Abstract
The facultative anaerobe group B Streptococcus (GBS) is an opportunistic pathogen of pregnant women, newborns, and the elderly. Although several virulence factors have been identified, environmental factors that regulate the pathogenicity of GBS have not been well characterized. Using the dynamic in vitro attachment and invasion system (DIVAS), we examined the effect of oxygen on the ability of GBS to invade immortalized human epithelial cells. GBS type III strain M781 invaded human epithelial cells of primitive neurons, the cervix, the vagina, and the endometrium in 5- to 400-fold higher numbers when cultured at a cell mass doubling time (td) of 1.8 h than at a slower td of 11 h. Invasion was optimal when GBS was cultured at a td of 1.8 h in the presence of ≥5% oxygen and was significantly reduced without oxygen. Moreover, GBS grown in a chemostat under highly invasive conditions (td of 1.8 h, with oxygen) was more virulent in neonatal mice than was GBS grown under suboptimal invasion conditions (td of 1.8 h, without oxygen), suggesting a positive association between in vitro invasiveness with DIVAS and virulence.
The ability of bacterial pathogens to adapt to often-changing environments within a single host is critical to their growth and survival. Nutrients such as carbon, nitrogen, and oxygen and environmental stimuli such as temperature and pH may regulate certain traits, including those involved with the ability of a commensal to become a frank pathogen. In humans, group B Streptococcus (GBS) is found as a part of the normal colonic microflora. It transiently colonizes rectal and/or vaginal surfaces in ∼30% of the female population (7). Although GBS can colonize these sites without causing harm, it can also ascend from the vagina to the cervix, where it is responsible for a wide array of associated morbidity (1), and it has also been associated with midgestation abortion (10). In addition, GBS can be vertically transmitted from a vaginally colonized mother to her newborn, in whom it can cause pneumonia, sepsis, and meningitis. GBS meningitis can be fatal, and 25 to 50% of survivors have neurological deficiencies that range from partial sensory loss to profound mental retardation, blindness, and deafness (1). Clinical manifestations of GBS disease in nonpregnant adults include skin, soft tissue, or bone infections; bacteremia without focus; urosepsis; pneumonia; and peritonitis (2).
We recently developed a new technology to study bacteria-host cell interactions (9). The dynamic in vitro attachment and invasion system (DIVAS) combines the advantages of controlling conditions of bacterial growth with those of perfusion tissue culture for studying bacterial attachment and invasion in vitro. Using DIVAS, we found that the ability of GBS to invade respiratory epithelial cells is regulated by the rate of bacterial growth. GBS held at a fast mass doubling time (td) readily invaded these epithelial cells, whereas GBS held at a relatively slower td was significantly less invasive. Growth rate-dependent regulation of GBS invasiveness was independent of the limiting nutrient used to achieve steady-state growth and of GBS capsular polysaccharide phenotype (9).
For this study, we sought to determine, by using DIVAS, whether oxygen has a role in regulating the ability of GBS to invade immortalized human epithelial cells of the lung, vagina, cervix, endometrium, and primitive neurons and also whether in vitro invasiveness correlates with virulence.
MATERIALS AND METHODS
Bacterial strain.
GBS type III strain M781, originally isolated from an infant with meningitis, was used for this study (20).
Cell lines.
Human cervical epithelial cells, ME-180 cells (ATCC HTB-33), were maintained in McCoy's 5a cell culture medium with 10% fetal bovine serum (FBS). Human type II alveolar epithelial carcinoma A549 cells were maintained as described previously (9). PFSK-1 cells (ATCC CRL-2060), a human neuroepithelial cell line (4), were propagated in RPMI 1640 culture medium containing 1% l-glutamine and 10% FBS in culture dishes coated with poly-d-lysine, which has been determined to be necessary for optimal adherence. KLE cells (ATCC CRL-1622), a human endometrial carcinoma cell line, were maintained in a 1:1 mixture of Dulbecco's modified Eagle's culture medium and Ham's F12 culture medium with 10% FBS. VK2 cells, an immortalized human vaginal epithelial cell line, were maintained in keratinocyte-SFM culture medium with epidermal growth factor, bovine pituitary extract, penicillin G-streptomycin, and calcium chloride (3).
All cell cultures were incubated at 37°C with 5% CO2. Spent medium was replaced every 2 to 3 days and 1 day before use of the cells. Confluent cells were split 1:4 with 0.25% trypsin-EDTA to release the cells. For DIVAS, cells were subcultured into 12.5-cm2 vented culture flasks or 60- by 15-mm culture dishes. For static invasion assays, cells were subcultured into 24-well culture plates. Immediately before the invasion experiments, monolayers were washed once with phosphate-buffered saline (PBS), and fresh RPMI 1640 containing 10% FBS was added.
DIVAS.
DIVAS was assembled as described previously (9). In brief, a 1-liter chemostat vessel (Applikon, Foster City, Calif.) with a working volume of 0.5 liter was used to grow GBS in chemically defined medium with glucose limitation at a td of 1.8 h (fast growth) or 11 h (slow growth). Temperature (37°C), pH (pH 7.3), and mixing rate (400 rpm) were maintained at constant levels throughout the experiments. Levels of dissolved oxygen (DO2) were monitored with a polarographic DO2 probe (Applikon) and controlled by the addition of a mixture of compressed nitrogen and oxygen. Initial experiments with added oxygen were conducted by supplying air to the reaction vessel with an air compressor. All growth parameters (pH, temperature, mixing rate, medium inflow rate, and DO2) were computer controlled with a BioController (model ADI 1030; Applikon).
Confluent cells in 12.5-cm2 vented culture flasks were washed twice with 3.0 ml of PBS. To use DIVAS with eukaryotic cells grown in 60-mm-diameter culture dishes, we manufactured an apparatus to accommodate four plates, each with inlet and outlet ports to allow for perfusion of bacteria. The use of DIVAS with tissue culture cells grown in 60-mm-diameter plates was validated with GBS type III strain M781 and A549 respiratory epithelial cells. As measured with A549 cells cultured in flasks (9), GBS invaded significantly better at the fast (td of 1.8 h) than at the slow (td of 11.0 h) cell mass doubling time (data not shown). Thus, DIVAS could be used with cells grown either in flasks or in tissue culture plates in the new apparatus. The flasks or plates were then placed on a rotary shaker at 20 rpm in a 37°C incubator. Perfusion of GBS proceeded for 2 h, after which the flasks or plates were disconnected from the chemostat. Cell monolayers were washed with PBS, extracellular GBS was killed with antibiotics, eukaryotic cells were disrupted, and internalized GBS was quantified by standard plate counts as described previously (9).
Conventional static invasion assay.
To study the effect of multiplicity of infection (MOI) on the degree of invasion, we used the conventional static invasion assay (16). In brief, 10 ml of GBS maintained in the chemostat at a td of 1.8 h with 0 or 12% DO2 was collected and pelleted by centrifugation (4,225 × g) at 4°C for 15 min, and cells were suspended in RPMI 1640. A549 cells (average of 4.25 × 105 cells/well), cultured to confluency in 24-well plates, were infected at MOIs (ratios of GBS to A549 cells) of 0.1, 1, and 10 and placed in a 5% CO2 incubator at 37°C. After 2 h of incubation, infected monolayers were washed three times with PBS before incubation in RPMI medium containing 10% FBS plus gentamicin (100 μg/ml) and penicillin (5 μg/ml) for an additional 2 h to kill extracellular bacteria. The monolayers were washed three times with PBS, 0.2 ml of 0.25% trypsin-EDTA was added, and the mixture was incubated for 5 min. Triton X-100 (0.8 ml of 0.025% solution) was then added to the monolayer. The monolayers were incubated at room temperature for an additional 1 min and the intracellular GBS was liberated by repeated use of a pipette. The number of intracellular GBS was determined by viable plate counts and the percentage of invasive GBS was calculated with the formula (CFU of intracellular GBS/CFU of original inoculum) × 100.
Transmission electron microscopy.
Confluent A549 cells (3.5 × 106 viable cells in total) in 60-mm-diameter dishes were perfused for 2 h with GBS held at steady state at a td of 1.8 h with 0 or 5% DO2 in DIVAS as described above. After the wells were washed with 3 ml of PBS, cells were fixed with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4) and then postfixed in 1% osmium tetraoxide. After samples were dehydrated through a graded alcohol series, the cells were embedded in TAAB 812 resin (Marivac, Halifax, Nova Scotia, Canada). Thin sections were prepared with a diamond knife on an ultramicrotome, stained with uranyl acetate and lead citrate, and examined with a JEOL 1200EX electron microscope operating at 80 kV.
Infection of neonatal mice.
Neonatal CD-1 outbred mouse pups (24- to 72-h old) were inoculated intraperitoneally with GBS grown in a chemostat and held at steady state at a td of 1.8 h with 0 or 5% DO2 by use of two continuous culture systems. GBS used to inoculate pups was obtained directly from the chemostat or diluted 1:10 with filtered (0.22-μm pore size) medium obtained from the chemostat. Each pup received 50 μl of inoculum containing 3.0 × 105 CFU (n = 8 pups) or 3.0 × 106 CFU (n = 7 pups) of GBS grown with 0% DO2 or 3.2 × 105 CFU (n = 13 pups) or 3.2 × 106 CFU (n = 11 pups) of GBS grown with 5% DO2. Pup survival was monitored for 96 h. Animal studies were reviewed and approved by the Harvard Medical Area Standing Committee on Animals.
Statistical analyses.
The significance of GBS internalization for two different variables was determined by use of the unpaired t test with the Welch correction. Analysis of GBS internalization by more than two variables was assessed by use of one-way analysis of variance with the Bonferroni multiple comparisons test. P values of <0.05 were considered significant. These statistical analyses were performed on log-transformed data with Statview (version 5.0; SAS Institute, Cary, N.C.). Kaplan-Meier survival plots and survival analyses were performed with Instat (version 3.0a; GraphPad, San Diego, Calif.).
RESULTS
Growth rate-regulated invasion of eukaryotic tissue cells by GBS.
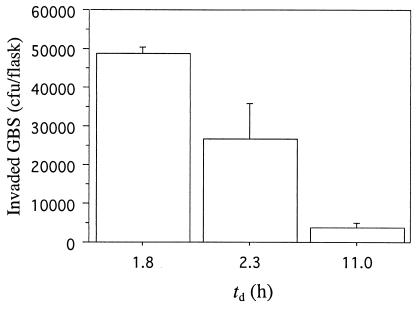
To determine whether GBS exhibits growth rate-dependent invasion of established tissue culture cells other than A549 respiratory epithelial cells (9), we performed invasion experiments with GBS grown in continuous culture (with added air) at a fast (td of 1.8 h) and a slow (td of 11.0 h) cell mass doubling time, using PFSK-1 and ME-180 cells. GBS invaded PFSK-1 cells in significantly (P < 0.001) higher numbers when held at the high growth rate than when held at low growth rates (Fig. 1). When held at a td of 1.8 h, significantly more GBS invaded PFSK-1 cells than when held at a td of 2.3 h (P < 0.05) or 11 h (Fig. 1). The mean numbers (± standard deviations) of GBS strain M781 CFU that invaded ME-180 cells were 59,425 ± 21,026 and 150 ± 94, respectively, when GBS was maintained at the high growth rate or the low growth rate (P < 0.0001).
FIG. 1.
Growth rate-dependent internalization of PFSK-1 neuroepithelial cells by GBS type III strain M781 grown at tds of 1.8 h (n = 2), 2.3 h (n = 4), and 11.0 h (n = 2). Data are means and standard deviations of two measures per determination.
Effect of oxygen on GBS invasiveness.
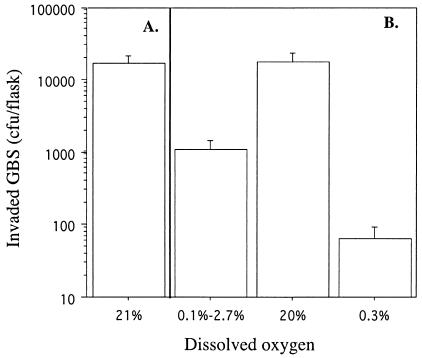
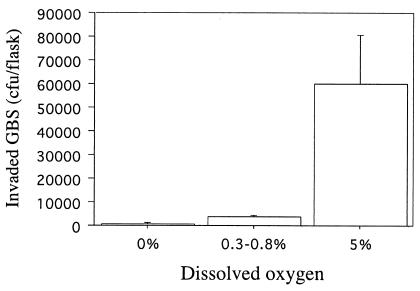
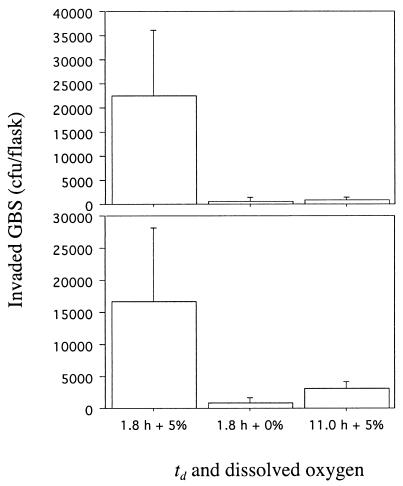
To determine if oxygen availability contributed to GBS invasiveness, we used DIVAS with GBS maintained at steady-state growth (td of 1.8 h) with and without added O2. GBS grown with air (21% DO2) readily invaded A549 cells (Fig. 2A). To assess whether the influence of oxygen on invasion was reversible, we performed three experiments in succession. (i) After GBS achieved steady-state growth with no added oxygen, oxygen was pulsed into the reaction vessel briefly during the 2-h invasion period, resulting in DO2 levels between 0.1 and 2.7%. (ii) The culture was then allowed to achieve steady-state growth with 20% DO2. (iii) Finally, the culture was allowed to achieve steady-state growth with 0.3% DO2. Significantly (P < 0.001) more GBS invaded A549 cells when cultured with 20% than with 0.3% DO2 (Fig. 2B). A brief pulse of oxygen (DO2 level, 0.1 to 2.7%) resulted in GBS invasion in numbers intermediate between those achieved when GBS was grown at the two extremes of DO2 (Fig. 2B). These results suggested an important role for oxygen in the ability of GBS to become invasive and showed that exposure to oxygen for a short period may be sufficient to alter the ability of GBS to be invasive. The requirement for oxygen to regulate GBS invasiveness was not limited to lung epithelial cells. The numbers of GBS grown at a td of 1.8 h with 0 or 5% DO2 that invaded ME-180 cells were 565 ± 243 and 27,600 ± 19,470, respectively (P = 0.006). Similarly, GBS invaded PFSK-1 cells in the presence of 5% DO2, but invaded in significantly (P < 0.001) lower numbers in the absence of DO2 (Fig. 3). The mean number of GBS CFU that invaded PFSK-1 cells was 71-fold higher when GBS was grown at 5% rather than 0% DO2. It is interesting that a brief pulse of O2 into the reaction vessel during the 2-h invasion of PFSK-1 cells, which resulted in a flux of DO2 of 0.3 to 0.8%, resulted in a statistically significant (P < 0.001) increase in invasion. The mean and standard deviation of the CFU of invasive GBS increased from 845 ± 152 at 0% DO2 to 3,547 ± 584 at 0.3 to 0.8% DO2 (Fig. 3). Optimal invasion of VK2 and KLE cells by GBS required both fast growth and oxygen (Fig. 4). Invasion of these cells was suboptimal when GBS was maintained at a td of 1.8 h and 0% DO2 or at a lower growth rate (td of 11.0 h), even in the presence of oxygen (Fig. 4).
FIG. 2.
Invasion of A549 respiratory epithelial cells by GBS type III strain M781. (A) The invasion assay was performed with GBS grown in a chemostat at a cell mass doubling time of 1.8 h with 21% DO2. (B) Three invasion assays were performed in series with GBS grown in a chemostat at DO2 levels of 0.1 to 2.7, 20, and 0.3%. Data are means and standard deviations of two measures each for eight determinations.
FIG. 3.
Invasion of PFSK-1 neuroepithelial cells by GBS type III strain M781 grown at a cell mass doubling time of 1.8 h with 0%, trace (0.3 to 0.8%), and 5% DO2. Data are means and standard deviations of two measures each for four determinations.
FIG. 4.
Invasion of VK2 vaginal epithelial cells (top) and KLE endometrial epithelial cells (bottom) by GBS grown at a td of 1.8 h with 0 and 5% DO2 and at a td of 11.0 h with 5% DO2. Data are means and standard deviations of two measures each for four determinations.
Microscopy.
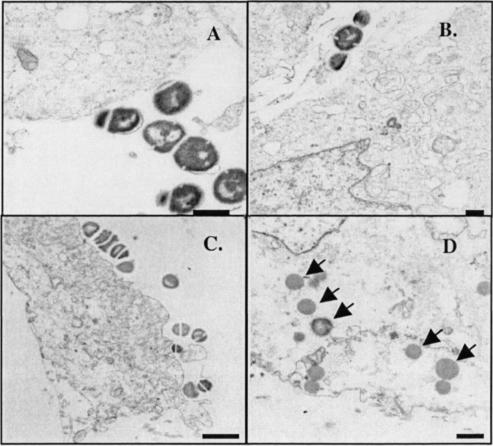
Electron micrographs revealed a close association of GBS cultured at a high rate of growth, with and without added oxygen, with the outer surfaces of respiratory cells (Fig. 5A and C). However, large numbers of intracellular GBS were seen only when GBS was grown in the presence of oxygen (Fig. 5D).
FIG. 5.
Representative transmission electron micrographs of respiratory epithelial cells infected with GBS type III strain M781 grown at a cell mass doubling time of 1.8 h with 0% (A and B) and 5% (C and D) DO2. Although GBS cells grown under the two conditions apparently adhere to the respiratory cells (A and C), the number of GBS cells located intracellularly (indicated by arrows) is high only when GBS is grown with 5% DO2 (D). Bars: 500 nm (A), 500 nm (B), 1 μm (C), and 500 nm (D). Magnification: ×15,000 (A), ×10,000 (B), ×6,500 (C), and ×10,000 (D).
Invasiveness as a function of MOI.
Because bacteria are slowly perfused over confluent monolayers of eukaryotic cells in DIVAS, establishing a specific MOI is difficult. To ensure that the increase in invasion observed with GBS grown in the presence of oxygen was not due to an increase in the number of cells per volume, as noted by direct plate counts and an increase in cell biomass, we performed the conventional static invasion assay at different MOIs with GBS grown in a chemostat. At each of the three MOIs tested, GBS maintained at a high growth rate with 12% DO2 invaded A549 cells in significantly higher numbers than did GBS grown without oxygen at the same MOI (Table 1). These results corroborate those obtained with DIVAS and also imply that the differences in invasion were due to physiological conditions of GBS and not to perfusion of higher cell numbers.
TABLE 1.
Invasion of A549 respiratory epithelial cells by GBS type III strain M781 grown at a td of 1.8 h with 0 or 12% DO2
| MOI | % (mean ± standard deviation) of invading GBS cells that were grown at the indicated DO2 levela
|
P | |
|---|---|---|---|
| 0% | 12% | ||
| 0.1 | 1.8 ± 1.0 | 12.0 ± 5.8 | 0.0002 |
| 1.0 | 2.2 ± 0.5 | 9.0 ± 2.8 | <0.0001 |
| 10.0 | 0.5 ± 0.1 | 2.8 ± 0.9 | <0.0001 |
n = 8 measurements.
Virulence of GBS in neonatal mice.
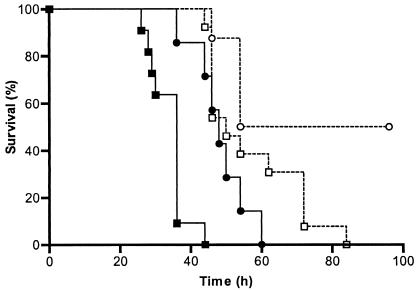
We hypothesized that although GBS administered to mice will change metabolically in response to available nutrients in the in vivo environment, the time required to kill the population of pups should reflect the ability of GBS to invade eukaryotic cells as measured in vitro, i.e., GBS held under more invasive growth conditions would translocate more efficiently from the peritoneum to the blood than would GBS grown under less invasive conditions, resulting in a more rapidly progressing infection and death. At the higher challenge dose (106 CFU/pup) tested, pups that received GBS grown with 5% DO2 died sooner (P = 0.0007) than pups that received GBS grown with 0% DO2 (Fig. 6). At the lower challenge dose (105 CFU/pup), all pups that received GBS grown with 5% DO2 died (Fig. 6), whereas only 50% of pups that received GBS grown without added oxygen died (P < 0.02).
FIG. 6.
Survival proportions of neonatal pups infected with GBS strain M781 grown in a chemostat at a cell mass doubling time of 1.8 h with 0% (circles) or 5% (squares) DO2. Pups were infected with a high dose (106 CFU/pup; closed symbols) or a lower dose (105 CFU/pup; open symbols) of GBS.
DISCUSSION
GBS attaches to and/or invades a variety of human tissues, including embryonic, fetal, and adult buccal epithelial cells (12, 21), embryonic chorioamnion cells (6, 22), vaginal epithelial cells (23), respiratory epithelial cells (8, 16, 18), umbilical vein endothelial cells (5), brain microvascular endothelial cells (13), and chorionic and amnionic epithelial cells (22). The environmental signals that regulate the invasiveness and pathogenic potential of GBS, however, are not well elucidated.
The results presented herein strongly suggest that oxygen is an important stimulus for increased invasiveness of GBS. Moreover, only brief periods of low levels of oxygen were necessary for reversal of the relatively poor invasiveness measured when GBS was grown in the absence of oxygen, suggesting a rapid response by GBS to available oxygen. What role could oxygen have in regulating invasiveness of this facultative anaerobe? Studies performed by Mickelson in the early 1970s (11) showed that GBS grown anaerobically in a complex medium, with energy source limitation, resulted in the generation of 2 mol of ATP per mol of glucose from substrate-level phosphorylation based on molar growth yield measurements. When GBS was grown aerobically (∼1 mol of oxygen was consumed per mol of glucose), molar growth yields indicated the generation of 5 mol of ATP per mol of glucose (about one-half of the ATP was generated by substrate-level phosphorylation and oxidative phosphorylation). In our studies, the average biomass yields of GBS obtained from growth in the chemostat at a td of 1.8 h with 0% or ≥5% DO2 were 0.345 ± 0.08 and 0.519 ± 0.15 mg/ml, respectively; these results are in agreement with those of Mickelson (11). It is tempting to speculate that the observed improvement in GBS invasiveness in the presence of oxygen during growth at a td of 1.8 h is linked to its ability to generate ATP.
Growth rate-dependent invasion by GBS of eukaryotic cells was not restricted to those from the respiratory epithelia but included those that originated from the vagina, cervix, endometrium, and primitive neurons. A trend towards enhanced invasion by GBS as a function of increasing growth rate existed, with optimal invasion of these cell lines measured in the presence of oxygen. Although the effect of growth conditions on GBS adherence to eukaryotic cells was not measured directly, electron micrographs showed an intimate association of GBS with the eukaryotic cell membrane regardless of the bacterial growth condition used. Many extracellular GBS cells showed multiple septum formation, which may be attributed to their rapid growth rate; however, no septa were visualized in internalized GBS, confirming that they do not divide intracellularly in epithelial cells (16). Also, the higher number of internalized GBS cells seen by electron microscopy when they were grown in the presence of oxygen is consistent with results obtained by the antibiotic exclusion assay and plate count method.
GBS grown in a chemostat under highly invasive conditions were also more virulent in neonatal mice. Unlike vaccine potency studies in which the GBS challenge is administered to pups in a rich medium to promote growth (14), we inoculated pups with GBS in the medium in which they were cultured in an effort to maintain initial nutrient conditions as long as possible. The higher in vitro invasiveness of GBS grown in the presence of oxygen corresponded to increased virulence in neonatal mice. This repeated finding suggests that the degree of invasiveness measured with DIVAS has relevance to virulence in this animal model of GBS disease.
In summary, we used DIVAS to expose metabolically stable GBS to immortalized epithelial cells representative of tissues it can encounter in vivo in an effort to gain a better understanding of how GBS regulates its pathogenic potential. GBS invaded all five epithelial cell lines tested in a growth rate-dependent manner, and invasion was optimal when GBS was grown in the presence of oxygen. Moreover, GBS grown in the chemostat under conditions that promoted optimal invasion (td of 1.8 h, with ≥5% DO2) was more virulent in neonatal mice than was GBS held under suboptimal invasion growth conditions.
It is worth noting that levels of pO2 in tissues that GBS can infect are dynamic. The normal pO2 of human fetal arterial blood is 30 mm Hg, which increases to 60 to 100 mm Hg after birth (15). The median pO2 in the cervix of nulliparous women is 48 mm Hg but is lower (mean and median of 16 mm Hg) in the normal cervix of parous women (19). Tissue pO2 values in regions of animal brains in situ range from 0 to 99 mm Hg, with dramatic changes in closely adjacent sites (17). Exposure to oxygen may be a powerful stimulus for upregulation of factors involved in GBS pathogenesis.
Acknowledgments
We thank Raina N. Fichorova for the VK-2 cell line, Phillip J. Albrecht for helpful discussions, and Kathleen McGovern for assistance with the mouse studies. We also thank Andrew B. Onderdonk and Dennis L. Kasper for critical review of the manuscript.
This work was supported by NIAID Contract AI-75326 and the William Randolph Hearst Award.
The contents of this publication do not necessarily reflect the views or policies of the Department of Health and Human Service, nor does the mention of trade names, commercial products, or organizations imply endorsements by the U.S. Government.
Editor: V. J. DiRita
REFERENCES
- 1.Baker, C., and M. Edwards. 1990. Group B streptococcal infections, p. 742-811. In J. Remington and J. Klein (ed.), Infectious diseases of the fetus and newborn infant. W. B. Saunders, Philadelphia, Pa.
- 2.Farley, M. M., R. C. Harvey, T. Stull, J. D. Smith, A. Schuchat, J. D. Wenger, and D. S. Stephens. 1993. A population-based assessment of invasive disease due to group B Streptococcus in nonpregnant adults. N. Engl. J. Med. 328:1807-1811. [DOI] [PubMed] [Google Scholar]
- 3.Fichorova, R. N., J. G. Rheinwald, and D. J. Anderson. 1997. Generation of papillomavirus-immortalized cell lines from normal human ectocervical, endocervical, and vaginal epithelium that maintain expression of tissue-specific differentiation proteins. Biol. Reprod. 57:847-855. [DOI] [PubMed] [Google Scholar]
- 4.Fults, D., C. Pedone, H. Morse, J. Rose, and R. McKay. 1992. Establishment and characterization of a human primitive neuroectodermal tumor cell line from the cerebral hemisphere. J. Neuropathol. Exp. Neurol. 51:272-280. [DOI] [PubMed] [Google Scholar]
- 5.Gibson, R. L., M. K. Lee, C. Soderland, E. Y. Chi, and C. E. Rubens. 1993. Group B streptococci invade endothelial cells: type III capsular polysaccharide attenuates invasion. Infect. Immun. 61:478-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldschmidt, J. C., Jr., and C. Panos. 1984. Teichoic acids of Streptococcus agalactiae: chemistry, cytotoxicity, and effect on bacterial adherence to human cells in tissue culture. Infect. Immun. 43:670-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hickman, M. E., M. A. Rench, P. Ferrieri, and C. J. Baker. 1999. Changing epidemiology of group B streptococcal colonization. Pediatrics 104:203-209. [DOI] [PubMed] [Google Scholar]
- 8.Hulse, M. L., S. Smith, E. Y. Chi, A. Pham, and C. E. Rubens. 1993. Effect of type III group B streptococcal capsular polysaccharide on invasion of respiratory epithelial cells. Infect. Immun. 61:4835-4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malin, G., and L. C. Paoletti. 2001. Use of a dynamic in vitro attachment and invasion system (DIVAS) to determine influence of growth rate on invasion of respiratory epithelial cells by group B Streptococcus. Proc. Natl. Acad. Sci. USA 98:13335-13340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDonald, H. M., and H. M. Chambers. 2000. Intrauterine infection and spontaneous midgestation abortion: is the spectrum of microorganisms similar to that in preterm labor? Infect. Dis. Obstet. Gynecol. 8:220-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mickelson, M. N. 1972. Glucose degradation, molar growth yields, and evidence for oxidative phosphorylation in Streptococcus agalactiae. J. Bacteriol. 109:96-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nealon, T. J., and S. J. Mattingly. 1984. Role of cellular lipoteichoic acids in mediating adherence of serotype III strains of group B streptococci to human embryonic, fetal, and adult epithelial cells. Infect. Immun. 43:523-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nizet, V., K. S. Kim, M. Stins, M. Jonas, E. Y. Chi, D. Nguyen, and C. E. Rubens. 1997. Invasion of brain microvascular endothelial cells by group B streptococci. Infect. Immun. 65:5074-5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paoletti, L. C. 2001. Potency of clinical group B streptococcal conjugate vaccines. Vaccine 19:2118-2126. [DOI] [PubMed] [Google Scholar]
- 15.Polin, R. A., and W. W. Fox (ed.). 1998. Fetal and neonatal physiology, 2nd ed. W. B. Saunders Co., Philadelphia, Pa.
- 16.Rubens, C. E., S. Smith, M. Hulse, E. Y. Chi, and G. van Belle. 1992. Respiratory epithelial cell invasion by group B streptococci. Infect. Immun. 60:5157-5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silver, I., and M. Erecinska. 1998. Oxygen and ion concentrations in normoxic and hypoxic brain cells, p. 7-16. In A. G. Hudetz and D. F. Bruley (ed.), Oxygen transport to tissue XX. Plenum Press, New York, N.Y. [DOI] [PubMed]
- 18.Tamura, G. S., and A. Nittayajarn. 2000. Group B streptococci and other gram-positive cocci bind to cytokeratin 8. Infect. Immun. 68:2129-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaupel, P. 1996. Oxygen transport in tumors, p. 341-351. In C. Ince, J. Kesecioglu, L. Telci, and K. Akpir (ed.), Oxygen transport to tissue XVII. Plenum Press, New York, N.Y.
- 20.Wessels, M. R., L. C. Paoletti, D. L. Kasper, J. L. DiFabio, F. Michon, K. Holme, and H. J. Jennings. 1990. Immunogenicity in animals of a polysaccharide-protein conjugate vaccine against type III group B Streptococcus. J. Clin. Investig. 86:1428-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wibawan, I. T., C. Lammler, and F. H. Pasaribu. 1992. Role of hydrophobic surface proteins in mediating adherence of group B streptococci to epithelial cells. J. Gen. Microbiol. 138:1237-1242. [DOI] [PubMed] [Google Scholar]
- 22.Winram, S. B., M. Jonas, E. Chi, and C. E. Rubens. 1998. Characterization of group B streptococcal invasion of human chorion and amnion epithelial cells in vitro. Infect. Immun. 66:4932-4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zawaneh, S. M., E. M. Ayoub, H. Baer, A. C. Cruz, and W. N. Spellacy. 1979. Factors influencing adherence of group B streptococci to human vaginal epithelial cells. Infect. Immun. 26:441-447. [DOI] [PMC free article] [PubMed] [Google Scholar]