Abstract
OBJECTIVES:
To examine the association of neuropsychiatric symptoms with risk of institutionalization and death.
DESIGN:
Analysis of longitudinal data
SETTING:
The Aging, Demographics, and Memory Study (ADAMS)
PARTICIPANTS:
A sample (n=537) of adults aged 71 or older with cognitive impairment drawn from the Health and Retirement Study (HRS)
MEASUREMENTS:
The presence of neuropsychiatric symptoms (delusions, hallucinations, agitation, depression, apathy, elation, anxiety, disinhibition, irritation, and aberrant motor behaviors) and caregiver distress were identified using the Neuropsychiatric Inventory. Cognitive category was assigned in the ADAMS by a consensus panel. Date of nursing home placement and death, functional limitations, medical comorbidity, and sociodemographics were obtained from the HRS and ADAMS.
RESULTS:
Overall, the presence of 1 or more neuropsychiatric symptoms was not associated with a significantly higher risk for either institutionalization or death during the 5-year study period. However, when assessing each symptom individually, the presence of depression, delusions, and agitation were each associated with a significantly higher risk of institutionalization (HR, 3.06; 95% CI, 1.09-8.59 for depression; HR, 5.74; 95% CI, 1.94-16.96 for clinically significant delusions; HR, 4.70; 95% CI, 1.07-20.70 for clinically significant agitation). The association of delusions and agitation with institutionalization was mediated by caregiver distress. The presence of depression and hallucinations were each associated with a significantly higher mortality (HR, 1.56; 95% CI, 1.08-2.26 for depression; HR, 2.59; 95% CI, 1.09-6.16 for clinically significant hallucinations).
CONCLUSION:
Some, but not all, neuropsychiatric symptoms are associated with a higher risk of institutionalization and death among those with cognitive impairment, and some are additionally influenced by caregiver distress. Interventions that better target and treat the neuropsychiatric symptoms of depression, delusions, agitation, and hallucinations, as well as caregiver distress, may help delay or prevent these negative clinical outcomes.
Keywords: neuropsychiatric symptoms, caregiver distress, institutionalization, mortality
INTRODUCTION
Neuropsychiatric symptoms, such as agitation, depression, apathy, delusions, and hallucinations, are highly prevalent in older adults with dementia and milder forms of cognitive impairment.1,2,3,4 Population-based studies have estimated that 40-50% of individuals with cognitive impairment without dementia (CIND) or mild cognitive impairment (MCI), and 50-60% of those with dementia have at least one neuropsychiatric symptom, compared to only 10-20% of those with normal cognitive function.1,2,3,4 The pattern of presentation of individual neuropsychiatric symptoms differs between those with CIND and those with dementia, with psychotic symptoms and aberrant motor behaviors being more prevalent among demented persons.4 A greater number of neuropsychiatric symptoms was independently associated with functional limitations among those with CIND and dementia, even after controlling for severity of cognitive impairment and other potentially confounding factors.4
Neuropsychiatric symptoms have important implications for patients, families, and policymakers because they may lead to increased caregiver distress,5,6 functional decline,7,8,9 earlier institutionalization,8,9,10,11,12 increased healthcare costs,13,14,15,16 and greater risk of mortality.8,17 Yaffe and colleagues found that the presence of any difficult symptoms/behaviors (e.g., psychotic symptoms, aggressive behaviors, wandering, waking up the caregiver at night) increased the likelihood of nursing home placement over 3 years of follow-up. Other characteristics, including African-American race, Hispanic ethnicity, living alone, ADL limitations, and advanced cognitive impairment also increased the risk of nursing home placement.12 This study did not examine whether specific individual neuropsychiatric symptoms are more or less strongly associated with the risk of institutionalization. Other studies have suggested that agitation, hallucinations, and aberrant motor behaviors are associated with a higher risk of institutionalization, but these studies are difficult to interpret due to lack of adjustment for co-occurring symptoms.8,9,10,11
The results of studies examining the association of neuropsychiatric symptoms with mortality are mixed, likely due to differences in methodology.8,9,11,17,18 Some studies suggest that hallucinations,8 depression17 and wandering18 are associated with higher mortality, although other studies did not find these associations.9
In order to better assess the relationship of neuropsychiatric symptoms with institutionalization and death, we used a nationally representative sample of older Americans to examine whether the presence of any neuropsychiatric symptoms, or particular individual symptoms, were associated with a higher risk of nursing home placement and death over 5 years of follow-up. We hypothesized that the presence of particular individual symptoms, such as depression17 and aberrant motor behaviors18, would be associated with these negative clinical outcomes, independent of the level of cognitive impairment.
METHODS
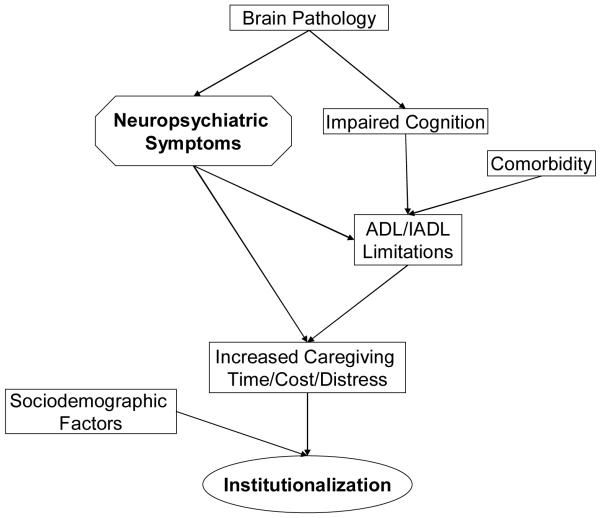
Conceptual Model for Higher Risk of Institutionalization in Older Adults with Neuropsychiatric Symptoms
The conceptual model underlying our analysis of the risk of institutionalization in older adults with neuropsychiatric symptoms is shown in Figure 1. Neuropsychiatric symptoms are common among those with CIND/MCI and dementia1,2,3,4 and probably associated with the brain pathology causing cognitive impairment.19,20 Cognitive impairment and medical comorbidities in older adults are strongly associated with a higher risk of limitations in activities of daily living which, in turn, are associated with an increased risk of nursing home placement.12 Neuropsychiatric symptoms may also be associated with a higher risk of functional limitations independent of cognitive impairment and chronic medical conditions.4 We hypothesized that neuropsychiatric symptoms may lead to increased supervision time and emotional distress among family caregivers,5,6 which may accelerate the decision for nursing home placement,12 independent of the severity of functional limitations. Sociodemographic factors, including race and ethnicity, wealth, or insurance coverage for long term care may also increase the likelihood of nursing home placement.12
Figure 1.
Conceptual Model of Higher Risk of Institutionalization in Older Adults with Neuropsychiatric Symptoms
Abbreviation: ADL, activity of daily living. IADL, instrumental activity of daily living
We developed a similar conceptual model for the possible association between neuropsychiatric symptoms and death (figure not shown). We hypothesized that neuropsychiatric symptoms may be associated with a higher risk of functional limitations,4 risky behaviors, or eating problems, which may lead to increased risk of death, independent of cognitive impairment and medical comorbidities.
Sample
We used data from the Aging, Demographics, and Memory Study (ADAMS) and the 2000, 2002, 2004, and 2006 waves of the Health and Retirement Study (HRS). The HRS is an ongoing biennial longitudinal survey of a nationally representative cohort of more than 20,000 U.S. adults aged 51 or older who reside both in the community and in nursing homes throughout the 48 contiguous United States.21 The HRS sample is selected using a multi-stage area probability sample design and population weights are constructed so that valid inferences can be drawn for the entire US aged 51+ population. Weights are constructed in a two-step process, where the first step develops post-stratified household weights using the initial sampling probabilities for each household, as well as birth year, race/ethnicity, and gender of household members. The second step uses these household weights to then construct post-stratified respondent-level weights which are scaled to yield weight sums corresponding to the number of individuals in the US population as measured by the US Census Bureau’s Current Population Survey (CPS) for the month of March in the year of data collection.22 The HRS is sponsored by the National Institute on Aging and performed by the Institute for Social Research at the University of Michigan.
The ADAMS is a sub-study of the HRS focused on identifying the prevalence and outcomes of cognitive impairment and dementia. The ADAMS sample was a stratified random subsample of 1770 individuals aged 71 years or older from 5 cognitive strata based on scores for the 35-point HRS cognitive scale (HRS cog)23 or proxy assessments of cognition from the 2000 or 2002 wave of the HRS.24 The ADAMS further stratified the 3 highest cognitive strata by age (age 71 to 79 years vs. ≥80 years) and sex to ensure adequate numbers in each subgroup.24 109 individuals (13%) in the ADAMS sample resided in nursing homes at the time of the ADAMS assessment. Population weights for nursing home residents were derived using data from the 2000 Census and the Centers for Medicare and Medicaid Services (CMS) Minimum Data Set (MDS).24 Full details of the ADAMS sample design and selection procedures are described elsewhere.24,25,26 The initial assessments of ADAMS subjects occurred between July 2001 and December 2003, on average, 13.3 months (SD, 6.9) after the most recent HRS interview. The study flow and additional details on participation rates have been reported previously.26 A total of 856 of the 1770 individuals selected for the sample (mean age 81.5 years) completed the initial ADAMS assessment (56% of the non-deceased subjects). We did not include 16 individuals for whom the Neuropsychiatric Inventory (NPI) was not completed and 303 individuals with normal cognition (n=537 for current analyses). To minimize the potential bias due to selective nonparticipation, the ADAMS performed a response propensity analysis and developed nonresponse adjustments to the ADAMS sample selection weights.24 The ADAMS then constructed population sample weights to take into account the probabilities of selection in the stratified sample design and to adjust for differential nonparticipation in the ADAMS.24
The ADAMS data are publicly available and can be obtained from the HRS Web site (http://hrsonline.isr.umich.edu). The institutional review boards at Duke University Medical Center and the University of Michigan approved all study procedures, and study participants or their surrogates provided informed consent.
Measurements
Cognitive Evaluation
In the ADAMS, a nurse and a neuropsychology technician assessed all participants at their residence for cognitive impairment. The full details of the assessment and diagnostic procedures are described elsewhere.25.26 During the assessment, the participant completed a battery of neuropsychological measures; a self-reported depression measure; a standardized neurological examination; a blood pressure measurement; collection of buccal DNA samples for apolipoprotein E (APOE) genotyping; and a 7-minute, videotaped segment covering portions of the cognitive status and neurological examinations. Proxy informants provided information about the participant’s cognitive impairment, functional limitations, neuropsychiatric symptoms, and medical history. The informant was usually a spouse or child (73%), and informants lived with the participant in just over half of the cases (53%). The ADAMS consensus expert panel of neuropsychologists, neurologists, geropsychiatrists, and internists reviewed all information collected during the in-home assessment and assigned cognitive diagnoses. Diagnoses were within three cognitive categories: normal cognitive function, cognitive impairment without dementia (CIND), and dementia. The Diagnostic and Statistical Manual of Mental Disorders, Revised Third Edition,27 and the Diagnostic and Statistical Manual of Mental Disorder, Fourth Edition criteria28 were used for diagnosis of dementia. CIND was defined as mild cognitive or functional impairment reported by the participant or informant that did not meet criteria for dementia, or performance on neuropsychological measures that was both below expectation and at least 1.5 SDs below published norms on any test within a cognitive domain (e.g., memory, orientation, language, executive function, praxis).
Those with dementia were classified by the stage or severity using the Clinical Dementia Rating (CDR) Scale,29,30,31 a widely used assessment tool that stages the severity of dementia based on information obtained from both the participant and informant during the course of the evaluation. As in prior studies,3,32 we defined mild dementia as CDR stage 0.5 or 1, moderate dementia as CDR stage 2, and severe dementia as CDR stages 3-5.
Neuropsychiatric Symptoms
The ADAMS assessed neuropsychiatric symptoms using the Neuropsychiatric Inventory (NPI). The NPI is a widely accepted measure of neuropsychiatric symptoms associated with cognitive impairment.33, 34 It collects information on symptoms during the past month in 10 domains -- delusions, hallucinations, agitation, depression, anxiety, elation, apathy, disinhibition, irritability, and aberrant motor behaviors -- using a structured interview of a knowledgeable informant. For each symptom reported by the informant, additional information is obtained on the frequency (4-point scale) and severity (3-point scale) of the symptom. We defined symptoms as clinically significant if the product of the frequency and severity score of the reported symptom was 4 or higher.35 Psychometric properties of the NPI have been previously reported.33 The NPI has been validated in prior studies and has also been shown to have very good reliability (Cronbach’s alpha was 0.88 for internal consistency reliability).33
Caregiver Distress
In the NPI, caregivers were asked to rate the emotional or psychological distress they experienced in relation to each symptom they reported on a 6-point scale: 0 (Not at all distressing), 1 (Minimally distressing), 2 (Mildly distressing), 3 (Moderately distressing), 4 (Severely distressing), and 5 (Very Severely or Extremely distressing).34 We used the total score to identify overall caregiver distress, and included this measure in our analytical models. We categorized caregiver distress into 4 ordinal levels based on the total score (0, 1-5, 6-10, ≥11). The psychometric properties (e.g., reliability, validity) of this scale have been assessed previously, and were found to be adequate.34
Determining Institutionalization, Mortality, and the Date of Outcomes
For respondents who reported living in a nursing home at the time of a follow-up survey, the time to admission was established by calculating the time from the ADAMS assessment until nursing home admission. Only the first nursing home admission was evaluated; hence, multiple nursing home stays were not addressed. In addition, admissions to a nursing home that were brief enough to be completed between surveys were not included.36 Since older adults with terminal illness may enter a nursing home just prior to death, we excluded from the institutionalization analysis 6 individuals who entered a nursing home within 1 month of death. The same method was used to compute the time from the ADAMS assessment until death. We censored individuals after 5 years of follow-up or at death.
Sociodemographic Characteristics
We obtained data on participants’ age (71-79, 80-89, ≥90 years), sex, race (white, black, other), and years of formal education (<12, 12, >12 years) from the ADAMS. Household net worth was categorized by quartile, and marital and living status (married/partnered living together, unmarried living with other, unmarried living alone, living in nursing home) were determined using data from the 2000 and 2002 waves of the HRS. We determined whether respondents had Medicaid and / or long-term care insurance using data from the 2000 or 2002 waves of the HRS.
Functional Limitations
The ADAMS assessed the number of limitations in activities of daily living (ADLs) and instrumental activities of daily living (IADLs) using an informant questionnaire. The ADLs assessed were getting across a room, dressing, bathing, eating, transferring and toileting. The IADLs assessed were preparing meals, grocery shopping, making telephone calls, taking medications, and handling finances. We categorized the number of limitations using 3 ordinal levels (0, 1 or 2, 3 or more) for both ADLs and IADLs.
Chronic Medical Conditions
The HRS collects data on the presence of chronic medical conditions (heart disease, chronic lung disease, diabetes, cancer, musculoskeletal conditions, stroke, and psychiatric problems) in each wave of the survey.37 Respondents report whether a physician has ever diagnosed each condition. We used data on chronic conditions from either the 2000 or 2002 wave of the HRS and included them in the analysis as dichotomous variables.
Other Covariates
The ADAMS obtained detailed information regarding the medications that respondents were taking at the time of the interview. We chose antipsychotics, antidepressants, and cholinesterase inhibitors as medications potentially associated with nursing home placement and death, and included dichotomous variables indicating their current use in the analyses.
Statistical Analysis
We compared sociodemographic characteristics, total number of neuropsychiatric symptoms and presence of individual neuropsychiatric symptoms according to cognitive category using chi-square tests. Since one goal of the analysis was to identify whether the presence of any neuropsychiatric symptoms was independently associated with an increased risk of institutionalization or death, we estimated separate Cox proportional hazards models with nursing home placement and death as the dependent variables to determine adjusted hazard ratios (HRs) for these outcomes over 5 years of follow-up. The analysis for the risk of institutionalization was adjusted for sociodemographics, long-term care insurance, and cognitive category. We also examined whether the significant relationship between neuropsychiatric symptoms and institutionalization was mediated by caregiver distress and whether the relationship was confounded by functional limitations by additionally controlling for these variables. The analysis for mortality was adjusted for sociodemographics, medical comorbidities, relevant medications, and cognitive category. We then estimated similar Cox models to examine whether individual neuropsychiatric symptoms were associated with a higher risk of these clinical outcomes, adjusting for other co-occurring symptoms. We computed adjusted hazard ratios (HRs) to compare the relative strength of the association of each neuropsychiatric symptom with 5-year institutionalization or death. To test the hypothesis that the level of cognitive impairment modifies the relationship of neuropsychiatric symptoms and these clinical outcomes, we tested for significant interactions between cognitive category and neuropsychiatric symptoms (e.g., total number of neuropsychiatric symptoms X cognitive category, the presence of delusions X cognitive category). None of these interaction terms was statistically significant, so we did not include them in the final regression models. We repeated the analysis with the variables for clinically significant neuropsychiatric symptoms (frequency score times severity score >=4) and compared these results with those from the previous analyses for the presence/absence of any neuropsychiatric symptoms.
We assessed the proportional hazards assumption with both graphical and goodness-of-fit testing procedures and confirmed that it was not violated.38 All analyses were weighted and adjusted for the complex sampling design (stratification, clustering, and nonresponse) of both the ADAMS and the HRS.23 STATA, version 10.1 (Stata Corp, College Station, TX) was used for data analysis. All reported p values are two-tailed, and a p value < 0.05 was considered statistically significant.
RESULTS
Characteristics of Study Sample
Table 1 shows the sociodemographic characteristics and the presence of neuropsychiatric symptoms of the study sample stratified by cognitive category (e.g., CIND, mild dementia, moderate dementia, and severe dementia). Those with more advanced cognitive impairment were older, more likely to be women, and more likely to live in a nursing home. More severe cognitive impairment was associated with an increased risk for the presence of neuropsychiatric symptoms.
Table 1.
Sample Characteristics by Cognitive Category
| CIND | Dementia | P-value† | |||
|---|---|---|---|---|---|
| Mild | Moderate | Severe | |||
| Sample (Weighted %*) | 238 (61.6) | 153 (21.1) | 64 (6.9) | 82 (10.4) | |
| Age | <0.001 | ||||
| 71-79y | 94 (42.9) | 36 (24.5) | 13 (26.3) | 10 (10.2) | |
| 80-89y | 109 (44.7) | 83 (61.3) | 26 (35.5) | 45 (70.1) | |
| ≥90y | 35 (12.4) | 34 (14.2) | 25 (38.2) | 27 (19.7) | |
| Sex | 0.006 | ||||
| Female | 120 (54.5) | 92 (58.1) | 49 (77.7) | 67 (83.1) | |
| Male | 118 (45.6) | 61 (41.9) | 15 (22.3) | 15 (16.9) | |
| Race | 0.156 | ||||
| White | 173 (85.6) | 105 (79.1) | 49 (86.8) | 64 (77.3) | |
| Black | 52 (10.4) | 42 (16.3) | 11 (9.4) | 14 (7.9) | |
| Other | 13 (4.0) | 6 (4.6) | 4 (3.8) | 4 (14.8) | |
| Education | 0.090 | ||||
| <12y | 143 (42.7) | 100 (58.1) | 34 (38.6) | 40 (39.2) | |
| =12y | 53 (30.5) | 33 (27.5) | 14 (22.0) | 21 (29.4) | |
| >12y | 42 (26.8) | 20 (14.4) | 16 (39.4) | 21 (31.4) | |
| Household Net Worth | 0.180 | ||||
| Quartile 1 (low) | 110 (37.5) | 77 (53.2) | 38 (45.1) | 42 (56.7) | |
| Quartile 2 | 60 (21.9) | 37 (21.6) | 11 (18.6) | 15 (18.8) | |
| Quartile 3 | 41 (21.5) | 23 (14.6) | 6 (24.7) | 13 (11.0) | |
| Quartile 4 (high) | 27 (19.1) | 15 (10.6) | 9 (11.5) | 12 (13.5) | |
| Living Situation | <0.001 | ||||
| Living Alone | 79 (32.5) | 53 (41.1) | 19 (24.6) | 11 (17.4) | |
| Living With Spouse | 105 (43.2) | 46 (29.2) | 11 (11.4) | 11 (12.4) | |
| Living With Others | 44 (19.5) | 40 (20.5) | 16 (18.6) | 18 (21.0) | |
| Living In Nursing Home | 10 (4.8) | 14 (9.2) | 18 (45.4) | 42 (49.2) | |
| NPI Symptom(s) | 0.002 | ||||
| 0 | 151 (55.4) | 71 (53.7) | 17 (24.0) | 21 (28.7) | |
| 1 | 42 (21.1) | 31 (20.9) | 14 (21.7) | 18 (17.4) | |
| ≥2 | 45 (23.5) | 51 (25.4) | 33 (54.3) | 43 (53.9) | |
| Delusions | 7 (4.0) | 16 (6.6) | 21 (39.7) | 20 (27.6) | <0.001 |
| Hallucinations | 6 (2.2) | 13 (6.8) | 13 (30.6) | 22 (20.6) | <0.001 |
| Agitation | 27 (13.5) | 29 (12.9) | 20 (24.0) | 34 (41.3) | 0.003 |
| Depression | 51 (29.5) | 45 (25.1) | 22 (35.9) | 16 (28.9) | 0.773 |
| Apathy | 23 (14.4) | 24 (13.2) | 15 (24.7) | 29 (42.0) | 0.001 |
| Elation | 1 (0.1) | 0 (0) | 0 (0) | 4 (5.9) | <0.001 |
| Anxiety | 19 (9.1) | 29 (12.5) | 20 (29.8) | 12 (11.2) | 0.066 |
| Disinhibition | 9 (9.6) | 14 (5.9) | 11 (31.5) | 10 (8.6) | 0.039 |
| Irritation | 29 (16.0) | 23 (11.1) | 13 (17.4) | 17 (15.5) | 0.648 |
| Aberrant Motor Behaviors | 4 (2.4) | 13 (10.7) | 11 (13.0) | 24 (31.0) | 0.001 |
Note.
Values in parentheses are weighted percentages derived by using the ADAMS sample weights to adjust for the complex sampling design of the ADAMS.
P-values were derived from the χ2 test for association between the indicated variable and the cognitive category.
Abbreviation: NPI, Neuropsychiatric Inventory. CIND, cognitive impairment without dementia.
Neuropsychiatric Symptoms and 5-year Risk of Institutionalization
84 individuals already living in a nursing home at the time of the ADAMS assessment were excluded from the present analyses, leaving a sample size of 453. Table 2 shows the results of Cox proportional hazards regression for the association between presence of any neuropsychiatric symptoms (e.g., total number of symptoms, individual symptoms) and 5-year risk of institutionalization among those with CIND and dementia. The first column of the table shows the adjusted HRs for 5-year risk of institutionalization in those with neuropsychiatric symptoms compared to those without. Based on all NPI responses, individuals with 1 or more neuropsychiatric symptoms did not have a significantly higher risk of institutionalization over the 5-year period, compared to those without neuropsychiatric symptoms. Among specific neuropsychiatric symptoms, those with depression had a significantly higher risk of institutionalization controlling for the other co-occurring symptoms. This association was not diminished even after controlling for caregiver distress (the second column). HRs of those with delusions and depression increased substantially after controlling for functional limitations (the third column).
Table 2.
Presence of Any Symptoms and 5-year Risk of Institutionalization
| Presence of Any Symptoms | |||
|---|---|---|---|
| HR* (95% CI) | |||
| NPI Symptom(s) | Base Model | Adjusted for Caregiver Distress | Adjusted for Functional Limitations |
| 0 | Reference | Reference | Reference |
| 1 | 2.55 (0.89-7.24) | 3.20 (0.92-11.15) | 2.47 (0.88-6.91) |
| ≥2 | 2.81 (0.91-8.58) | 4.36 (0.75-25.32) | 2.71 (0.77-9.53) |
| Cognitive Category | 1.84 (1.15-2.49) | 1.86 (1.19-2.93) | 1.71 (1.06-2.77) |
| Caregiver Distress | - | 0.77 (0.43-1.36) | - |
| ADL Limitations | - | - | 0.73 (0.42-1.27) |
| IADL Limitations | - | - | 1.23 (0.77-1.99) |
| Delusions | 2.95 (0.59-14.72) | 2.93 (0.52-16.30) | 4.26 (1.29-14.05) |
| Hallucinations | 0.06 (0.01-0.75) | 0.06 (0.00-0.73) | 0.05 (0.00-0.62) |
| Agitation | 1.27 (0.41-3.88) | 1.26 (0.42-3.73) | 1.52 (0.50-4.56) |
| Depression | 3.06 (1.09-8.59) | 3.03 (1.00-9.84) | 3.47 (1.06-11.29) |
| Apathy | 1.47 (0.24-8.83) | 1.45 (0.17-11.83) | 1.54 (0.42-5.64) |
| Elation‡ | - | - | - |
| Anxiety | 0.33 (0.06-1.75) | 0.33 (0.06-1.78) | 0.10 (0.00-1.37) |
| Disinhibition | 0.39 (0.07-1.95) | 0.39 (0.06-2.33) | 0.74 (0.17-3.09) |
| Irritation | 0.81 (0.39-1.68) | 0.79 (0.33-1.91) | 1.18 (0.64-2.19) |
| Aberrant Motor Behaviors | 3.76 (0.49-28.41) | 3.73 (0.50-27.69) | 4.78 (0.69-33.1) |
| Cognitive Category | 1.79 (1.06-3.03) | 1.78 (1.12-2.84) | 1.54 (0.88-2.68) |
| Caregiver Distress | - | 1.02 (0.43-2.41) | - |
| ADL Limitations | - | - | 0.58 (0.33-1.00) |
| IADL Limitations | - | - | 1.46 (0.87-2.45) |
HR derived by using a Cox proportional hazards regression model with the 5-year risk of institutionalization as the dependent variable. 95% confidence intervals are in parentheses. The model adjusts for cognitive category (e.g., CIND, mild/moderate/severe dementia), sociodemographic characteristics, and long-term care insurance.
Unable to estimate due to small sample size
Abbreviation: NPI, Neuropsychiatric Inventory. ADL, activity of daily living. IADL, instrumental activity of daily living.
Table 3 shows the results of Cox proportional hazards regression for the association between clinically significant neuropsychiatric symptoms (e.g., total number of symptoms, individual symptoms) and 5-year risk of institutionalization among those with CIND and dementia. Among specific neuropsychiatric symptoms, those with clinically significant delusions and agitation had a significantly higher risk of institutionalization, controlling for the other co-occurring symptoms. HRs of those with clinically significant delusions and agitation decreased substantially after controlling for caregiver distress suggesting mediation of this relationship through caregiver distress, while the HRs increased substantially after controlling for functional limitations (the second and third column, respectively).
Table 3.
Clinically Significant Symptoms and 5-year Risk of Institutionalization
| Clinically Significant Symptoms† | |||
|---|---|---|---|
| HR* (95% CI) | |||
| NPI Symptom(s) | Base Model | Adjusted for Caregiver Distress | Adjusted for Functional Limitations |
| 0 | Reference | Reference | Reference |
| ≥1 | 2.36 (0.87-6.42) | 2.77 (0.36-21.36) | 2.30 (0.86-6.18) |
| Cognitive Category | 1.77 (1.27-2.47) | 1.78 (1.27-2.50) | 1.67 (1.08-2.57) |
| Caregiver Distress | - | 0.90 (0.40-2.01) | - |
| ADL Limitations | - | - | 0.75 (0.43-1.30) |
| IADL Limitations | - | - | 1.21 (0.79-1.85) |
| Delusions | 5.74 (1.94-16.96) | 3.87 (0.96-15.58) | 7.13 (2.67-19.02) |
| Hallucinations | 0.14 (0.01-2.68) | 0.25 (0.00-8.75) | 0.18 (0.01-4.36) |
| Agitation | 4.70 (1.07-20.70) | 3.41 (0.85-13.58) | 5.77 (1.00-33.51) |
| Depression | 2.09 (0.30-14.47) | 1.41 (0.11-17.15) | 1.44 (0.10-19.42) |
| Apathy | 0.24 (0.08-0.74) | 0.15 (0.04-0.60) | 0.22 (0.08-0.63) |
| Elation‡ | - | - | - |
| Anxiety | 0.32 (0.04-2.33) | 0.21 (0.02-1.56) | 0.24 (0.03-1.95) |
| Disinhibition | 0.08 (0.00-1.40) | 0.06 (0.00-0.74) | 0.22 (0.02-1.91) |
| Irritation | 0.52 (0.16-1.66) | 0.35 (0.08-1.39) | 0.49 (0.11-2.03) |
| Aberrant Motor Behaviors | 2.99 (0.47-19.01) | 2.37 (0.34-16.17) | 3.29 (0.44-24.18) |
| Cognitive Category | 1.93 (1.27-2.93) | 1.89 (1.27-2.81) | 1.83 (1.13-2.98) |
| Caregiver Distress | - | 1.67 (0.71-3.95) | - |
| ADL Limitations | - | - | 0.94 (0.43-2.02) |
| IADL Limitations | - | - | 1.03 (0.60-1.76) |
HR derived by using a Cox proportional hazards regression model with the 5-year risk of institutionalization as the dependent variable. 95% confidence intervals are in parentheses. The model adjusts for cognitive category (e.g., CIND, mild/moderate/severe dementia), sociodemographic characteristics, and long-term care insurance.
Clinically significant symptoms is defined as a frequency score times severity score of >=4.
Unable to estimate due to small sample size
Abbreviation: NPI, Neuropsychiatric Inventory. ADL, activity of daily living. IADL, instrumental activity of daily living
Neuropsychiatric Symptoms and 5-year Risk of Death
Table 3 shows the results of Cox proportional hazards regression for the association between neuropsychiatric symptoms (e.g., total number of symptoms, individual symptoms) and 5-year risk of death among those with CIND and dementia. The first column of the table shows the adjusted HRs for 5-year risk of death in those with neuropsychiatric symptoms compared to those without. The second column of the table shows the adjusted HRs for 5-year risk of death in those presenting with clinically significant symptoms compared to those without clinically significant symptoms. Based on all NPI responses, individuals with 1 or more neuropsychiatric symptoms, even clinically significant symptoms, did not have a significantly higher 5-year risk of death, compared to those without neuropsychiatric symptoms. Those with the specific symptom of depression and those with clinically significant hallucinations had a significantly higher 5-year risk of death controlling for the other co-occurring symptoms.
DISCUSSION
In this study using a nationally representative sample of older adults with CIND and dementia, the total number of neuropsychiatric symptoms, even clinically significant symptoms, was not associated with a higher risk of either institutionalization or death over 5 years of follow-up, after adjusting for potentially confounding factors including severity of cognitive impairment. However, as we hypothesized, there were several particular neuropsychiatric symptoms – depression, delusions, and agitation- that were strongly associated with a higher risk of nursing home placement or death, even after adjusting for the other co-occurring symptoms.
Regarding the association of neuropsychiatric symptoms with risk of institutionalization, our results are different than some prior studies, probably due to methodological differences. Two studies reported that those with one or more difficult behaviors had a significantly higher risk of institutionalization (HR, 1.47; 95% CI, 1.10-1.97;9 HR, 1.30; 95% CI, 1.11-1.5212) during their study periods (3 years12 and 4.4 years9, respectively). These prior studies assessed the presence of fewer difficult behaviors (e.g. psychotic symptoms, wandering, and aggression) which might be more strongly associated with risk of institutionalization. In addition, less complete adjustment for confounders and more statistical power in those prior studies may have contributed to the detection of a significant relationship. To our knowledge, ours is the first study to examine the association of individual neuropsychiatric symptoms with risk of institutionalization while adjusting for other co-occurring symptoms. Prior studies have suggested that hallucinations8 and wandering9 were associated with a higher risk of nursing home placement.
Our study highlights the possible mediating roles of caregiver distress and functional limitations in the association between neuropsychiatric symptoms and the risk of institutionalization. We found that depression was associated with the increased risk of institutionalization independent of caregiver distress, while the association of clinically significant delusions and agitation with institutionalization was mediated by caregiver distress. These findings suggest that some neuropsychiatric symptoms may increase the risk of institutionalization independently from the distress they cause caregivers. Functional limitations may confound the association of some neuropsychiatric symptoms and the risk of institutionalization, likely due to their independent association with particular symptoms.4
The independent association of depression and institutionalization that we found highlights the importance of identification and treatment of depression in older adults with cognitive impairment and dementia. The mediating role of caregiver distress between agitation / delusions and institutionalization suggests that interventions targeted at supporting caregivers and reducing caregiver burden when agitation and delusions are present in care recipients may be especially valuable to both caregiver and patients.
Depression has been shown to be associated with increased risk of death.17, 39 Our study provides additional confirmation of this relationship, using the NPI to determine presence of depression. Cognitive impairment and depression may combine to increase mortality risk in older adults via pathways that include failure to thrive, frailty, poor chronic disease management, and social isolation.17, 40 Impairment of visuospatial and executive function have been shown to be associated with increased mortality and perhaps to mediate the association of depression and mortality through autonomic dysfunction.41, 42 As a prior study suggested, our study found that clinically significant hallucinations were associated with mortality. This may be due to a residual confounding effect of severity of cognitive impairment or presence of delirium43, 44, and may also due to an increased mortality risk associated with dementia with Lewy bodies, a sub-type of dementia characterized by frequent hallucinations.45
The strengths of this study include a nationally representative population-based sample that included the range of cognition in those who have CIND or dementia, the use of a well-validated comprehensive assessment of neuropsychiatric symptoms, and the ascertainment of the date of important clinical outcomes by using longitudinal data from the HRS. A number of potential limitations should also be considered when interpreting the results. For the neuropsychiatric symptoms with low prevalence, we may have failed to detect a significant association due to low statistical power. There may be residual confounding due to omission of unmeasured factors related to both neuropsychiatric symptoms and the outcomes, even though we included a wide range of potentially confounding factors. Measurement error may have occurred even though we used the NPI which has good psychometric characteristics.33 Ascertainment of date of nursing home admission may also be subject to measurement error due suboptimal reliability and recall bias.
In summary, our findings suggest that depression, delusions, and agitation are associated with an increased risk of institutionalization in those with CIND and dementia, while depression and hallucinations are associated with an increased risk of death. The association of delusions and agitation with risk of institutionalization may depend on the level of caregiver distress associated with these symptoms. Further studies should assess whether interventions to better target and treat these neuropsychiatric symptoms and caregiver distress in those with CIND and dementia may help delay or prevent these negative clinical outcomes.
Table 4.
Neuropsychiatric Symptoms and 5-year Risk of Death
| NPI Symptom(s) | Presence of Any Symptoms | Clinically Significant Symptoms† |
|---|---|---|
| HR* (95% CI) | HR* (95% CI) | |
| 0 | Reference | Reference |
| 1 | 0.79 (0.53-1.17) | 1.03 (0.73-1.45) |
| ≥2 | 1.00 (0.66-1.51) | |
| Cognitive Category | 1.73 (1.47-2.02) | 1.69 (1.42-2.02) |
| Delusions | 0.88 (0.48-1.62) | 0.84 (0.42-1.66) |
| Hallucinations | 1.24 (0.74-2.07) | 2.59 (1.09-6.16) |
| Agitation | 0.91 (0.64-1.29) | 0.95 (0.50-1.82) |
| Depression | 1.56 (1.08-2.26) | 1.61 (0.95-2.74) |
| Apathy | 1.18 (0.65-2.16) | 1.45 (0.83-2.52) |
| Elation‡ | - | - |
| Anxiety | 0.67 (0.35-1.27) | 0.98 (0.52-1.86) |
| Disinhibition | 0.57 (0.24-1.34) | 0.38 (0.08-1.74) |
| Irritation | 1.24 (0.73-2.11) | 1.03 (0.57-1.86) |
| Aberrant Motor Behaviors | 0.92 (0.50-1.66) | 0.86 (0.48-1.56) |
| Cognitive Category | 1.77 (1.50-2.10) | 1.65 (1.38-1.97) |
Note.
HR derived by using a Cox proportional hazards regression model with the 5-year risk of death as the dependent variable. 95% confidence intervals are in parentheses.
The model adjusts for cognitive category (e.g., CIND, mild/moderate/severe dementia), sociodemographic characteristics, relevant medications, and medical comorbidities.
Clinically significant symptoms is defined as a frequency score times severity score of >=4.
Unable to estimate due to small sample size
Abbreviation: NPI, Neuropsychiatric Inventory.
ACKNOWLEDGMENT
Conflict of Interest Disclosures:
The National Institute on Aging (NIA) provided funding for the Heath and Retirement Study and the Aging, Demographics, and Memory Study (U01 AG09740). Additional funding support was provided by NIA grants R01 AG027010 and R01 AG030155.
Sponsor’s Role:
The funding agencies had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
REFERENCES
- 1.Lyketsos CG, Lopez O, Jones B, et al. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment. JAMA. 2002;288:1475–1483. doi: 10.1001/jama.288.12.1475. [DOI] [PubMed] [Google Scholar]
- 2.Geda YE, Roberts RO, Knopman DS, et al. Prevalence of neuropsychiatric symptoms in mild cognitive impairment and normal cognitive aging: population-based study. Arch Gen Psychiatry. 2008;65:1193–1198. doi: 10.1001/archpsyc.65.10.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lyketsos CG, Steinberg M, Tschanz JT, et al. Mental and behavioral disturbances in dementia: findings from the Cache County Study on Memory in Aging. Am J Psychiatry. 2000;157:708–714. doi: 10.1176/appi.ajp.157.5.708. [DOI] [PubMed] [Google Scholar]
- 4.Okura T, Plassman BL, Steffens DC, et al. Prevalence of neuropsychiatric symptoms and their association with functional limitations in older adults in the United States: The Aging, Demographics, and Memory Study. J Am Geriatr Soc. 2010;58:330–337. doi: 10.1111/j.1532-5415.2009.02680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.González-Salvador MT, Arango C, Lyketsos CG, et al. The stress and psychological morbidity of the Alzheimer patient caregiver. Int J Geriatr Psychiatry. 1999;14:701–710. doi: 10.1002/(sici)1099-1166(199909)14:9<701::aid-gps5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 6.Logsdon RG, Teri L, McCurry SM, et al. Wandering: A significant problem among community-residing individuals with Alzheimer’s disease. J Gerontol B Psychol Sci Soc Sci. 1998;53:294–299. doi: 10.1093/geronb/53b.5.p294. [DOI] [PubMed] [Google Scholar]
- 7.Lyketsos CG, Steele C, Baker L, et al. Major and minor depression in Alzheimer’s disease: prevalence and impact. J Neuropsychiatry Clin Neurosci. 1997;9:556–561. doi: 10.1176/jnp.9.4.556. [DOI] [PubMed] [Google Scholar]
- 8.Scarmeas N, Brandt J, Albert M, et al. Delusions and hallucinations are associated with worse outcome in Alzheimer disease. Arch Neurol. 2005;62:1601–1608. doi: 10.1001/archneur.62.10.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scarmeas N, Brandt J, Blacker D, et al. Disruptive behavior as a predictor in Alzheimer disease. Arch Neurol. 2007;64:1755–1761. doi: 10.1001/archneur.64.12.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steele C, Rovner B, Chase GA, et al. Psychiatric symptoms and nursing home placement of patients with Alzheimer’s disease. Am J Psychiatry. 1990;147:1049–1051. doi: 10.1176/ajp.147.8.1049. [DOI] [PubMed] [Google Scholar]
- 11.Stern Y, Tang MX, Albert MS, et al. Predicting time to nursing home care and death in individuals with Alzheimer disease. JAMA. 1997;277:806–812. [PubMed] [Google Scholar]
- 12.Yaffe K, Fox P, Newcomer R, et al. Patient and caregiver characteristics and nursing home placement in patients with dementia. JAMA. 2002;287:2090–2097. doi: 10.1001/jama.287.16.2090. [DOI] [PubMed] [Google Scholar]
- 13.Beeri MS, Werner P, Davidson M, et al. The cost of behavioral and psychological symptoms of dementia (BPSD) in community dwelling Alzheimer’s disease patients. Int J Geriatr Psychiatry. 2002;17:403–408. doi: 10.1002/gps.490. [DOI] [PubMed] [Google Scholar]
- 14.Jönsson L, Eriksdotter Jönhagen M, Kilander L, et al. Determinants of costs of care for patients with Alzheimer’s disease. Int J Geriatr Psychiatry. 2006;21:449–459. doi: 10.1002/gps.1489. [DOI] [PubMed] [Google Scholar]
- 15.Herrmann N, Lanctôt KL, Sambrook R, et al. The contribution of neuropsychiatric symptoms to the cost of dementia care. Int J Geriatr Psychiatry. 2006;21:972–976. doi: 10.1002/gps.1594. [DOI] [PubMed] [Google Scholar]
- 16.Moore MJ, Zhu CW, Clipp EC. Informal costs of dementia care: Estimates from the National Longitudinal Caregiver Study. J Gerontol B Psychol Sci Soc Sci. 2001;56:S219–228. doi: 10.1093/geronb/56.4.s219. [DOI] [PubMed] [Google Scholar]
- 17.Mehta KM, Yaffe K, Langa KM, et al. Additive effects of cognitive function and depressive symptoms on mortality in elderly community-living adults. J Gerontol A Biol Sci Med Sci. 2003;58:M461–467. doi: 10.1093/gerona/58.5.m461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larson EB, Shadlen MF, Wang L, et al. Survival after initial diagnosis of Alzheimer disease. Ann Intern Med. 2004;140:501–509. doi: 10.7326/0003-4819-140-7-200404060-00008. [DOI] [PubMed] [Google Scholar]
- 19.Starkstein SE, Vázquez S, Petracca G, et al. A SPECT study of delusions in Alzheimer’s disease. Neurology. 1994;44:2055–2059. doi: 10.1212/wnl.44.11.2055. [DOI] [PubMed] [Google Scholar]
- 20.Förstl H, Burns A, Luthert P, et al. Clinical and neuropathological correlates of depression in Alzheimer’s disease. Psychol Med. 1992;22:877–884. doi: 10.1017/s0033291700038459. [DOI] [PubMed] [Google Scholar]
- 21.Juster FT, Suzman R. An overview of the Health and Retirement Study. J Hum Resour. 1995;30:S135–S145. [Google Scholar]
- 22.Health and Retirement Study, Sample Evolution: 1992-1998. Accessed at http://hrsonline.isr.umich.edu/sitedocs/surveydesign.pdf on 11 August 2009.
- 23.Ofstedal MB, Fisher G, Herzog AR. Documentation of cognitive functioning measures in the Health and Retirement Study. University of Michigan; Ann Arbor, MI: 2005. Accessed at http://hrsonline.isr.umich.edu/docs/userg/dr-006.pdf on 12 December 2008. [Google Scholar]
- 24.Heeringa SG, Fisher GG, Hurd MD, et al. Aging, Demographics, and Memory Study (ADAMS). Sample design, weighting, and analysis for ADAMS. 2009 Accessed at http://hrsonline.isr.umich.edu/sitedocs/userg/ADAMSSampleWeights_Jun2009.pdf on 29 August 2009.
- 25.Langa KM, Plassman BL, Wallace RB, et al. The Aging, Demographics, and Memory Study: Study design and methods. Neuroepidemiology. 2005;25:181–191. doi: 10.1159/000087448. [DOI] [PubMed] [Google Scholar]
- 26.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med. 2008;148:427–434. doi: 10.7326/0003-4819-148-6-200803180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. ed 3. American Psychiatric Association; Washington: 1987. revised. [Google Scholar]
- 28.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. ed 4 American Psychiatric Association; Washington: 1994. [Google Scholar]
- 29.Hughes CP, Berg L, Danziger WL, et al. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 30.Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 31.Clinical Dementia Rating (CDR) Scale. Alzheimer’s Research Center, Washington University; St. Louis: Accessed at http://alzheimer.wustl.edu/cdr/PDFs/CDR_OverviewTranscript-Revised.pdf on 29 April 2009. [Google Scholar]
- 32.Steffens DC, Maytan M, Helms MJ, et al. Prevalence and clinical correlates of neuropsychiatric symptoms in dementia. Am J Alzheimers Dis Other Demen. 2005;20:367–373. doi: 10.1177/153331750502000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cummings JL, Mega M, Gray K, et al. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 34.Kaufer DI, Cummings JL, Christine D, et al. Assessing the impact of neuropsychiatric symptoms in Alzheimer’s disease: The Neuropsychiatric Inventory Caregiver Distress Scale. J Am Geriatr Soc. 1998;46:210–215. doi: 10.1111/j.1532-5415.1998.tb02542.x. [DOI] [PubMed] [Google Scholar]
- 35.Schneider LS, Tariot PN, Lyketsos CG, et al. NIMH-CATIE: Alzheimer’s Disease Clinical Trial Methodology. Am J Geriatr Psychitry. 2001;9:346–360. [PubMed] [Google Scholar]
- 36.Banaszak-Holl J, Fendrick AM, Foster NL, et al. Predicting nursing home admission: estimates from a 7-year follow-up of a nationally representative sample of older Americans. Alzheimer Dis Assoc Disord. 2004;18:83–89. doi: 10.1097/01.wad.0000126619.80941.91. [DOI] [PubMed] [Google Scholar]
- 37.Fisher GG, Faul JD, Weir DR, et al. Documentation of Chronic Disease Measures in the Heath and Retirement Study (HRS/AHEAD) Accessed at http://hrsonline.isr.umich.edu/docs/userg/dr-009.pdf on 12 December 2008.
- 38.Kleinbaum DG. Survival Analysis: A Self-Learning Text. Springer; New York: 1996. [Google Scholar]
- 39.Arfken CL, Lichtenberg PA, Tancer ME. Cognitive impairment and depression predict mortality in medically ill older adults. J Gerontol A Biol Sci Med Sci. 1999;54:M152–156. doi: 10.1093/gerona/54.3.m152. [DOI] [PubMed] [Google Scholar]
- 40.Sarkisian CA, Lachs MS. “Failure to thrive” in older adults. Ann Intern Med. 1996;124:1072–1078. doi: 10.7326/0003-4819-124-12-199606150-00008. [DOI] [PubMed] [Google Scholar]
- 41.Royall DR, Palmer RF, Chiodo LK, et al. Clock-drawing potentially mediates the effect of depression on mortality: Replication in three cohorts. Int J Geriatr Psychiatry. 2008;23:821–829. doi: 10.1002/gps.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Royall DR, Chiodo LK, Mouton C, et al. Cognitive predictors of mortality in elderly retirees: Results from the Freedom House study. Am J Geriatr Psychiatry. 2007;15:243–251. doi: 10.1097/01.JGP.0000240824.84867.02. [DOI] [PubMed] [Google Scholar]
- 43.Leslie DL, Zhang Y, Holford TR, et al. Premature death associated with delirium at 1-year follow-up. Arch Intern Med. 2005;165:1657–1662. doi: 10.1001/archinte.165.14.1657. [DOI] [PubMed] [Google Scholar]
- 44.Kiely DK, Marcantonio ER, Inouye SK, et al. Persistent Delirium Predicts Greater Mortality. J Am Geriatr Soc. 2008;16:664–673. doi: 10.1111/j.1532-5415.2008.02092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams MM, Xiong C, Morris JC, et al. Survival and mortality differences between dementia with Lewy bodies vs Alzheimer disease. Neurology. 2006;67:1935–1941. doi: 10.1212/01.wnl.0000247041.63081.98. [DOI] [PubMed] [Google Scholar]