The ability to create materials with well-controlled structures on the nanometer length scale is of intense interest for a variety of applications,[1,2] including controlled drug delivery[3] and biomedical devices.[4] Preparing nanoscale objects using self-assembly and templated growth techniques has been described in some recent reviews.[2,5] For example, porous membranes can be used to synthesize desired materials within the pores.[4,6] Conducting polymers are of considerable interest for a variety of biomedical applications.[7] Their response to electrochemical oxidation or reduction can produce a change in conductivity, color,[8,9] and volume.[10] A change in the electronic charge is accompanied by an equivalent change in the ionic charge, which requires mass transport between the polymer and electrolyte.[11] When counterions enter a polymer it expands and when they exit it contracts. The extent of expansion or contraction depends on the number and size of ions exchanged.[12] Electrochemical actuators using conducting polymers based on this principle have been developed by several investigators.[13–15] They can be doped with bioactive drugs, and can be used in actuators such as microfluidic pumps.[16,17] The precisely controlled local release of anti-inflammatory drugs at desired points in time is important for treating the inflammatory response of neural prosthetic devices in the central and peripheral nervous systems.[18] Here we report on a method to prepare conducting-polymer nanotubes that can be used for precisely controlled drug release. The fabrication process involves electrospinning of a biodegradable polymer, into which a drug has been incorporated, followed by electrochemical deposition of a conducting-polymer around the drug-loaded, electrospun biodegradable polymers. The conducting-polymer nanotubes significantly decrease the impedance and increase the charge capacity of the recording electrode sites on microfabricated neural prosthetic devices. The drugs can be released from the nanotubes in a desired fashion by electrical stimulation of the nanotubes; this process presumably proceeds by a local dilation of the tube that then promotes mass transport.
Microelectrode neural probes facilitate the functional stimulation or recording of neurons in the central nervous system and peripheral nervous system. We have prepared conducting polymers such as polypyrrole (PPy) and poly(3,4-ethylenedioxythiophene) (PEDOT) in templated nanostructures for neural prosthesis applications.[19,20] Minimizing the electrode impedance is an important requirement for obtaining high-quality signals (high signal-to-noise ratio).[21] We have shown previously that PPy and PEDOT can decrease the impedance of the recording sites.[19,20,22] Here we show that PEDOT nanotubes that have a well-defined internal and external surface texture further decrease the electrode impedance by increasing the effective surface area for ionic-to-electronic charge transfer to occur at the interface between brain tissue and the recording site. We demonstrate that the release of dexamethasone can be precisely controlled by external electrical stimulation of PEDOT nanotubes. In order to produce the nanotubular conducting polymers, nanofibers of biodegradable poly(l-lactide) (PLLA) or poly(lactide-co-glycolide) (PLGA) were first electrospun onto the surface of a neural probe followed by electrochemical deposition of conducting polymers around the electrospun nanofibers. In a final step, the fiber templates can be removed or allowed to slowly degrade, providing additional means of controlled delivery of biologically active agents incorporated into the fibers themselves (Fig. 1).
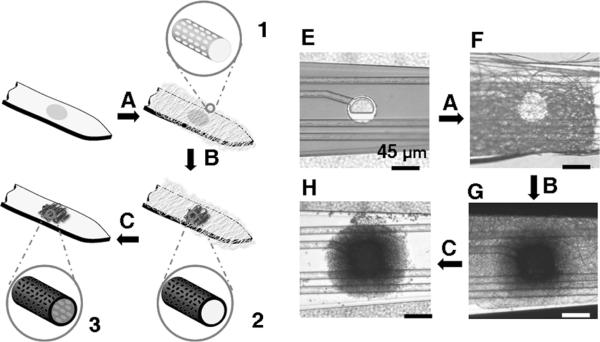
Figure 1.
Schematic diagrams illustrating the surface modification of neural microelectrodes to create nanotubular PEDOT: A) electrospinning of biodegradable polymer (PLGA) fibers with well-defined surface texture (1) on the probe tip, B) electrochemical polymerization of conducting polymers (PEDOT) (2) around the electrospun fibers, and C) dissolving the electrospun core fibers to create nanotubular conducting polymers (3). Optical micrograph of: E) the gold electrode site, F) the electrode site after electrospinning showing the coverage of the PLGA electrospun nanoscale fibers, G) the electrode after electrochemical deposition of PEDOT on the gold site and around the electrospun fibers, and H) the electrode after removal of the core nanoscale fiber templates.
PLLA and PLGA were considered as suitable polymers for the template since they can be readily processed into nano-scale fibers, are stable during electrochemical deposition of the conducting-polymer coating, and can be easily removed under conditions that leave the wall material intact.[23] PLLA/PLGA nanoscale fibers and dexamethasone-incorporated PLGA nanoscale fibers were collected on the microfabricated electrode tips and gold-coated silicon wafers, respectively. The diameters of the nanoscale fibers ranged from 40–500 nm with the majority being in the range 100–200 nm (Fig. 2A). The nanoscale fibers were distributed randomly on the surface of the probe (Fig. 1F). The thickness of the resulting fiber mesh depended on the duration of the electrospinning process. When using a volatile solvent such as chloroform and dichloromethane, a well-defined surface texture was observed on the surface of the electrospun fibers (Fig. 2A).[24] This rough surface morphology is useful for templating a similar structure into the subsequently deposited PEDOT coating.
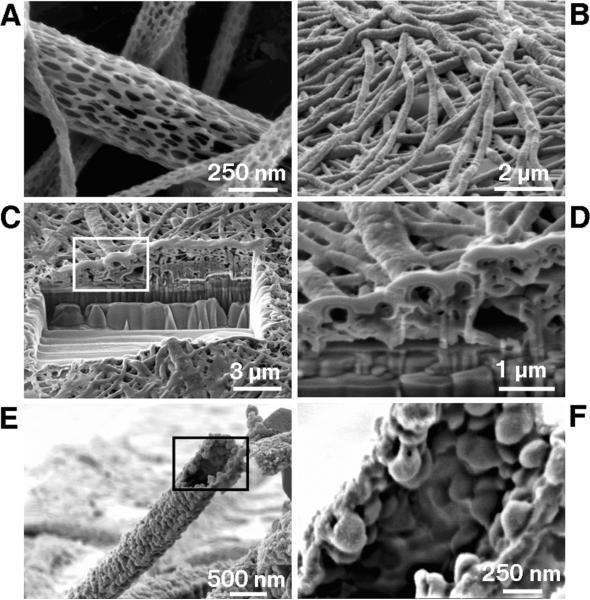
Figure 2.
Scanning electron micrographs of PLGA nanoscale fibers and PEDOT nanotubes. A) diameters of the PLGA fibers were distributed over the range 40–500 nm with the majority being between 100–200 nm. B) Electropolymerized PEDOT nanotubes on the electrode site of an acute neural probe tip after removing the PLGA core fibers. C) A section of (B) cut with a FIB showing the silicon substrate layer and PEDOT nanoscale fiber coating. D) Higher-magnification image of (C) showing the PEDOT nanotubes crossing each other. E) A single PEDOT nanotube which was polymerized around a PLGA nanoscale fiber, followed by dissolution of the PLGA core fiber. This image shows the external texture at the surface of the nanotube. F) Higher-magnification image of a single PEDOT nanotube demonstrating the textured morphology that has been directly replicated from the external surface of the electrospun PLGA fiber templates. The average wall thickness of PEDOT nanotubes varied from 50–100 nm, with the nanotube diameters ranging from 100 to 600 nm.
Most of our work has focused on the oxidative electrochemical polymerization of PPy, PEDOT, and PEDOT derivatives.[19,20,22,25] After preparation of the PLGA and PLGA/dexamethasone nanoscale fibers, PEDOT doped with phosphate-buffered saline (PBS) was electrochemically polymerized on the electrode sites. After PEDOT deposition, the PLGA fibers were removed by soaking in dichloromethane for 5 min. Figures 1E–H display optical micrographs of acute neural probes during the surface modification process. PEDOT was grown on the gold metal electrode sites and then grown up and around the PLGA nanoscale fibers by creating three-dimensional (3D) nanotubular structures of PEDOT (Fig. 2B). Focused-ion-beam (FIB) instruments are useful for local nanoscale machining and structural characterization.[26] We used the FIB to make cross sections of the PEDOT nano-fibers deposited on the microfabricated electrodes. As shown in Figures 2C,D the PEDOT nanofibers are hollow as expected from the impedance results. Figures 2E,F show the hollow fiber morphology of PEDOT with well-defined internal and external texture. The internal texture is replicated from the external texture of PLLA/PLGA electrospun nano-scale fibers.[27] The wall thickness of the PEDOT nanotubes varies from 50 to 100 nm, and the nanotube diameter ranges from 100 to 600 nm. By controlling the polymerization time, we could reproducibly prepare tubular structures with thin or thick walls by using shorter or longer deposition times, respectively.
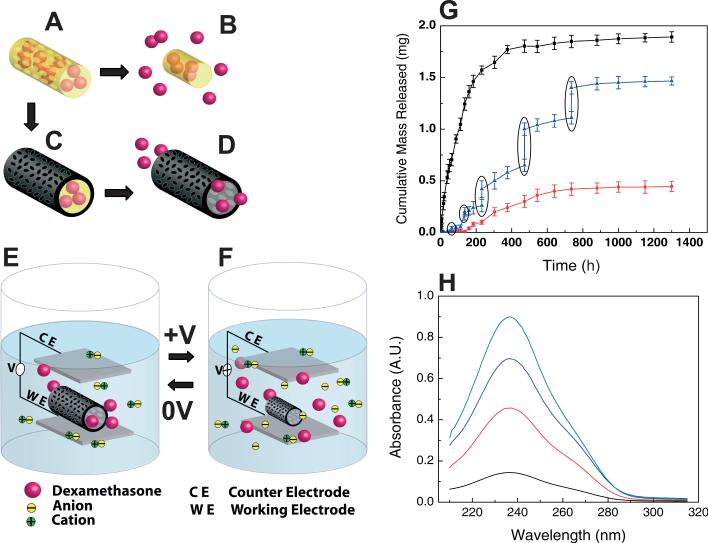
Since it has been reported that conducting polymers actuate under applied voltages,[11,15] we hypothesized that these nanotubes could be used to deliver small amounts of bioactive species. This task can be accomplished either by passive delivery resulting from controlled degradation of the PLLA/PLGA or other matrix polymer used in the core (Figs. 3C,D)[28] or by actively actuating the drug-loaded nanotubes with an applied electrical field (Fig. 3F). UV spectrophotometry was used to determine the release profile of dexamethasone from the PLGA nanoscale fibers and PEDOT nanotubes. We monitored the absorption at a wavelength of 237 nm, which corresponds to the absorbance peak of dexamethasone. The amount of incorporated dexamethasone within PLGA nano-scale fibers on each sample was approximately 2 mg. We found that about 75 % of the dexamethasone loaded in PLGA nanoscale fibers was released after seven days as a result of fast hydrolytic degradation by means of bulk erosion and backbone cleavage of PLGA fibers. Adding the PEDOT sheath layer around the drug-loaded electrospun PLGA nanoscale fibers dramatically slowed down the rate of drug release. Less than 25 % of the dexamethasone was released after 54 days, presumably because the diffusion of dexamethasone through the walls of the PEDOT nanotubes was difficult, requiring the molecules to migrate to the tube ends before being released. On the other hand, a small portion of drug diffused into the solution in the absence of any PEDOT actuation, while the reminder was trapped inside the PEDOT nanotubes. We successfully demonstrated controlled release of the drug by electrical stimulation of the PEDOT nanotubes. To electrochemically control the nanotube actuation, we used an Autolab PGSTAT 12 galvanostat/potentiostat with a conventional four-electrode configuration. We used platinum wire as the counter electrode and Ag/AgCl as the reference electrode. The drug-loaded PEDOT nanotubes were actuated by applying a positive voltage of 1 V with a scan rate of 0.1 V s–1 for 10 s (charge density 0.8 C cm–2) at five specific times. As illustrated in Figure 3F, during reduction of the PEDOT nanotubes (positive voltage bias), electrons were injected into the chains and positive charges in the polymer chains were compensated. To maintain overall charge neutrality, negatively charged counterions were expelled towards the solution and the nanotubes contract.[12,29] PEDOT contraction then produced a mechanical force creating pressure within the nanotubes. The hydrodynamic force inside the nanotubes caused expulsion of PLGA degradation products and dexamethasone, presumably either through the ends of PEDOT nanotubes or though openings or cracks on the surface of the nanotubes created by actuation. A control experiment was also carried out to show that dexamethasone did not exhibit significant diffusion though the PEDOT nanotube walls. In this experiment, dexamethasone was incorporated inside the PEDOT film layers; no dexamethasone elution into the media was detected. After electrical excitation a significant increase in the amount of dexamethasone released was observed (Fig. 3G). The externally applied voltage bias provides a means of controlling the release of the drug, as has been seen for other conducting polymers.[17] Here, the expansion and contraction of the nanotube provides an additional means for controlling the kinetics of drug release. It was anticipated that the amount of drug release would be directly related to the contraction force and the duration of the contraction, as controlled by the externally applied voltage.
Figure 3.
Schematic illustration of the controlled release of dexamethasone: A) dexamethasone-loaded electrospun PLGA, B) hydrolytic degradation of PLGA fibers leading to release of the drug, and C) electrochemical deposition of PEDOT around the dexamethasone-loaded electrospun PLGA fiber slows down the release of dexamethasone (D). E) PEDOT nanotubes in a neutral electrical condition. F) External electrical stimulation controls the release of dexamethasone from the PEDOT nanotubes due to contraction or expansion of the PEDOT. By applying a positive voltage, electrons are injected into the chains and positive charges in the polymer chains are compensated. To maintain overall charge neutrality, counterions are expelled towards the solution and the nanotubes contract. This shrinkage causes the drugs to come out of the ends of tubes. G) Cumulative mass release of dexamethasone from: PLGA nanoscale fibers (black squares), PEDOT-coated PLGA nanoscale fibers (red circles) without electrical stimulation, and PEDOT-coated PLGA nanoscale fibers with electrical stimulation of 1 V applied at the five specific times indicated by the circled data points (blue triangles). H) UV absorption of dexamethasone-loaded PEDOT nanotubes after 16 h (black), 87 h (red), 160 h (blue), and 730 h (green). The UV spectra of dexamethasone have peaks at a wavelength of 237 nm. Data are shown with a ± standard deviation (n = 15 for each case).
Electrochemical impedance spectroscopy was used to explore the conductivity of the polymer coatings over the frequency range 1 Hz–100 kHz. The impedances at 1 kHz are particularly important because they correspond to the characteristic frequency of neuronal-action potentials.[30] The electrode's impedance across all frequencies was moderately increased by electrospinning of the non-conductive layer of PLGA fibers. Specifically the impedance at 1 kHz was increased by about two orders of magnitude (Fig. 4A). However, this impedance significantly decreased with subsequent deposition of the conducting-polymer nanotubes; specifically the impedance at 1 kHz was decreased by about four orders of magnitude, a net decrease of two orders of magnitude from an unmodified electrode (Fig. 4A). By monitoring the impedance as a function of deposition time at 1 kHz, we found that the impedance initially decreased dramatically and then slowly increased, as has been seen previously for other conducting-polymer films.[19,20,31]
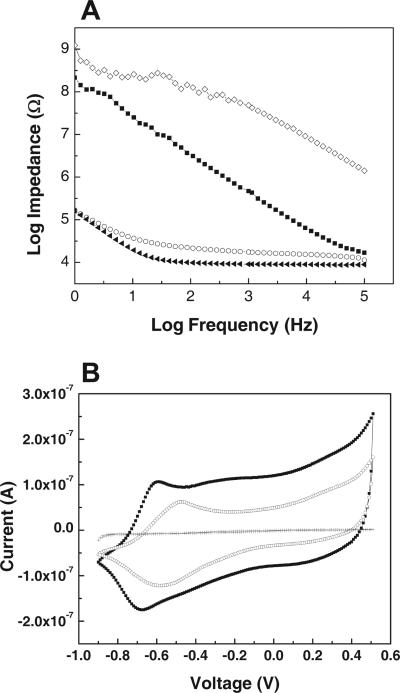
Figure 4.
Impedance spectroscopy and CV: A) impedance spectrum of an acute neural probe site over a frequency range of 1–105 kHz: bare gold (filled squares), with electrospun PLGA fiber templates (diamonds), with PLGA fibers and electrochemical polymerized PEDOT (circles), or with PEDOT nanotubes by removing the PLGA core fibers (filled triangles). B) CV of an acute neural probe: bare gold (crosses), with PLGA electrospun fiber templates and electrochemical deposition of PEDOT (circles), or with PEDOT nanotube fibers prepared by removing the PLGA core fibers (filled squares). The potential was swept from –0.9 to 0.5 V at a scan rate of 100 mV s–1.
The initial impedance of the bare gold sites was 800 ± 20 kΩ for acute (1250 μm2) and 4 ± 0.08 MΩ for chronic (1600 μm2) probes at 1 kHz. The corresponding values of impedance decreased to a minimum of 8 ± 2kΩ (acute probes, after 18 μC of total deposition charge) and 1.0 ± 0.2 kΩ (chronic probes, after 18 μC of total deposition charge) by growing PEDOT around the PLGA nanoscale fibers. These values decreased further to 4 ± 1kΩ (acute) and 800 ± 85 Ω (chronic) after removing the PLGA template fibers and creating nanotubular PEDOT (Fig. 4A). These extremely low values of electrode impedance are expected to significantly enhance the performance of these probes in vivo. This method has resulted in the largest decrease of the 1 kHz impedance multiplied by electrode area (5 MΩ × μm2 for acute and 1.3 MΩ × μm2 for chronic) of any coating design developed to date in our laboratory or reported in literature.[31]
Cyclic voltammetry (CV) was used to investigate the charge-transfer capacity through the electrodes after surface modification with PEDOT nanotubes. The microelectrode was swept through a potential of –0.9 to 0.5 V at a scan rate of 100 mV s–1. During CV, the coating undergoes reduction and oxidation due to movement of counterions in and out of the PEDOT coatings. The oxidization and reduction reactions involve current flow through PEDOT that appears as peaks (Ipc and Ipa, cathodic and anodic current peaks, respectively) on the CV graph. As shown in Figure 4B, the peak value increased from 0.16 to 0.25 μA by removing the PLLA/PLGA core fibers from the PEDOT shell fibers, evidently due to the corresponding increase in the effective surface area. The charge-transfer capacity was also calculated from the integrated area of the CV graph. The charge-transfer capacity of the electrode site increased from 0.001 ± 10–4 to 2.8 ± 0.5 μC (about three orders of magnitude) by growing PEDOT around the PLLA/PLGA nanofibers. This value increased significantly to 4.9 ± 0.6 μC after making the PEDOT nanotubes. Scanning electron microscopy images of the electrode sites show the growth of PEDOT around the PLGA fibers after electrochemical deposition. PEDOT nucleated on gold at the electrode site and grew around the PLLA/PLGA nanoscale fibers creating a 3D mesh of PEDOT nanoscale fibers (Fig. 2B).
In summary, we have demonstrated that the impedance of the neural microelectrodes can be significantly decreased (by about two orders of magnitude) and the charge-transfer capacity significantly increased (about three orders of magnitude) by creating conducting-polymer nanotubes on a gold electrode surface. We can precisely release individual drugs and bioactive molecules at desired points in time by using electrical stimulation of the nanotubes. We believe this method provides a generally useful means for creating low impedance, biologically active polymer coatings, which will facilitate integration of electronically active devices with living tissues. Other potential biomedical applications of these molecule-eluting, electrically active polymer nanotubes include highly localized stimulation of neurite outgrowth and guidance for neural tissue regeneration, and spatially and temporally controlled drug delivery for ablation of specific cell populations. We are actively investigating the use of these materials for functionalizing microelectrodes on neural prostheses and biosensors. However, we also expect this technique to be of interest for application within a broad range of fields such as organic chemistry, biomedical engineering, and pharmacology.
Experimental
Materials
PLLA (RESOMER L 210) with an inherent viscosity of 3.3–4.3 dL g–1 was purchased from Boehringer Ingelheim Pharma GmbH & Co. (KG, Germany). PLGA 75/25 with an inherent viscosity of 0.5–0.6 dL g–1 was prepared from Alkermes Inc. Dexamethasone (molecular weight: 392.5 g mol–1) was purchased from Alexis Corporation. 3,4-Ethylenedioxythiophene (EDOT, BAYTRON® M) with a molecular weight of 142.17 g mol–1 was received from H.C. Starck Inc. (Newton, MA). PBS (pH 7.4) was purchased from Mediatech Inc.
Fabrication of Electrospun Nanoscale Fiber Templates
PLLA/ PLGA and PLGA nanoscale fibers loaded with dexamethasone were prepared by electrospinning. The PLLA solution was prepared by dissolving 0.72 g PLLA in 10 mL of chloroform at a temperature of 50 °C for 10 h in order to have a homogenous solution with PLLA concentration of 4 % (w/v). The PLGA solutions consisting of 2.7 g PLGA and 10 mL chloroform were stirred at a temperature of 55 °C for 5 h to obtain a homogenous solution with a concentration of 15 % (w/v). A mixture of 2.5 g PLGA and 0.675 g dexamethasone was added to 9 mL chloroform and this solution was stirred at a temperature of 55 °C for 10 h to prepare a homogenous solution with a concentration of 15 % (w/v) PLGA and 3.75 % dexamethasone. PLGA and dexamethasone-loaded PLGA nanoscale fibers were directly deposited on ten microfabricated electrode arrays and forty five gold-coated silicon wafers (2 cm × 2 cm), respectively by electrospinning. The electrospinning process was carried out in an electrical field of 0.6 kV cm–1 with a flow rate of 0.25 mL h–1. The neural probes were held at a distance of 11 cm from the syringe needle.
Electrochemical Deposition of Conducting Polymers
The electro-chemical process was performed for acute and chronic probes on each electrode site by using an Autolab PGSTAT 12 (EcoChemie, Utrecht, The Netherlands) in galvanostatic mode with a conventional four-electrode configuration at room temperature. EDOT monomer (21.4 μL) was added to 10 mL of PBS and stirred at room temperature to obtain a EDOT concentration of 0.02 M. The solution was purged with nitrogen gas for 20 min. The working and sensing electrodes were connected to the electrode site. The reference and counter electrode were connected to a platinum wire in the EDOT–PBS solution. EDOT was polymerized on each electrode site and around the PLGA- and PLGA-loaded dexamethasone nanoscale fibers at a current density of 0.9 mA cm–2 for 30 min. After electrochemical deposition was complete, the PLGA core fibers were removed by soaking the probe tips in dichloromethane for 10 min.
Electrochemical Impedance Spectroscopy
An Autolab PGSTAT 12 and Frequency Response Analyzer software were used to record impedance spectra of electrode sites for ten neural probes. A solution of 0.1 M PBS (pH 7) was used as the electrolyte in a four-electrode cell. The working electrode was connected to the electrode site through a connector. The counter electrode was connected to a platinum foil that was placed in a glass container. An Ag/AgCl reference electrode and the neural microelectrode tip were immersed in a glass container of the electrolyte. An alternating current sinusoidal signal, 5 mV in amplitude, was used to record the impedance over a frequency range of 1–105 Hz.
Cyclic Voltammetry
CV was performed for ten neural probes using an Autolab 12 instrument in a four-electrode configuration, as described earlier. A scan rate of 100 mV s–1 was used, and the potential applied to the working electrode was swept between –0.9 and 0.5 V. Before each CV curve was recorded, several cycles were swept to insure that the film had reached a stable state. The data was captured by using general purpose electrochemical software (GPES).
In-Vitro Release Study
The release of dexamethasone from PLGA and PEDOT-coated PLGA nanoscale fibers (PEDOT nanotubes) for fifteen samples (total forty five samples) was monitored as a function of incubation time in PBS at 37 °C. The samples were immersed in 50 mL of PBS (pH 7.4). At specific times the concentration of dexamethasone in the solution was determined by a DU UV-vis Spectrophotometer (Beckman Coulter Inc.) at 237 nm.
Electrical Stimulation of Drug-Loaded PEDOT Nanotubes
To electrochemically control the nanotube actuation, an Autolab, PGSTAT 12 (EcoChemie, Utrecht, The Netherlands) galvanostat/potentiostat, was used with a conventional four-electrode configuration. We used platinum wire as the counter electrode and Ag/AgCl as the reference electrode. The drug-loaded PEDOT nanotubes were actuated by applying a positive voltage of 1 V with scan rate of 0.1 V s–1 for 10 s at five specific times for fifteen samples.
Footnotes
The authors express their appreciation to Matt Meier, David Lin, Prof. Daryl Kipke, and Dr. David Pellinen. Helpful comments on the manuscript were provided by Jeffrey Hendricks, Dr. Sarah Richardson-Burns, and Prof. Michael Mayer. This research was supported by the National Institute of Health (NIH) National Institute of Neurological Disorders and Stroke (NINDS) (NO1-NS-1–2338). Partial support was also provided by the National Science Foundation (DMR-0084 304 and DMR-0518 079), and the NASA Bioscience and Engineering Institute (NBEI). This research was facilitated by the Neural Engineering Laboratory, the Center for Neural Communications Technology, and the Electron Microbeam Analysis Laboratory (EMAL) at the University of Michigan.
Contributor Information
Mohammad Reza Abidian, Department of Biomedical Engineering University of Michigan, Ann Arbor Ann Arbor, MI 48109-2136 (USA).
Dong-Hwan Kim, Department of Biomedical Engineering University of Michigan, Ann Arbor Ann Arbor, MI 48109-2136 (USA).
David C. Martin, Departments of Materials Science and Engineering, Biomedical Engineering, and Macromolecular Science and Engineering University of Michigan H. H. Dow Building Room 2022 Ann Arbor, MI 48109-2136 (USA) milty@umich.edu
References
- 1.Devoret MH, Esteve D, Urbina C. Nature. 1992;360:547. [Google Scholar]
- 2.Ozin GA. Adv. Mater. 1992;4:612. [Google Scholar]
- 3.Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R. Science. 1994;263:1600. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- 4.Parthasarathy RV, Martin CR. Nature. 1994;369:298. doi: 10.1038/369298a0. [DOI] [PubMed] [Google Scholar]
- 5.Whitesides GM, Mathias JP, Seto CT. Science. 1991;254:1312. doi: 10.1126/science.1962191. [DOI] [PubMed] [Google Scholar]
- 6.Martin CR. Science. 1994;266:1961. doi: 10.1126/science.266.5193.1961. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt CE, Shastri VR, Vacanti JP, Langer R. Proc. Natl. Acad. Sci. U. S. A. 1997;94:8948. doi: 10.1073/pnas.94.17.8948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groenendaal BL, Jonas F, Freitag D, Pielartzik H, Reynolds JR. Adv. Mater. 2000;12:481. [Google Scholar]
- 9.Smela E. Adv. Mater. 1999;11:1343. [Google Scholar]
- 10.Smela E, Inganas O, Lundstrom I. Science. 1995;268:1735. doi: 10.1126/science.268.5218.1735. [DOI] [PubMed] [Google Scholar]
- 11.Smela E. Adv. Mater. 2003;15:481. [Google Scholar]
- 12.Gandhi MR, Murray P, Spinks GM, Wallace GG. Synth. Met. 1995;73:247. [Google Scholar]
- 13.Baughman RH, Shacklette RL, Elsenbaumer RL, Plichta EJ, Becht C. Bredas JL, Chance RR, editors. Conjugated Polymeric Materials: Opprtunities in Electronics, Optoelectronics, and Molecular Electronics. 1990. p. 559. NATO ASI Series E: Applied Science.
- 14.Pei QB, Inganas O. Adv. Mater. 1992;4:277. [Google Scholar]
- 15.Otero TF, Cortes MT. Adv. Mater. 2003;15:279. [Google Scholar]
- 16.Adeloju SB, Shaw SJ, Wallace GG. Anal. Chim. Acta. 1997;341:155. [Google Scholar]
- 17.Pernaut JM, Reynolds JR. J. Phys. Chem. B. 2000;104:4080. [Google Scholar]
- 18.Reinhard CS, Radomsky ML, Saltzman WM, Hilton J, Brem H, Controlled Release J. 1991;16:331. [Google Scholar]
- 19.Kim DH, Abidian M, Martin DC. J. Biomed. Mater. Res. 2004;71A:577. doi: 10.1002/jbm.a.30124. [DOI] [PubMed] [Google Scholar]
- 20.Yang JY, Martin DC. Sens. Actuators, B. 2004;101:133. [Google Scholar]
- 21.Kipke DR, Vetter RJ, Williams JC, Hetke JF. IEEE T Neur. Sys. Reh. 2003;11:151. doi: 10.1109/TNSRE.2003.814443. [DOI] [PubMed] [Google Scholar]
- 22.Cui XY, Hetke JF, Wiler JA, Anderson DJ, Martin DC. Sens. Actuators, A. 2001;93:8. [Google Scholar]
- 23.Zong XH, Kim K, Fang DF, Ran SF, Hsiao BS, Chu B. Polymer. 2002;43:4403. [Google Scholar]
- 24.Bognitzki M, Czado W, Frese T, Schaper A, Hellwig M, Stein-hart M, Greiner A, Wendorff JH. Adv. Mater. 2001;13:70. [Google Scholar]
- 25.Yang JY, Martin DC. Sens. Actuators, A. 2004;113:204. [Google Scholar]
- 26.White H, Pu Y, Rafailovich M, Sokolov J, King AH, Giannuzzi LA, Urbanik-Shannon C, Kempshall BW, Eisenberg A, Schwarz SA, Strzhemeckny YM. Polymer. 2001;42:1613. [Google Scholar]
- 27.Bognitzki M, Hou HQ, Ishaque M, Frese T, Hellwig M, Schwarte C, Schaper A, Wendorff JH, Greiner A. Adv. Mater. 2000;12:637. [Google Scholar]
- 28.Kim K, Luu YK, Chang C, Fang DF, Hsiao BS, Chu B, Hadjiargyrou M. J. Controlled Release. 2004;98:47. doi: 10.1016/j.jconrel.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Jager EWH, Inganas O, Lundstrom I. Science. 2000;288:2335. doi: 10.1126/science.288.5475.2335. [DOI] [PubMed] [Google Scholar]
- 30.Kandel ER, Schwarts JH, Jessel TM. Principles of Neural Science. Appleton & Lange; Norwalk, CT: 1991. [Google Scholar]
- 31.Nyberg T, Inganas O, Jerregard H. Biomed. Microdevices. 2002;4:43. [Google Scholar]