobulins using DNA PLOT (V Base index; MRC Center for Protein Engineering, Cambridge, United Kingdom).
Infection and survival studies.
The protective efficacies of D11 and NAD were evaluated in a systemic and an intratracheal (i.t.) model of type 8 pneumococcal infection. The efficacy of D11 was previously established in a systemic model (56). This model was based on the mouse pneumococcal serum potency assay used to standardize antisera for treatment of pneumococcal pneumonia in the preantibiotic era (9). Complement-deficient mice were used to evaluate the efficacy of NAD, because the same mice were used to determine the efficacy of D11 (56). Six- to 8-week-old C4-deficient mice (C4−/−) (12, 56), bred at the Institute for Animal Studies of the Albert Einstein College of Medicine, were used. In the systemic model, groups of seven C4−/− mice received 10 μg of NAD, the control IgA, or PBS intraperitoneally (i.p.) 1 h prior to i.p. infection with 50 CFU of type 8 pneumococci. The i.t. infection was performed as described previously (15) and as follows: groups of 10 C4−/− mice were anesthetized i.p. with 6.5 mg of sodium pentobarbital (Abbott Laboratories, North Chicago, Ill.)/kg of body weight, a tracheal incision was made, and each mouse was given 20 CFU of pneumococci with either PBS, 1 μg of control IgM or D11, or 10 μg of control IgA or NAD. After injection, the incision was sutured. The number of CFU administered to each mouse was confirmed by plating the inoculum onto a Trypticase agar plate containing 5% sheep's blood (Becton Dickinson, Franklin Lakes, N.J.) and incubating the plates overnight at 37°C, 5% CO2. Mice were checked twice daily for survival, and the number of surviving mice was recorded. The concentration of D11 used corresponded to the amount of MAb that was previously shown to protect 86% of mice against death from systemic disease (56). Based on the relative avidities of IgM and IgA, 10:1, the amounts of each MAb represent comparable masses. Zhong et al. have established that mice die with high levels of bacteremia in the systemic (56) and i.t. models (data not shown).
Epitope specificities.
The epitope specificities of NAD and D11 were compared by inhibition ELISAs on type 8 PS-coated polystyrene plates as described elsewhere (12). Titration curves were used to determine the concentration of each MAb that resulted in 50% binding to plates coated with 10 μg of PS 8/ml, 1.25 μg of D11/ml, and 2.5 μg of NAD/ml (data not shown). For inhibition studies, the plates were coated with 10 μg of type 8 PS/ml in PBS, blocked with 1% bovine serum albumin-PBS, washed, and incubated with an equal volume of a constant concentration of each MAb (1.25 μg of D11/ml and 2.5 μg of NAD/ml) and serial dilutions of the other beginning at 10 μg/ml. The plates were incubated at 37°C for 1 h, washed, and incubated with alkaline phosphatase-labeled goat-anti human IgA for NAD or IgM for D11 (Southern Biotechnology, Birmingham, Ala.). The plates were developed with the substrate p-nitrophenyl phosphate (Sigma-Aldrich) and read in an MRX microplate reader (Dynex, Chantilly, Va.) to determine the optical density at 405 nm.
C3 deposition assay.
The ability of NAD and D11 to deposit C3 onto solid-phase type 8 PS via activation of the classical or the alternative complement pathway was determined by ELISA as described previously (12). Briefly, polystyrene ELISA plates were coated with 10 μg of type 8 PS/ml and incubated at 37°C for 1 h with a solution consisting of a final concentration of 10 μg/ml of either MAb or of a control IgM or IgA MAb with 10 and 5% factor B-depleted (FB−) or C2-depleted (C2−) human serum (HS; Calbiochem). After washing, the plates were incubated at 37°C for 1 h with goat anti-human C3 (ICN Biomedicals, Aurora, Ohio), washed, and incubated with alkaline phosphatase-labeled rabbit anti-goat IgG (Calbiochem). After washing, the plates were developed with p-nitrophenylphosphate substrate (Sigma-Aldrich) and read as described above.
Opsonophagocytic killing assay.
The ability of D11 and NAD to mediate opsonophagocytic killing (opsonophagocytosis) of type 8 pneumococci by human PMNs was determined as described elsewhere (29), with some modifications. Venous blood obtained from a healthy volunteer was diluted in Hank's balanced salt solution (Mediatech, Herndon, Va.), and the PMNs were isolated by density gradient centrifugation as described previously (57). PMNs from the same donor were used for the opsonophagocytic killing assays and cytokine determinations (see below). Trypan blue exclusion analysis showed greater than 90% cell viability of PMNs used in the assays. The opsonophagocytic killing assays were performed in a total volume of 100 μl; 2 × 103 CFU of type 8 pneumococci were cocultured at room temperature for 30 min with a 1-μg/ml solution of D11 or the control IgM or a 10-μg/ml solution of NAD or the control IgA and then incubated for 1 h at 37°C with 106 PMNs (effector/target ratio [E:T], 500:1) and 10% by volume of either PBS, C2−, FB−, or HS (Sigma-Aldrich). The experiments were also performed with 0.1- and 1-μg/ml solutions of D11 and the control IgM or 0.1- and 1-μg/ml solutions of NAD and the control IgA, each with 10, 5, and 1% HS. Preliminary studies (data not shown) demonstrated clumping and/or agglutination of the pneumococci with 10 μg of D11/ml, and this concentration was not used. Dilutions of the cultures were spread onto blood agar plates (Becton Dickinson) and incubated at 37°C overnight in 5% CO2, and the number of CFU were counted. The number of CFU in cocultures from each of the type 8-specific MAbs was compared to that of its respective isotype control for each of the complement sources used by one-way analysis of variance (ANOVA) and Bonferroni's multiple comparison test (GraphPad Prism 3.03; San Diego, Calif.).
Cytokine quantitation.
Interleukin-6 (IL-6) and IL-8 release from human PMNs was determined using a modification of the method described by Retini et al. (33). The PMNs were diluted to a concentration of 107 cells/ml in RPMI (Sigma-Aldrich) supplemented with 10% fetal calf serum (Omega, Tarzana, Calif.), and 100 μl of this solution was placed into the wells of 48-well plastic culture dishes (Falcon, Franklin Lakes, N.J.). Preliminary studies revealed that maximal IL-6 and IL-8 production was greatest after 18 h of incubation (data not shown); therefore, this time was used to collect supernatants for cytokine determinations. PMNs were cocultured in a volume of 200 μl for 18 h at 37°C in 5% CO2 with 5 × 106 CFU (E:T, 1:5) of heat-killed type 8 pneumococci, a 10-μg/ml solution of D11 or the control IgM, or of NAD or the control IgA, and 10% by volume of either unheated or heat-inactivated C2−, FB−, or HS. Sera were heat inactivated at 56°C for 30 min. Cytokine determinations were also performed with complement sources at E:T ratios of 500:1; 50:1, and 1:5 with 106 PMNs and at 1:50 and 1:500 with 105 or 104 PMNs. The positive control was PMNs cocultured with 10 μg of lipopolysaccharide (Escherichia coli 055:B5; Sigma-Aldrich)/ml. Supernatants were collected from the cocultures, and the concentrations of IL-6 and IL-8 in the supernatants were determined with ELISA-based cytokine detection kits (R&D Systems, Minneapolis, Minn.) according to the manufacturer's instructions. Care was exercised to ensure that the experiments were performed under endotoxin-free conditions, and all reagents were tested for endotoxin with the Limulus amebocyte lysate test (Sigma-Aldrich) before use. The Limulus amebocyte lysate test showed that endotoxin was not present in the reagents used. The levels of IL-8 and IL-6 in supernatants from cocultures with each of the type 8-specific MAbs were compared to the respective isotype control for each of the heated and unheated complement sources used by a one-way ANOVA and Bonferroni's multiple comparison test.
Cell viability assay.
The viability of PMNs was evaluated by trypan blue staining for each of the E:T ratios at which IL-8 and IL-6 secretion was determined to ascertain whether or not there was an association between changes in the levels of these mediators and cell viability. Trypan blue exclusion was used because it is a reliable and useful method that correlates well with other methods to determine cell viability (3, 13). For these experiments, aliquots of cocultures containing heat-killed type 8 pneumococci, the type-specific MAbs or control MAbs, and PMNs and HS were mixed with 0.4% trypan blue (Sigma-Aldrich) and examined by light microscopy. The number of stained and unstained cells was counted using a hemocytometer, and the percentage of viable cells was determined as follows: (number of unstained [viable] cells/total number of cells) × 100.
Statistical analysis.
Comparisons between the effects of the type-specific and control MAbs in the opsonophagocytosis, cytokine, and cell viability assays were performed with a one-way ANOVA and Bonferroni multiple comparison test. The goal of these studies was to assess the ability of each type-specific MAb to mediate opsonophagocytosis or affect chemokine or cytokine secretion compared to a control MAb. Statistical comparisons were not made between conditions with different complement sources because these sera were not standardized and were likely to have differences in addition to the specific complement component that was lacking. The results of the infection experiments were analyzed using the Kaplan-Meier log rank survival test as our investigators have done previously (12, 56). All statistical tests were performed using GraphPad Prism 3.03.
Nucleotide sequence accession numbers.
The GenBank accession numbers for the VH and VL gene sequences of NAD are AF332144 and AF332145, respectively.
RESULTS
Nucleotide sequence analysis of NAD.
NAD uses the VH3 gene segment VH3-23 and the κ light chain gene segment DPK18/A17.
Infection and survival studies.
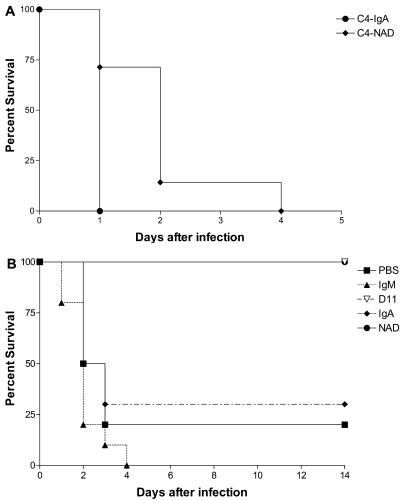
The median survival of the NAD-treated C4−/− mice was significantly longer, 2 days, than that of control IgA-treated mice, whose median survival was 1 day (P = 7.2 × 10−3) (Fig. 1A). D11 treatment had previously been shown to protect 86% of C4−/− mice from death in the systemic infection model (56). D11 and NAD were both protective against infection compared to their respective isotype controls and PBS (Fig. 1B). The median survival of PBS-treated mice was 2.5 days (P = 3 × 10−4, compared to D11 and NAD). The median survival times of control IgM- and IgA-treated mice were 2.0 and 2.5 days, respectively (P < 1 × 10−4 and P = 1.2 × 10−3, compared to D11 and NAD, respectively). D11- and NAD-treated mice survived 14 days, at which time they were euthanized.
FIG. 1.
Survival of C4−/− mice after infection with type 8 pneumococci. (A) Mice were infected i.p. with type 8 pneumococci 1 h after i.p. administration of 10 μg of NAD or control IgA. (B) Mice were infected i.t. with type 8 pneumococci mixed with PBS, with D11 or control IgM (1 μg), or with NAD or control IgA (10 μg). The symbols representing each of the mouse groups are depicted in the legend. The median survival of the C4−/− mice in the i.p./i.p. model was 1 day for IgA and 2 days for NAD (P = 7.2 × 10−3 comparing NAD to IgA). In the i.t. model, the median survival of the C4−/− mice was 2.5 days for PBS, 2 days for IgM, and 2.5 days for IgA (P = 3 × 10−4 and P < 1 × 10−4 for the comparisons between PBS and D11 and IgM and D11, respectively; P = 3 × 10−4 and 1.2 × 10−3 comparing PBS to NAD and IgA to NAD, respectively). All statistical comparisons were performed with the Kaplan-Meier log rank survival test.
Epitope specificity.
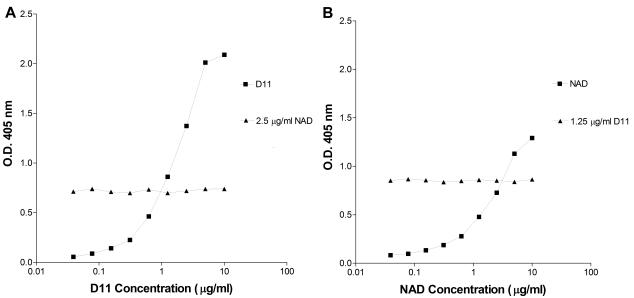
Neither NAD nor D11 inhibited the binding of the other MAb to type 8 PS (Fig. 2). In the presence of a constant concentration of the other MAb, the titration curves of NAD and D11 were the same as they were in the absence of the other MAb. These findings are most consistent with the conclusion that D11 and NAD have different type 8 PS specificities.
FIG. 2.
Epitope specificities of the MAbs to type 8 PS. The specificity of D11 (IgM) and NAD (IgA) were studied in a competition ELISA in which the binding of each MAb was detected separately. (A) Results of the IgA detection of a fixed concentration of NAD added to serial dilutions of D11. (B) Results of the IgM detection of a fixed concentration of D11 added to serial dilutions of NAD. The squares represent D11, and the triangles represent NAD.
Complement fixation.
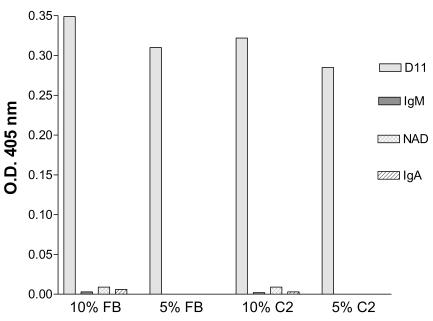
D11 led to C3 deposition onto type 8 PS by activation of the classical (FB−) and alternative (C2−) complement pathways (Fig. 3). NAD, the control IgM, and the control IgA did not result in detectable C3 deposition with any complement source (Fig. 3).
FIG. 3.
MAb-mediated C3 deposition on type 8 PS. D11- and NAD-mediated C3 deposition onto type 8 PS was evaluated by detection of type 8-bound C3 by ELISA. D11 results are depicted with gray bars; NAD results are depicted with hatched bars.
Opsonophagocytosis.
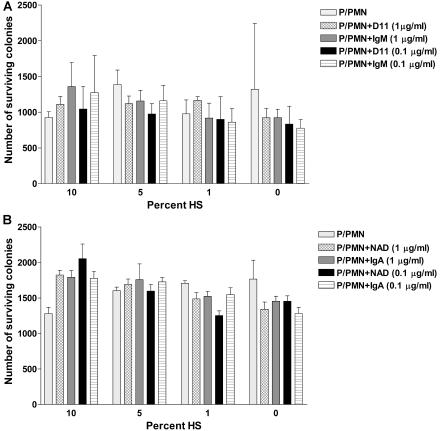
The ability of NAD and D11 to promote killing of type 8 pneumococci by human PMNs was evaluated under the same conditions in three individual experiments and in additional experiments with different MAb concentrations and complement sources and concentrations. In one experiment, there was a statistically significant reduction in the number of CFU with 10% C2− or HS with D11 and NAD (Table 1). However, in the other two experiments, there was no statistically significant killing with D11, although NAD resulted in a significant degree of killing with HS (Table 1). Experiments performed using other concentrations of the MAbs and complement sources did not reveal significant killing with either specific MAb compared to its respective control (Fig. 4). Although a human MAb to type 8 PS known to be opsonic (e.g., a positive control MAb) was not available, these findings are most consistent with the conclusion that D11 and NAD promote minimal PMN-mediated killing (less than 50% of the inoculum) with human complement sources over the range of conditions studied.
TABLE 1.
Results of opsonophagocytic killing assays with human PMNs, type 8 pneumococci, human complement sources, and human IgM and IgA MAbs to type 8 PS
| Conditiona | CFUb
|
||
|---|---|---|---|
| Expt 1 | Expt 2 | Expt 3 | |
| P/PMN/no complement/no MAb | 440 ± 120 | 1,300 ± 200 | 1,300 ± 400 |
| P/PMN/no complement/D11 | 390 ± 60 | 1,100 ± 230 | 1,100 ± 110 |
| P/PMN/no complement/IgM | 440 ± 34 | 1,300 ± 320 | 1,200 ± 460 |
| P/PMN/FB−/no MAb | 600 ± 140 | 1,500 ± 320 | 760 ± 51 |
| P/PMN/FB−/D11 | 340 ± 89 | 1,100 ± 110 | 720 ± 270 |
| P/PMN/FB−/IgM | 410 ± 59 | 1,100 ± 250 | 710 ± 220 |
| P/PMN/C2−/no MAb | 600 ± 120 | 1,300 ± 220 | 910 ± 200 |
| P/PMN/C2−/D11 | 440 ± 100* | 1,100 ± 99 | 610 ± 170 |
| P/PMN/C2−/IgM | 690 ± 63 | 1,300 ± 130 | 780 ± 210 |
| P/PMN/HS/no MAb | 430 ± 71 | 750 ± 180 | 1,100 ± 240 |
| P/PMN/HS/D11 | 120 ± 50* | 510 ± 190 | 550 ± 250 |
| P/PMN/HS/IgM | 430 ± 81 | 770 ± 250 | 890 ± 220 |
| P/PMN/no complement/no MAb | 440 ± 120 | 1,300 ± 200 | 1,300 ± 400 |
| P/PMN/no complement/NAD | 340 ± 80 | 1,400 ± 140 | 1,100 ± 270 |
| P/PMN/no complement/IgA | 490 ± 120 | 1,300 ± 240 | 1,000 ± 240 |
| P/PMN/FB−/no MAb | 600 ± 140 | 1,500 ± 320 | 760 ± 51 |
| P/PMN/FB−/NAD | 450 ± 150 | 1,000 ± 250 | 590 ± 150 |
| P/PMN/FB−/IgA | 650 ± 120 | 1,100 ± 270 | 620 ± 130 |
| P/PMN/C2−/no MAb | 600 ± 120 | 1,300 ± 220 | 910 ± 200 |
| P/PMN/C2−/NAD | 430 ± 100* | 1,300 ± 310 | 810 ± 120 |
| P/PMN/C2−/IgA | 730 ± 92 | 1,500 ± 240 | 930 ± 290 |
| P/PMN/HS/no MAb | 430 ± 71 | 750 ± 180 | 1,100 ± 240 |
| P/PMN/HS/NAD | 240 ± 90 | 750 ± 220* | 1,000 ± 140 |
| P/PMN/HS/IgA | 360 ± 89 | 1,100 ± 230 | 1,200 ± 300 |
P, type 8 pneumococci; PMN, human PMNs; FB−, factor B-deficient serum; C2−, C2-deficient serum; HS, human serum.
CFU of type 8 pneumococci; values represent mean ± standard deviation of duplicate samples for each condition. *, P < 0.05, comparing type-specific MAb to isotype control MAbs using one-way ANOVA and Bonferroni multiple comparison test.
FIG. 4.
MAb-mediated opsonophagocytic killing of type 8 pneumococci. The numbers of CFU resulting from the incubation of type 8 cells with human PMNs, D11, NAD, or isotype controls were compared in an opsonophagocytic killing assay, as shown on the y axis, for each of the concentrations of HS shown on the x axis. No statistical differences were detected (one-way ANOVA). HS contains intact classical and alternative complement pathways. P, type 8 pneumococci; P/PMN, type 8 pneumococci and human PMNs.
PMN IL-8 secretion.
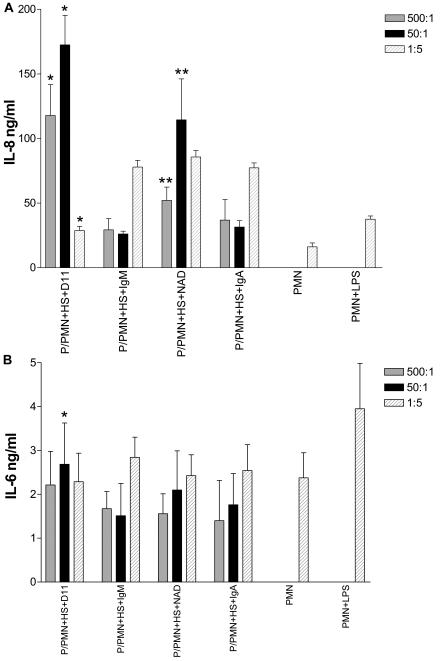
At an E:T ratio of 1:5, supernatants from PMNs cocultured with type 8 pneumococci and the specific MAbs had lower levels of IL-8 than those cocultured with control MAbs (Table 2). In each of four independent experiments, there was a significantly lower level of IL-8 with either FB− or HS and D11 compared to the control IgM and with C2− and D11 compared to the control IgM in three of four experiments. In one of three independent experiments with heated complement sources, the level of IL-8 with D11 was significantly different than with the control IgM; in one experiment, there was a significant decrease in IL-8 secretion with heated FB− and D11 compared to the control IgM, and in one experiment there was a significant increase in IL-8 with heated HS and D11 compared to that with the control IgM (Table 2). In all three experiments, the level of IL-8 was higher with D11 and heated HS compared to that with unheated HS, and there was no significant difference in the level of IL-8 with D11 and heated HS compared to that with unheated FB− and C2− serum in two of the three experiments (Table 2). Hence, compared to the control IgM, D11 was associated with a complement-dependent decrease in IL-8 and with an increase in IL-8 in the absence of complement (heated complement source).
TABLE 2.
IL-8 levels in 18-h cocultures of human PMNs, type 8 pneumococci, human complement sources, and human IgM and IgA MAbs to type 8 PS
| Conditiona | IL-8 (ng/ml)b
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Expt 1
|
Expt 2
|
Expt 3
|
Expt 4
|
|||||
| Unheated | Heated | Unheated | Heated | Unheated | Heated | Unheated | Heated | |
| P/PMN/FB−/no MAb | 57 ± 4 | 12 ± 2 | 110 ± 28 | 34 ± 14 | 54 ± 32 | 65 ± 17 | 25 ± 9 | |
| P/PMN/FB−/D11 | 18 ± 2c,d | 35 ± 3 | 67 ± 14c | 100 ± 35 | 52 ± 8c | 37 ± 11c | 32 ± 19c | |
| P/PMN/FB−/IgM | 41 ± 2 | 35 ± 7 | 130 ± 15 | 140 ± 55 | 300 ± 110 | 120 ± 42d | 57 ± 19 | |
| P/PMN/C2−/no MAb | 2 ± 0 | 0 ± 0 | 110 ± 30 | 67 ± 40 | 110 ± 52 | 18 ± 16 | 38 ± 18 | |
| P/PMN/C2−/D11 | 14 ± 5c | 12 ± 8 | 73 ± 23c | 110 ± 37 | 160 ± 56c | 14 ± 13d | 51 ± 7 | |
| P/PMN/C2−/IgM | 35 ± 6d | 12 ± 2 | 150 ± 32 | 140 ± 48 | 280 ± 94 | 20 ± 19d | 44 ± 9 | |
| P/PMN/HS/no MAb | 19 ± 6 | 2 ± 1 | 150 ± 39 | 88 ± 32 | 180 ± 51 | 69 ± 10 | 41 ± 7 | |
| P/PMN/HS/D11 | 9 ± 2c,d | 23 ± 2 | 45 ± 18c,d | 180 ± 150 | 75 ± 37c | 36 ± 10c,d | 100 ± 49c | |
| P/PMN/HS/IgM | 41 ± 10d | 26 ± 3 | 120 ± 43d | 240 ± 120 | 250 ± 80 | 85 ± 27 | 60 ± 30 | |
| P/PMN/FB−/no MAb | 250 ± 55 | 250 ± 68 | 54 ± 32 | 65 ± 17 | 25 ± 9 | |||
| P/PMN/FB−/NAD | 87 ± 19d | 150 ± 31 | 160 ± 49 | 47 ± 11c | 60 ± 8 | |||
| P/PMN/FB−/IgA | 120 ± 17d | 250 ± 36 | 180 ± 41 | 150 ± 32d | 66 ± 5 | |||
| P/PMN/C2−/no MAb | 120 ± 70 | 72 ± 54 | 100 ± 52 | 18 ± 16 | 38 ± 18 | |||
| P/PMN/C2−/NAD | 92 ± 16c,d | 210 ± 74 | 230 ± 67 | 47 ± 20c | 69 ± 7 | |||
| P/PMN/C2−IgA | 170 ± 53 | 200 ± 59 | 320 ± 130 | 110 ± 29d | 59 ± 10 | |||
| P/PMN/HS/no MAb | 120 ± 70 | 180 ± 18 | 180 ± 51 | 69 ± 10 | 41 ± 7 | |||
| P/PMN/HS/NAD | 94 ± 16c,d | 170 ± 25 | 260 ± 105 | 37 ± 7c,d | 73 ± 17 | |||
| P/PMN/HS/IgA | 200 ± 44 | 200 ± 41 | 180 ± 90 | 120 ± 30d | 62 ± 17 | |||
P, type 8 pneumococci; PMN, human PMNs; FB−, factor B-deficient serum; C2−, C2-deficient serum; HS, human serum.
IL-8 levels in secreted supernatants from cocultures performed with unheated and heat-inactivated complement sources; values represent mean ± standard deviation of duplicate samples for each condition.
P < 0.05 comparing type-specific MAb to isotype control MAbs, using one-way ANOVA and Bonferroni multiple comparison test.
P < 0.05 comparing conditions with heated and unheated complement sources, using one-way ANOVA and Bonferroni multiple comparison test.
In two of the three experiments, the level of IL-8 was significantly lower with C2− serum and HS and NAD compared to that with the control IgA (Table 2). In one experiment, NAD resulted in a lower IL-8 level than that with the control IgA with FB− serum (Table 2). In each of two experiments with heated HS and C2−, there was no significant difference in the amount of IL-8 with NAD compared to that with the control IgA. In one experiment, NAD and heated FB− was significantly lower than control IgA. The levels of IL-8 with heated FB−, C2−, and HS compared to that in their unheated counterparts were higher in one experiment, and they were higher with heated HS in the other. Hence, compared to the control IgA, NAD was associated with a complement-dependent decrease in IL-8 and with an increase in IL-8 with inactive complement sources.
Effect of the E:T ratio on PMN IL-8 secretion.
The influence of the E:T ratio on IL-8 secretion was examined in cocultures with HS. The level of PMN IL-8 with D11 or NAD was dependent on the E:T ratio (Fig. 5A). At E:T ratios of 500:1 or 50:1, the amount of IL-8 was higher with both D11 and NAD than with their respective isotype controls, whereas at 1:5, D11 resulted in a decrease in IL-8 compared to the control IgM. The isotype control MAbs did not significantly alter the IL-8 level at any of the E:T ratios examined. Hence, the influence of D11 and NAD on IL-8 secretion was specific and a function of the E:T ratio.
FIG. 5.
The effect of the E:T ratio on type 8-stimulated IL-8 and secretion from human PMNs. The amount of IL-8 (A) and IL-6 (B) in supernatants secreted by PMNs cocultured with killed type 8 pneumococci (P), D11, NAD, or the isotype controls (IgM or IgA) with HS was determined by ELISA. Symbols in panel A: *, P < 0.001, comparing D11 to IgM; **, P < 0.05, comparing NAD to IgA. Symbol in panel B: *, P < 0.05 comparing D11 to IgM. The error bars represent standard deviations of replicate samples. All statistical comparisons were performed with a one-way ANOVA and the Bonferroni multiple comparison test.
PMN IL-6 secretion.
Coculture of PMNs and type 8 pneumococci with D11 or the isotype control IgM resulted in increased PMN IL-6 levels compared to conditions without a MAb in unheated complement sources in two of the three experiments (Table 3). Compared to conditions with no MAb, there was an increase in IL-6 with NAD and the control IgA and C2− and HS (Table 3). The level of IL-6 was also increased in heated complement sources with NAD, except in one experiment where the IL-6 level was lower than that with heated HS with no MAb. In the experiments at different E:T ratios, D11 resulted in a higher level of IL-6 than the control IgM at a ratio of 50:1 (Fig. 5B). Hence, both specific and nonspecific MAbs increased the IL-6 level, and the increase in IL-6 secretion was dependent on the presence of an intact complement pathway for IgM, but not IgA, MAbs.
TABLE 3.
IL-6 levels in 18-h cocultures of human PMNs, type 8 pneumococci, human complement sources, and human IgM and IgA MAbs to type 8 PS
| Conditiona | IL-6 (ng/ml)b
|
||||
|---|---|---|---|---|---|
| Expt 1
|
Expt 2
|
Expt 3
|
|||
| Unheated | Heated | Unheated | Unheated | Heated | |
| P/PMN/FB−/no MAb | 2.2 ± 0.4 | 1.3 ± 0.5 | 2.5 ± 2.1 | 2.6 ± 1.1 | 3.0 ± 1.5 |
| P/PMN/FB−/D11 | 5.0 ± 0.8 | 5.0 ± 0.9 | 6.1 ± 1.0 | 3.1 ± 1.1 | 2.8 ± 1.0 |
| P/PMN/FB−/IgM | 5.0 ± 0.6 | 4.5 ± 3.7 | 3.4 ± 1.1 | 2.4 ± 1.2 | 3.9 ± 1.4 |
| P/PMN/C2−no MAb | 1.8 ± 0.4 | 0.7 ± 0.5 | 1.6 ± 1.7 | 1.7 ± 0.7 | 1.3 ± 1.2 |
| P/PMN/C2−/D11 | 5.2 ± 0.8 | 3.7 ± 2.3 | 7.3 ± 1.8 | 1.9 ± 1.8 | 3.3 ± 1.3 |
| P/PMN/C2−/IgM | 4.0 ± 0.6 | 3.2 ± 2.8 | 7.9 ± 2.4 | 2.6 ± 1.4 | 3.1 ± 1.2 |
| P/PMN/HS/no MAb | 2.8 ± 0.4 | 2.2 ± 2.4 | 3.0 ± 1.4 | 2.0 ± 1.6 | 3.2 ± 2.5 |
| P/PMN/HS/D11 | 4.0 ± 1.8d | 7.5 ± 0.5c | 4.8 ± 1.8 | 3.2 ± 1.4 | 3.6 ± 1.6 |
| P/PMN/HS/IgM | 5.7 ± 2.3 | 3.8 ± 0.4 | 6.0 ± 1.2 | 3.2 ± 1.4 | 2.9 ± 1.4 |
| P/PMN/FB−/no MAb | 1.0 ± 0.9 | 1.1 ± 0.5 | 2.5 ± 2.1 | 2.6 ± 1.1 | 3.0 ± 1.5 |
| P/PMN/FB−/NAD | 4.0 ± 1.0 | 3.0 ± 0.5 | 3.3 ± 1.6 | 3.2 ± 1.6 | 3.5 ± 1.4 |
| P/PMN/FB−/IgA | 3.7 ± 0.6d | 2.2 ± 0.4 | 4.7 ± 2.3 | 3.1 ± 1.3 | 3.1 ± 1.5 |
| P/PMN/C2−/no MAb | 2.6 ± 0.5 | 0.3 ± 0.3 | 1.6 ± 1.7 | 1.7 ± 0.7 | 1.3 ± 1.2 |
| P/PMN/C2−/NAD | 5.7 ± 1.0d | 2.7 ± 0.5 | 5.8 ± 0.8 | 2.7 ± 1.7 | 3.4 ± 1.5 |
| P/PMN/C2−/IgA | 5.7 ± 0.9d | 2.0 ± 0.5 | 6.0 ± 1.7 | 2.2 ± 1.0 | 3.6 ± 1.9 |
| P/PMN/HS/no MAb | 3.5 ± 0.7 | 1.4 ± 0.9 | 3.0 ± 1.4 | 2.0 ± 1.6 | 3.2 ± 2.5 |
| P/PMN/HS/NAD | 5.0 ± 0.6c | 5.0 ± 1.0c | 5.5 ± 1.3 | 3.3 ± 1.3 | 2.8 ± 1.0 |
| P/PMN/HS/IgA | 6.2 ± 0.8d | 3.3 ± 0.5 | 7.1 ± 2.7 | 3.1 ± 1.4 | 2.0 ± 1.6 |
P, type 8 pneumococci; PMN, human PMNs; FB−, factor B-deficient serum; C2−, C2-deficient serum; HS, human serum.
IL-6 levels in secreted supernatants from cocultures performed with unheated and heat-inactivated complement sources; values represent mean ± standard deviation of duplicate samples for each condition.
P < 0.05 comparing type-specific MAb to isotype control MAbs, using one-way ANOVA and Bonferroni multiple comparison test.
P < 0.05 comparing conditions with heated and unheated complement sources, using one-way ANOVA and Bonferroni multiple comparison test.
Cell viability studies.
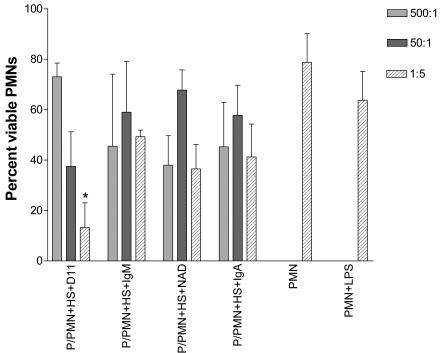
Cell viability was assessed with trypan blue staining after coculturing PMNs with pneumococci in HS with the MAbs at different E:T ratios. At an E:T ratio of 1:5, D11 was associated with a significantly lower proportion of viable PMNs than the control IgM (Fig. 6). At E:T ratios of 1:5 and 1:50 (data not shown), compared to when no MAb was present, the addition of either the specific or nonspecific MAbs (except IgM at 1:50) resulted in significantly less PMN viability (P < 1 × 10−2; Bonferroni multiple comparison test).
FIG. 6.
PMN viability after coculture with type 8 and type-specific and nonspecific MAbs. Results shown are the viability of PMNs incubated with type 8 pneumococci (P), D11, NAD, or the isotype controls (IgM or IgA) with HS as determined by trypan blue staining. *, P < 1 × 10−3, comparing D11 to IgM. All statistical comparisons were performed with a one-way ANOVA and the Bonferroni multiple comparison test.
DISCUSSION
The studies reported herein were undertaken to gain a better understanding of type-specific IgM and IgA efficacy against type 8 pneumococci. The results show that the type-specific MAbs to type 8 PS, NAD (42) and D11 (56), an IgA and IgM, respectively, each modulated PMN IL-8 secretion in vitro and were highly protective against i.t. challenge with type 8 pneumococci. Each MAb promoted statistically significant type 8 killing with human PMNs in only one out of three experiments, but the degree of killing was markedly less than that reported for type-specific IgG (35, 36, 50) or serum IgA to another serotype (type 14) (21). The minimal degree of D11-mediated opsonophagocytic killing was not due to an inability to deposit C3 on type 8 PS. Since D11 mediated C3 deposition on solid-phase PS 8 in FB− and C2− serum, Zhong et al. have previously shown that D11 binds type 8 pneumococci by electron microscopy (56). The different opsonophagocytic killing abilities observed for NAD and serum IgA to type 14 PS could reflect type-specific differences, the different conditions or complement sources used, or the increased ability of polyclonal, polymeric IgA to promote alternative complement pathway activation (21, 37). NAD consists of monomers and dimers (data not shown) and did not promote C3 deposition in this or a previous study that used another method (42). The results of the opsonophagocytosis experiments did not differ with the use of different conditions, some of which were similar to those used to study immune sera (45), and there was no evidence for a prozone-like phenomenon in which high amounts of antibody inhibit antibody function (16, 47, 49). These findings suggest that D11 and NAD are minimally opsonic. We could not assess whether opsonic human type-specific IgM or IgA MAbs to PS 8 can mediate similar effects, since such reagents are not available.
Zhong et al. have previously shown that D11 protected 86% of C4−/− mice against systemic type 8 infection (56). Herein, we show that all NAD-treated mice died after systemic infection, although survival was prolonged in NAD-treated mice compared to control mice. The different efficacies of the MAbs against systemic challenge may be due to their differences in isotype, specificity, and gene usage, or a combination of these factors. NAD was isolated from a child 1 year after pneumococcal PS vaccination, a time when vaccine-elicited antibodies would be unlikely in the circulation (19) and uses VH3-23, the predominant VH gene segment in the circulating human antibody repertoire (46). VH3-23 is used by naturally occurring antibodies that share antigenic mimicry with microbial polysaccharides (26), antibodies to 23F PS (58), and Haemophilus influenzae type b capsular PS (4).
In contrast to their different efficacies against systemic challenge, D11 and NAD were equally and highly protective against i.t. challenge in C4−/− mice. Hence, our data show that the classical complement pathway is dispensable for D11- and NAD-mediated protection against i.t. challenge with type 8 pneumococci. Since the classical complement pathway was required for protection against pneumococci by naturally occurring IgM (7), our results suggest that type-specific antibodies with certain specificities may have a superior capacity to activate the alternative complement pathway. Chang et al. have previously shown that type-specific IgM MAbs to type 3 PS are highly protective against type 3 in mice with only an intact alternative complement pathway (C4−/− mice), but only certain MAbs were protective against systemic challenge in mice with alternative complement pathway deficiency (12). Since NAD and D11 have different type 8 PS specificities and different efficacies in C4−/− mice, the systemic model in C4−/− mice may discriminate antibodies with different PS specificities, with the caveat that isotype can also influence antibody specificity for capsular PS antigens (27).
Despite involving different infection models and serotypes, our observations suggest specificity is a key determinant of type-specific IgM and IgA efficacy against pneumococci. Specificity for defined haptens determined IgM efficacy in experimental intraabdominal sepsis (6), and antibody-dependent activation of the alternative complement pathway is mediated by the portion of the antibody that confers antigen specificity, the F(ab′)2 (5, 54). Since NAD was as protective as D11 in i.t. infection, type 8 PS specificity, or binding to a given type 8 determinant, might have different biological consequences in the lung. For example, the milieu of the lung might provide mediators and/or opsonins that enhance IgA-mediated antipneumococcal activity (21, 51).
D11 and NAD both reduced PMN IL-8 secretion compared to their respective controls. Different serotypes of live and killed pneumococci have been shown to stimulate IL-8 release from human cells in vitro to varying degrees (1, 2, 17, 25). To our knowledge, it has not been reported previously that type 8 pneumococci can trigger IL-8 release or that IL-8 release can be regulated by type-specific antibodies to pneumococci. However, there is precedent for antibody-mediated downregulation of cellular IL-8 secretion by MAbs to a herpes simplex virus glycoprotein (44) and endotoxin (38). Downregulation of IL-8 has been implicated as a mechanism to dampen the inflammatory response to pneumococci (25), and a MAb to IL-8 (30) was shown to reduce the inflammatory response to experimental pneumococcal meningitis. These observations suggest that further investigation of whether downregulation of IL-8 contributes to IgM-mediated protection against type 8 pneumococci is warranted. Interestingly, certain macrolide antibiotics also reduce secretion of IL-8 by human mononuclear cells and PMNs exposed to pneumococci in vitro (31, 41), but the clinical and biological significance of these observations for protection against human disease requires further investigation.
Our results show that the effects of D11 and NAD on type 8 PMN IL-8 release were complement dependent in the presence of either the alternative or the classical complement pathway and were also a function of the E:T ratio. Compared to control MAbs, both type-specific MAbs downregulated type 8-induced PMN IL-8 release at an E:T ratio of 1:5 but increased IL-8 secretion at ratios of 500:1 and 50:1. Therefore, rather than being a fixed microbial characteristic, type 8-mediated IL-8 induction was dynamic in the presence of the type-specific MAbs, displaying a prozone-like phenomenon that was maximal when PMNs dominated. Notably, 500:1 is close to the ratio that is used to assess type-specific antibody-mediated opsonophagocytosis. Since we were not able to study a highly opsonic MAb as a control, we do not know if such an antibody would modulate IL-8 secretion in a similar manner. However, it is interesting to speculate that increased IL-8 levels might trigger cellular mechanisms that lead to microbial clearance (1).
IL-6 secretion was increased by both the type-specific and nonspecific MAbs compared to conditions without a MAb. Therefore, the effects of the MAbs on IL-8 secretion were limited to the type-specific MAbs, whereas their effects on IL-6 were nonspecific. IL-6 can be elevated in sepsis (18), but it has also been shown to have an antiinflammatory effect in vivo (55), which is underscored by the association between murine IL-6 deficiency and an increased inflammatory response to pneumococcal pneumonia (52). At present, the biological significance of our findings is uncertain, as they require validation in vivo. Nonetheless, our data show for the first time that type-specific IgM and IgA to PS 8 modulate PMN IL-8 secretion in a specific manner. The mechanism by which type-specific MAbs might modulate IL-8 secretion remains to be determined. One possible mechanism involves antibody-mediated interference with the function of the C3 binding protein on pneumococci, which is known to induce IL-8 release from epithelial cells (25). Such an effect could arise through the induction of morphological changes in capsular structure upon antibody binding to a specific determinant (48).
We found that PMN viability was reduced after coculture with type 8 pneumococci and D11, but not NAD. This phenomenon was observed at an E:T ratio that was also associated with decreased IL-8 release, 1:5. Notably, cell viability was not decreased at higher E:T ratios, which are the ratios generally used to establish type-specific opsonophagocytosis, e.g., 500:1 (21, 29). Killed pneumococci can induce PMN apoptosis, and apoptosis has been shown to result in decreased secretion of proinflammatory cytokines, including IL-8 (59). At present, we do not know if the decreased PMN viability we observed is due to apoptosis and/or another mechanism of cell death. Irrespective of the mechanism, the decrease in PMN viability was most likely antibody mediated, because cell viability did not vary at any E:T ratio without antibody. The biological relevance of this finding requires further investigation, since the data were obtained under defined in vitro conditions and both specific and nonspecific MAbs reduced cell viability.
Our data suggest a novel mechanism of action for type-specific IgM and IgA to type 8 PS, namely, modulation of the PMN IL-8 response to pneumococci. The features that characterize such “modulating” antibodies and their biological significance in vivo require further investigation. Modulating antibodies could work with complement and/or other components of innate immunity to mediate resistance in the naïve host (7), or they could reduce the inflammatory effect of microbial products and FcR-mediated activation in the immune host (10, 11, 14, 32). Investigation of the questions raised by our findings may lead to a better understanding of the mechanisms of IgM and IgA efficacy against pneumococci.
Acknowledgments
This work was supported by grants from the National Institutes of Health (5F31GM20775 to T.B. and RO1-AI35370, RO1-AI45459, and RO1-AI44374 to L.P.).
We thank Ulrike Buchwald for critical review of the manuscript.
Editor: T. R. Kozel
REFERENCES
- 1.Arva, E., and B. Andersson. 1999. Induction of phagocyte-stimulating and Th1-promoting cytokines by in vitro stimulation of human peripheral blood mononuclear cells with Streptococcus pneumoniae. Scand. J. Immunol. 49:417-423. [DOI] [PubMed] [Google Scholar]
- 2.Arva, E., and B. Andersson. 1999. Kinetics of cytokine release and expression of lymphocyte cell-surface activation markers after in vitro stimulation of human peripheral blood mononuclear cells with Streptococcus pneumoniae. Scand. J. Immunol. 49:237-243. [DOI] [PubMed] [Google Scholar]
- 3.Basha, G., P. Yap, and F. Penninckx. 1996. Comparative study of classical, colorimetric and immunologic staining methods for the assessment of tumor cell viability. Tumour Biol. 17:354-361. [DOI] [PubMed] [Google Scholar]
- 4.Beck, J. G., K. H. Low, M. Burnett, L. Xu, S. Suleyman, K. M. Thompson, L. Sullivan, J. B. Natvig, and G. V. Pinchuk. 2000. Analysis of “natural” and vaccine-induced Haemophilis influenzae type B capsular polysaccharide serum antibodies for 3H1, a V3-23-associated idiotope. Immunol. Lett. 72:171-177. [DOI] [PubMed] [Google Scholar]
- 5.Bjornson, A. B., and P. A. Detmers. 1995. The pentameric structure of IgM is necessary to enhance opsonization of Bacteroides thetaiotaomicron and Bacteroides fragilis via the alternative complement pathway. Microb. Pathog. 29:117-128. [DOI] [PubMed] [Google Scholar]
- 6.Boes, M., A. P. Prodeus, T. Schmidt, M. C. Carroll, and J. Chen. 1999. A critical role of natural immunoglobulin M in immediate defense against systemic bacterial infection. J. Exp. Med. 188:2381-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown, J. S., T. Hussell, S. M. Gilliland, D. W. Holden, J. C. Paton, M. R. Ehrenstein, M. J. Walport, and M. Botto. 2002. The classical pathway is the dominant complement pathway required for innate immunity to Streptococcus pneumoniae infection in mice. Proc. Natl. Acad. Sci. USA 99:16969-16974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruunsgaard, H., P. Skinhoj, J. Qvist, and F. K. Pedersen. 1999. Elderly humans show prolonged in vivo inflammatory activity during pneumococcal infections. J. Infect. Dis. 180:551-554. [DOI] [PubMed] [Google Scholar]
- 9.Buchwald, U. K., and L. Pirofski. 2003. Immune therapy for infectious diseases at the dawn of the 21st century: the past, present and future of antibody therapy, therapeutic vaccination and biological response modifiers. Curr. Pharm. Des. 9:945-968. [DOI] [PubMed] [Google Scholar]
- 10.Casadevall, A., and L. A. Pirofski. 2003. Antibody mediated regulation of cellular immunity and the inflammatory response. Trends Immunol. 24:474-478. [DOI] [PubMed] [Google Scholar]
- 11.Casadevall, A., and L. A. Pirofski. 2003. Exploiting the redundancy in the immune system: vaccines can mediate protection by eliciting “unnatural” immunity. J. Exp. Med. 197:1401-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang, Q., Z. Zhong, A. Lees, M. Pekna, and L. Pirofski. 2002. Structure-function relationships for human antibodies to pneumococcal capsular polysaccharide from transgenic mice with human immunoglobulin loci. Infect. Immun. 70:4977-4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dailey, D. C., A. Kaiser, and R. H. Schloemer. 1987. Factors influencing the phagocytosis of Clostridium difficile by human polymorphonuclear leukocytes. Infect. Immun. 55:1541-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deo, Y. M., R. F. Graziano, R. Repp, and J. G. J. van de Winkel. 1997. Clinical significance of IgG Fc receptors and FcγR-directed immunotherapies. Immunol. Today 18:127-135. [DOI] [PubMed] [Google Scholar]
- 15.Feldmesser, M., and A. Casadevall. 1997. Effect of serum IgG1 to Cryptococcus neoformans glucuronoxylomannan on murine pulmonary infection. J. Immunol. 158:790-799. [PubMed] [Google Scholar]
- 16.Goodner, K., and F. L. Horsfall. 1935. The protective action of type I antipneumococcus serum in mice. I. Quantitative aspects of the mouse protection test. J. Exp. Med. 62:359-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hachicha, M., P. Rathanaswami, P. H. Naccache, and S. R. McColl. 1998. Regulation of chemokine gene expression in human peripheral blood neutrophils phagocytosing microbial pathogens. J. Immunol. 160:449-454. [PubMed] [Google Scholar]
- 18.Hack, C. E., L. A. Aarden, and L. G. Thijs. 1997. Role of cytokines in sepsis. Adv. Immunol. 66:101-195. [DOI] [PubMed] [Google Scholar]
- 19.Heilmann, C., and F. K. Pedersen. 1986. Quantitation of blood lymphocytes secreting antibodies to pneumococcal polysaccharides after in vivo antigenic stimulation. Scand. J. Immunol. 23:189-194. [DOI] [PubMed] [Google Scholar]
- 20.Hill, W. C., and J. B. Robbins. 1966. Horse anti-pneumococcal immunoglobulin. II. Specific mouse protective activity. Proc. Soc. Exp. Biol. Med. 123:105-108. [DOI] [PubMed] [Google Scholar]
- 21.Janoff, E. N., C. Fasching, J. M. Orenstein, J. B. Rubins, N. L. Opstad, and A. P. Dalmasso. 1999. Killing of Streptococcus pneumoniae by capsular polysaccharide-specific polymeric IgA, complement, and phagocytes. J. Clin. Investig. 104:1139-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jarvis, G. A., and J. M. Griffiss. 1991. Human IgA1 blockade of IgG-initiated lysis of Neisseria meningitidis is a function of Fab binding to the polysaccharide capsule. J. Immunol. 147:1962-1967. [PubMed] [Google Scholar]
- 23.Jones, J. N. K., and M. B. Perry. 1957. The structure of the type VIII pneumococcus specific polysaccharide. J. Am. Chem. Soc. 79:2787-2793. [Google Scholar]
- 24.Leibovitz, E., R. Dagan, J. H. Laver, L. Piglansky, S. Raiz, M. R. Abboud, D. M. Fliss, A. Leiberman, and A. Barzilai. 2000. Interleukin 8 in middle ear fluid during acute otitis media: correlation with etiology and bacterial eradication. Arch. Dis. Child. 82:165-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madsen, M., Y. Lebenthal, Q. Cheng, B. L. Smith, and M. K. Hostetter. 2000. A pneumococcal protein that elicits interleukin-8 from pulmonary epithelial cells. J. Infect. Dis. 181:1330-1336. [DOI] [PubMed] [Google Scholar]
- 26.Mageed, R. A., I. J. Harmer, S. L. Wynn, S. P. Moyes, B. R. Maziak, and M. Bruggemann. 2001. Rearrangement of the human heavy chain variable region gene V3-23 in transgenic mice generates antibodies reactive with a range of antigens on the basis of VHCDR3 and residues intrinsic to the heavy chain variable region. Clin. Exp. Immunol. 123:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLean, G. R., M. Torres, N. Elguezabal, A. Nakouzi, and A. Casadevall. 2002. Isotype can affect the fine specificity of an antibody for a polysaccharide antigen. J. Immunol. 169:1379-1386. [DOI] [PubMed] [Google Scholar]
- 28.Musher, D. M. 1992. Infections caused by Streptococcus pneumoniae: clinical spectrum, pathogenesis, immunity, and treatment. Clin. Infect. Dis. 14:801-809. [DOI] [PubMed] [Google Scholar]
- 29.Nahm, M. H., J. V. Olander, and M. Magyarlaki. 1997. Identification of cross-reactive antibodies with low opsonophagocytic activity for Streptococcus pneumoniae. J. Infect. Dis. 176:698-703. [DOI] [PubMed] [Google Scholar]
- 30.Ostergaard, C., R. V. Yieng-Kow, T. Benfield, N. Frimodt-Moller, F. Espersen, and J. D. Lundgren. 2000. Inhibition of leukocyte entry into the brain by selectin blocker fucoidin decreases interleukin-1 (IL-1) levels but increases IL-8 levels in cerebrospinal fluid during experimental pneumococcal meningitis in rabbits. Infect. Immun. 68:3153-3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Purswani, M., S. Eckert, H. Arora, R. Johann-Liang, and G. J. Noel. 2000. The effect of three broad-spectrum antimicrobials on mononuclear cell responses to encapsulated bacteria: evidence for downregulation of cytokine mRNA transcription by trovafloxacin. J. Antimicrob. Chemother. 46:921-929. [DOI] [PubMed] [Google Scholar]
- 32.Ravetch, J. V., and S. Bolland. 2001. IgG Fc receptors. Annu. Rev. Immunol. 19:275-290. [DOI] [PubMed] [Google Scholar]
- 33.Retini, C., A. Vecchiarelli, C. Monari, C. Tascini, F. Bistoni, and T. R. Kozel. 1996. Capsular polysaccharide of Cryptococcus neoformans induces proinflammatory cytokine release by human neutrophils. Infect. Immun. 64:2897-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robbins, J. B., R. Schneerson, and S. C. Szu. 1995. Perspective. Hypothesis: serum IgG antibody is sufficient to confer protection against infectious diseases by inactivating the inoculum. J. Infect. Dis. 171:1387-1398. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez, M. E., W. L. van der Pol, L. A. M. Sanders, and J. G. J. van de Winkel. 1999. Crucial role of FcγRIIa (CD32) in assessment of functional anti-Streptococcus pneumoniae antibody activity in human sera. J. Infect. Dis. 179:423-433. [DOI] [PubMed] [Google Scholar]
- 36.Romero-Steiner, S., D. Libutti, L. B. Pais, et al. 1997. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin. Diagn. Lab. Immunol. 4:415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roos, A., L. H. Bouwman, D. J. van Gijlswijk-Janssen, M. C. Farber-Krol, G. L. Stahl, and M. R. Daha. 2001. Human IgA activates the complement system via the mannan-binding lectin pathway. J. Immunol. 167:2861-2868. [DOI] [PubMed] [Google Scholar]
- 38.Rothenburger, M., R. Soeparwata, M. C. Deng, C. Schmid, E. Berendes, T. D. Tjan, M. J. Wilhelm, M. Erren, D. Bocker, and H. H. Scheld. 2001. Prediction of clinical outcome after cardiac surgery: the role of cytokines, endotoxin, and anti-endotoxin core antibodies. Shock 16:44-50. [DOI] [PubMed] [Google Scholar]
- 39.Russell, M. W., D. A. Sibley, E. B. Nikolova, M. Tomana, and J. Mestecky. 1996. IgA antibody as a non-inflammatory regulator of immunity. Biochem. Soc. Trans. 25:466-470. [DOI] [PubMed] [Google Scholar]
- 40.Russell, N., J. R. Corvalan, M. L. Gallo, C. G. Davis, and L. Pirofski. 2000. Production of protective human anti-pneumococcal antibodies by transgenic mice with human immunoglobulin loci. Infect. Immun. 68:1820-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schultz, M. J., P. Speelman, S. Zaat, S. J. H. van Deventer, and T. van der Poll. 1998. Erythromycin inhibits tumor necrosis factor alpha and interleukin 6 production induced by heat-killed Streptococcus pneumoniae in whole blood. Antimicrob. Agents Chemother. 42:1605-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steinitz, M., S. Tamir, M. Ferne, and A. Goldfarb. 1986. A protective human monoclonal IgA antibody produced in vitro: anti-pneumococcal antibody engendered by Epstein-Barr virus-immortalized cell line. Eur. J. Immunol. 16:187-193. [DOI] [PubMed] [Google Scholar]
- 43.Steinitz, M., S. Tamir, and A. Goldfarb. 1984. Human anti-pneumococci antibody produced by an Epstein Barr virus (EBV)-immortalized cell line. J. Immunol. 132:877-882. [PubMed] [Google Scholar]
- 44.Su, Y.-H., X.-T. Yan, J. E. Oakes, and R. N. Lausch. 1996. Protective antibody therapy is associated with reduced chemokine transcripts in herpes simplex virus type 1 corneal infection. J. Virol. 70:1277-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun, Y., Y. I. Hwang, and M. Nahm. 2001. Avidity, potency, cross-reactivity of monoclonal antibodies to pneumococcal capsular polysaccharide serotype 6B. Infect. Immun. 69:336-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suzuki, I., L. Pfister, A. Glas, C. Nottenburg, and E. C. B. Milner. 1995. Representation of rearranged VH gene segments in the human adult antibody repertoire. J. Immunol. 154:3902-3911. [PubMed] [Google Scholar]
- 47.Taborda, C., and A. Casadevall. 2001. Immunoglobulin M efficacy against Cryptococcus neoformans: mechanism, dose dependence, and prozone-like effects in passive protection experiments. J. Immunol. 166:2100-2107. [DOI] [PubMed] [Google Scholar]
- 48.Taborda, C. P., and A. Casadevall. 2002. CR3 (CD11b/CD18) and CR4 (CD11c/CD18) are involved in complement-independent antibody-mediated phagocytosis of Cryptococcus neoformans. Immunity 16:791-802. [DOI] [PubMed] [Google Scholar]
- 49.Taborda, C. P., J. Rivera, O. Zaragoza, and A. Casadevall. 2003. More is not necessarily better: prozone-like effects in passive immunization with IgG. J. Immunol. 170:3621-3630. [DOI] [PubMed] [Google Scholar]
- 50.Usinger, W. R., and A. H. Lucas. 1999. Avidity as a determinant of the protective efficacy of human antibodies to pneumococcal capsular polysaccharides. Infect. Immun. 67:2366-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van der Pol, W., G. Vidarsson, H. A. Vile, J. G. Van de Winkel, and M. E. Rodriguez. 2000. Pneumococcal capsular polysaccharide-specific IgA triggers efficient neutrophil effector functions via Fc alpha RI (CD89). J. Infect. Dis. 182:1139-1145. [DOI] [PubMed] [Google Scholar]
- 52.van der Poll, T., C. V. Keogh, X. Guirao, W. A. Buurman, M. Kopf, and S. F. Lowry. 1997. Interleukin-6 gene-deficient mice show impaired defense against pneumococcal pneumonia. J. Infect. Dis. 176:439-444. [DOI] [PubMed] [Google Scholar]
- 53.Wang, E., M. Simard, N. Ouellet, Y. Bergeron, D. Beauchamp, and M. G. Bergeron. 2000. Modulation of cytokines and chemokines, limited pulmonary vascular bed permeability, and prevention of septicemia and death with ceftriaxone and interleukin-10 in pneumococcal pneumonia. J. Infect. Dis. 182:1255-1259. [DOI] [PubMed] [Google Scholar]
- 54.Winkelstein, J. A., and H. S. Shin. 1974. The role of immunoglobulin in the interaction of pneumococci and the properdin pathway: evidence for its specificity and lack of requirement for the Fc portion of the molecule. J. Immunol. 112:1635-1642. [PubMed] [Google Scholar]
- 55.Xing, Z., J. Gauldie, H. Baumann, M. Jordana, X. F. Lei, and M. K. Achong. 1998. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J. Clin. Investig. 101:311-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhong, Z., T. Burns, Q. Chang, M. Carroll, and L. Pirofski. 1999. Molecular and functional characteristics of a protective human monoclonal antibody to serotype 8 Streptococcus pneumoniae capsular polysaccharide. Infect. Immun. 67:4119-4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhong, Z., and L. Pirofski. 1998. Antifungal activity of a human antiglucuronoxylomannan antibody. Clin. Diagn. Lab. Immunol. 5:58-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou, J., K. R. Lottenbach, S. J. Barenkamp, A. H. Lucas, and D. C. Reason. 2002. Recurrent variable region gene usage and somatic mutation in the human antibody response to the capsular polysaccharide of Streptococcus pneumoniae type 23F. Infect. Immun. 70:4083-4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zysk, G., L. Bejo, B. K. Schneider-Wald, R. Nau, and H. P. Heinz. 2000. Induction of necrosis and apoptosis of neutrophil granulocytes by Streptococcus pneumoniae. Clin. Exp. Immunol. 122:61-66. [DOI] [PMC free article] [PubMed] [Google Scholar]