Abstract
Single, double, and triple mutants of an enterobactin-deficient mutant strain of Salmonella enterica serovar Typhimurium were constructed that were defective in the expression of the iron-regulated outer membrane proteins (IROMPs) FepA, IroN, and Cir, which are proposed to function as catecholate receptors. Uptake of naturally occurring and chemically synthesized catecholate molecules by these mutants was assessed in standard growth promotion assays. Unique patterns of uptake were identified for each IROMP; specifically, FepA and IroN were confirmed to be required for transport of enterobactin, and all three proteins were shown to function as receptors for the enterobactin breakdown product 2,3-dihydroxybenzoylserine. The fepA, iroN, and cir alleles were transduced to enterobactin-proficient strains of S. enterica serovar Typhimurium and S. enterica serovar Enteritidis, and the resulting phenotypes were confirmed by analysis of outer membrane protein profiles, by sensitivity to KP-736, a catecholate-cephalosporin conjugate, and by growth promotion tests on egg white agar. Intragastric infections of mice with the S. enterica serovar Typhimurium strains indicated that the parental strain and the fepA iroN double mutant were similarly virulent but that the fepA iroN cir triple mutant was significantly attenuated. Moreover, in mixed infections, the fepA iroN mutant showed similar cecal colonization and invasion of the liver to the parental strain, while the triple mutant showed significantly reduced cecal colonization and no measurable spread to the liver. Infections of 4-day-old chicks with S. enterica serovar Enteritidis strains also indicated that mutation of the fepA iroN genes did not significantly reduce cecal colonization and systemic spread compared with those of the parental strain. The results indicate that, while enterobactin uptake is not essential for the virulence of S. enterica serovars in mouse and chicken infection models, the ability to take up 2,3-dihydroxybenzoylserine via any of the three catecholate siderophore receptors appears to play an important role, since the S. enterica serovar Typhimurium triple mutant was significantly attenuated in the mouse model. Salmochelins appear not to be involved in the virulence of S. enterica.
The transport and recycling of iron in vertebrates are achieved by the iron-binding glycoproteins transferrin and lactoferrin and their cognate receptors. In humans, transferrin-bound iron accounts for approximately 0.7% of total iron. However, serum transferrin is normally only about 30% iron saturated, and so, since transferrin has a very high affinity for ferric ions (ca. 1 × 102 to 6 × 1022 M−1), the levels of iron in low-molecular-mass complexes in equilibrium with transferrin-bound iron are extremely low. Moreover, in response to the presence of invading microorganisms, free iron levels in blood and tissue fluids of a host organism are reduced still further in a set of reactions collectively known as the hypoferremic response. Transferrin-bound iron is not readily available for bacterial use, but the growth of serum-exposed bacteria can be facilitated by supplementation with excess iron or with iron-binding compounds, such as siderophores (36).
Under iron-limited conditions, Salmonella enterica expresses a number of siderophore systems that may be involved in acquiring iron from transferrin. In common with many other species of the family Enterobacteriaceae, S. enterica uses the catecholate siderophore enterobactin and its stable breakdown products, the linear trimeric, dimeric, and monomeric forms of 2,3-dihydroxybenzoylserine (DHBS3, DHBS2, and DHBS1, respectively) (50). Two other DHBS derivatives, salmochelins 1 and 2 (having two and three DHBS moieties, respectively, bridged by glucose residues), have recently been reported (16). In addition, some Salmonella strains of subspecies III and VI possess a high pathogenicity island that encodes the phenolate siderophore yersiniabactin and its uptake system (30). Some strains also make the hydroxamate siderophore aerobactin (8, 26, 34), and another hydroxamate-type siderophore has been detected but not further characterized up to now (33). In addition, S. enterica practices siderophore piracy. For example, the fungal siderophore ferrichrome can provide iron for growth in iron-limited environments (23, 24), as can the ferrioxamines, hydroxamate siderophores produced by various bacterial species. Indeed, ferrioxamine E produced by Pantoea agglomerans (5) and Hafnia alvei (40) is taken up by virtually all clinical serovars of S. enterica subspecies I, II, and IIIa via the FoxA receptor protein and is a highly effective semiselective supplement for the diagnosis of contamination of foods (21, 41). A number of other hydroxamate siderophores (e.g., schizokinen and rhodotorulic acid) and natural and synthetic catecholate siderophores (e.g., serratiochelin and the myxochelins) can also be used by S. enterica to supply iron (2, 38, 39; R. Reissbrodt and W. Rabsch, unpublished data). In addition, the primary metabolites α-keto acids and α-hydroxy acids may act as surrogate siderophores for Salmonella (20, 37).
In addition to hydroxamate siderophore receptor proteins, S. enterica serovar Typhimurium expresses three outer membrane proteins of approximately 83, 78, and 74 kDa under conditions of iron starvation (10, 12). The largest of these so-called iron-regulated outer membrane proteins (IROMPs), FepA, was identified over 30 years ago as a receptor for ferri-enterobactin (28, 30). Much more recently, the 78-kDa IROMP, designated IroN, was shown to be an alternative ferri-enterobactin receptor (3, 35). The iroN gene is present in all phylogenetic linkages of S. enterica (at 57 centisomes [Cs] on the S. enterica serovar Typhimurium chromosome and at 4 Cs in serovar Typhi [3]),but not in Salmonella bongori. The iroN gene was also detected in uropathogenic Escherichia coli strains (16, 19). The nature of the third major IROMP is not yet clear. However, an S. enterica serovar Typhimurium MudJ mutant, designated AR8412, has been reported (47) in which the insertion mutation maps to 46 Cs, the same location as the cir gene in the E. coli chromosome. The cir gene product of E. coli is known to be the receptor for colicin I, but since S. enterica serovars are not sensitive to colicin I, the existence of a Salmonella Cir-like protein has not been confirmed. It is interesting that IROMPs may also function as receptors for siderophore-antibiotic conjugates (6, 9).
Here we report the use of previously described fepA and iroN mutations (35) to construct double and triple mutants with the putative cir::MudJ allele in an enterobactin-deficient strain of S. enterica serovar Typhimurium. We have characterized the ability of these mutants to acquire iron from a range of natural and synthetic catecholate compounds in order to construct an uptake profile for each of the three receptor proteins. In addition, we have transferred the mutations to enterobactin-proficient mouse- and chicken-virulent strains of S. enterica as backgrounds for in vivo virulence assays.
MATERIALS AND METHODS
Bacteria, growth media, and antibiotic sensitivity.
The S. enterica strains used in this study are listed in Table 1. Bacteria were routinely cultured aerobically at 37°C in Luria-Bertani (LB) broth or on LB agar plates with antibiotics added as required at the following concentrations: kanamycin, 50 mg/liter; chloramphenicol, 30 mg/liter; ampicillin, 100 mg/liter; tetracycline, 20 mg/liter. For Bioscreen C analysis of growth, bacteria were cultured in a nutrient-rich medium containing the following: nutrient broth, 8.0 g/liter; tryptone, 3.0 g/liter; yeast extract, 2.0 g/liter; NaCl, 5.0 g/liter; bovine serum, 5 ml; FeCl3 · 6H2O, 40 mg (pH 7.2 ± 0.1) (all nutrients from BD, Heidelberg, Germany). To measure KP-736 susceptibility, cultures grown overnight in LB were diluted in saline to approximately 105 CFU/ml, dispensed into wells of a microtiter plate containing serial dilutions of KP-736 (0.125 to 8.0 μg/ml), and incubated at 37°C for 18 h. MICs were read as the lowest concentration of antibiotic that prevented visible bacterial growth. E. coli NCTC10418 was used as a control strain for these assays.
TABLE 1.
Strains of S. enterica used in this study
| Serovar and strain | Characteristic(s) | Source or reference |
|---|---|---|
| Serovar Typhimurium | ||
| ATCC 14028 | Wild type | American Type Culture Collection |
| AJB64 | ATCC 14028 NalriroN::pGP704 aroA::Tn10 | 3 |
| AR8412 | ATCC 14028 cir::MudJ | 47 |
| AIR49 | ATCC 14028 iroBC::kan aroA::Tn10 | 16 |
| R18 | galE854 lky-1 fur-1 rspL1266 | 12 |
| TA2700 | LT2 ent fhuC | J. B. Neilands (32) |
| TT10423 | LT7 proAB47/F′ 128 pro+lac+zzf-1831::Tn10dTc | 11 |
| WR1169 | As TA2700 but cir::MudJ | This study |
| WR1223 | As TA2700 but iroN::pGP704 | This study |
| WR1244 | As TA2700 but iroN::pGP704 cir::MudJ | This study |
| WR1315 | As TA2700 but fepA::Tn10dTc ent+ | This study |
| WR1316 | As TA2700 but fepA::Tn10dTc | This study |
| WR1330 | As TA2700 but fepA::Tn10dTc iroN::pGP704 cir::MudJ | This study |
| WR1332 | As TA2700 but fepA::Tn10dTc iroN::pGP704 | This study |
| WR1334 | As TA2700 but fepA::Tn10dTc cir::MudJ | This study |
| WR1726 | As ATCC 14028 but fepA::Tn10dTc | This study |
| WR1727 | As ATCC 14028 but fepA::Tn10dTc iroN::pGP704 | This study |
| WR1728 | As ATCC 14028 but fepA::Tn10dTc iroN::pGP704 cir::MudJ | This study |
| WR1729 | As ATCC 14028 but fepA::Tn10dTc iroN::pGP704 iroBC::kan | This study |
| Serovar Enteritidis | ||
| SE147 | Wild type, phage type 4, virulent to chicken | 26 |
| SE147Nalr | As SE147 but nalidixic acid resistant | 26 |
| WR1425 | As SE147Nalr but fepA::Tn10dTc | This study |
| WR1434 | As SE147Nalr but fepA::Tn10dTc iroN::pGP704 | This study |
| WR1529 | As SE147Nalr but tonB::MudJ | This study |
| WR1530 | As SE147Nalr but cir::MudJ | This study |
Analysis of IROMPs.
Strains to be tested were freshly cultivated on tryptic soy agar (BD) containing 200 to 400 μM 2,2′bipyridyl (depending on the mutant) and appropriate antibiotics. Outer membrane proteins were isolated and analyzed by polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate (SDS-PAGE) as previously described (25). Gels were stained with Coomassie blue (25) or with alkaline silver nitrate solution (45).
Analysis of LPS profiles.
Bacteria were grown on tryptic soy agar at 37°C for 20 h, and lipopolysaccharides (LPS) were isolated as previously described (1). Electrophoresis was performed by the method of Lugtenberg et al. (25), and gels were stained with alkaline silver nitrate solution (45).
Isolation of Tn10dTc insertions linked to the ent operon.
Tn10dTc insertion mutants were isolated as described previously (11, 31). Transduction was performed with the high-frequency generalized transducing phage mutant P22HT105/1 int201. To isolate a fepA mutant of S. enterica serovar Typhimurium, phage stocks were prepared on large pools of random Tn10dTc insertion mutants of the ent+ strain TT10423 (11) and used to transduce the ent recipient strain TA2700 (24), screening for siderophore-overproducing transductants on chromazurol S (CAS) agar (42) containing tetracycline (CAS-TET). Of about 130 transductants tested, one carrying the allele zbd-129::Tn10dTc produced a larger than normal indicator zone on CAS-TET plates, and when subsequently purified, showed greater growth stimulation of strain TA2700 in a standard growth promotion assay than wild-type (ent+) strains, suggesting a mutation in a siderophore receptor gene (42). SDS-PAGE of outer membrane preparations of this strain showed loss of the 83-kDa FepA protein. A new phage lysate was prepared on the strain carrying zbd-129::Tn10dTc and used to transduce the transposon insertion into strain TA2700, with screening on CAS-TET agar to identify transductants with the genotypes ent+ (designated WR1315) and ent (designated WR1316).
Growth promotion tests.
The ability of natural and chemically synthesized catecholate siderophores to promote the growth of enterobactin-deficient mutants of S. enterica serovar Typhimurium was determined on standard low-iron siderophore assay plates (Vogel-Bonner medium supplemented with 200 μM 2,2′-bipyridyl) as described previously (37). For derivatives of enterobactin-proficient strains of S. enterica serovars Typhimurium and Enteritidis, growth promotion was checked on egg white nutrient medium (20). Double-strength nutrient agar containing 100 μM 2,2′-bipyridyl was mixed with 45% (vol/vol) fresh hen's egg white, and the mutants to be tested were seeded into this mixture. The presence of ovotransferrin and other proteins in the egg white prevents enterobactin-mediated overgrowth of the test strains, thus allowing the assessment of growth-stimulatory properties of reagents added on filter paper disks.
Growth characterization of S. enterica mutants in Bioscreen C.
Strains to be tested were freshly cultivated, using appropriate antibiotics for selection, in nutrient-rich medium supplemented with bovine serum and excess iron. Ten microliters of a dilution containing 105 CFU of each strain per ml was inoculated into 290-μl aliquots of the same culture medium in 20 replicate wells of a Bioscreen C apparatus (Labsystems, Helsinki, Finland). Growth at 37°C was monitored for 22 h.
Bacterial growth in chicken serum.
Bacterial strains were freshly cultivated on nutrient agar with or without appropriate antibiotics; bacterial growth was suspended in saline, and viable counts were determined by plating serial dilutions onto nutrient agar. Twenty microliters of each dilution was inoculated into 1 ml of pooled sterile chicken serum as previously described (4) and incubated without agitation at 37°C. Samples (0.1 ml) of each culture were plated directly, after 1:100 dilution onto nutrient agar after incubation for 4, 6, and 9 h, and after standing overnight at 37°C.
Experimental infections.
Female BALB/c mice that were 6 to 8 weeks old and housed in specific-pathogen-free conditions were infected with S. enterica serovar Typhimurium strain ATCC 14028 and its derivatives. Bacteria grown overnight in LB without shaking at 37°C were harvested by centrifugation and resuspended in sterile phosphate-buffered saline. Groups of two mice were inoculated intragastrically with approximately 1:1 or 1:100 mixtures of strains at total inocula of 104 to 106 CFU. Samples of cecum and liver were aseptically removed from mice that had died or from mice sacrificed 7 days after infection. Homogenized organ samples were diluted in phosphate-buffered saline, plated on deoxycholate-citrate agar (SIFIN, Berlin, Germany), and incubated at 37°C overnight; 250 colonies were subcultured onto LB agar plates containing appropriate antibiotics to determine the proportions of mutant bacteria in the recovered populations.
Specific-pathogen-free White Leghorn chicks were hatched at the facilities of the Bundesforschungsanstalt für Viruskrankheiten der Tiere, Jena, Germany, from eggs obtained from Charles River Deutschland GmbH, Extertal, Germany. Experimental groups were kept in separate rooms. Commercial feed (powder form without antibiotics or other additives) and drinking water were both available ad libitum. Virulence of derivatives of S. enterica serovar Enteritidis strain SE147Nalr was tested in highly susceptible 4-day-old chicks; strains were administered orally by crop gavage to groups of four birds at a dose of 1 × 103 to 2 × 103 CFU/bird in a volume of 0.1 ml. Doses were estimated by measuring extinction at 600 nm against a calibration graph determined for each strain used and subsequently confirmed by plate counting on nutrient agar (SIFIN). The virulence characteristics of the parent strain have been described previously (27). The ability of the strains to colonize the gut and to invade internal organs was evaluated on day 4 after infection by determining viable counts in the ceca and livers of the birds as described previously (27). Homogenized organ samples were diluted in phosphate-buffered saline, plated on deoxycholate-citrate agar (SIFIN) supplemented with sodium nalidixate (50 μg/ml), and incubated at 37°C overnight.
Viable counts were expressed as logarithms of CFU per milliliter. For the purposes of statistical analysis, a viable count of log10 of <1.47 (the limit for direct plate detection) obtained from a sample that became positive only after enrichment was given a log10 score of 1.0, while a sample that yielded no growth after enrichment was given a log10 score of 0. Data were evaluated by analysis of variance. P values of <0.05 were regarded as statistically significant (software was from Statgraphics Plus, Inc., Rockville, Md.).
Sources of specialized chemicals.
Catecholate siderophores were chemically synthesized by L. Heinisch, Hans-Knöll-Institut, Jena, Germany. Myxochelins were synthesized and characterized by W. Trowitzsch-Kienast, Technische Fachhochschule, Berlin, Germany. Corynebactin and protochelin were isolated from Corynebacterium glutamicum by H. Budzikiewicz, Institute of Organic Chemistry, Köln, Germany. Amonabactins P2 and T2 were provided by A. Stintzi, Department of Chemistry, Berkeley, Calif. The catecholate-cephalosporin conjugate KP-736 was provided by Y. Tatsumi, Episome Institute Kogure, Fujimi-mura, Seta-gun, Gumna-ken, Japan.
RESULTS
Genetic characterization of IROMP mutants.
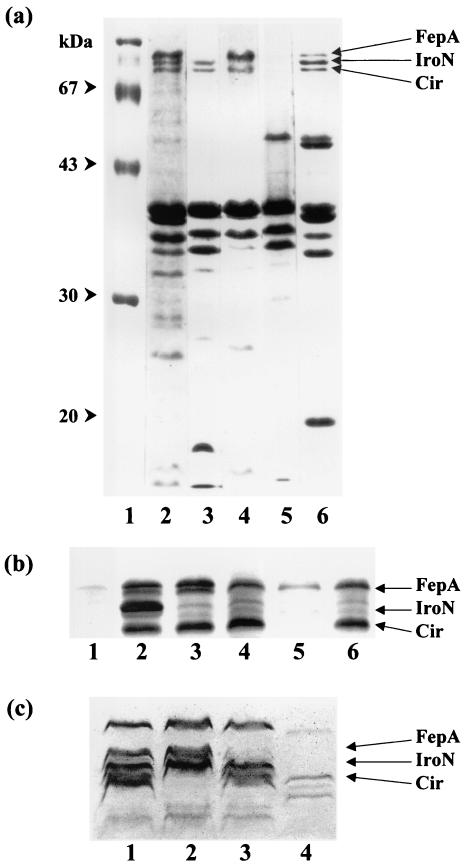
This study involves the construction and characterization of mutants carrying transposon insertion mutations in three genes encoding IROMPs proposed to act as receptors for ferric catecholate compounds. S. enterica serovar Typhimurium strains WR1315 and WR1316, isolated as described in Materials and Methods, are isogenic ent+ and ent transductants carrying Tn10dTc. The outer membrane protein profile lacked a protein band of 83 kDa corresponding to FepA (Fig. 1a); the mutation in S. enterica serovar Typhimurium strains WR1315 and WR1316 is therefore designated fepA::Tn10dTc. Addition of plasmid pITS449, which carries the E. coli fepA gene, to fepA mutant strains restored their ability to express the 83-kDa IROMP (35).
FIG. 1.
SDS-PAGE of outer membrane proteins of S. enterica strains carrying mutations in the fepA, iroN, and cir genes. (a) Lane 1, molecular size markers; lane 2, TA2700; lane 3, WR1316 (fepA); lane 4, WR1223 (iroN); lane 5, WR1330 (fepA iroN cir); lane 6, R18 (fur). (b) Lane 1, ATCC 14028 cultivated under iron-rich conditions; lane 2, ATCC 14028 cultivated under iron-restricted conditions; lane 3, WR1726 (iroN); lane 4, WR1727 (fepA iroN); lane 5, WR1728 (iroN fepA cir); lane 6, WR1729 (fepA iroBC). (c) Lane 1, SE147Nalr; lane 2, WR1530 (cir); lane 3, WR1425 (fepA); lane 4, WR1434 (fepA iroN). Gels shown in panels a and c were stained with Coomassie blue; the gel in panel b was stained with an alkaline silver nitrate solution.
S. enterica serovar Typhimurium strain AJB64, carrying the insertion mutation iroN::pGP704, has been reported previously (3). For this study, the iroN::pGP704 allele was transduced from strain AJB64 to TA2700, selecting for ampicillin resistance; the resulting strain, designated WR1223, lacked the 78-kDa outer membrane protein band (Fig. 1a) corresponding to the IROMP IroN (3).
S. enterica serovar Typhimurium strain AR8412 (47) carries a Fur-regulated MudJ insertion mutation that maps to 46 Cs on the S. enterica serovar Typhimurium chromosome, equivalent to the cir (colicin I receptor) locus of E. coli K-12 (14). Salmonella strains are naturally resistant to colicin I, so this protein cannot be used to identify or select cir mutants of S. enterica as it can for E. coli. Both species are, however, sensitive to the 1,5-dihydroxy-4-pyridone-substituted cephalosporin KP-736, whose uptake is known to be Cir dependent in E. coli (44). Transduction of the MudJ insertion from strain AR8412 to S. enterica serovar Typhimurium strain TA2700 and to fepA, iroN, and fepA iroN derivatives of TA2700 (strains WR1316, WR1223, and WR1332, respectively), with selection for kanamycin resistance, resulted in significantly increased MICs for KP-736 among the transductants (Table 2). The mutation carried by KP-736-resistant strains is therefore designated cir::MudJ. Moreover, KP-736 resistance of the tonB mutant strain WR1529 (Table 2) indicates that the activity of the IROMP Cir is TonB dependent, as would be expected of an active transport system. Note that the triple mutant strain WR1330 was deficient in all three high-molecular-weight outer membrane proteins expressed by the fur mutant control strain R18 (Fig. 1a).
TABLE 2.
Susceptibility of S. enterica cir mutants to KP-736
| Strain | Genotypea
|
MIC (μg/ml) | ||
|---|---|---|---|---|
| fepA | iroN | cir | ||
| TA2700 | + | + | + | ≤0.125 |
| WR1169 | + | + | − | 2 |
| WR1316 | − | + | + | ≤0.125 |
| WR1334 | − | + | − | 2 |
| WR1223 | + | − | + | ≤0.125 |
| WR1244 | + | − | − | 2 |
| WR1332 | − | − | + | ≤0.125 |
| WR1330 | − | − | − | 4 |
| WR1529 (tonB) | + | + | + | 4 |
The fepA, iroN, and cir genotypes are shown as follows: +, wild type; −, mutant.
Role of IROMPs in the uptake of natural and synthetic catechols.
The use of the enterobactin-deficient S. enterica serovar Typhimurium strain TA2700 as the background for construction of single, double, and triple mutants enabled us to perform standard growth promotion assays to identify uptake routes for a range of siderophores and related compounds (Table 3). Enterobactin, the principal siderophore of S. enterica, is recognized by either one of the two IROMPs FepA and IroN (34). Thus, while the single fepA (WR1316) and iroN (WR1223) mutant strains grew in the presence of enterobactin, the fepA iroN double mutant WR1332 did not. The enterobactin breakdown product DHBS1 can apparently use any of the three outer membrane proteins for uptake, since only the fepA iroN cir triple mutant WR1330 failed to respond in growth promotion tests with this compound. The different responses observed in these growth promotion assays indicate that the preparation of enterobactin used was not significantly contaminated with DHBS.
TABLE 3.
Growth promotion of S. enterica serovar Typhimurium and S. enterica serovar Enteritidis fepA, iroN, and cir mutants by natural catecholate siderophores and chemically synthesized catecholate compounds
| Catecholate | Growth of straina
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Typhimurium | TA2700 | WR1316 | WR1223 | WR1169 | WR1332 | WR1244 | WR1334 | WR1330 | |
| Typhimurium | ATCC 14028 | WR1726 | WR1727 | WR1728 | |||||
| Enteritidis | SE147Nalr | WR1425 | WR1530 | WR1434 | |||||
| fepA | + | − | + | + | − | + | − | − | |
| iroN | + | + | − | + | − | − | + | − | |
| cir | + | + | + | − | + | − | − | − | |
| Enterobactin | + | + | + | + | − | + | + | − | |
| DHBS | + | + | + | + | + | + | + | − | |
| Amonabactin P2 | + | + | + | + | + | − | + | − | |
| Amonabactin T2 | + | + | + | + | + | − | + | − | |
| Corynebactin | + | + | − | + | − | − | + | − | |
| Myxochelin A | + | + | + | + | + | + | + | − | |
| Myxochelin B | + | + | + | + | + | + | + | − | |
| Myxochelin C | + | − | + | + | − | + | − | − | |
| Protochelin | + | + | + | + | + | + | + | − | |
| N,N′-bis-(2,3-dihydroxybenzoyl)-l-lysine | + | + | + | + | + | + | + | − | |
| N,N′-bis-(2,3-diacetoxybenzoyl)-l-lysine | + | + | + | + | + | + | + | − | |
| N,N′-bis-(2,3-dihydroxybenzoyl)-l-serine | + | + | + | + | − | + | + | − | |
| N,N′-bis-(2,3-dihydroxybenzoyl)-d-ornithine | + | + | − | + | − | − | + | − | |
| Fe(II)SO4 | + | + | + | + | + | + | + | + | |
Growth promotion of S. enterica serovar Typhimurium strain TA2700 and its derivatives was assessed on Vogel-Bonner medium supplemented with 200 μM 2,2′-bipyridyl. Growth promotion of S. enterica serovar Typhimurium strain ATCC 14028 and its derivatives and of S. enterica serovar Enteritidis strain SE147Nalr and its derivatives was assessed on egg white agar plates. The fepA, iroN, and cir genotypes of the strains are shown as follows: +, wild type; −, mutant. Symbols: +, a halo of growth around filter paper disks to which the compounds had been applied; −, no growth. All strains listed in a column had the same genotype and showed the same growth characteristics.
Of a number of catecholate siderophores made by other bacteria, myxochelin A, myxochelin B, and protochelin could use any of the three receptors, while amonabactins P2 and T2 could use IroN or Cir but not FepA. Corynebactin was specific for IroN, while myxochelin C used FepA as its sole receptor (Table 3). Addition of the E. coli fepA gene to fepA mutant strains on recombinant plasmid pITS449 restored their ability to grow in the presence of myxochelin C (35). Similarly, among a group of chemically synthesized catecholate molecules tested, N,N′-bis-(2,3-dihydroxybenzoyl)-l-lysine and N,N′-bis-(2,3-diacetoxybenzoyl)-l-lysine utilized any of the three receptors, N,N′-bis-(2,3-dihydroxybenzoyl)-l-serine used IroN or FepA, and N,N′-bis-(2,3-dihydroxybenzoyl)-d-ornithine used IroN exclusively. None of the compounds we have tested showed specificity for the Cir protein. The triple mutant WR1330 did not obtain iron for growth from any of these catecholate molecules, although it was able to grow in the presence of excess iron (Table 3). Growth promotion assays using these compounds therefore gave characteristic patterns by which receptor mutants could be uniquely recognized (Table 4).
TABLE 4.
Catecholate uptake systems of S. enterica
| IROMP | Receptor for:
|
|
|---|---|---|
| Natural catecholate siderophore | Chemically synthesized catecholate siderophore | |
| FepA | Enterobactin | N,N′-bis-(2,3-dihydroxybenzoyl)-l-lysine |
| DHBS | N,N′-bis-(2,3-diacetoxybenzoyl)-l-lysine | |
| Myxochelins A, B, and C | N,N′-bis-(2,3-dihydroxybenzoyl)-l-serine | |
| Protochelin | ||
| IroN | Enterobactin | N,N′-bis-(2,3-dihydroxybenzoyl)-l-lysine |
| DHBS | N,N′-bis-(2,3-diacetoxybenzoyl)-l-lysine | |
| Amonabactins P2 and T2 | N,N′-bis-(2,3-dihydroxybenzoyl)-l-serine | |
| Corynebactin | N,N′-bis-(2,3-dihydroxybenzoyl)-d-ornithine | |
| Myxochelins A and B | ||
| Protochelin | ||
| Cir | DHBS | N,N′-bis-(2,3-dihydroxybenzoyl)-l-lysine |
| Amonabactins P2 and T2 | N,N′-bis-(2,3-diacetoxybenzoyl)-l-lysine | |
| Myxochelins A and B | ||
| Protochelin | ||
Characterization of fepA, iroN, and cir mutants of enterobactin-proficient strains.
The mutations fepA::Tn10dTc, iroN::pGP704, and cir::MudJ were characterized in detail in derivatives of S. enterica serovar Typhimurium strain TA2700 in order to facilitate standard growth promotion assays as described. However, because this background is enterobactin deficient, these mutants were not appropriate for infection studies. The mutations were therefore transferred by transduction to the mouse-virulent S. enterica serovar Typhimurium type strain ATCC 14028 and to a nalidixic-acid resistant mutant of the phage type 4 chicken-virulent strain of S. enterica serovar Enteritidis SE147 (48). Because these strains are both enterobactin proficient, standard growth promotion tests on low-iron medium could not be used. Instead, fepA, iroN, and cir derivatives of strains ATCC 14028 and SE147Nalr were tested in a more technically difficult assay involving egg white agar (see Materials and Methods). Results obtained in these backgrounds by this method confirmed data from standard growth promotion assays with TA2700 derivatives for each combination of mutations tested (Table 3). Additionally, susceptibility tests indicated that, while strains ATCC 14028 and SE147Nalr were highly sensitive to KP-736, transductants carrying the cir::MudJ allele showed significantly increased resistance to the catechol-cephalosporin conjugate (data not shown). Electrophoretic analysis of IROMPs in the various mutants confirmed the absence of 83-, 78-, and 74-kDa bands corresponding to mutations in fepA, iroN, and cir, respectively (Fig. 1b and c).
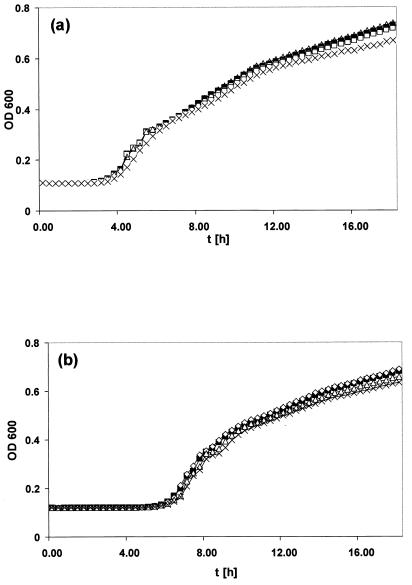
LPS profiles of S. enterica serovar Typhimurium ATCC 14028 and the mutant derivatives were identical (Fig. 2), suggesting that changes in the expression of IROMPs did not cause generalized structural alterations to the cell membranes. Moreover, all the strains showed the same serotype (4,5,12:i:1,2). Bioscreen C analysis indicated that the growth characteristics of mutants WR1727 (fepA iroN) and WR1728 (fepA iroN cir) were essentially identical with those of the parental strain ATCC 14028 (Fig. 3). A similar analysis of S. enterica serovar Enteritidis SE147Nalr derivatives indicated that the growth characteristics of mutants WR1425 (fepA), WR1434 (fepA iroN), and WR1530 (cir) were essentially identical with those of the parental strain, with lag phases of approximately 5.5 h and OD620 (optical density at 620 nm) maxima of 0.85 in stationary phase. All these strains were able to utilize ferrioxamine E and ferrichrome, as measured by cross-feeding tests (38), indicating that the expression and function of the IROMPs FoxA and FhuA are unaffected by the mutations in fepA, iroN, or cir.
FIG. 2.
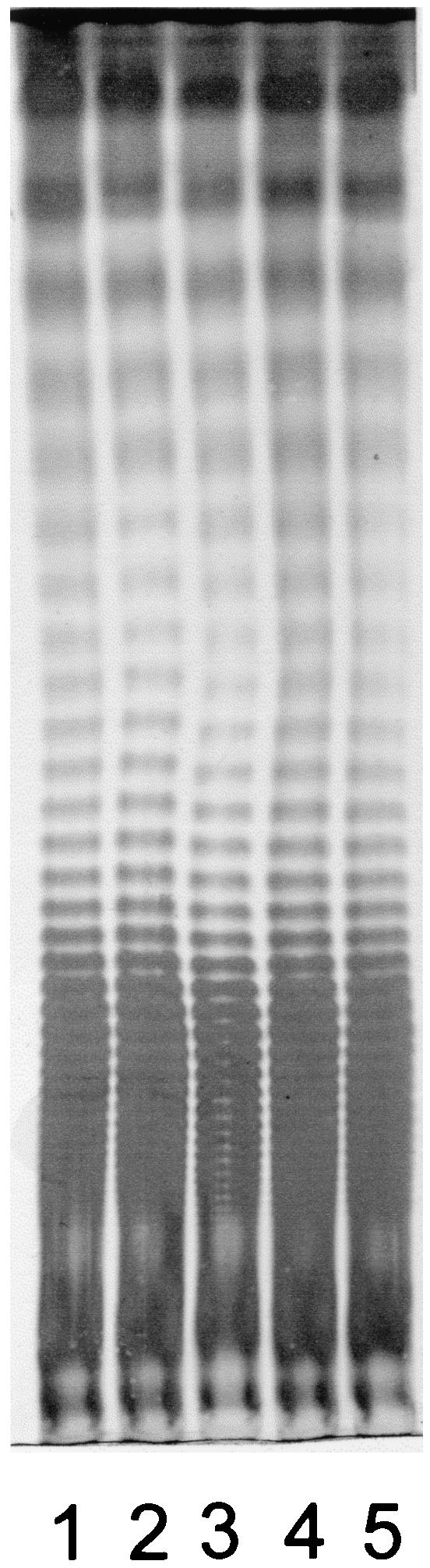
LPS profiles of S. enterica serovar Typhimurium strain ATCC 14028 and derivatives defective in IROMPs. Lane 1, ATCC 14028; lane 2, WR1726 (iroN); lane 3, WR1727 (fepA iroN); lane 4, WR1728 (iroN fepA cir); lane 5, WR1729 (fepA iroBC). The gel was stained with an alkaline silver nitrate solution.
FIG. 3.
Bioscreen C analysis of the growth of S. enterica serovar Typhimurium strain ATCC 14028 and S. enterica serovar Enteritidis strain SE147Nalr and their derivatives deficient in IROMPs over time (t). (a) Growth of strains ATCC 14028 (▪), WR1726 (iroN) (□), WR1727 (fepA iroN) (▵), and WR1728 (fepA iroN cir) (×). (b) Growth of strains SE147Nalr (□), WR1425 (fepA) (▵), WR1530 (cir) (⋄), and WR1434 (fepA iroN) (×). Culture densities (OD600) were monitored at 37°C for 18 h, and each line represents the average data from 20 replicate wells.
Mouse virulence of S. enterica serovar Typhimurium mutants.
The input and recovered populations after intragastric infection with mixed inocula of S. enterica serovar Typhimurium ATCC 14028 and its mutant derivatives were compared to determine whether mutations that reduced the uptake of enterobactin and DHBS affected the ability of this strain to cause lethal systemic infections in mice. Preliminary experiments indicated that similar doses of strains ATCC 14028 and WR1727 (iroN fepA) were required for morbidity and mortality of infected mice, but that the triple mutant strain WR1728 was significantly attenuated, requiring doses more than 103-fold higher to induce equivalent effects. Data from mixed inoculation of mice (Table 5) confirmed this pattern of virulence. Approximately equal numbers of strains ATCC 14028 and WR1727 were recovered from the ceca (to determine intestinal colonization) and livers (as a measure of systemic invasion) of mice infected with an approximately 1:1 mixture of these two strains. Conversely, no colonies of mutant strain WR1728 (iroN fepA cir) were recovered after infection with an approximately 1:1 mixture of this strain with ATCC 14028. Moreover, even with an inoculum mixture in which WR1728 was present in 100-fold excess, no bacteria of this strain were recovered from the ceca, and only one colony was recovered from the livers of infected animals.
TABLE 5.
Recovery of bacteria from the liver and cecum of mice infected with derivatives of S. enterica serovar Typhimurium strain ATCC 14028
| Inoculum mixture | Proportion (%) of parent strain in inoculum | Challenge dose (CFU/mouse)a | Proportion (%) of parent strain recovered from:
|
|
|---|---|---|---|---|
| Cecum | Liver | |||
| WR1727 (fepA iroN) + ATCC 14028 | 54 | 104 | 48 | 46 |
| 105 | 49 | 52 | ||
| WR1728 (fepA iroN cir) + ATCC 14028 | 46 | 104 | >96 | >96 |
| 105 | >96 | >96 | ||
| 1 | 105 | >96 | >96 | |
| 106 | >96 | 96 | ||
| WR1729 (fepA iroN iroBC) + ATCC 14028 | 52 | 104 | 49 | 49 |
| 105 | 51 | 52 | ||
Groups of two mice were inoculated with inoculum mixtures at the doses indicated.
The presence of the iroBC mutation, which results in deficiency in salmochelin production, in the ATCC 14028 background had no significant effect on virulence. When mice were infected with a 1:1 mixture of strains ATCC 14028 and WR1729, approximately equal numbers of both strains were recovered from the ceca and livers, suggesting that the inability to produce salmochelins had no effect on the infection process.
Chicken virulence of IROMP mutants of S. enterica serovar Enteritidis.
S. enterica serovar Enteritidis strain SE147Nalr and mutant derivatives WR1425 (fepA) and WR1434 (fepA iroN) were orally inoculated into groups of 4-day-old chicks, the severity of infection being assessed by enumeration of bacteria in the ceca (to determine intestinal colonization) and livers (as a measure of systemic invasion) (Table 6). Note that the fepA iroN double mutant WR1434, which growth promotion data indicated should be unable to take up enterobactin, showed the same level of virulence in this model, with respect to both cecal colonization and translocation to the liver, as the parental strain SE147Nalr and the fepA single mutant strain WR1425.
TABLE 6.
Recovery of bacteria from the liver and cecum of chicks infected with fepA, iroN, and cir mutants of S. enterica serovar Enteritidis strain SE147Nalr
| Inoculum strain (genotype) | Challenge dose (CFU/bird)a | Recovery of bacteria (log10 CFU/ml)b from:
|
|
|---|---|---|---|
| Cecum | Liver | ||
| SE147Nalr | 2.5 × 103 | 9.36 | 4.08 |
| WR1425 (fepA) | 1.74 × 103 | 9.44 | 3.88 |
| WR1434 (fepA iroN) | 2.5 × 103 | 9.02 | 3.86 |
Groups of four birds were inoculated with each strain at the doses indicated.
Standard errors for counts were ±0.24 for the cecum and ±0.14 for the liver.
Growth of IROMP mutants of S. enterica serovar Typhimurium and Enteritidis in chicken serum.
The parental strains ATCC 14028 and SE147Nalr grew better in chicken serum than any of the IROMP mutant derivatives and generated larger colonies when plated. All the mutants with the exception of strain WR1728 were able to overcome the inhibitory effects of the serum and grew significantly, especially during overnight incubation (Table 7). The triple mutant WR1728, however, did not grow even from higher inocula than the other strains and indeed showed markedly reduced viable counts after overnight incubation.
TABLE 7.
Growth of fepA, iroN, and cir mutants of S. enterica serovars Typhimurium and Enteritidis in chicken serum
| Strain (genotype) | Inoculum size (CFU) | Viable counts (CFU) after incubation for:
|
||
|---|---|---|---|---|
| 4 h | 9 h | overnight | ||
| ATCC 14028 | 3.7 × 102 | 5.5 × 102 | 2.2 × 104 | >107 |
| WR1726 (fepA) | 3.4 × 102 | 4.6 × 102 | 5.8 × 102 | >107 |
| WR1727 (fepA iroN) | 3.0 × 102 | 5.0 × 102 | 2.1 × 103 | >107 |
| WR1728 (fepA iroN cir) | 6.4 × 102 | 5.5 × 102 | 3.2 × 102 | 0.3 × 102 |
| WR1729 (fepA iroBC) | 4.0 × 102 | 5.6 × 102 | 6.3 × 102 | >107 |
| SE147Nalr | 3.7 × 102 | 1.8 × 103 | 3.7 × 103 | >107 |
| WR1425 (fepA) | 5.7 × 102 | 9.3 × 102 | 2.0 × 103 | >107 |
| WR1434 (fepA iroN) | 3.2 × 102 | 8.0 × 102 | 1.7 × 103 | >107 |
DISCUSSION
Iron is an essential element for almost all living systems. However, most of the iron in the biological fluids of vertebrates is bound by transferrin or lactoferrin, and much of the intracellular iron exists in the red blood cells. In establishing an infection, therefore, microorganisms depend on their ability to use the various forms of complexed iron to overcome the nonspecific defenses of the host in order to promote bacterial multiplication. One of the major mechanisms that enable pathogenic bacteria to survive and proliferate within the vertebrate host is the production of iron-sequestering siderophores and the synthesis of their cognate transport systems (36).
Salmonella serovars synthesize the catecholate siderophore enterobactin, a cyclic trimer of DHBS that has among the highest affinities for ferric iron of any natural compound (17, 29). The enterobactin precursor 2,3-dihydroxybenzoic acid and the breakdown products of enterobactin, DHBS1, DHBS2, and DHBS3, also possess iron-binding and transport capabilities and so can be utilized by Salmonella serovars as sources of iron (49).
There is conflicting evidence for a role for enterobactin in the virulence of Salmonella. On the one hand, Yancey and coworkers (50) reported that S. enterica serovar Typhimurium mutants defective in enterobactin synthesis were much less virulent in mice than the ent+ parent strain and that intraperitoneal administration of 300 μg of enterobactin together with the ent bacteria restored virulence, as measured by an apparent decrease in the 50% lethal dose. Similarly, an ent mutant of S. enterica serovar Typhi showed restricted growth in human Mac 6 monocytic cells (13). Consistent with this are studies showing that aro mutants of S. enterica serovar Typhimurium were avirulent for mice (18). Such mutants are unable to make chorismate via the aromatic biosynthetic pathway and therefore cannot make aromatic amino acids or the enterobactin precursor 2,3-dihydroxybenzoic acid. On the other hand, Benjamin and colleagues determined that, although enterobactin-deficient mutants were unable to multiply in mouse serum, their virulence in several mouse strains was not reduced (4); similar net growth was observed in the spleens and livers of inbred and F1 hybrid mouse lines experimentally infected with ent+ and ent strains of bacteria.
The receptors for enterobactin and other siderophores belong to a family of proteins whose transport activities are dependent on the function of TonB (7, 43). Thus, tonB mutants of S. enterica serovar Typhimurium, like ent mutants, were unable to grow in pooled mouse serum samples in which wild-type strains grew well. Furthermore, the tonB mutation attenuated S. enterica serovar Typhimurium for infection of mice by the intragastric but not the intraperitoneal route (46). These data are consistent with the proposal that intracellular pathogens may not require high-affinity iron-gathering processes for virulence (22). Indeed, it may be that enterobactin is more important for the survival of enterobacterial pathogens in the gut or environmental niches than for pathogenic processes occurring in the blood and tissues of higher organisms.
We sought to resolve this discrepancy by the use of mutants defective in the three major IROMPs proposed to act as receptors for catecholate-mediated iron uptake. Single, double, and triple fepA, iroN, and cir mutants of an enterobactin-deficient strain of S. enterica serovar Typhimurium were characterized in terms of their outer membrane protein profiles and their responses in growth promotion tests with a number of natural and chemically synthesized catecholate compounds. The cir mutation was additionally characterized by reduced uptake of the siderophore-cephalosporin conjugate KP-736. Here we confirm previous reports (3, 35) that FepA and IroN are both able to transport enterobactin across the outer membrane of S. enterica, and we additionally show that FepA, IroN, and Cir are all involved in the uptake of DHBS. Monomeric DHBS1 has also been demonstrated to stimulate the growth of E. coli strains under iron-limited conditions by acting as a siderophore that utilizes the outer membrane receptor proteins FepA, Fiu, and to a minor extent, Cir (15). Note that this is the first report of cir mutants of Salmonella and also the first rigorous analysis of the compounds that can use the FepA, IroN, and Cir proteins as receptors for iron transport (Table 4).
Having characterized the fepA, iroN, and cir mutations in the enterobactin-deficient background of S. enterica serovar Typhimurium strain TA2700, we transferred the mutations into enterobactin-proficient backgrounds in order to check their effects on virulence. S. enterica serovar Typhimurium derivatives were used to infect mice, while S. enterica serovar Enteritidis were assayed in experimental infections of 4-day-old chicks. Both approaches showed unequivocally that fepA iroN double mutants, which growth promotion tests indicated were unable to take up enterobactin, had the same virulence characteristics as the parent strains; therefore, enterobactin is not a virulence factor for S. enterica serovars Typhimurium and Enteritidis. A role for salmochelins is also excluded, because these molecules use IroN primarily as a receptor. Moreover, the virulence of an iroBC mutant, which does not synthesize salmochelins (16), is similar to that of the parental strain ATCC 14028. On the other hand, the S. enterica serovar Typhimurium fepA iroN cir triple mutant was markedly attenuated in the mouse model and showed reduced capacities for cecal colonization and systemic spread. Analysis of the growth of the various strains in chicken serum gave a similar pattern. Growth of single and double mutants was weaker than for the parent strains but was nevertheless significant, particularly with overnight incubation; only the triple mutant WR1728 did not grow, presumably because it was unable to utilize enterobactin breakdown products to overcome iron stress. Since the parent strain ATCC 14028 and all the mutant derivatives behaved identically in terms of their antigenicity (LPS profile and O and H serotypes), growth characteristics in the Bioscreen C, and full functionality of hydroxamate siderophore uptake systems, it seems probable that the effects of the fepA, iroN, and cir mutations analyzed in this study are essentially restricted to the cognate catecholate siderophore receptors.
Growth promotion assays indicate that several catecholate compounds besides DHBS, including myxochelin A, myxochelin B, and protochelin, can use any of the three IROMPs for uptake into S. enterica. Bearing in mind, however, that enterobactin is the only siderophore made by the S. enterica derivatives used in these studies, among the compounds mentioned, only the enterobactin degradation products DHBS1 to DHBS3 are likely to be present in our assay systems. It is probable, therefore, that full virulence is due to the ability to acquire iron complexed with DHBS. During the course of infection, S. enterica passes through several compartments containing different potential sources of iron for bacterial growth. Particular iron uptake systems, such as enterobactin, may not be effective in all conditions and consequently may not act as virulence factors in the animal model.
Acknowledgments
We thank Philip E. Klebba, University of Oklahoma, Norman, for the E. coli fepA plasmid pITS449; L. Heinisch for chemically synthesized catecholate siderophores; W. Trowitzsch-Kienast for myxochelins A, B, and C; H. Budzikiewicz for corynebactin and protochelin; A. Stintzi for amonabactins P2 and T2; and Y. Tatsumi for KP-736. We are particularly indebted to Renée Tsolis and Andreas Bäumer, Texas A&M University, College Station, for critically reading the manuscript and for their generous support of our research over many years. We are also very grateful to Julie Morrissey for discussions, suggestions, and critical reading of the manuscript and to Andreas Kresse for help with preparation of the figures. We thank Waltraut Jacobi, Ilse Rienäcker, Petra Schweinitz, Brigitte Tannert, and Annette Weller for skillful technical assistance.
Editor: F. C. Fang
REFERENCES
- 1.Achtmann, M., A. Mercer, A. Pohl, M. Heuzenroeder, W. Aaronson, A. Sutton, and R. P. Silver. 1983. Six widespread bacterial clones among Escherichia coli K1 isolates. Infect. Immun. 39:315-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambrosi, H.-D., V. Hartmann, D. Pistorius, R. Reissbrodt, and W. Trowitzsch-Kienast. 1998. Myxochelins B, C, D, E and F: a new structural principle for powerful siderophores imitating nature. Eur. J. Org. Chem. 1998:541-551. [Google Scholar]
- 3.Bäumler, A. J., T. L. Norris, T. Lasco, W. Voigt, R. Reissbrodt, W. Rabsch, and F. Heffron. 1998. IroN, a novel outer membrane siderophore receptor characteristic of Salmonella enterica. J. Bacteriol. 180:1446-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benjamin, W. H., Jr., C. L. Turnbough, Jr., B. S. Posey, and D. E. Briles. 1985. The ability of Salmonella typhimurium to produce the siderophore enterobactin is not a virulence factor in mouse typhoid. Infect. Immun. 50:392-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berner, I., D. Konetschny-Rapp, G. Jung, and G. Winkelmann. 1988. Characterization of ferrioxamine E as the principal siderophore of Erwinia herbicola (Enterobacter agglomerans). Biol. Metals 1:51-56. [DOI] [PubMed] [Google Scholar]
- 6.Braun, V., and M. Braun. 2002. Active transport of iron and siderophore antibiotics. Curr. Opin. Microbiol. 5:194-201. [DOI] [PubMed] [Google Scholar]
- 7.Braun, V., and K. Hantke. 1991. Genetics of bacterial iron transport, p. 107-139. In G. Winkelmann (ed.), Handbook of microbial iron chelates. CRC Press, Boca Raton, Fla.
- 8.Colonna, B., M. Nicoletti, P. Visca, M. Casalino, P. Valenti, and F. Maimone. 1985. Composite IS1 elements encoding hydroxamate-mediated iron uptake in Flme plasmids from epidemic Salmonella spp. J. Bacteriol. 162:307-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curtis, N. A., R. L. Eisenstadt, S. J. East, R. J. Cornford, L. A. Walker, and A. J. White. 1988. Iron-regulated outer membrane proteins of Escherichia coli K-12 and mechanism of action of catechol-substituted cephalosporins. Antimicrob. Agents Chemother. 32:1879-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Earhardt, C. F. 1996. Uptake and metabolism of iron and molybdenum, p. 1075-1090. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. American Society for Microbiology, Washington, D.C.
- 11.Elliott, T., and J. R. Roth. 1988. Characterization of Tn10d-Cam: a transposition-defective Tn10 specifying chloramphenicol resistance. Mol. Gen. Genet. 213:332-338. [DOI] [PubMed] [Google Scholar]
- 12.Ernst, J. F., R. L. Bennett, and L. I. Rothfield. 1978. Constitutive expression of the iron-enterochelin and ferrichrome uptake systems in mutant strains of Salmonella typhimurium. J. Bacteriol. 135:928-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorbacheva, V. Y., G. Faundez, H. P. Godfrey, and F. C. Cabello. 2001. Restricted growth of ent and tonB mutants of Salmonella enterica serovar Typhi in human Mac 6 monocytic cells. FEMS Microbiol. Lett. 196:7-11. [DOI] [PubMed] [Google Scholar]
- 14.Hancock, R. E. W., K. Hantke, and V. Braun. 1976. Iron transport in Escherichia coli K-12: involvement of the colicin B receptor and of a citrate-inducible protein. J. Bacteriol. 127:1370-1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hantke, K. 1990. Dihydroxybenzoylserine—a siderophore for E. coli. FEMS Microbiol. Lett. 67:5-8. [DOI] [PubMed] [Google Scholar]
- 16.Hantke, K., G. Nicholson, W. Rabsch, and G. Winkelmann. 2003. Salmochelins, siderophores of Salmonella enterica and uropathogenic Escherichia coli strains, are recognized by the outer membrane receptor IroN. Proc. Natl. Acad. Sci. USA 100:3677-3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris, W. R., C. J. Carrano, S. R. Cooper, S. R. Sofen, A. E. Avdeef, J. V. McArdle, and K. N. Raymond. 1979. Coordination chemistry of microbial iron transport compounds. 19. Stability constants and electrochemical behavior of ferric enterobactin and model complexes. J. Am. Chem. Soc. 101:6097-6104. [Google Scholar]
- 18.Hoiseth, S. K., and B. A. D. Stocker. 1981. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature 291:238-239. [DOI] [PubMed] [Google Scholar]
- 19.Johnson, J. R., T. A. Russo, P. I. Tarr, U. Carlino, S. S. Bilge, J. C. Vary, Jr., and A. L. Stell. 2000. Molecular epidemiological and phylogenetic associations of two novel putative virulence genes, iha and iroNE. coli, among Escherichia coli isolates from patients with urosepsis. Infect. Immun. 68:3040-3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kingsley, R., W. Rabsch, M. Roberts, R. Reissbrodt, and P. H. Williams. 1996. TonB-dependent iron supply in Salmonella by a-ketoacids and a-hydroxyacids. FEMS Microbiol. Lett. 140:65-70. [DOI] [PubMed] [Google Scholar]
- 21.Kingsley, R. A., R. Reissbrodt, W. Rabsch, J. M. Ketley, R. M. Tsolis, P. Everest, G. Dougan, A. J. Bäumler, M. Roberts, and P. H. Williams. 1999. Ferrioxamine-mediated iron(III) utilization by Salmonella enterica. Appl. Environ. Microbiol. 65:1610-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Konopka, K., and J. B. Neilands. 1984. Effect of serum albumin on siderophore mediated utilization of transferrin iron. Biochemistry 23:2122-2127. [DOI] [PubMed] [Google Scholar]
- 23.Luckey, M., and J. B. Neilands. 1976. Iron transport in Salmonella typhimurium LT-2: prevention by ferrichrome of adsorption of bacteriophages ES18 and ES18.h1 to a common cell envelope receptor. J. Bacteriol. 127:1036-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luckey, M., J. R. Pollack, R. Wayne, B. N. Ames, and J. B. Neilands. 1972. Iron uptake in Salmonella typhimurium: utilization of exogenous siderochromes as iron carriers. J. Bacteriol. 111:731-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lugtenberg, B., J. Meiers, R. Peters, P. van der Hoeck, and L. van Alphen. 1975. Electrophoretic resolution of the major outer membrane proteins of Escherichia coli K12 into four bands. FEBS Lett. 58:254-258. [DOI] [PubMed] [Google Scholar]
- 26.McDougall, S., and J. B. Neilands. 1984. Plasmid- and chromosome-coded aerobactin synthesis in enteric bacteria: insertion sequences flank operon in plasmid-mediated systems. J. Bacteriol. 159:300-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Methner, U., S. Al-Shabibi, and H. Meyer. 1995. Experimental oral infection of specific pathogen-free laying hens with Salmonella enteritidis strains. J. Vet. Med. Ser. B 42:471-480. [DOI] [PubMed] [Google Scholar]
- 28.Neilands, J. B. 1982. Microbial envelope proteins related to iron. Annu. Rev. Microbiol. 36:285-309. [DOI] [PubMed] [Google Scholar]
- 29.O'Brien, G., and F. Gibson. 1970. The structure of enterochelin and related 2, 3-dihydroxy-N-benzoylserine conjugates from Escherichia coli. Biochim. Biophys. Acta 215:393-402. [DOI] [PubMed] [Google Scholar]
- 30.Oelschlaeger, T. A., D. Zhang, S. Schubert, E. Carniel, W. Rabsch, H. Karch, and J. Hacker. 2003. The high pathogenicity island is absent in human pathogens of Salmonella enterica subspecies I but present in isolates of subspecies III and VI. J. Bacteriol. 185:1107-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pollack, J. R., N. Ames, and J. B. Neilands. 1970. Iron transport in Salmonella typhimurium mutants blocked in the biosynthesis of enterobactin. J. Bacteriol. 104:635-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rabsch, W. 1998. Characterization of the catecholate indicator strain S. typhimurium TA2700 as an ent fhuC double mutant. FEMS Microbiol. Lett. 163:79-84. [DOI] [PubMed] [Google Scholar]
- 33.Rabsch, W., P. Paul, and R. Reissbrodt. 1987. A new hydroxamate siderophore for iron supply of Salmonella. Acta Microbiol. Hung. 34:85-92. [PubMed] [Google Scholar]
- 34.Rabsch, W., and R. Reissbrodt. 1985. Biotest zum Nachweis von Hydroxamat Fe-Chelatoren (Aerobactin). J. Basic Microbiol. 25:663-667. [DOI] [PubMed] [Google Scholar]
- 35.Rabsch, W., W. Voigt, R. Reissbrodt, R. M. Tsolis, and A. J. Bäumler. 1999. Salmonella typhimurium IroN and FepA proteins mediate uptake of enterobactin but differ in their specificity for other siderophores. J. Bacteriol. 181:3610-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ratledge, C., and L. G. Dover. 2000. Iron metabolism in pathogenic bacteria. Annu. Rev. Microbiol. 54:881-941. [DOI] [PubMed] [Google Scholar]
- 37.Reissbrodt, R., R. Kingsley, W. Rabsch, W. Beer, M. Roberts, and P. H. Williams. 1997. Iron-regulated excretion of α-keto acids by Salmonella typhimurium. J. Bacteriol. 179:4538-4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reissbrodt, R., and W. Rabsch. 1988. Further differentiation of Enterobacteriaceae by means of siderophore-pattern-analysis. Zentbl. Bakteriol. Mikrobiol. Hyg. Abt. 268:306-317. [DOI] [PubMed] [Google Scholar]
- 39.Reissbrodt, R., and W. Rabsch. 1994. Ability of catecholate derivatives to promote growth of gram-negative bacteria, p. 209-235. In R. Bergeron (ed.), The development of iron chelators for clinical use. CRC Press, Boca Raton, Fla.
- 40.Reissbrodt, R., W. Rabsch, A. Chapeaurouge, G. Jung, and G. Winkelmann. 1990. Isolation and identification of ferrioxamine G and E in Hafnia alvei. Biol. Metals 3:54-60. [Google Scholar]
- 41.Reissbrodt, R., E. Vielitz, E. Kormann, W. Rabsch, and H. Kühn. 1996. Ferrioxamine E supplemented pre-enrichment and enrichment media improve various isolation methods for Salmonella. Int. J. Food Microbiol. 29:81-91. [DOI] [PubMed] [Google Scholar]
- 42.Schwyn, B., and J. B. Neilands. 1987. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 160:47-56. [DOI] [PubMed] [Google Scholar]
- 43.Skare, J. T., B. M. M. Ahmer, C. L. Seachord, R. P. Darveau, and K. Postle. 1993. Energy transduction between membranes: TonB, a cytoplasmic membrane protein, can be chemically cross-linked in vivo to the outer membrane receptor FepA. J. Biol. Chem. 268:16302-16308. [PubMed] [Google Scholar]
- 44.Tatsumi, J., T. Maejima, and S. Mitsuhashi. 1995. Mechanism of tonB-dependent transport of KP-736, a 1,5-dihydroxy-4-pyridone substituted cephalosporin, into Escherichia coli K-12 cells. Antimicrob. Agents Chemother. 39:613-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsai, C. M., and C. E. Frasch. 1982. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 119:115-119. [DOI] [PubMed] [Google Scholar]
- 46.Tsolis, R. M., A. J. Bäumler, F. Heffron, and I. Stojilikovic. 1996. Contribution of TonB- and Feo-mediated iron uptake to growth of Salmonella typhimurium in the mouse. Infect. Immun. 64:4549-4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsolis, R. M., A. J. Bäumler, I. Stojilikovic, and F. Heffron. 1995. Fur regulon of Salmonella typhimurium: identification of new iron-regulated genes. J. Bacteriol. 177:4628-4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ward, L. R., J. D. H. de Sa, and B. Rowe. 1987. A phage typing scheme for Salmonella enteritidis. Epidemiol. Infect. 99:291-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilkins, T. D., and C. E. Lankford. 1970. Production by Salmonella typhimurium of 2,3-dihydroxybenzoylserine, and its stimulation of growth in human serum. J. Infect. Dis. 121:129-136. [DOI] [PubMed] [Google Scholar]
- 50.Yancey, R. J., S. A. L. Breeding, and C. E. Lankford. 1979. Enterochelin (enterobactin): virulence factor for Salmonella typhimurium. Infect. Immun. 24:174-180. [DOI] [PMC free article] [PubMed] [Google Scholar]