Abstract
The mechanisms by which the immune response can eradicate gastric Helicobacter infection are unknown. We hypothesized that Helicobacter-induced activation of the complement system could promote both inflammation and eradication of Helicobacter from the stomach. In vitro studies demonstrated that Helicobacter felis activates complement in normal mouse serum but not in serum from Rag2−/− mice, indicating that H. felis activates complement through the classical pathway. Next, we infected complement-depleted wild-type control and interleukin-10-deficient (IL-10−/−) mice with H. felis. Helicobacter infection of wild-type mice elicited a mild, focal gastritis and did not alter serum complement levels. Infection of IL-10−/− mice with H. felis elicited severe gastritis. After the initial colonization, the IL-10−/− mice completely cleared Helicobacter from the stomach by day 8. In contrast to wild-type mice, H. felis-infected IL-10−/− mice had a marked increase in serum complement levels. Complement depletion of wild-type mice did not affect the intensity of gastric inflammation or the extent of Helicobacter colonization compared to that for the wild-type control mice. In contrast, complement depletion of Helicobacter-infected IL-10−/− mice decreased the severity of gastritis, decreased the Helicobacter-induced infiltration of neutrophils into the stomach, and delayed the clearance of bacteria. In vitro studies of stimulated splenocytes and neutrophils from IL-10−/− mice produced a twofold increase in complement production compared to that for wild-type mice. Pretreatment with IL-10 inhibited this increase. These studies identify a role for complement in the local immune response to gastric Helicobacter in IL-10−/− mice and suggest a role for IL-10 in the regulation of complement production.
Helicobacter pylori is a human gastric pathogen that can cause gastritis, peptic ulcer disease, and gastric cancer. The organism was first associated with peptic ulcer disease in 1984 (25), and since that time there has been an exponential increase in our knowledge of the role of Helicobacter in disease. However, despite these advances in the understanding of the biology of H. pylori, the mechanisms leading to eradication of this noninvasive bacterium remain poorly understood.
In recent years, evidence has accumulated to suggest that in both human patients and animal models the host immune response is an important determinant of the outcome of the infection. H. pylori-infected individuals express proinflammatory cytokines in their gastric mucosa (49), and it has been proposed that Helicobacter induces a Th1-type CD4+ T-cell immune response (12, 26). Indeed, T cells are required for eradication of gastric infection in animal models of Helicobacter infection (38). However, other components of the immune response may have an important role in control of gastric Helicobacter infection. For example, Helicobacter infection also induces a prominent neutrophilic infiltration (8), and it has recently been reported that depletion of neutrophils in an animal model of Helicobacter infection resulted in delayed clearance of bacteria and a blunted immune response to Helicobacter (20).
Several lines of evidence suggest that the complement system may have an important role in H. pylori-induced gastritis. H. pylori can activate complement in vitro (7). Moreover, complement is activated in H. pylori-positive gastritis (6). C3b, soluble terminal complement complexes, and C3b-opsonised H. pylori have been found in the gastric mucus of H. pylori-infected individuals (6). However, the extent to which activation of complement promotes Helicobacter-induced gastritis and clearance of gastric Helicobacter infection remains unknown.
Because of the difficulty of performing invasive studies in humans, much of our understanding of the immune basis of H. pylori-related disease comes from studies with animal models. We have developed a murine model of Helicobacter felis gastritis in interleukin-10-deficient (IL-10−/−) mice that mimics many features of chronic H. pylori infection. Based on 16S rRNA sequence analysis, H. felis is genetically quite similar to H. pylori (33), and this organism has been demonstrated to efficiently colonize rodent gastric mucosa (10). H. felis infection in mice with a targeted disruption of the IL-10 gene (IL-10−/− mice), a key antiinflammatory and immune regulatory cytokine, results in severe inflammation with the development of Th1-type CD4+ T cells (5). IL-10−/− mice infected with H. felis develop a chronic gastritis that simulates many of the features of human H. pylori infection, including preneoplastic epithelial changes that occur within 4 weeks of infection (5), paralleling the epithelial changes which may take up to 1 to 2 years in H. felis-infected wild-type (WT) mice (23) and which may also occur (over a period of decades) in patients chronically infected with H. pylori. Moreover, we have found that H. felis is rapidly cleared from IL-10−/− mice (20). Therefore, H. felis-infected IL-10−/− mice represent a useful model for study of the immune mechanisms that lead to Helicobacter-induced pathology and clearance of Helicobacter from the stomach.
The aim of this study was to evaluate the role of complement on the course of H. felis-induced gastritis in IL-10−/− mice. We observed that complement depletion decreased the ability of IL-10−/− mice to clear H. felis infection. Moreover, complement depletion significantly decreased the severity of H. felis-induced gastritis. These studies suggest an important role for complement in the immune and inflammatory responses to Helicobacter in IL-10−/− mice.
MATERIALS AND METHODS
Mice.
Healthy 6-week-old IL-10−/− mice on a 129/SvEv background were used for this study (3). Helicobacter-free WT 129/SvEv mice were purchased from Taconic Farms (Germantown, N.Y.). The mice were maintained in microisolator cages under specific-pathogen-free conditions at the animal care facility at the University of Iowa. All mice were maintained in accordance with the guidelines of the University of Iowa Animal Care and Use Committee.
Bacteria.
H. felis (ATCC 49179) was obtained from the American Type Culture Collection (Rockville, Md.). The bacteria were grown as described previously (28). Briefly, the bacteria were grown on brucella agar plates with trimethoprim-vancomycin-polymyxin B (Remel, Lenexa, Kans.) under microaerophilic conditions at 37°C for 2 days. Confluent plates of bacteria were harvested, and the number of organisms was determined by absorption at an optical density at 450 nm (OD450), with one OD unit corresponding to 109 bacteria (28). The bacteria were positively identified on the basis of morphology and the presence of urease enzyme activity and via PCR amplification of the 16S rRNA gene by using Helicobacter-specific primers (35).
Infection with H. felis.
H. felis (1 × 108 bacteria in 100 μl of phosphate-buffered saline [PBS]) was instilled by gavage by using a 23-gauge feeding needle (Popper and Sons, New Hyde Park, N.Y). The mice received three inoculations over a period of 5 days, with 1 day separating each inoculation. The mice were made to fast overnight prior to the inoculations.
Complement depletion.
To characterize the role of complement during gastric H. felis infection, mice were decomplemented by using cobra venom anticomplementary protein (Naja Naja kaouthia; Sigma, St. Louis, Mo.), hereafter referred to as CVF (cobra venom factor). WT mice received intraperitoneal injections of 0.8 μg of CVF/g of body weight every 48 h (44). IL-10−/− mice received intraperitoneal injections of 3.0 μg of CVF/g of body weight every 24 h. To evaluate the efficiency of CVF in decomplementation of WT and IL-10−/− mice, blood samples for assessment of C3 levels were obtained by tail vein bleeding prior to injection of CVF, 24 h after injection of CVF, and at the completion of the experiment (day 8). Each mouse served as its own control, and the pretreatment value was set to 100%. Two days after the start of the treatment, the levels of C3 were decreased to 5 to 9% and remained at this level for the duration of the experiment. Inoculation with H. felis began 1 day after initiation of CVF treatment. The mice were evaluated for colonization of H. felis and severity of inflammation 8 days after the initiation of infection.
C5 depletion.
To characterize the effect of C5 depletion during gastric H. felis infection, WT and IL-10−/− mice were treated with intraperitoneal injections of BB5.1 (40 mg/kg every other day), a monoclonal antibody directed against C5 (47). WT and IL-10−/− mice treated with the same dose and schedule of the isotype control antibody (135.8) served as the controls. Anti-C5 treatment began 2 days before H. felis infection. Serum samples for determining C5 levels were obtained before and after anti-C5 treatment, as well as 7 days after H. felis infection. Sera were assessed for efficiency of C5 depletion using a hemolytic assay as described previously (46). Briefly, mouse serum samples were diluted to 10% (vol/vol) with gelatin Veronal buffer 2+ (Sigma) and added (50 μl/well) to 96-well microtiter plates containing 50 μl of human C5-deficient serum (Quidel, San Diego, Calif.). The plates were incubated for 30 min at room temperature. Erythrocyte preparation and hemolytic assays were then performed as previously described (36). Each mouse served as its own control, and the pretreatment value was set to 100%. Anti-C5 treatment reduced the levels of C5 to 5% of the pretreatment control value for the duration of the experiment. Inoculation with H. felis began one day after initiation of CVF treatment. The mice were evaluated for colonization of H. felis and severity of inflammation 8 days after initiation of infection.
Complement activation assay.
Normal nonimmune serum was obtained from healthy, uninfected 129/SvEv mice. Antibody-deficient nonimmune serum was obtained from healthy 129/SvEv/Rag2−/− mice. Serum (90 μl) was incubated with H. felis suspension (10 μl, approximately 3 × 106 CFU) or lipopolysaccharide (LPS) (10 μl; final concentration, 10 to 100 ng/ml) at 37°C. EDTA (pH 8.0) was added to a final concentration of 10 mmol/liter after 15, 30, or 60 min to terminate complement activation. Serum incubated with 10 mmol of EDTA/liter was used as the negative control. The mixtures were centrifuged at 14,000 × g for 5 min, and the supernatants were stored at −80°C until assessment of C3 levels was performed.
Assessment of C3 levels in serum.
A sandwich enzyme-linked immunosorbent assay (ELISA) was used to quantify C3 in mouse sera (13). Briefly, 96-well microtiter plates were coated with immunoglobulin G anti-mouse C3 (1:3000) (Cappel), washed with PBS, and incubated for 2 h at 37°C with serial dilutions of mouse sera. The plates were washed with PBS containing 0.05% Tween 80 and then incubated with affinity-purified peroxidase-labeled goat anti-mouse C3 diluted 1:500 in PBS (Cappel). After incubation with the peroxidase substrate (o-phenylenediamine dihydrochloride; Sigma) the plates were read at OD492. A titration of pooled normal mouse serum obtained from uninfected WT 129/SvEv mice was used to generate a standard curve. One unit of C3 was defined as the amount of C3 contained in a 1/256,000 dilution of the standard pooled mouse serum (11, 18).
Gastric colonization by H. felis.
To assess the effect of decomplementation on gastric colonization of H. felis, WT and IL-10−/− mice were inoculated with H. felis as described above. Decomplementation with CVF was initiated 1 day prior to inoculation with H. felis and continued throughout the duration of the experiment. The day of the first inoculation of H. felis is referred to as day 1. Stomachs were assessed for colonization with H. felis on day 8. Each in vivo experiment testing the effect of complement depletion on IL-10−/− mice consisted of six WT mice (as a positive control for Helicobacter infection), six IL-10−/− control mice, and six CVF-treated IL-10−/− mice. To test the effect of CVF on Helicobacter infection in WT mice, CVF was administered to six WT mice and the results were compared with those for six WT control mice.
The presence of H. felis was determined on histologic sections stained by using a modified Steiner method (Sigma). Eight longitudinal cross sections of the stomach from each WT or IL-10−/− mouse were examined. The number of infected glands and the number of bacteria in each gland were counted in each section to determine the number of bacteria per section. The data are presented as the mean number of bacteria for all mice in each group, with the value from the section with the highest number of bacteria being used. In WT mice, gastric colonization was uniform and bacterial counts did not vary significantly between sections. With IL-10−/− control (non-CVF-treated) mice, bacteria were not present in any of the multiple stomach cross sections. In a previous study (20), PCR analysis of stomach DNA from infected WT and IL-10−/− mice (day 8 postinoculation) for the 16S rRNA gene with Helicobacter-specific primers (35) and reisolation cultures of Helicobacter validated the quantification obtained with the histochemical method.
Histologic analysis.
Stomachs from WT and IL-10−/− mice were fixed and flattened in 95% ethanol, routinely processed, sectioned at 6 μm, and stained with hematoxylin and eosin (H&E) for light microscopic examination. For each stomach, eight longitudinal sections extending from the gastroesophageal junction to the duodenum were examined. Sections were examined by the same pathologist (R. G. Lynch) without knowledge of the identity of the samples. Because lesions were multifocal and of variable severity, the grade given to any section of stomach took into account the number of lesions as well as their severity. Scores from 0 to 6 were based on the following criteria: grade 0, no change from normal tissue; grade 1, unifocal mild cellular infiltration in the lamina propria, usually located in the distal stomach or at the junction of the squamous and glandular epithelium; grade 2, few multifocal lesions of moderate inflammatory cell infiltrates in the lamina propria; grade 3, lesions over a large area of the mucosa, more frequent than for grade 2; grade 4, more severe lesions than for grade 3 over most of the section; grade 5, moderate inflammation, often involving the submucosa but rarely transmural, with inflammatory cells consisting of a mixture of mononuclear cells and neutrophils and with moderate epithelial metaplasia; grade 6, diffuse and severe inflammation, including transmural inflammation, with inflammatory cells consisting of a mixture of mononuclear cells and neutrophils and with epithelial metaplasia and ulcerations.
Quantification of neutrophil percentage in peripheral blood.
Peripheral blood smears were prepared before CVF administration, 24 h after CVF administration, and at the completion of the experiment (day 8). The smears were stained with Wright-Giemsa, and 100-cell differentials were performed to determine the percentage of circulating neutrophils.
Quantification of neutrophils in stomach.
A myeloperoxidase assay was used to quantify the degree of neutrophil infiltration in the stomachs of the H. felis-infected WT and IL-10−/− mice. The glandular portion of the stomach was weighed and subsequently homogenized in a solution of PBS with 0.5% hexadecyltrimethylammonium bromide.The samples were freeze-thawed three times and centrifuged at 10,000 × g for 20 min. The supernatants were diluted 1:2 in 50 mM NaPO4 buffer, and 20 μl of sample was added to 180 μl of O-dianisidine HCl (0.2 mg/ml in NaPO4 buffer) with or without 0.0006% H2O2. The plates were read at OD450. The values were multiplied by 2.655 × 10−4 to calculate the IU/sample (40) and were normalized to the weight of the tissue.
Isolation and culture of peritoneal neutrophils.
Peritoneal neutrophils were elicited in WT and IL-10−/− mice by an intraperitoneal injection (2 ml) of freshly prepared 2% glycogen (Sigma) in sterile isotonic saline (2). Four hours later, the mice were euthanized and the peritoneal cavity was irrigated three times with PBS. The collected peritoneal exudates were centrifuged at 1500 × g for 7 min. Cytospin slides were prepared and stained with Wright-Geimsa staining. The cells were 88 to 91% neutrophils. Viability was 92 to 95%, as assessed by trypan blue staining. Prior to cell culture, red blood cells were lysed by using a red blood cell lysis buffer (8.26 g of NH4Cl/liter, 37 mg of EDTA-2Na/liter, and 1.0 g of KHCO3/liter). Isolated peritoneal neutrophils from uninfected WT or IL-10−/− mice were cultured at 3 × 106 cells/ml in RPMI 1640 supplemented with 10% fetal calf serum (complement-deactivated)-2 mM l-glutamine-0.05 mM 2-mercaptoethanol-100 U of penicillin/ml-100 U of streptomycin/ml in 48-well tissue culture plates (Costar, Corning, N.Y.). The cells were incubated in medium alone or in medium supplemented with 12-O-tetradecanoylphorbol-13-acetate (TPA). In some experiments, IL-10−/− neutrophils were pretreated with 20 ng of recombinant IL-10 (rIL-10)/ml for 4 h before being cultured and activated with TPA. Supernatants from triplicate cultures were harvested after 20 h and stored at −80°C before being analyzed for C3 levels.
Spleen cell culture.
Spleen cells from IL-10−/− control or H. felis-infected WT mice were cultured at 5 × 106 cells/ml in RPMI 1640 supplemented with 10% fetal calf serum-2 mM l-glutamine-0.05 mM 2-mercaptoethanol-100 U of penicillin/ml-100 U of streptomycin/ml in 12-well tissue culture plates (Costar). The cells were incubated in medium alone or in medium supplemented with H. felis sonicate at 1.0 μg of protein/ml. Supernatants from triplicate cultures were harvested after 48 h and stored at −80°C before being analyzed for C3 levels.
Statistics.
The data are expressed as means ± standard deviations (SDs). Significant differences between experimental groups were evaluated by the nonparametric Mann-Whitney U test. A P value of <0.05 was considered statistically significant.
RESULTS
H. felis fixes complement in vitro.
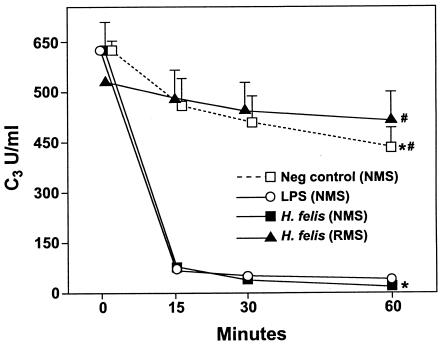
It has previously been reported that IL-10−/− mice rapidly clear gastric Helicobacter infection, whereas in WT mice the gastric burden of Helicobacter increases with time (20). As it has previously been reported that H. pylori is capable of fixing complement (7), we hypothesized that complement may play a role in eradication of gastric Helicobacter. In order to assess the mechanisms underlying Helicobacter eradication in IL-10−/− mice, we first tested whether H. felis would fix complement (Fig. 1). Incubation of H. felis with normal (uninfected) mouse serum resulted in a rapid decline of C3 levels, indicating that H. felis, like H. pylori (7), can fix complement. In contrast, when H. felis was incubated with serum from Rag2−/− mice (which have no serum antibodies and thus cannot activate the classical pathway), C3 levels did not differ from those of the control mice. This finding demonstrates that H. felis can activate complement through the classical pathway (Fig. 1), although a specific anti-Helicobacter antibody does not appear to be required.
FIG. 1.
Activation of complement in vitro by H. felis. Serum from control WT or Rag2−/− mice was incubated with H. felis suspension or LPS for various lengths of time, and C3 levels were subsequently measured by ELISA at various time points. The results are expressed as OD492 ± SD and represent the mean values of the results for a triplicate of a given sample. LPS (positive control), rapidly activated complement; NMS, normal mouse serum; RMS, Rag2−/− mouse serum. An asterisk (*) indicates a P value of <0.001 compared to the results for the control, and a number sign (#) indicates a P value of <0.001 compared to the results for normal mouse serum incubated with H. felis. The results are representative of three independent experiments.
Serum complement levels in H. felis-infected IL-10−/− and WT mice.
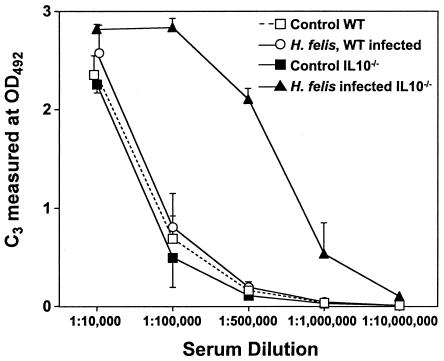
To further assess the role of complement in H. felis-infected mice, we tested whether gastric H. felis infection would alter the levels of complement in serum in WT and IL-10−/− mice. Infection of WT mice with H. felis did not significantly change levels of C3 in serum (Fig. 2). In contrast, levels of C3 in serum were significantly higher in IL-10−/− mice after 1 week of H. felis infection than in uninfected IL-10−/− control mice (Fig. 2).
FIG. 2.
Levels of complement in serum in control and H. felis-infected WT and IL-10−/− mice. C3 levels were determined 8 days after inoculation with H. felis. Levels of C3 in serum are expressed as OD492 in a dilution series of serum. The results are expressed as means ± SDs and represent the mean values of the results for 10 mice within a given group. The results are representative of 10 independent experiments.
Effects of decomplementation on clearance of H. felis infection in WT and IL-10−/− mice.
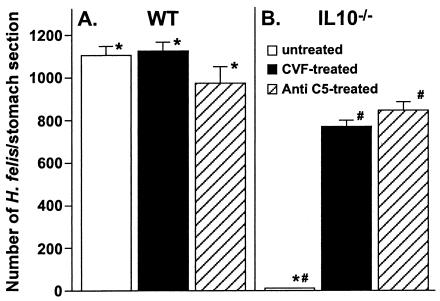
We next assessed the extent to which decomplementation would alter the ability of IL-10−/− mice to clear the gastric Helicobacter infection. Bacterial colonization in both WT and IL-10−/− mice with or without CVF-mediated decomplementation was determined. After 8 days of H. felis infection, all H. felis-infected WT mice were densely colonized (Fig. 3A and 4A). Complement depletion of WT mice did not alter the level of gastric H. felis colonization (Fig. 3A). IL-10−/− control mice were able to completely clear the gastric Helicobacter infection by day 8 (Fig. 3B and 4C). However, CVF-mediated decomplementation of IL-10−/− mice resulted in persistent H. felis infection, with a level of H. felis colonization that was approximately 70% that of WT mice at the same time point (Fig. 3B and 4E). In one experiment, complement depletion was extended to 14 days. Complement depletion of WT mice for 14 days had no effect on colonization by H. felis. In contrast, IL-10−/− mice depleted of complement for 14 days had persistent infection with H. felis at a level that was approximately 51% that of WT mice at the same time point (data not shown). We also assessed the effect of decomplementation mediated by anti-C5 treatment on H. felis infection in WT and IL-10−/− mice. Depletion of complement by anti-C5 treatment had no effect on H. felis infection in WT mice at the time point assessed (Fig. 3A). In contrast, IL-10−/− mice depleted of complement by anti-C5 treatment had persistent infections that were approximately 80% of the level of infection in WT stomach at the same time point (Fig. 3B).
FIG. 3.
Effect of decomplementation on clearance of H. felis infection in WT and IL-10−/− mice. WT mice received intraperitoneal injections of 0.8 μg of CVF/g of body weight every 48 h, and IL-10−/− mice received 3.0 μg of CVF/g every 24 h. The control mice were injected with vehicle. Anti-C5 was given as intraperitoneal injections of 40 mg/kg every 48 h. CVF and anti-C5 injections began 2 days prior to inoculation with H. felis and continued for the duration of the experiment. One week after inoculation, the mice were sacrificed for analysis of H. felis colonization. The results are expressed as the means ± SDs of the results for 4 to 6 mice within a given group. An asterisk (*) indicates a P value of <0.001 compared to the results for WT, and a number sign (#) indicates a P value of <0.001 compared to the results for untreated IL-10−/− mice. The results are representative of three independent experiments.
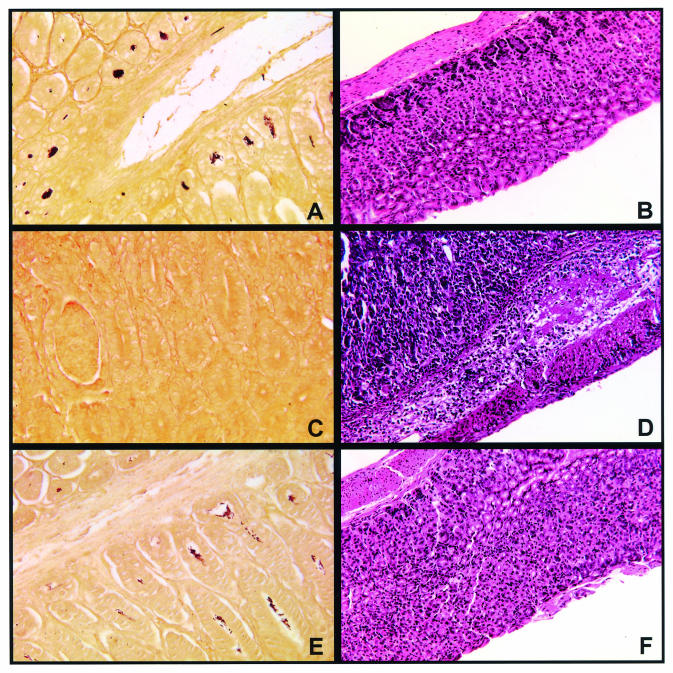
FIG. 4.
H. felis colonization and histopathology of H. felis-infected WT and IL-10−/− mice. Gastric sections from WT and IL-10−/− mice were stained by using a modified Steiner method for the detection of H. felis or with H&E for light microscopic examination. Histologic evaluation was performed 8 days after inoculation with H. felis. (A) Steiner stain of H. felis-infected WT mouse (20× magnification). Note the dense colonization of the gastric glands. (B) H&E stain of H. felis-infected WT mouse (20× magnification). Note the minimal inflammatory response. (C) Steiner stain of H. felis-infected IL-10−/− mouse (20× magnification). Note the absence of Helicobacter. (D) H&E stain of H. felis-infected IL-10−/− mouse (20× magnification). The lamina propria is heavily infiltrated with both mononuclear cells and neutrophils. (E) Steiner stain of H. felis-infected CVF-treated IL-10−/− mouse (20× magnification). Note the colonization of the gastric glands with Helicobacter. (F) H&E stain of H. felis-infected CVF-treated IL-10−/− mouse (20× magnification). Note the absence of cellular infiltrate in the lamina propria.
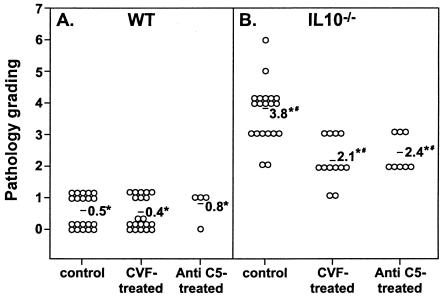
Effect of decomplementation on H. felis-induced gastritis in WT and IL-10−/− mice.
Since decomplementation resulted in persistent H. felis infection in IL-10−/− mice, we next assessed the effect of decomplementation on the development of Helicobacter-induced gastritis. As has been reported in previous studies (5), infection of IL-10−/− mice with Helicobacter results in rapid development of severe gastric inflammation. After 8 days of infection, H. felis-infected IL-10−/− stomachs developed pathological lesions that consisted of diffuse inflammation with intensive cellular infiltration (Fig. 4D and 5B). In contrast, infected WT stomachs showed either no inflammation or a very mild degree of inflammation that was unifocal in nature and distal in location (Fig. 4B and 5A). Decomplementation did not result in any alteration to the minimal gastritis that develops in H. felis-infected WT mice. In contrast, decomplementation of IL-10−/− mice resulted in a 45% decrease in the severity of H. felis-induced pathology (Fig. 4F and 5B). Similarly, decomplementation mediated by anti-C5 treatment in H. felis-infected IL-10−/− mice resulted in a 30% decrease in the severity of H. felis-induced pathology (Fig. 5B).
FIG. 5.
Effect of complement depletion on H. felis-induced gastritis in WT and IL-10−/− mice. Histopathologic evaluation was performed 8 days after inoculation with H. felis. The average pathological score is indicated for each group. An asterisk (*) indicates a P value of <0.001 compared to the results for the WT, and a number sign (#) indicates a P value of <0.001 compared to the results for the IL-10−/− control mice. The results are representative of three independent experiments.
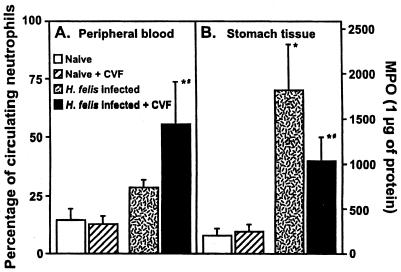
Effect of decomplementation on the neutrophil response in H. felis-infected IL-10−/− mice.
As it has previously been found that depletion of neutrophils delayed Helicobacter clearance and significantly reduced Helicobacter-induced gastritis in IL-10−/− mice (20), we assessed the effect of decomplementation on the neutrophil response in H. felis-infected IL-10−/− mice. CVF treatment of (control) noninfected IL-10−/− mice did not induce a significant increase in the percentage of circulating neutrophils (data not shown). In contrast, CVF treatment of infected IL-10−/− mice resulted in a threefold increase in the percentage of circulating neutrophils (Fig. 6A). We then assessed the effect of decomplementation on the level of neutrophil infiltration in the stomachs of H. felis-infected IL-10−/− mice. Despite the increased percentage of circulating neutrophils in the CVF-treated mice, decomplementation resulted in a 45% decrease in neutrophil infiltration in the stomachs of the H. felis-infected IL-10−/− mice compared to that for the H. felis-infected IL-10−/− control mice (Fig. 6B).
FIG. 6.
(A) Effect of CVF-mediated decomplementation on the percentage of circulating neutrophils in control and H. felis-infected IL-10−/− mice. The percentage of circulating neutrophils was determined at day 8 of H. felis infection or after 8 days of CVF treatment. Smears were stained with Wright-Giemsa, and 100-cell differentials were performed to determine the percentage of circulating neutrophils. (B) Effect of decomplementation on the level of neutrophil infiltration of the stomachs of H. felis-infected IL-10−/− mice, as measured by myeloperoxidase activity. An asterisk (*) indicates a P value of <0.001 compared to the results for the IL-10−/− control mice, and a number sign (#) indicates a P value of <0.001 compared to the results for H. felis-infected non-CVF-treated IL-10−/− mice. The results are representative of two independent experiments. MPO, myeloperoxidase activity.
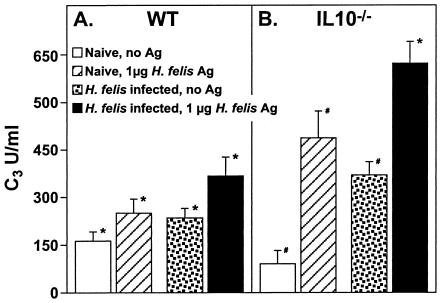
Complement levels in spleen cell cultures from control and H. felis-infected WT and IL-10−/−mice.
We next assessed the level of complement production induced in spleen cell cultures of H. felis-infected WT and IL-10−/− mice. Splenocytes from control and H. felis-infected WT and IL-10−/− mice were cultured with and without sonicated H. felis antigen, and culture supernatants were evaluated for the concentration of C3. Although splenocytes from naïve IL-10−/− mice produced a level of C3 that was 60% of that produced by splenocytes from naïve WT mice (Fig. 7A, B), when stimulated with H. felis antigen, splenocytes from naïve IL-10−/− mice produced a twofold-higher level of C3 than that produced by splenocytes from naïve WT mice (Fig. 7A and B). Stimulated splenocytes from H. felis-infected IL-10−/− mice produced a 10-fold-higher level of C3 than that produced by splenocytes of the unstimulated naïve IL-10−/− mice; this level was also twofold higher than that produced by stimulated splenocytes of H. felis-infected WT mice.
FIG. 7.
C3 production by splenocytes from WT and IL-10−/− mice. (A) Spleen cells were cultured at 5 × 106 cells/ml in complete medium or medium supplemented with H. felis antigen as described in Materials and Methods. After 48 h of culture, supernatants were harvested and analyzed for C3 level by ELISA. Data at each time point are expressed as means ± SDs of the results of observations of 10 mice per group. An asterisk (*) indicates a P value of <0.001 compared to the results for the WT control mice, a number sign (#) indicates a P value of <0.0001 compared to the results for the IL-10−/− control mice, and a plus sign (+) indicates a P value of <0.001 compared to the results for Ag- stimulated H. felis-infected WT mice. The results are representative of four independent experiments.
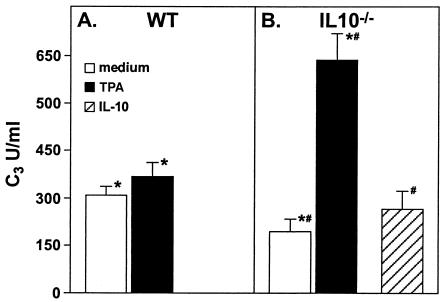
Complement secretion by naïve WT and IL-10−/− peritoneal neutrophils.
Since neutrophils are important mediators of the immune response to H. felis in IL-10−/− mice (20) and are also an important source of complement in inflammation (30, 31), we evaluated the levels of complement secretion by neutrophils isolated from the peritoneal exudates of both naïve IL-10−/− and WT mice. Neutrophils from WT and IL-10−/− mice were cultured with and without TPA stimulation, and culture supernatants were evaluated for the concentration of C3 20 h later. Interestingly, neutrophils from naïve IL-10−/− mice produced 50% of the level of complement secreted by neutrophils from naïve WT mice. However, TPA stimulation induced an approximately fivefold increase in complement secretion from neutrophils of IL-10−/− mice but not from those of WT mice (Fig. 8A and B). When neutrophils from IL-10−/− mice were pretreated with rIL-10, TPA stimulation did not induce a significant increase in complement level (Fig. 8A and B).
FIG. 8.
C3 secretion by peritoneal neutrophils from WT and IL-10−/− mice. Glycogen-elicited neutrophils from uninfected WT or IL-10−/− mice were cultured in control medium or medium supplemented with TPA. Supernatants from triplicate cultures were harvested after 20 h. C3 levels in the samples were evaluated by ELISA. In some experiments, IL-10−/− neutrophils were pretreated with rIL-10 before culture and activation with TPA. C3 levels are plotted as OD492. Results are expressed as means ± SDs and represent the mean value of the results for four mice within a given group. An asterisk (*) indicates a P value of <0.001 compared to the results for WT neutrophils, and a number sign (#) indicates a P value of <0.001 compared to the results for the IL-10−/− control neutrophils. The results are representative of three independent experiments.
DISCUSSION
We have used H. felis-infected WT and IL-10-deficient mice to further define the role of complement in the immune response to gastric Helicobacter infection. Our studies show that H. felis is capable of fixing complement in vitro. We found that infection of IL-10−/− mice with H. felis resulted in a large increase in the levels of complement in serum in IL-10−/− mice. Depletion of complement did not alter the level of gastric colonization or inflammation in WT mice. In contrast, complement depletion of IL-10−/− mice resulted in persistence of the gastric Helicobacter infection and a significant reduction in Helicobacter-induced gastric inflammation, as well as reduced neutrophil infiltration of the stomach. Helicobacter-stimulated production of complement was significantly increased in spleen cells from H. felis-infected IL-10-deficient mice compared to that for infected WT mice, and stimulation of neutrophils from IL-10−/− mice resulted in higher levels of C3 secretion than for WT mice. Taken together, these results suggest that complement is a central mediator of Helicobacter-induced inflammation and clearance of gastric Helicobacter in IL-10−/− mice.
Several lines of evidence have suggested a role for complement in the immune-inflammatory response to gastric Helicobacter infection. The complement system is a key mediator of the immune response to multiple extracellular organisms, including, for example, pneumococci (42), Haemophilus influenzae (29), Candida (24), and Leishmania major (39). Moreover, evidence of complement activation has been demonstrated in gastric biopsies from individuals infected with H. pylori (6). The complement products were present only in those areas associated with Helicobacter, suggesting that H. pylori induced local activation of complement. In the present study, we found that H. felis is capable of complement fixation in the presence of normal serum antibodies but not in Rag2−/− serum, which does not have antibodies. These results show that H. felis, just like H. pylori (7), activates complement through the classical pathway in the absence of a specific antibody to Helicobacter. The mechanism for this phenomenon is not known but could be secondary to activation of complement via low levels of antiendotoxin antibody. Alternatively, the activation of complement may be secondary to antibodies to conserved proteins, such as heat shock proteins. Indeed, we have detected antibody to HSP-70 from H. felis in uninfected mice (data not shown). The finding that H. felis can activate complement suggested that complement may have a role in the immune response to gastric Helicobacter infection.
The potential role of the complement cascade in the development of H. felis-induced gastritis was studied by depleting complement levels in WT and IL-10−/− mice by using anticomplementary CVF. CVF is a peptide fragment of cobra C3 that is capable of activating the alternative complement pathway, but unlike C3b, it is not cleaved by factors H and I (44). As a result, it continues to activate the alternative pathway in an unregulated way, leading to the depletion of several proteins that are necessary for complement function. Persistent treatment with CVF results in severe depletion of C3, factor B, and C5 through C9 and, subsequently, total abolishment of complement activity (17). Complement depletion of WT mice did not affect Helicobacter clearance or development of the minimal gastritis seen in these mice at the time point tested. At this early time point, WT mice have minimal inflammation in response to Helicobacter. With time (months), WT mice can develop significant gastric inflammation and clear the organism. It is possible that complement plays a more important role at this later time point. In contrast to the results for WT mice, complement depletion of IL-10−/− mice resulted in significantly less gastritis in H. felis-infected IL-10−/− mice. Importantly, this finding was associated with a decreased ability to clear H. felis in the decomplemented IL-10−/− mice, as high numbers of H. felis were present in CVF-treated IL-10−/− mice whereas control IL-10−/− mice effectively cleared the Helicobacter. Although the overall pathology grading of anti-C5-treated H. felis-infected IL-10−/− mice demonstrated a 40% decrease in the severity of gastritis, when the cardia, body, and pylorus of the stomach were evaluated separately for pathology, we found that the bodies of the stomachs of all anti-C5-treated mice were completely free of pathology (grade 0), and the number of H. felis organisms was comparable to that for WT mice (data not shown). These data suggest that complement activation plays a role in the eradication of Helicobacter and the development of gastritis in Helicobacter-infected IL-10−/− mice.
To control for the possibility that CVF might have induced another mechanism that altered the course of H. felis infection in IL-10−/− mice, we also depleted complement in H. felis-infected IL-10−/− mice by using a monoclonal antibody specific for the C5 component of complement. This antibody blocks the cleavage of C5 and thus prevents the generation of the potent proinflammatory factors C5a and C5b-9 (47). Anti-C5 treatment induced a similar effect to that of CVF, as the anti-C5-treated IL-10−/− mice did not clear the H. felis infection and had a reduced level of gastritis. Overall, absence of complement prevented clearance of gastric Helicobacter and decreased Helicobacter-induced inflammation in IL-10−/− mice.
There are multiple mechanisms by which complement activation may affect the immune and inflammatory responses to gastric Helicobacter infection. In this study, we demonstrate that H. felis can fix complement in the presence of antibody; thus, H. felis clearance in IL-10−/− mice may be due to direct complement lysis. Several lines of evidence suggest that evasion of complement is important for maintenance of chronic gastric Helicobacter infection. Both H. pylori and H. felis produce the urease enzyme, and the ammonia produced by this enzyme can directly inhibit complement activation (37). In addition, H. pylori is known to survive complement lysis by binding of GPI-anchored protectin (CD59) (34). The enhanced clearance of H. felis from IL-10−/− mice may be secondary to direct bacterial lysis due to the increased levels of complement induced by infection in these mice. However, complement proteins have pleiotropic effects on the immune system and may have indirectly enhanced gastritis and Helicobacter eradication through effects on the immune response to Helicobacter.
Depletion of complement may have impaired Helicobacter clearance in IL-10−/− mice by interfering with homing of the neutrophils into the infected stomach. Complement is a major component of innate immunity and is involved in early protective immune responses against pathogens that occur before the induction of acquired T-cell and B-cell immunity (14). Complement is an important regulator of neutrophil function. For example, C3a (1) and C5a (16) promote chemotaxis of neutrophils toward the site of inflammation; as would be expected, homing and/or extravasation of neutrophils is significantly decreased in CVF-treated rats and mice (43). For example, CVF treatment was reported to reduce accumulation of neutrophils at the site of lung injury (15). Neutrophils are a prominent component of infiltration in the H. pylori-infected stomachs. A previous study found that depletion of neutrophils in IL-10−/− mice delayed clearance of Helicobacter and significantly reduced Helicobacter-induced gastritis (20). Therefore, we hypothesized that decomplementation of IL-10−/− mice could impair migration and extravasation of neutrophils into the stomach, resulting in decreased inflammation and impaired clearance of Helicobacter. Indeed, decomplemented IL-10−/− mice that were infected with H. felis showed a significant increase in neutrophil percentages in peripheral blood, but the myeloperoxidase levels in the stomach showed a significant decrease in neutrophil infiltration. These data suggest that in the absence of complement, emigration of neutrophils into the stomach is diminished, which may lead to a decrease in the ability of mice to clear the infection and to diminished inflammation and pathology.
Absence of complement proteins might indirectly affect the acquired immune system. In the absence of complement, there may be less targeting of microbial antigen to antigen-presenting cells, such as dendritic cells, impairing T-cell activation as a result of impaired antigen presentation. The role of complement in direct regulation of T-cell responses is not completely worked out. A negative regulatory role for complement in cellular immunity was suggested recently by demonstrating that the cross-linking of membrane cofactor protein (CD46) led to suppression of IL-12 production (21). However, murine and human T cells also express complement receptors, and it has been proposed that activated complement functions to facilitate recruitment of T cells to areas of inflammation (22, 27, 41, 48). The impaired recruitment of T cells into the stomach may have contributed to the decreased clearance of H. felis in the decomplemented IL-10−/− mice.
Interestingly, epithelial pathology was not connected to the absolute number of gastric Helicobacter. It is well established that Helicobacter produces bacterial products (e.g., VacA cytotoxin) that have cytotoxic effects on the epithelium (32). However, in this study (and in other animal models of Helicobacter infection) (12), the development of gastric pathology is clearly related to the immune response rather than to the presence of Helicobacter. Interestingly, in our study, the IL-10−/− control mice cleared the Helicobacter infection within 1 week and developed significant gastritis. In contrast, the complement-depleted IL-10−/− mice continued to harbor the bacterium yet had less histologic damage, despite having increased numbers of gastric Helicobacter. The decrease in pathology may have been due to the decreased infiltration of neutrophils. We have found that neutrophil depletion reduced gastritis in H. felis-infected IL-10−/− mice (20). Neutrophils have been postulated to be important mediators of the gastric pathology seen in H. pylori infection, due to their production of tissue-damaging degradative enzymes and reactive oxygen species (45).
Of note, we uncovered a previously unappreciated relationship between IL-10 and the complement system. We found that in vitro stimulation of spleen cells from IL-10−/− mice resulted in a twofold increase in C3 production compared to that for stimulated WT spleen cells. Neutrophils have been identified as a source of C3 at the site of inflammation. Within a few hours after stimulation, neutrophils are able to release stored C3 (30, 31). In this study, we have shown that TPA-stimulated neutrophils from naïve IL-10−/− mice produce twofold more C3 in culture than that produced by stimulated WT neutrophils. This finding implies that in the absence of IL-10, neutrophils might store higher levels of their C3 to be secreted in response to stimulation. Interestingly, IL-10 was able to block the TPA-induced secretion, suggesting a role for IL-10 in the regulation of complement secretion.
Absence of IL-10 may lead to increased complement production due to increased levels of inflammatory cytokines (9). Multiple studies have demonstrated that inflammatory cytokines, such as tumor necrosis factor alpha, IL-1, IL-6, and gamma interferon, can induce increased production of complement from several cell types (19). It has previously been demonstrated that LPS stimulation in the absence of IL-10 results in marked increases in inflammatory cytokine production (e.g., tumor necrosis factor alpha, IL-1, IL-6, and gamma interferon) (4), and we hypothesize that increased inflammatory cytokine production in IL-10-deficient mice may contribute to an increase in the levels of complement in serum. Corresponding to the increased ability of IL-10−/− mice to produce complement, we found that IL-10−/− mice required a sixfold higher dose of CVF to deplete complement than did WT mice.
In summary, our studies indicate that the complement system has a pivotal role in the regulation of the immune and inflammatory response to gastric Helicobacter in IL-10−/− mice. We found that Helicobacter infection of IL-10−/− mice resulted in a marked increase in the levels of complement in serum. Moreover, depletion of complement resulted in decreased gastritis and a decreased ability of IL-10−/− mice to clear gastric Helicobacter infection. These phenomena were correlated with a decrease in neutrophil infiltration into the gastric mucosa. Taken together, these data suggest that complement activation with subsequent induction of neutrophil infiltration may play a key role in the development of gastritis and eradication of gastric Helicobacter infection in IL-10−/− mice.
Acknowledgments
This work was supported by Public Health Service grant R29 CA-76129 from the National Cancer Institute (D.J.B).
Editor: T. R. Kozel
REFERENCES
- 1.Ames, R. S., D. Lee, J. J. Foley, A. J. Jurewicz, M. A. Tornetta, W. Bautsch, B. Settmacher, A. Klos, K. F. Erhard, R. D. Cousins, A. C. Sulpizio, J. P. Hieble, G. McCafferty, K. W. Ward, J. L. Adams, W. E. Bondinell, D. C. Underwood, R. R. Osborn, A. M. Badger, and H. M. Sarau. 2001. Identification of a selective nonpeptide antagonist of the anaphylatoxin C3a receptor that demonstrates antiinflammatory activity in animal models. J. Immunol. 166:6341-6348. [DOI] [PubMed] [Google Scholar]
- 2.Baron, E. J., and R. A. Proctor. 1982. Elicitation of peritoneal polymorphonuclear neutrophils from mice. J. Immunol. Methods 49:305-313. [DOI] [PubMed] [Google Scholar]
- 3.Berg, D. J., N. Davidson, R. Kuhn, W. Muller, S. Menon, G. Holland, L. Thompson-Snipes, M. W. Leach, and D. Rennick. 1996. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4+ TH1-like responses. J. Clin. Investig. 98:1010-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berg, D. J., R. Kuhn, K. Rajewsky, W. Muller, S. Menon, N. Davidson, G. Grunig, and D. Rennick. 1995. Interleukin-10 is a central regulator of the response to LPS in murine models of endotoxic shock and the Shwartzman reaction but not endotoxin tolerance. J. Clin. Investig. 96:2339-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berg, D. J., N. A. Lynch, R. G. Lynch, and D. M. Lauricella. 1998. Rapid development of severe hyperplastic gastritis with gastric epithelial dedifferentiation in Helicobacter felis-infected IL-10−/− mice. Am. J. Pathol. 152:1377-1386. [PMC free article] [PubMed] [Google Scholar]
- 6.Berstad, A. E., P. Brandtzaeg, R. Stave, and T. S. Halstensen. 1997. Epithelium related deposition of activated complement in Helicobacter pylori associated gastritis. Gut 40:196-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berstad, A. E., K. Hogasen, G. Bukholm, A. P. Moran, and P. Brandtzaeg. 2001. Complement activation directly induced by Helicobacter pylori. Gastroenterology 120:1108-1116. [DOI] [PubMed] [Google Scholar]
- 8.Blaser, M. J. 1992. Hypotheses on the pathogenesis and natural history of Helicobacter pylori-induced inflammation. Gastroenterology 102:720-727. [DOI] [PubMed] [Google Scholar]
- 9.Boros, D. L., and J. R. Whitfield. 1998. Endogenous IL-10 regulates IFN-gamma and IL-5 cytokine production and the granulomatous response in Schistosomiasis mansoni-infected mice. Immunology 94:481-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dick-Hegedus, E., and A. Lee. 1991. Use of a mouse model to examine anti-Helicobacter pylori agents. Scand. J. Gastroenterol. 26:909-915. [DOI] [PubMed] [Google Scholar]
- 11.Di Renzo, L., M. R. De Cristofaro, A. Zicari, R. Viola, G. Pontieri, and M. Lipari. 1994. IFN gamma and TNF alpha cause an increased release of C3 by murine macrophages. Immunol. Lett. 42:167-172. [DOI] [PubMed] [Google Scholar]
- 12.Eaton, K. A., M. Mefford, and T. Thevenot. 2001. The role of T cell subsets and cytokines in the pathogenesis of Helicobacter pylori gastritis in mice. J. Immunol. 166:7456-7461. [DOI] [PubMed] [Google Scholar]
- 13.Faried, H. F., T. Tachibana, and T. Okuda. 1993. The secretion of the third component of complement (C3) by human polymorphonuclear leucocytes from both normal and systemic lupus erythematosus cases. Scand. J. Immunol. 37:19-28. [DOI] [PubMed] [Google Scholar]
- 14.Fearon, D. T., and R. M. Locksley. 1996. The instructive role of innate immunity in the acquired immune response. Science 272:50-53. [DOI] [PubMed] [Google Scholar]
- 15.Ferrante, A., A. J. Martin, E. J. Bates, D. H. Goh, D. P. Harvey, D. Parsons, D. A. Rathjen, G. Russ, and J. M. Dayer. 1993. Killing of Staphylococcus aureus by tumor necrosis factor-alpha-activated neutrophils. The role of serum opsonins, integrin receptors, respiratory burst, and degranulation. J. Immunol. 151:4821-4828. [PubMed] [Google Scholar]
- 16.Foreman, K. E., M. M. Glovsky, R. L. Warner, S. J. Horvath, and P. A. Ward. 1996. Comparative effect of C3a and C5a on adhesion molecule expression on neutrophils and endothelial cells. Inflammation 20:1-9. [DOI] [PubMed] [Google Scholar]
- 17.Frank, M. M. 1995. Animal models for complement deficiencies. J. Clin. Immunol. 15:113S-121S. [DOI] [PubMed] [Google Scholar]
- 18.Garred, P., T. E. Mollnes, and T. Lea. 1988. Quantification in enzyme-linked immunosorbent assay of a C3 neoepitope expressed on activated human complement factor C3. Scand. J. Immunol. 27:329-335. [DOI] [PubMed] [Google Scholar]
- 19.Gerritsma, J. S., A. F. Gerritsen, M. De Ley, L. A. van Es, and M. R. Daha. 1997. Interferon-gamma induces biosynthesis of complement components C2, C4 and factor H by human proximal tubular epithelial cells. Cytokine 9:276-283. [DOI] [PubMed] [Google Scholar]
- 20.Ismail, H. F., P. Fick, J. Zhang, R. G. Lynch, and D. J. Berg. 2003. Depletion of neutrophils in IL-10−/− mice delays clearance of gastric Helicobacter infection and decreases the Th1 immune response to Helicobacter. J. Immunol. 170:3782-3789. [DOI] [PubMed] [Google Scholar]
- 21.Karp, C. L., M. Wysocka, L. M. Wahl, J. M. Ahearn, P. J. Cuomo, B. Sherry, G. Trinchieri, and D. E. Griffin. 1996. Mechanism of suppression of cell-mediated immunity by measles virus. Science 273:228-231. [DOI] [PubMed] [Google Scholar]
- 22.Kaya, Z., M. Afanasyeva, Y. Wang, K. M. Dohmen, J. Schlichting, T. Tretter, D. Fairweather, V. M. Holers, and N. R. Rose. 2001. Contribution of the innate immune system to autoimmune myocarditis: a role for complement. Nat. Immunol. 2:739-745. [DOI] [PubMed] [Google Scholar]
- 23.Lee, A. 1995. Helicobacter infections in laboratory animals: a model for gastric neoplasias? Ann. Med. 27:575-582. [DOI] [PubMed] [Google Scholar]
- 24.Marodi, L., H. M. Korchak, and R. B. Johnston, Jr. 1991. Mechanisms of host defense against Candida species. I. Phagocytosis by monocytes and monocyte-derived macrophages. J. Immunol. 146:2783-2789. [PubMed] [Google Scholar]
- 25.Marshall, B. J., and J. R. Warren. 1984. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet i:1311-1315. [DOI] [PubMed] [Google Scholar]
- 26.Mohammadi, M., S. Czinn, R. Redline, and J. Nedrud. 1996. Helicobacter-specific cell-mediated immune responses display a predominant Th1 phenotype and promote a delayed-type hypersensitivity response in the stomachs of mice. J. Immunol. 156:4729-4738. [PubMed] [Google Scholar]
- 27.Nataf, S., N. Davoust, R. S. Ames, and S. R. Barnum. 1999. Human T cells express the C5a receptor and are chemoattracted to C5a. J. Immunol. 162:4018-4023. [PubMed] [Google Scholar]
- 28.Nedrud, J. G., and T. G. Blanchard. 2000. Helicobacter animal models, p. 19.8.1-19.8.26. In J. E. Coligan, A. M. Kruisbeek, D. H. Margulies, E. M. Shevach, and W. Strober (ed.), Current protocols in immunology. John Wiley and Sons, New York, N.Y. [DOI] [PubMed]
- 29.Noel, G. J., D. M. Mosser, and P. J. Edelson. 1990. Role of complement in mouse macrophage binding of Haemophilus influenzae type b. J. Clin. Investig. 85:208-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okuda, T. 1991. Murine polymorphonuclear leukocytes synthesize and secrete the third component and factor B of complement. Int. Immunol. 3:293-296. [DOI] [PubMed] [Google Scholar]
- 31.Okuda, T., and T. Tachibana. 1992. Secretion and function of the third component of complement (C3) by murine leukocytes. Int. Immunol. 4:681-690. [DOI] [PubMed] [Google Scholar]
- 32.Papini, E., B. Satin, M. de Bernard, M. Molinari, B. Arico, C. Galli, J. R. Telford, R. Rappuoli, and C. Montecucco. 1998. Action site and cellular effects of cytotoxin VacA produced by Helicobacter pylori. Folia Microbiol. 43:279-284. [DOI] [PubMed] [Google Scholar]
- 33.Paster, B. J., A. Lee, J. G. Fox, F. E. Dewhirst, L. A. Tordoff, G. J. Fraser, J. L. O'Rourke, N. S. Taylor, and R. Ferrero. 1991. Phylogeny of Helicobacter felis sp. nov., Helicobacter mustelae, and related bacteria. Int. J. Syst. Bacteriol. 41:31-38. [DOI] [PubMed] [Google Scholar]
- 34.Rautemaa, R., H. Rautelin, P. Puolakkainen, A. Kokkola, P. Karkkainen, and S. Meri. 2001. Survival of Helicobacter pylori from complement lysis by binding of GPI-anchored protectin (CD59). Gastroenterology 120:470-479. [DOI] [PubMed] [Google Scholar]
- 35.Riley, L. K., C. L. Franklin, R. R. Hook, Jr., and C. Besch-Williford. 1996. Identification of murine helicobacters by PCR and restriction enzyme analyses. J. Clin. Microbiol. 34:942-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rinder, C. S., H. M. Rinder, B. R. Smith, J. C. Fitch, M. J. Smith, J. B. Tracey, L. A. Matis, S. P. Squinto, and S. A. Rollins. 1995. Blockade of C5a and C5b-9 generation inhibits leukocyte and platelet activation during extracorporeal circulation. J. Clin. Investig. 96:1564-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rokita, E., A. Makristathis, E. Presterl, M. L. Rotter, and A. M. Hirschl. 1998. Helicobacter pylori urease significantly reduces opsonization by human complement. J. Infect. Dis. 178:1521-1525. [DOI] [PubMed] [Google Scholar]
- 38.Roth, K. A., S. B. Kapadia, S. M. Martin, and R. G. Lorenz. 1999. Cellular immune responses are essential for the development of Helicobacter felis-associated gastric pathology. J. Immunol. 163:1490-1497. [PubMed] [Google Scholar]
- 39.Scharton-Kersten, T., and P. Scott. 1995. The role of the innate immune response in Th1 cell development following Leishmania major infection. J. Leukoc. Biol. 57:515-522. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki, K., H. Ota, S. Sasagawa, T. Sakatani, and T. Fujikura. 1983. Assay method for myeloperoxidase in human polymorphonuclear leukocytes. Anal. Biochem. 132:345-352. [DOI] [PubMed] [Google Scholar]
- 41.Tsuji, R. F., I. Kawikova, R. Ramabhadran, M. Akahira-Azuma, D. Taub, T. E. Hugli, C. Gerard, and P. W. Askenase. 2000. Early local generation of C5a initiates the elicitation of contact sensitivity by leading to early T cell recruitment. J. Immunol. 165:1588-1598. [DOI] [PubMed] [Google Scholar]
- 42.Tuomanen, E., B. Hengstler, O. Zak, and A. Tomasz. 1986. The role of complement in inflammation during experimental pneumococcal meningitis. Microb. Pathog. 1:15-32. [DOI] [PubMed] [Google Scholar]
- 43.Ulich, T. R., K. M. Bannister, and C. B. Wilson. 1987. Inhibition of the neutrophilic infiltrate of experimental tubulointerstitial nephritis in the Brown-Norway rat by decomplementation. Clin. Immunol. Immunopathol. 42:288-297. [DOI] [PubMed] [Google Scholar]
- 44.Vogel, C. W. 1991. Cobra venom factor: the complement-activating protein of cobra venom, p. 147-188. Handbook of natural toxins. Marcel Decker, Inc., New York, N.Y.
- 45.Wallace, J. L. 1991. Possible mechanisms and mediators of gastritis associated with Helicobacter pylori infection. Scand. J. Gastroenterol. 187(Suppl.):65-70. [PubMed] [Google Scholar]
- 46.Wang, Y., Q. Hu, J. A. Madri, S. A. Rollins, A. Chodera, and L. A. Matis. 1996. Amelioration of lupus-like autoimmune disease in NZB/WF1 mice after treatment with a blocking monoclonal antibody specific for complement component C5. Proc. Natl. Acad. Sci. USA 93:8563-8568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang, Y., S. A. Rollins, J. A. Madri, and L. A. Matis. 1995. Anti-C5 monoclonal antibody therapy prevents collagen-induced arthritis and ameliorates established disease. Proc. Natl. Acad. Sci. USA 92:8955-8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Werfel, T., K. Kirchhoff, M. Wittmann, G. Begemann, A. Kapp, F. Heidenreich, O. Gotze, and J. Zwirner. 2000. Activated human T lymphocytes express a functional C3a receptor. J. Immunol. 165:6599-6605. [DOI] [PubMed] [Google Scholar]
- 49.Yamaoka, Y., M. Kita, T. Kodama, N. Sawai, K. Kashima, and I. Imanishi. 1995. Expression of cytokine mRNA in gastric mucosa with Helicobacter pylori infection. Scand. J. Gastroenterol. 30:1153-1159. [DOI] [PubMed] [Google Scholar]