Abstract
Infection of mice with Salmonella enterica serovar Typhimurium induces strong Th1 T-cell responses that are central to the control of the infection. In the present study, we examined the role of B cells in the development of Th1 T-cell responses to Salmonella by using gene-targeted B-cell-deficient mice (Igh-6−/− mice). The development of Th1 T-cell responses in Igh-6−/− mice was impaired in the early stage of a primary infection. This impairment persisted throughout the course of the disease. The ability of T cells to produce the Th1 cytokine gamma interferon and the frequency at which they did so were lower in Igh-6−/− mice than in control mice. We also observed a transient switch toward Th2 cytokine production in Igh-6−/− mice. Thus, B cells are important for the induction of protective Th1 T-cell responses in the early phase of a Salmonella infection. Activated B cells express high levels of major histocompatibility complex and costimulatory molecules and are nearly as effective as dendritic cells in their antigen-presenting cell (APC) activity. However, their importance as APCs in infection and their role in initiating and/or maintaining T-cell responses are unknown. Here, we show that B cells upregulate costimulatory molecules upon in vitro stimulation with S. enterica serovar Typhimurium and that they can present Salmonella antigens to Salmonella-specific CD4+ T cells. Our results show that B cells are important for the development of T-cell responses in the early stage of a Salmonella infection and that this property may be due to their ability to present antigens to T cells.
Salmonella infections are still a serious health problem worldwide. Infection of mice with Salmonella enterica serovar Typhimurium results in a systemic infection that closely resembles human typhoid fever caused by the related S. enterica serovar Typhi (12, 40). Infection of mice with S. enterica serovar Typhimurium therefore is a widely accepted and valuable model for human typhoid fever.
After oral uptake, S. enterica serovar Typhimurium crosses the intestinal epithelium and spreads to the spleen and liver, where the bacteria replicate. Bacterial growth in mice is controlled by the Slc11a1 (formerly Nramp1) gene (67). The expression of functional SLC11A1 molecules in phagocytes is a critical component during the early phase of an anti-Salmonella response, since mouse strains with a natural mutation of Slc11a1 fail to adequately restrain the initial multiplication of S. enterica serovar Typhimurium (67). Other T-cell-independent mechanisms, such as tumor necrosis factor alpha, interleukin 12 (IL-12), IL-18, gamma interferon (IFN-γ), and nitric oxide production, also are involved in the early phase of resistance to infection (25, 30, 42, 43, 46, 62, 65).
In a primary infection, the clearance of Salmonella from infected tissues is controlled by the acquisition of T-cell-specific immunity. Conversely, both T and B lymphocytes are involved in protection against a secondary infection. However, the exact function and the relative importance of B- and T-cell-mediated immunity in this process are still a matter of debate (12, 40). Data are available showing that antibodies or T cells alone can confer only a moderate level of protection against salmonellosis. Passive transfer of immune serum or B cells alone can protect innately resistant mice against virulent salmonellae or susceptible mice against moderately virulent organisms (6, 14, 26). Additionally, adoptive transfer of immune T cells can protect mice against infection with very low doses of virulent salmonellae or against infection with moderately virulent organisms (21). In contrast, adoptive transfer of both immune serum and T cells can protect innately susceptible mice against highly virulent salmonellae (45), indicating that both humoral immunity and cell-mediated immunity are required for resistance to virulent salmonellae in Slc11a1s animals.
In addition to antibody production, B cells display a wide range of other functions in the immune system, including antigen presentation and cytokine production (22, 54). The use of attenuated bacteria (29) allows analysis of the immune response against S. enterica serovar Typhimurium in susceptible mice, particularly of the Slc11a1s background. Recent reports indicated that gene-targeted B-cell-deficient mice displayed increased susceptibility to infection by some agents but not by others (3, 28, 33, 35, 47, 69). In the murine model of Salmonella infection, it was shown that B-cell-deficient mice could control and clear a primary infection (44, 53). In spite of this finding, mice were unable to mount long-lasting, antigen-specific Th1 protective T-cell immunity. However, it is unclear from these reports whether T-cell immunity to Salmonella is initiated normally but the memory response is defective in the absence of B cells or whether B cells are needed for both the initiation and the maintenance of functional T-cell responses.
The role of B cells in the establishment (4, 37) and/or persistence (1, 5, 66) of a stable T-cell memory pool is still debatable. Since the extent of T-cell expansion during a primary response may have an impact on the development of a persistent memory population, we wondered whether B cells play an essential role in the development of T-cell responses in Salmonella infection. It has long been considered that B cells play an important, although not obligatory, role as antigen-presenting cells (APC) in priming T-cell responses. Mamula and Janeway (41) suggested that helper T-cell-activated B cells can express the necessary costimulatory signals for naive T-cell activation, thereby initiating a positive feedback loop and diversifying the immune response. Although primary CD4+-T-cell responses can develop in mice that lack B cells (15, 63), the depletion of B cells from normal mice can reduce the magnitude of the responses, implying that B cells may contribute to the level of T-cell priming (24, 31, 34, 55). Furthermore, studies confirming that antigen-bearing B cells induce naive CD4+-T-cell proliferation in vivo (8, 64) as well as studies of the progressive migration of subsets of cells within lymphoid tissues during the CD4+-T-cell response to antigen suggest that precisely regulated interactions with APC influence the magnitude of the immune response (18, 56, 64).
In the present study, we analyzed the development of the protective Th1 T-cell response in Salmonella infection in B-cell-deficient mice. We further investigated the antigen presentation ability of naive versus antigen-experienced B cells as well as the efficiencies of different APC populations isolated from C57BL/6 and Igh-6−/− mice in presenting bacterial antigens to T cells. Our experiments established that Th1 T-cell responses are impaired from the early stage of Salmonella infection, implying that B cells play an essential role in the initiation of T-cell-mediated protection against this bacterium.
MATERIALS AND METHODS
Animals.
C57BL/6 mice were purchased from Harlan Olac Ltd. (Bicester, United Kingdom). B-cell-deficient mice with a C57BL/6 background and homozygous for a targeted mutation in the gene for immunoglobulin heavy chain 6 (Igh-6) that disrupts the immunoglobulin μ-chain gene (32) were bred at the Clinical Veterinary Medicine Animal Unit, Cambridge, United Kingdom. Initial breeding mice were from either Jackson Laboratory (Bar Harbor, Maine) or K. Rajewsky (Boston, Mass.). Age-matched groups of mice were used when they were more than 8 weeks old.
Bacteria and infections.
S. enterica serovar Typhimurium SL3261 is an aroA attenuated live vaccine strain with an intravenous (i.v.) 50% lethal dose (LD50) for C57BL/6 mice of 107 CFU (27). S. enterica serovar Typhimurium C5 is a virulent strain (30) with an i.v. LD50 of <10 CFU and an oral LD50 of approximately 106 CFU for C57BL/6 mice. S. enterica serovar Typhimurium SL3261-GFP was constructed by electroporation of pGFP into S. enterica serovar Typhimurium SL3261. pGFP was a gift from A. Papaconstantinopoulou (Imperial College, London, United Kingdom) and contains the gene for the fluorescence-activated cell sorting (FACS)-optimized mutant of green fluorescent protein (GFP) (9) expressed from the lacZ promoter in a pUC19-derived plasmid. S. enterica serovar Typhimurium SL3261-GFP was grown in the presence of ampicillin (100 μg/ml; Sigma, Poole, United Kingdom).
For i.v. inoculation, S. enterica serovar Typhimurium strain SL3261 was grown overnight at 37°C as a stationary culture in Luria-Bertani (LB) broth (Difco, West Molesey, United Kingdom). Aliquots were snap-frozen and stored in liquid nitrogen. The inoculum was diluted in phosphate-buffered saline (PBS), and 8-week-old mice were immunized with 5 × 105 SL3261 bacteria injected into a lateral tail vein. The number of viable bacteria in the inoculum was checked by pour plating on LB agar plates.
Antibodies, tissue culture reagents, and cell lines.
Mouse monoclonal antibodies (MAbs) to CD16/CD32 (purified), T-cell receptor αβ (TCRαβ), CD3, CD4, CD8, CD19, CD11b, CD11c, CD69, CD80, CD86, CD40, IA/IE, IFN-γ, and IL-4, isotype controls, and other reagents used for flow cytometry and intracellular cytokine staining (ICCS) were purchased from BD PharMingen (Cowley, United Kingdom). Unless otherwise stated, antibodies were directly conjugated to fluorescein isothiocyanate, phycoerthythrin, or Cy-Chrome. Purified and biotinylated anti-IFN-γ antibodies (R4-6A2 and XMG1.2, respectively) and purified and biotinylated anti-IL-4 antibodies (11B11 and BVD6-24G2, respectively) were used for and ELISPOT techniques. The following reagents were used for tissue culturing: phorbol myristate acetate (PMA) (5 ng/ml; Sigma), ionomycin (1.25 μM; Sigma), cytochalasin D (10 μg/ml; Sigma), mitomycin C (25 μg/ml; Sigma), and anti-CD28 (1 μg/ml). All cells were cultured in RF10 complete medium, consisting of RPMI 1640 (Sigma) supplemented with 10% fetal bovine serum (Sigma), 2 mM glutamine (Sigma), and 0.05 mM 2-mercaptoethanol (Sigma). L929, a granulocyte-macrophage colony-stimulating factor-producing cell line, was purchased from the American Type Culture Collection, Manassas, Va. (catalog no. CRL-2148). The X63-Ag8 cell line used to produce supernatants for culturing bone marrow-derived dendritic cells (BMDC) was kindly provided by A. Knight (Institute for Cell, Animal and Population Biology, University of Edinburgh, Edinburgh, United Kingdom).
CD4+ T cells and CD19+ B cells were positively enriched with magnetic bead-conjugated antibodies as instructed by the manufacturer (Miltenyi Biotec, Camberley, United Kingdom). CD4+ T cells and CD19+ B cells were found to be >95 and 99% pure, respectively, as assessed by flow cytometry with a FACSCalibur flow cytometer (Becton Dickinson).
Antigens.
Salmonella antigens were prepared as described previously (23). Briefly, an overnight stationary culture of Salmonella strains SL3261 and C5 in LB broth was pelleted, washed once in PBS containing 5 mM EDTA (Sigma), and washed once more in PBS. The bacteria were either heat inactivated (C5/HK bacteria) or sonicated on ice. Cellular debris was removed by centrifugation at 13,000 × g for 20 min. The supernatant was filtered through a 0.22-μm-pore-size filter (Sartorius, Epsom, United Kingdom) and stored at −70°C. Alkali-treated antigen (C5/NaOH) was prepared by the addition of NaOH up to 0.25 M; the mixture was incubated at 37°C for 3 h before it was neutralized with HCl and filtered. The protein concentrations of the antigens were determined by using a bicinchoninic acid kit (Pierce Biochemicals, Rockford, Ill.) according to the manufacturer's instructions.
ICCS.
ICCS for IFN-γ and IL-4 was done with a Cytofix/Cytoperm Plus kit as instructed by the manufacturer (BD PharMingen). Briefly, splenocytes (5 × 106cells/ml) were stimulated with either medium alone, C5/NaOH (10 μg/ml), or PMA and ionomycin in the presence of 1 μg of anti-CD28 antibody/ml. Brefeldin A (1 μl/ml) was added 6 h after the initiation of the culture. Cells were collected 18 h later and stained for surface markers (CD4, CD8, and CD69). Cells were fixed for 30 min, washed, permeabilized for 10 min, washed again, and incubated with anticytokine antibodies (IFN-γ and IL-4) for 30 min. Cells were washed and analyzed with a FACSCalibur flow cytometer. Cytokine-positive CD4+ cells as well as cytokine-positive T cells that were also positive for the early activation marker CD69 were counted. Presented are the percentages of cytokine-positive cells detected when cultured in the presence of antigens, corrected for background levels measured in the absence of antigens.
ELISPOT assay.
Positively selected splenic CD4+ T cells were stimulated as described above in the presence of syngeneic splenic APC for 24 h. Cells were then transferred to ELISPOT 96-well nitrocellulose-based plates (MultiScreen HA; Millipore, Watford, United Kingdom) for a further 24 or 48 h before measurement of the production of IL-4 or IFN-γ, respectively. The IFN-γ and IL-4 ELISPOT assays were performed as previously described (59). Briefly, plates were coated with 100 μl of capture mouse anti-mouse IFN-γ or IL-4 MAbs (4 μg/ml) in PBS overnight at 4°C. Wells were washed with PBS and saturated with RF10 medium. The CD4+-T-cell cultures were seeded at serial dilutions (1 × 103 to 50 × 103 cells/well) overnight in duplicate. The plates were then washed with PBS and incubated with biotinylated anti-IFN-γ or IL-4 MAbs (1 μg/ml) for 2 h at 37°C. The plates were washed several times with PBS, and alkaline phosphatase-labeled ExtrAvidin (1:1,000 dilution; Sigma) was added for 1 h at 37°C. The plates were washed again with PBS and treated with a chromogenic alkaline phosphatase-conjugated substrate (Sigma Fast; Sigma). After 30 min, the plates were washed under running tap water and air dried overnight. Spots were visualized through a dissecting microscope (magnification, ×40). Only large spots with fuzzy borders were scored as IFN-γ or IL-4 spot-forming cells. The data represent the numbers of IFN-γ or IL-4 spot-forming cells detected per 106 cells when cultured in the presence of antigens, corrected for background levels measured in the absence of antigens.
BMM and dendritic cells.
Bone marrow-derived macrophages (BMM) were prepared from C57BL/6 mice as described elsewhere (70). Briefly, bone marrow cells were extracted from femurs and tibias and cultured for 6 days at 3 × 105cells/ml in bacterium-grade petri dishes containing RPMI 1640 supplemented with 10% fetal bovine serum, 5% horse serum (Sigma), 2 mM glutamine, 0.05 mM 2-mercaptoethanol, 40 μg of gentamicin (Sigma)/ml, 1 mM sodium pyruvate (Sigma), and 20% conditioned medium from L929 cells. The adherent population was washed with PBS, detached from the plastic with cold PBS containing 0.1% EDTA, washed twice with PBS, and used on days 6 to 14. BMM were uniformly CD11b+CD11c−CD19−TCRαβ −, as confirmed by flow cytometry.
For BMDC, extracted cells were cultured in RF10 medium supplemented with 20% X63-Ag8 supernatant and were used on days 6 to 8 of culturing. The BMDC phenotype was confirmed by FACS analysis. The cells were uniformly CD11b+CD11c+CD19−TCRαβ−.
Infection of BMM and CD19+ B cells.
S. enterica serovar Typhimurium SL3261 and SL3261-GFP were grown overnight as stationary cultures in LB broth at 37°C. BMM (2 × 106 cells/well) were grown overnight at 37°C in RF10 medium in six-well plates (Nunc, Roskilde, Denmark). CD19+ B cells isolated from naive or immunized C57BL/6 mice were plated at 2 × 106 cells/well and either stimulated overnight with 1 μg of S. enterica serovar Typhimurium lipopolysaccharide (LPS) (Sigma)/ml or used on the same day without stimulation. The cells were infected at multiplicities of infection (MOI) of 1:1, 10:1, 50:1, 100:1, 200:1, and 500:1 for 2 or 24 h in the presence or absence of 10 μg of cytochalasin D/ml. The number of viable bacteria per cell was assessed by pour plating on LB agar plates after the cells were lysed with 1% Triton X-100 (Sigma) for 5 min at room temperature. Alternatively, FACS analysis was used to monitor B-cell and macrophage infection with S. enterica serovar Typhimurium SL3261-GFP. To examine the upregulation of costimulatory molecules, cells were stained for major histocompatibility complex (MHC) class II (IA and IE), CD80, CD86, and CD40 before and after 2 or 24 h of incubation with S. enterica serovar Typhimurium SL3261 or stimulation with LPS.
Generation of Salmonella-specific CD4+-T-cell lines and T-cell proliferation assays.
Salmonella-specific CD4+-T-cell lines were generated from mice infected 5 to 8 weeks previously with S. enterica serovar Typhimurium SL3261. Splenocytes were removed and cultured in RF10 medium in the presence of Salmonella C5/NaOH. CD4+-T-cell lines were maintained by periodic restimulation with mitomycin C-treated (30 min at 37°C) syngeneic spleen cells and Salmonella C5/NaOH (5 μg/ml); this step was followed by expansion in recombinant murine IL-2 (5 and 0.5 ng/ml; R&D Systems Europe Ltd., Oxon, United Kingdom). T-cell lines were uniformly CD3+CD4+CD8−TCRαβ+, as confirmed by flow cytometry. The proliferation of CD4+ T cells (105) was measured by using 96-well plates in the presence of mitomycin C-treated splenocytes (105), B cells (105), or BMM (103) for 72 h at 37°C. APC were either infected with S. enterica serovar Typhimurium SL3261 or incubated with Salmonella antigens (C5/NaOH at 5 μg/ml or C5/HK bacteria). DNA synthesis was measured by pulse-labeling triplicate wells for the last 18 h with 0.37 MBq of [3H]thymidine/well (Amersham International, Little Chalfont, United Kingdom). [3H]thymidine incorporation was measured by harvesting on glass-fiber mats with a Tomtek 96-well harvester and counting by liquid scintillation with a Microbeta 1450 counter (Wallac, Perkin-Elmer). Results of proliferation assays are given as a stimulation index (SI) (experimental counts per minute divided by background counts per minute), and responses were considered positive when the SI was >3.
Statistical analysis.
Student's t test was used to determine the significance of differences between control and experimental groups, and differences were considered significant for P values of <0.05.
RESULTS
B-cell-deficient mice show reduced IFN-γ production from CD4+ and CD8+ T cells after Salmonella infection.
IFN-γ is the prototype Th1 cytokine and plays a central role in protective immunity against Salmonella infection in mice (51). It was previously shown that CD4+ T cells from Igh-6−/− mice 3.5 months postimmunization have an impaired ability to produce Th1 cytokines in response to in vitro stimulation with Salmonella antigens (44). To evaluate whether this impairment was due either to deficient initiation or to deficient persistence of T-cell immunity, we compared the kinetics of the development of Th1 immune responses in Igh-6−/− and C57BL/6 mice in Salmonella infection. For ICCS, the expression of CD69, an early activation marker, was used as a marker for antigen-specific IFN-γ-producing cells (60, 61).
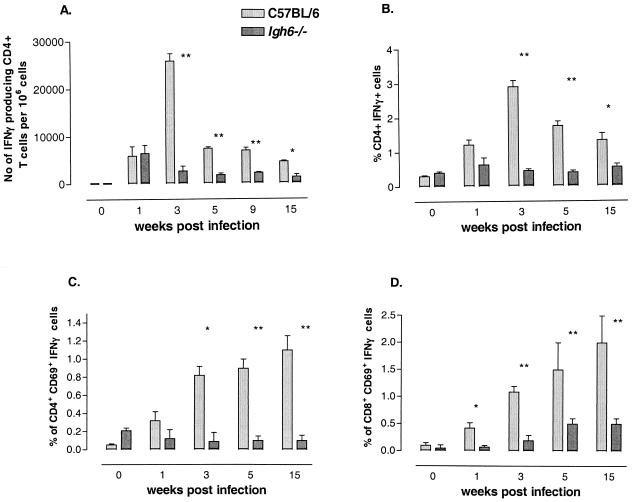
During the first week of infection, there was no difference in the frequency of IFN-γ-secreting CD4+ T cells between Igh-6−/− and C57BL/6 mice after in vitro stimulation with Salmonella C5/NaOH, as measured by an ELISPOT assay (Fig. 1A). This observation was confirmed when intracellular IFN-γ expression in CD4+ T cells after in vitro stimulation with Salmonella C5/NaOH was measured (Fig. 1B). A similar pattern was also seen when the percentage of cells positive for both IFN-γ and CD69 was analyzed (Fig. 1C). However, the frequency of CD8+ T cells positive for both IFN-γ and CD69 was already lower in the first week of infection in Igh-6−/− mice than in C57BL/6 mice (P < 0.05) (Fig. 1D). We measured IFN-γ production 3, 5, and 15 weeks after infection in Igh-6−/− and C57BL/6 mice. As shown in Fig. 1, the production of IFN-γ by CD4+ (Fig. 1A to C) and CD8+ (Fig. 1D) T cells was lower in Igh-6−/− mice than in C57BL/6 control mice throughout the infection.
FIG. 1.
Antigen-specific IFN-γ production by T cells from B-cell-deficient mice. (A) CD4+ T cells were isolated from C57BL/6 or Igh-6−/− naive mice or from mice sacrificed at the indicated times after immunization with S. enterica serovar Typhimurium SL3261. Cells were stimulated with Salmonella C5/NaOH and APC, and IFN-γ production was measured by an ELISPOT assay as indicated in Materials and Methods. (B) Whole splenocytes from C57BL/6 or Igh-6−/− naive mice or from mice sacrificed at the indicated times after immunization with S. enterica serovar Typhimurium SL3261 were stimulated with C5/NaOH in the presence of anti-CD28 antibodies and brefeldin A and stained for IFN-γ expression as indicated in Materials and Methods. CD4+ T cells were analyzed for IFN-γ production. (C) CD4+ T cells were gated, and only cytokine-positive T cells that were also positive for the early activation marker CD69 were counted. (D) CD8+ T cells were analyzed for IFN-γ expression in the same manner as CD4+ T cells in panel C. For all experiments, the averages and standard deviations for three individual mice at each time point are shown. The levels of IFN-γ produced by CD4+ and CD8+ T cells from Igh-6−/− mice were consistently lower than the levels produced by T cells from C57BL/6 mice. A single asterisk denotes a P value of <0.05; double asterisks denote a P value of <0.01. The data shown here are representative of two independent experiments.
CD4+ and CD8+ T cells from naive Igh-6−/− and C57BL/6 mice did not produce any IFN-γ after in vitro stimulation with Salmonella C5/NaOH, suggesting that the IFN-γ production seen in infected animals was antigen specific. Furthermore, CD4+ and CD8+ T cells from uninfected Igh-6−/− mice and C57BL/6 control mice produced similar levels of IFN-γ in response to polyclonal stimulation with PMA and ionomycin (data not shown), suggesting that T cells from Igh-6−/− mice have the same intrinsic capacity to produce IFN-γ as do T cells from C57BL/6 mice. However, T cells from infected Igh-6−/− mice produced lower levels of IFN-γ than did cells from C57BL/6 mice after stimulation with PMA and ionomycin (data not shown). This difference disappeared once the infection was cleared.
These results show that B cells play a role in the development of Th1 T-cell responses from the early stage of a Salmonella infection.
B-cell-deficient mice show increased IL-4 production from T cells early after Salmonella infection.
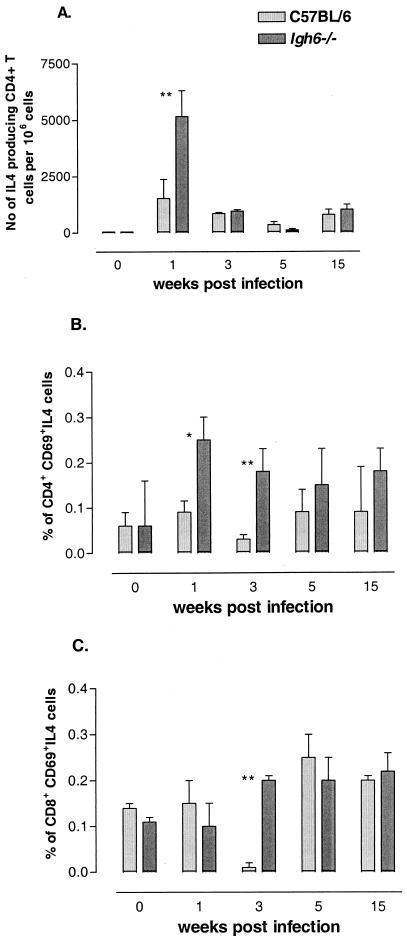
We analyzed the production of IL-4 by CD4+ and CD8+ T cells after 1, 3, 5, and 15 weeks of infection with Salmonella in Igh-6−/− and C57BL/6 mice. Naive Igh-6−/− and C57BL/6 mice showed similar, low levels of IL-4 production in response to in vitro stimulation with Salmonella C5/NaOH (Fig. 2 ) or PMA and ionomycin (data not shown). However, at 1 week after infection with S. enterica serovar Typhimurium, CD4+ T cells from Igh-6−/−mice showed higher levels of IL-4 production than did CD4+ T cells from C57BL/6 mice (P < 0.05) (Fig. 2A and B). This difference in IL-4 production by CD4+ T cells in Igh-6−/− and C57BL/6 mice was even more pronounced at 3 weeks after infection, as measured by ICCS (P < 0.01) (Fig. 2B). Using the ELISPOT assay, we were unable to detect differences in the production of IL-4 by CD4+ T cells at 3 weeks postinfection (Fig. 2A); this finding might reflect a difference in sensitivity between the two techniques. In contrast, elevated levels of IL-4 were observed in CD8+ T cells (P < 0.01) from Igh-6−/− mice at 3 weeks but not 1 week postinfection (Fig. 2C). The levels of IL-4 produced at later time points (5 and 15 weeks) of infection were similar in Igh-6−/− and C57BL/6 mice.
FIG. 2.
Antigen-specific IL-4 production by T cells from B-cell-deficient mice. (A) CD4+ T cells were isolated from C57BL/6 or Igh-6−/− naive mice or from mice sacrificed at the indicated times after immunization with S. enterica serovar Typhimurium SL3261. Cells were stimulated with Salmonella C5/NaOH and APC, and IL-4 production was measured by an ELISPOT assay as indicated in Materials and Methods. (B) Whole splenocytes from C57BL/6 or Igh-6−/− naive mice or from mice sacrificed at the indicated times after immunization with S. enterica serovar Typhimurium SL3261 were stimulated with C5/NaOH in the presence of anti-CD28 antibodies and brefeldin A and subjected to ICCS as indicated in Materials and Methods. CD4+ T cells were gated,and only cytokine-positive T cells that were also positive for the early activation marker CD69 were counted. (C) CD8+ T cells were analyzed for IL-4 expression in the same manner as CD4+ T cells in panel B. For all experiments, the averages and standard deviations for three individual mice at each time point are shown. The levels of IL-4 produced by CD4+ and CD8+ T cells from Igh-6−/− mice were transiently higher than those produced by T cells from C57BL/6 mice early after infection. A single asterisk denotes a P value of <0.05; double asterisks denote a P value of <0.01. The data shown here are representative of two independent experiments.
Polyclonal stimulation with PMA and ionomycin induced higher levels of production of IL-4 by CD4+ T cells but not CD8+ T cells in Igh-6−/− mice compared with C57BL/6 mice after Salmonella infection (data not shown).
Taken together, these results show that Igh-6−/−mice produce higher levels of IL-4 transiently early in infection with S. enterica serovar Typhimurium than do C57BL/6 mice. These results, coupled with the reduced IFN-γ production in Igh-6−/− mice, show that the early T-cell response is transiently skewed toward Th2 cytokine production.
Expression of costimulatory molecules on B cells.
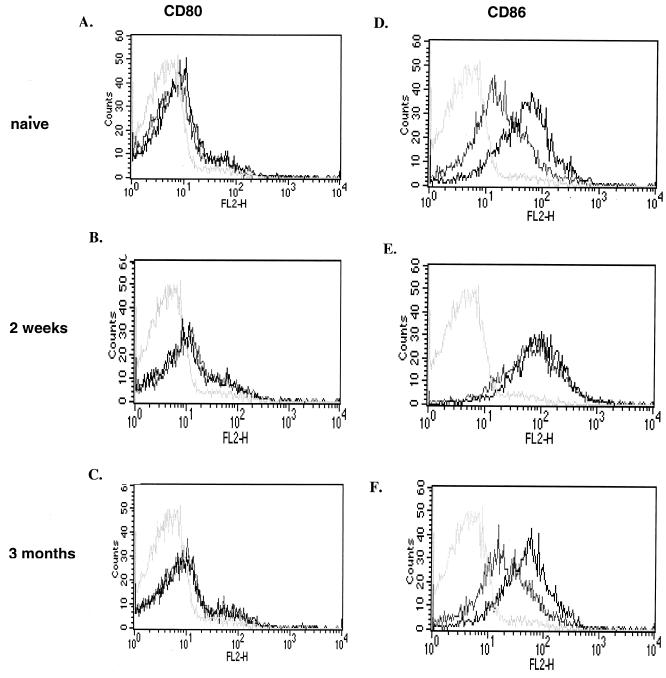
To determine whether incubation with S. enterica serovar Typhimurium SL3261 affected the B-cell expression of costimulatory molecules, we measured the levels of CD80 (B7.1), CD86 (B7.2), MHC class II (IA and IE), and CD40 on B cells before and after incubation either with live S. enterica serovar Typhimurium SL3261 at different MOI or with LPS. Incubation with S. enterica serovar Typhimurium SL3261 at an MOI of 1:1 for 2 h had the most dramatic effect on CD86 molecule expression. At a later time point of infection (24 h) and at different MOI, the effects of Salmonella and LPS on the upregulation of CD86 were less pronounced (data not shown).
After 2 h of incubation with 10 μg of LPS/ml (data not shown) or S. enterica serovar Typhimurium SL3261 at an MOI of 1:1, only CD86 expression was upregulated on B cells (Fig. 3), whereas constitutive CD80 (Fig. 3) and CD40 (data not shown) expression remained unchanged. B cells expressed MHC class II (IA and IE) constitutively, and the levels of MHC class II expression did not increase after stimulation (data not shown).
FIG. 3.
Phenotypes of S. enterica serovar Typhimurium-stimulated B cells. CD19+ B cells were isolated from naive mice or from mice sacrificed 2 weeks or 3 months after immunization with S. enterica serovar Typhimurium SL3261. The CD80 and CD86 expression phenotypes of B cells after culturing for 2 h with S. enterica serovar Typhimurium (black lines) were compared with those of cells cultured in medium alone (grey lines). Isotype control staining was identical for stimulated and unstimulated B cells (light grey lines). The data shown here are representative of two independent experiments.
To investigate whether the in vivo status had an effect on the upregulation of surface molecules in vitro, B cells isolated from naive mice or mice that had been immunized 2 weeks or 3 months previously were incubated for 2 h with S. enterica serovar Typhimurium SL3261 at an MOI of 1:1. Figure 3 shows that the B-cell surface expression of CD86 was upregulated after stimulation with live S. enterica serovar Typhimurium on naive B cells and resting B cells taken from mice immunized 3 months previously (Fig. 3D and F). In contrast, B cells taken 2 weeks after immunization showed high levels of CD86 with no further increase after in vitro stimulation with S. enterica serovar Typhimurium (Fig. 3E). In addition, there were no detectable changes in CD80 expression under any of the experimental conditions described above (Fig. 3A to C). These results indicate that S. enterica serovar Typhimurium can induce upregulation of the costimulatory molecule CD86 on B cells both in vitro and in vivo.
Infection of B cells with S. enterica serovar Typhimurium.
We further investigated whether B cells can be infected with Salmonella in vitro and serve as a source of APCs for Salmonella-specific CD4+-T-cell lines. B cells and BMM were infected with S. enterica serovar Typhimurium SL3261, and the infection was tested by FACS analysis (Fig. 4A) and by pour plating on LB agar plates (Fig. 4B). Infection of B cells was compared to that of BMM. Data represent the percentage of Salmonella SL3261-GFP-positive cells in the absence of cytochalasin D, corrected for the percentage of Salmonella SL3261-GFP-positive cells in the presence of cytochalasin D. Cytochalasin D treatment stops actin rearrangement and thus provides a measure of extracellular bacteria. BMM were readily infected with S. enterica serovar Typhimurium SL3261-GFP, showing 46% of cells infected (Fig. 4A). In contrast, only 0.02% of naive B cells and 1.5% of B cells isolated from mice that had been immunized 2 weeks earlier contained internal S. enterica serovar Typhimurium SL3261-GFP at the same MOI (50:1) for 2 h (Fig. 4A), indicating that naive B cells and B cells early after Salmonella infection were refractory to infection by Salmonella. However, B cells from mice that had been immunized 3 months earlier were more readily infected (7%) with S. enterica serovar Typhimurium SL3261-GFP (Fig. 4A), suggesting a better ability of antigen-specific B cells to associate with Salmonella.
FIG. 4.
Infection of B cells with S. enterica serovarTyphimurium. CD19+ B cells were isolated from naive mice or from mice sacrificed 2 weeks or 3 months after immunization with S. enterica serovar Typhimurium SL3261. (A) Percentages of B cells and BMM associated with S. enterica serovar Typhimurium SL3261-GFP, as measured by FACS. (B) Numbers of viable bacteria per 100 B cells and 100 BMM, as measured by gentamicin protection assays. The data shown here are representative of two independent experiments.
Intracellular viable bacteria were found in both infected B cells and BMM. However, considerably higher bacterial numbers were present in BMM than in B cells (Fig. 4B).
Antigen presentation.
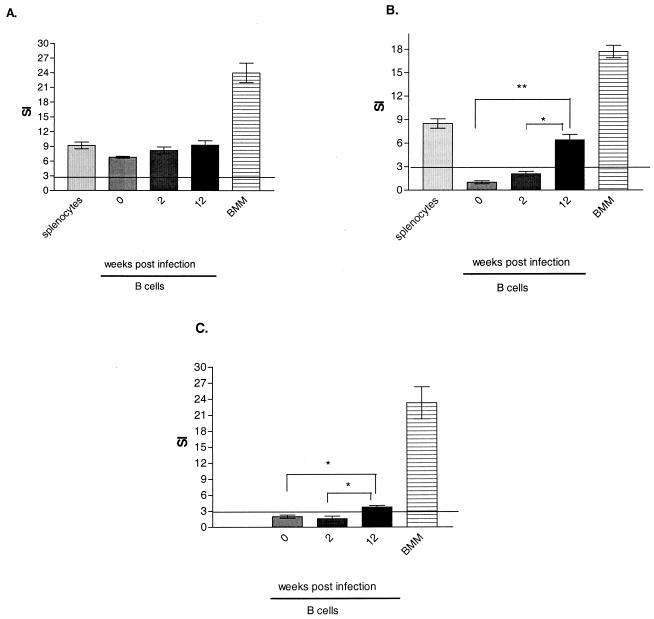
In order to determine whether B cells can present Salmonella antigens to Salmonella-specific CD4+-T-cell lines, we compared the antigen presentation ability of B cells from naive mice or mice that had been immunized with Salmonella either for 2 weeks or for 3 months with that of splenocytes or BMM. Proliferation was measured with a standard 72-h [3H]thymidine incorporation assay in the presence of either soluble (C5/NaOH) or heat-killed (C5/HK) Salmonella antigen. There was no significant difference between B cells from naive and immune mice and splenic APC (P > 0.05) in the ability to present soluble antigen; BMM induced the highest level of proliferation of Salmonella-specific CD4+-T-cell lines (Fig. 5A). Furthermore, both splenic APC and BMM were competent in presenting heat-killed antigen (Fig. 5B). In contrast, B cells taken from mice immunized 3 months earlier had a better ability to present particulate antigens to Salmonella-specific CD4+ T cells than did B cells taken from naive mice (P < 0.01) or from mice immunized 2 weeks earlier (P < 0.01) (Fig. 5B).
FIG. 5.
Responses of Salmonella-specific CD4+-T-cell lines to Salmonella antigens presented by B cells. CD19+ B cells were isolated from naive mice and from mice sacrificed 2 weeks or 3 months after immunization with S. enterica serovar Typhimurium SL3261. B cells were pulsed with soluble Salmonella C5/NaOH (A) or heat-killed particulate Salmonella C5/HK (B) or infected with live S. enterica serovar Typhimurium SL3261 (C). Their ability to present Salmonella antigens was compared with that of splenocytes and BMM. In all experiments, the proliferation of Salmonella-specific CD4+-T-cell lines was measured as described in Materials and Methods. Proliferation was considered positive when the SI was >3. A single asterisk denotes a P value of <0.05; double asterisks denote a P value of <0.01. The results presented here are representative of three different CD4+-T-cell lines tested in at least two independent experiments and are shown as averages and standard deviations.
B cells from naive and immunized animals as well as BMM were also tested for their ability to present Salmonella antigens and elicit proliferative responses from Salmonella-specific CD4+-T-cell lines after in vitro exposure to S. enterica serovar Typhimurium. S. enterica serovar Typhimurium SL3261-infected BMM were more efficient at inducing Salmonella-specific CD4+-T-cell proliferative responses than were B cells (Fig. 5C). As shown in Fig. 4, B cells from naive mice and B cells from mice immunized for 2 weeks could not internalize live Salmonella and, as expected, did not present antigens from live bacteria (Fig. 5C). However, B cells from animals immunized for 3 months had a better ability to present Salmonella antigens than did B cells from naive mice (P < 0.01) or from mice immunized for 2 weeks (P < 0.05) (Fig. 5C).
Taken together, these results suggest that B cells can act as APC for Salmonella-specific CD4+-T-cell lines. However, only B cells obtained from mice immunized 3 months earlier are capable of presenting both soluble and particulate antigens.
B-cell depletion decreases the ability of spleen cells to present Salmonella antigens to T cells.
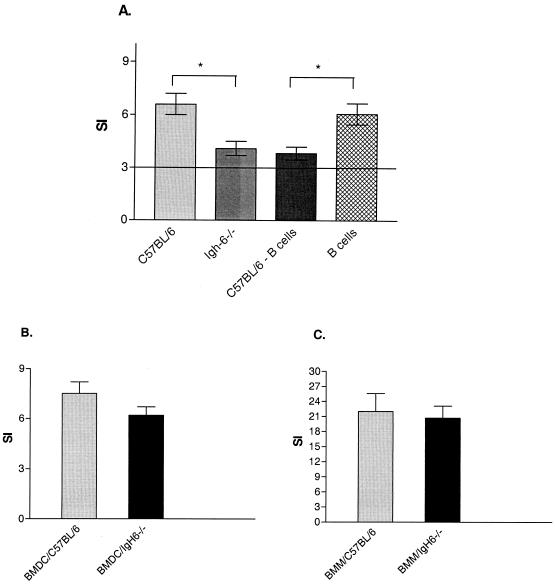
We assessed the ability of B cells and splenocytes from C57BL/6 and Igh-6−/− mice to present Salmonella antigens to T cells. As shown in Fig. 6A, splenocytes isolated from C57BL/6 mice have a significantly better ability to present Salmonella antigens to Salmonella-specific CD4+-T-cell lines than do splenocytes isolated from Igh-6−/− mice (P < 0.05) or B-cell-depleted splenocytes isolated from C57BL/6 mice (P < 0.05). However, there was no significant difference in antigen presentation ability between whole Igh-6−/− splenocytes and B-cell-depleted C57BL/6 splenocytes, indicating that B cells represent an important APC subpopulation within the spleen. FACS staining of C57BL/6 and Igh-6−/− splenocytes revealed no difference in the proportions of CD11b+ CD11c− cells, CD11b+ CD11c+ cells, and Ly6C+ cells within the spleen (data not shown). We also investigated whether other APC subpopulations (macrophages and dendritic cells) from Igh-6−/− mice have detectable intrinsic abnormalities in antigen presentation. BMM or BMDC isolated from C57BL/6 and Igh-6−/− mice showed no differences in the ability to present Salmonella antigens to Salmonella-specific CD4+-T-cell lines (Fig. 6B and C). Moreover, after Salmonella antigen stimulation, there were no differences in the expression of MHC class II, CD40, CD80, and CD86 molecules on BMM or BMDC isolated from C57BL/6 and Igh-6−/− mice (data not shown).
FIG. 6.
Responses of Salmonella-specific CD4+-T-cell lines to Salmonella antigens presented by different APC populations. (A) Splenocytes isolated from C57BL/6 mice were pulsed with Salmonella C5/NaOH, and their ability to present Salmonella antigens to Salmonella-specific CD4+-T-cell lines was compared with that of splenocytes isolated from Igh-6−/− mice, B-cell-depleted C57BL/6 splenocytes (C57BL/6 − B cells), or purified B cells. (B) Antigen presentation of Salmonella C5/NaOH to Salmonella-specific CD4+-T-cell lines by BMDC isolated from either C57BL/6 or Igh-6−/− mice. (C) Antigen presentation of Salmonella C5/NaOH to Salmonella-specific CD4+-T-cell lines by BMM isolated from either C57BL/6 or Igh-6−/− mice. An asterisk denotes a P value of <0.05. The results presented here are representative of four independent experiments and are shown as averages and standard deviations.
Taken together, these data show that the absence of B cells is sufficient to reduce T-cell responses to Salmonella antigens in the spleens of Igh-6−/− mice.
DISCUSSION
In mice, targeted mutation of the μ-chain gene causes a failure of B-cell maturation that leads to the absence of peripheral B cells (32). The use of B-cell-deficient mice allowed us to analyze the need for B cells in the development and persistence of T-cell-mediated immune responses against Salmonella infection. The most important finding in this study was the impairment of the development of the Th1 T-cell response to Salmonella that was detected in B-cell-deficient mice early after infection. We also observed transient upregulation of the Th2 cytokine IL-4 early after infection. We further showed that B cells upregulate CD86 in response to Salmonella and present Salmonella antigens to Salmonella-specific CD4+ T cells. Thus, our results show that B cells are important for the development of T-cell responses in the early stage of a Salmonella infection. It is possible that this effect is achieved through antigen presentation to activated T cells.
It was previously shown that CD4+ T cells from Igh-6−/− mice 3.5 months postimmunization have an impaired ability to produce Th1 cytokines in response to in vitro stimulation with Salmonella antigens (44). However, it was unclear whether this impairment of T-cell responses was due to the inadequate initiation of T-cell immunity or to the inability of the mice to maintain a memory response in the absence of B cells. We analyzed the kinetics of the development of Th1 immune responses in B-cell-deficient Igh-6−/−and C57BL/6 control mice. In the first week of infection, few CD4+ and CD8+ T cells from both Igh-6−/− and C57BL/6 mice were IFN-γ-producing cells, possibly because of the suppression of immune responses in the early phase of a Salmonella infection (13). Furthermore, at 3 weeks after infection, we found high frequencies of IFN-γ-producing CD4+ and CD8+ T cells in C57BL/6 mice, consistent with the finding that T-cell responses to Salmonella peak at about 3 to 4 weeks of infection (52; unpublished observations). The frequency of IFN-γ-producing T cells was at least three- to fivefold higher in C57BL/6 mice than in Igh-6−/− mice and remained elevated during the course of the infection. These data indicate that B cells are important for the induction of protective IFN-γ T-cell responses in the early phase of a Salmonella infection. We also observed a transient enhancement of IL-4 production in Igh-6−/− mice early in infection; this effect might have been a consequence of the low levels of Th1 cytokines produced in Igh-6−/− mice, as IFN-γ inhibits IL-4 production. The impairment of T-cell responses was not due to an intrinsic deficiency of T cells in B-cell-deficient mice, as unimmunized control mice showed normal levels of cytokine production and CD4+-T-cell proliferation in response to polyclonal stimulation (data not shown). The defective development of T-cell responses to Salmonella antigens in Igh-6−/− mice suggests that B cells play a crucial role in the initial phase of T-cell activation after Salmonella infection.
It is not clear why B-cell-deficient mice can clear a primary infection despite the reduced activation of Salmonella-specific T cells. It is possible that the low levels of T-cell activation seen in B-cell-deficient mice can control the growth and clearance of the attenuated S. enterica serovar Typhimurium SL3261 vaccine strain but not more virulent challenge strains.
B cells are involved in the initial phase of T-cell activation and may operate through antigen presentation and/or costimulation. Here we showed that only 2 h of exposure to live S. enterica serovar Typhimurium SL3261 or LPS induced the upregulation of CD86 expression on the surface of B cells. We also showed that B cells have an activated phenotype early in infection in vivo, implying that S. enterica serovar Typhimurium activates B cells in vivo on initial entry into the host. We hypothesized that the ability of a pathogen to activate B cells early in an immune response may determine whether B cells play a role in the expansion of effector and memory T cells. The notion that T cells are activated during the first day of infection (50) suggests that the early (24-h) expansion of T cells caused by the bacterial load would be similar in Igh-6−/− mice and C57BL/6 mice, as the bacterial counts were comparable in Igh-6−/− mice and C57BL/6 mice at 24 h after infection with S. enterica serovar Typhimurium SL3261 (44). Therefore, B cells may be required within the first weeks of a Salmonella infection to amplify T-cell immune responses. Further engagement of the B-cell receptor by antigen (7, 36) or ligation of CD40 by the CD40 ligand (CD154) that is expressed on activated T cells (19, 20) would rapidly elicit the expression of CD86 on B cells, an effect which would enable them to further amplify T-cell responses. Thus, although T cells may first encounter antigen presented on dendritic cells, shortly after a response is under way, B cells could become competent APC. Furthermore, as antigen-specific B cells proliferate, a bias toward the usage of B cells as APC by the expanding T-cell population in the presence of limiting numbers of dendritic cells would occur, providing a means to sustain the further proliferation of both T cells and B cells engaged in the response.
We further investigated whether different populations of B cells can capture Salmonella and present its antigens to Salmonella-specific CD4+-T-cell lines. We showed that only B cells from mice immunized 3 months earlier could be infected in vitro with S. enterica serovar Typhimurium; it was not possible to infect B cells from naive animals. The percentage of infected B cells was much lower than that of BMM. We demonstrated that only B cells from animals immunized with S. enterica serovar Typhimurium for 3 months could present in vitro both soluble and particulate antigens to Salmonella-specific CD4+-T-cell lines. These effects may depend on the presence of antigen-specific cells and may suggest higher numbers of antigen-specific cells that are capable of capturing and presenting antigens after 3 months of immunization.
It is still unclear how early T-cell immune responses to Salmonella can be affected by B cells if naive B cells cannot process and present whole bacteria. An intriguing possibility is that B cells access a source of nonparticulate antigen early in infection. In fact, it is well established that follicular dendritic cells can present antigen to activated B cells and evolving memory cells in germinal centers. It was recently shown that B cells can acquire antigen from dendritic cells after synapse formation (2, 11). Indeed, it is possible that in vivo naive B cells acquire antigen from other APC and further induce priming and clonal expansion of T cells. B cells that express antibodies specific for an antigen are highly efficient in the presentation of that antigen to T cells, and the binding of the antigen to its receptor on B cells signals functional changes in the class II presentation pathway (10). After in vivo antigen stimulation, activated B cells are present in higher numbers than are dendritic cells and macrophages, making them the choice candidate for interactions with T cells. Presentation of antigen to T cells could induce differentiation and diversification of these cells into effector T cells and even bias the differentiation of Th1 or Th2 CD4+ effector T cells through specific cytokine production (22).
It has been reported that the T-cell response is impaired in several bacterial and viral infections (3, 33, 35, 47, 69) in the absence of B cells. An absolute requirement for B cells as APC also has been reported for some autoimmune disease models (16, 57, 58) but not all of them (17, 68). These differences might be due to the nature of the immunizing antigen, i.e., peptide antigen versus whole proteins (39), and the pathogen. Microorganisms, unlike peptide antigens, can activate immunocompetent cells through a variety of pattern recognition receptors (49), resulting in different requirements for the presence of B cells.
In summary, our results provide evidence that B cells are essential for both the initiation and the development of protective Th1 cytokine production after S. enterica serovar Typhimurium infection. Studies demonstrating that antigen-driven selection of T cells with high-affinity T-cell receptors, which comprise the persistent memory pool, is completed within 24 h of primary infection (48) and studies showing that the transfer of B cells restores T-cell function and memory development (37, 38) are in line with our findings that B cells are important in the initial phase of the immune response. However, it is still unclear from this study whether B cells also are involved in the maintenance of T-cell memory in Salmonella infection and/or the modulation of the immune response via some other mechanism, such as (i) antibody modulation of antigen presentation, (ii) modulation of antigen presentation by a component(s) of the complement cascade, or (iii) targeting of bacterial antigens to specific compartments within lymphoid tissues. These possibilities are presently under investigation.
Acknowledgments
This work was supported by grants from the Wellcome Trust and the Medical Research Council (MRC).
We thank Barbara Blacklaws and Sangeeta Budhia for helpful discussions and critical reading of the manuscript and Fred Heath for help with the statistical analysis.
Editor: F. C. Fang
REFERENCES
- 1.Asano, M. S., and R. Ahmed. 1996. CD8 T cell memory in B cell-deficient mice. J. Exp. Med. 183:2165-2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Batista, F. D., D. Iber, and M. S. Neuberger. 2001. B cells acquire antigen from target cells after synapse formation. Nature 411:489-494. [DOI] [PubMed] [Google Scholar]
- 3.Bergmann, C. C., C. Ramakrishna, M. Kornacki, and S. A. Stohlman. 2001. Impaired T cell immunity in B cell-deficient mice following viral central nervous system infection. J. Immunol. 167:1575-1583. [DOI] [PubMed] [Google Scholar]
- 4.Bradley, L. M., J. Harbertson, G. C. Freschi, R. Kondrack, and P. J. Linton. 2000. Regulation of development and function of memory CD4 subsets. Immunol. Res. 21:149-158. [DOI] [PubMed] [Google Scholar]
- 5.Brundler, M. A., P. Aichele, M. Bachmann, D. Kitamura, K. Rajewsky, and R. M. Zinkernagel. 1996. Immunity to viruses in B cell-deficient mice: influence of antibodies on virus persistence and on T cell memory. Eur. J. Immunol. 26:2257-2262. [DOI] [PubMed] [Google Scholar]
- 6.Collins, F. M. 1974. Vaccines and cell-mediated immunity. Bacteriol. Rev. 38:371-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Constant, S., N. Schweitzer, J. West, P. Ranney, and K. Bottomly. 1995. B lymphocytes can be competent antigen-presenting cells for priming CD4+ T cells to protein antigens in vivo. J. Immunol. 155:3734-3741. [PubMed] [Google Scholar]
- 8.Constant, S. L. 1999. B lymphocytes as antigen-presenting cells for CD4+ T cell priming in vivo. J. Immunol. 162:5695-5703. [PubMed] [Google Scholar]
- 9.Cormack, B. P., R. H. Valdivia, and S. Falkow. 1996. FACS-optimized mutants of the green fluorescent protein (GFP). Gene 173:33-38. [DOI] [PubMed] [Google Scholar]
- 10.Davidson, H. W., P. A. Reid, A. Lanzavecchia, and C. Watts. 1991. Processed antigen binds to newly synthesized MHC class II molecules in antigen-specific B lymphocytes. Cell 67:105-116. [DOI] [PubMed] [Google Scholar]
- 11.Dustin, M. L., and L. B. Dustin. 2001. The immunological relay race: B cells take antigen by synapse. Nat. Immunol. 2:480-482. [DOI] [PubMed] [Google Scholar]
- 12.Eisenstein, T. K. 1999. Mucosal immune defence: the Salmonella typhimurium model, p. 51-109. In Y. Paterson (ed.), Intracellular bacterial vaccine vectors. Wiley-Liss Inc., New York, N.Y.
- 13.Eisenstein, T. K., N. Dalal, L. Killar, J. C. Lee, and R. Schafer. 1988. Paradoxes of immunity and immunosuppression in Salmonella infection. Adv. Exp. Med. Biol. 239:353-366. [DOI] [PubMed] [Google Scholar]
- 14.Eisenstein, T. K., L. M. Killar, and B. M. Sultzer. 1984. Immunity to infection with Salmonella typhimurium: mouse-strain differences in vaccine- and serum-mediated protection. J. Infect. Dis. 150:425-435. [DOI] [PubMed] [Google Scholar]
- 15.Epstein, M. M., F. Di Rosa, D. Jankovic, A. Sher, and P. Matzinger. 1995. Successful T cell priming in B cell-deficient mice. J. Exp. Med. 182:915-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falcone, M., J. Lee, G. Patstone, B. Yeung, and N. Sarvetnick. 1998. B lymphocytes are crucial antigen-presenting cells in the pathogenic autoimmune response to GAD65 antigen in nonobese diabetic mice. J. Immunol. 161:1163-1168. [PubMed] [Google Scholar]
- 17.Fillatreau, S., C. H. Sweenie, M. J. McGeachy, D. Gray, and S. M. Anderton. 2002. B cells regulate autoimmunity by provision of IL-10. Nat. Immunol. 3:944-950. [DOI] [PubMed] [Google Scholar]
- 18.Garside, P., E. Ingulli, R. R. Merica, J. G. Johnson, R. J. Noelle, and M. K. Jenkins. 1998. Visualization of specific B and T lymphocyte interactions in the lymph node. Science 281:96-99. [DOI] [PubMed] [Google Scholar]
- 19.Grewal, I. S., and R. A. Flavell. 1998. CD40 and CD154 in cell-mediated immunity. Annu. Rev. Immunol. 16:111-135. [DOI] [PubMed] [Google Scholar]
- 20.Grewal, I. S., and R. A. Flavell. 1996. The role of CD40 ligand in costimulation and T-cell activation. Immunol. Rev. 153:85-106. [DOI] [PubMed] [Google Scholar]
- 21.Guilloteau, L., D. Buzoni-Gatel, F. Bernard, I. Lantier, and F. Lantier. 1993. Salmonella abortusovis infection in susceptible BALB/cby mice: importance of Lyt-2+ and L3T4+ T cells in acquired immunity and granuloma formation. Microb. Pathog. 14:45-55. [DOI] [PubMed] [Google Scholar]
- 22.Harris, D. P., L. Haynes, P. C. Sayles, D. K. Duso, S. M. Eaton, N. M. Lepak, L. L. Johnson, S. L. Swain, and F. E. Lund. 2000. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat. Immunol. 1:475-482. [DOI] [PubMed] [Google Scholar]
- 23.Harrison, J. A., B. Villarreal-Ramos, P. Mastroeni, R. Demarco de Hormaeche, and C. E. Hormaeche. 1997. Correlates of protection induced by live Aro− Salmonella typhimurium vaccines in the murine typhoid model Immunology 90:618-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayglass, K. T., S. J. Naides, C. F. Scott, Jr., B. Benacerraf, and M. S. Sy. 1986. T cell development in B cell-deficient mice. IV. The role of B cells as antigen-presenting cells in vivo. J. Immunol. 136:823-829. [PubMed] [Google Scholar]
- 25.Hess, J., C. Ladel, D. Miko, and S. H. Kaufmann. 1996. Salmonella typhimurium aroA− infection in gene-targeted immunodeficient mice: major role of CD4+ TCR-α β cells and IFN-γ in bacterial clearance independent of intracellular location. J. Immunol. 156:3321-3326. [PubMed] [Google Scholar]
- 26.Hochadel, J. F., and K. F. Keller. 1977. Protective effects of passively transferred immune T- or B-lymphocytes in mice infected with Salmonella typhimurium. J. Infect. Dis. 135:813-823. [DOI] [PubMed] [Google Scholar]
- 27.Hoiseth, S. K., and B. A. Stocker. 1981. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature 291:238-239. [DOI] [PubMed] [Google Scholar]
- 28.Homann, D., A. Tishon, D. P. Berger, W. O. Weigle, M. G. von Herrath, and M. B. Oldstone. 1998. Evidence for an underlying CD4 helper and CD8 T-cell defect in B-cell-deficient mice: failure to clear persistent virus infection after adoptive immunotherapy with virus-specific memory cells from muMT/muMT mice. J. Virol. 72:9208-9216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hormaeche, C. E., H. S. Joysey, L. Desilva, M. Izhar, and B. A. Stocker. 1990. Immunity induced by live attenuated Salmonella vaccines. Res. Microbiol. 141:757-764. [DOI] [PubMed] [Google Scholar]
- 30.Hormaeche, C. E., P. Mastroeni, A. Arena, J. Uddin, and H. S. Joysey. 1990. T cells do not mediate the initial suppression of a Salmonella infection in the RES. Immunology 70:247-250. [PMC free article] [PubMed] [Google Scholar]
- 31.Janeway, C. A., Jr., J. Ron, and M. E. Katz. 1987. The B cell is the initiating antigen-presenting cell in peripheral lymph nodes. J. Immunol. 138:1051-1055. [PubMed] [Google Scholar]
- 32.Kitamura, D., J. Roes, R. Kuhn, and K. Rajewsky. 1991. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin μ chain gene. Nature 350:423-426. [DOI] [PubMed] [Google Scholar]
- 33.Kopf, M., F. Brombacher, and M. F. Bachmann. 2002. Role of IgM antibodies versus B cells in influenza virus-specific immunity. Eur. J. Immunol. 32:2229-2236. [DOI] [PubMed] [Google Scholar]
- 34.Kurt-Jones, E. A., D. Liano, K. A. HayGlass, B. Benacerraf, M. S. Sy, and A. K. Abbas. 1988. The role of antigen-presenting B cells in T cell priming in vivo. Studies of B cell-deficient mice. J. Immunol. 140:3773-3778. [PubMed] [Google Scholar]
- 35.Langhorne, J., C. Cross, E. Seixas, C. Li, and T. von der Weid. 1998. A role for B cells in the development of T cell helper function in a malaria infection in mice. Proc. Natl. Acad. Sci. USA 95:1730-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lenschow, D. J., A. I. Sperling, M. P. Cooke, G. Freeman, L. Rhee, D. C. Decker, G. Gray, L. M. Nadler, C. C. Goodnow, and J. A. Bluestone. 1994. Differential up-regulation of the B7-1 and B7-2 costimulatory molecules after Ig receptor engagement by antigen. J. Immunol. 153:1990-1997. [PubMed] [Google Scholar]
- 37.Linton, P. J., J. Harbertson, and L. M. Bradley. 2000. A critical role for B cells in the development of memory CD4 cells. J. Immunol. 165:5558-5565. [DOI] [PubMed] [Google Scholar]
- 38.Liu, Y., Y. Wu, L. Ramarathinam, Y. Guo, D. Huszar, M. Trounstine, and M. Zhao. 1995. Gene-targeted B-deficient mice reveal a critical role for B cells in the CD4 T cell response. Int. Immunol. 7:1353-1362. [DOI] [PubMed] [Google Scholar]
- 39.Lyons, J. A., M. San, M. P. Happ, and A. H. Cross. 1999. B cells are critical to induction of experimental allergic encephalomyelitis by protein but not by a short encephalitogenic peptide. Eur. J. Immunol. 29:3432-3439. [DOI] [PubMed] [Google Scholar]
- 40.Makela, P. H., and C. E. Hormaeche. 1997. Immunity to Salmonella, p. 143-166. In S. H. E. Kaufmann (ed.), Host response to intracellular pathogens. R. G. Landes Company, Austin, Tex.
- 41.Mamula, M. J., and C. A. Janeway, Jr. 1993. Do B cells drive the diversification of immune responses? Immunol. Today 14:151-152. [DOI] [PubMed] [Google Scholar]
- 42.Mastroeni, P., A. Arena, G. B. Costa, M. C. Liberto, L. Bonina, and C. E. Hormaeche. 1991. Serum TNF α in mouse typhoid and enhancement of a Salmonella infection by anti-TNF α antibodies. Microb. Pathog. 11:33-38. [DOI] [PubMed] [Google Scholar]
- 43.Mastroeni, P., J. A. Harrison, J. H. Robinson, S. Clare, S. Khan, D. J. Maskell, G. Dougan, and C. E. Hormaeche. 1998. Interleukin-12 is required for control of the growth of attenuated aromatic-compound-dependent salmonellae in BALB/c mice: role of gamma interferon and macrophage activation. Infect. Immun. 66:4767-4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mastroeni, P., C. Simmons, R. Fowler, C. E. Hormaeche, and G. Dougan. 2000. Igh-6−/− (B-cell-deficient) mice fail to mount solid acquired resistance to oral challenge with virulent Salmonella enterica serovar Typhimurium and show impaired Th1 T-cell responses to Salmonella antigens. Infect. Immun. 68:46-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mastroeni, P., B. Villarreal-Ramos, and C. E. Hormaeche. 1993. Adoptive transfer of immunity to oral challenge with virulent salmonellae in innately susceptible BALB/c mice requires both immune serum and T cells. Infect. Immun. 61:3981-3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mastroeni, P., B. Villarreal-Ramos, and C. E. Hormaeche. 1993. Effect of late administration of anti-TNF α antibodies on a Salmonella infection in the mouse model. Microb. Pathog. 14:473-480. [DOI] [PubMed] [Google Scholar]
- 47.Matsuzaki, G., H. M. Vordermeier, A. Hashimoto, K. Nomoto, and J. Ivanyi. 1999. The role of B cells in the establishment of T cell response in mice infected with an intracellular bacteria, Listeria monocytogenes. Cell. Immunol. 194:178-185. [DOI] [PubMed] [Google Scholar]
- 48.McHeyzer-Williams, L. J., J. F. Panus, J. A. Mikszta, and M. G. McHeyzer-Williams. 1999. Evolution of antigen-specific T cell receptors in vivo: preimmune and antigen-driven selection of preferred complementarity-determining region 3 (CDR3) motifs. J. Exp. Med. 189:1823-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Medzhitov, R., and C. A. Janeway, Jr. 1998. Innate immune recognition and control of adaptive immune responses. Semin. Immunol. 10:351-353. [DOI] [PubMed] [Google Scholar]
- 50.Mercado, R., S. Vijh, S. E. Allen, K. Kerksiek, I. M. Pilip, and E. G. Pamer. 2000. Early programming of T cell populations responding to bacterial infection. J. Immunol. 165:6833-6839. [DOI] [PubMed] [Google Scholar]
- 51.Mittrucker, H. W., and S. H. Kaufmann. 2000. Immune response to infection with Salmonella typhimurium in mice. J. Leukoc. Biol. 67:457-463. [DOI] [PubMed] [Google Scholar]
- 52.Mittrucker, H. W., A. Kohler, and S. H. Kaufmann. 2002. Characterization of the murine T-lymphocyte response to Salmonella enterica serovar Typhimurium infection. Infect. Immun. 70:199-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mittrucker, H. W., B. Raupach, A. Kohler, and S. H. Kaufmann. 2000. Cutting edge: role of B lymphocytes in protective immunity against Salmonella typhimurium infection. J. Immunol. 164:1648-1652. [DOI] [PubMed] [Google Scholar]
- 54.Rivera, A., C. C. Chen, N. Ron, J. P. Dougherty, and Y. Ron. 2001. Role of B cells as antigen-presenting cells in vivo revisited: antigen-specific B cells are essential for T cell expansion in lymph nodes and for systemic T cell responses to low antigen concentrations. Int. Immunol. 13:1583-1593. [DOI] [PubMed] [Google Scholar]
- 55.Ron, Y., and J. Sprent. 1987. T cell priming in vivo: a major role for B cells in presenting antigen to T cells in lymph nodes. J. Immunol. 138:2848-2856. [PubMed] [Google Scholar]
- 56.Sallusto, F., and A. Lanzavecchia. 1999. Mobilizing dendritic cells for tolerance, priming, and chronic inflammation. J. Exp. Med. 189:611-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Serreze, D. V., H. D. Chapman, D. S. Varnum, M. S. Hanson, P. C. Reifsnyder, S. D. Richard, S. A. Fleming, E. H. Leiter, and L. D. Shultz. 1996. B lymphocytes are essential for the initiation of T cell-mediated autoimmune diabetes: analysis of a new “speed congenic” stock of NOD.Ig mu null mice. J. Exp. Med. 184:2049-2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Serreze, D. V., S. A. Fleming, H. D. Chapman, S. D. Richard, E. H. Leiter, and R. M. Tisch. 1998. B lymphocytes are critical antigen-presenting cells for the initiation of T cell-mediated autoimmune diabetes in nonobese diabetic mice. J. Immunol. 161:3912-3918. [PubMed] [Google Scholar]
- 59.Simmons, C. P., P. Mastroeni, R. Fowler, M. Ghaem-maghami, N. Lycke, M. Pizza, R. Rappuoli, and G. Dougan. 1999. MHC class I-restricted cytotoxic lymphocyte responses induced by enterotoxin-based mucosal adjuvants. J. Immunol. 163:6502-6510. [PubMed] [Google Scholar]
- 60.Thiel, A., and A. Radbruch. 1999. Antigen-specific cytometry. Arthritis Res. 1:25-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thiel, A., P. Wu, R. Lauster, J. Braun, A. Radbruch, and J. Sieper. 2000. Analysis of the antigen-specific T cell response in reactive arthritis by flow cytometry. Arthritis Rheum. 43:2834-2842. [DOI] [PubMed] [Google Scholar]
- 62.Tite, J. P., G. Dougan, and S. N. Chatfield. 1991. The involvement of tumor necrosis factor in immunity to Salmonella infection. J. Immunol. 147:3161-3164. [PubMed] [Google Scholar]
- 63.Topham, D. J., R. A. Tripp, A. M. Hamilton-Easton, S. R. Sarawar, and P. C. Doherty. 1996. Quantitative analysis of the influenza virus-specific CD4+ T cell memory in the absence of B cells and Ig. J. Immunol. 157:2947-2952. [PubMed] [Google Scholar]
- 64.Townsend, S. E., and C. C. Goodnow. 1998. Abortive proliferation of rare T cells induced by direct or indirect antigen presentation by rare B cells in vivo. J. Exp. Med. 187:1611-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Umezawa, K., T. Akaike, S. Fujii, M. Suga, K. Setoguchi, A. Ozawa, and H. Maeda. 1997. Induction of nitric oxide synthesis and xanthine oxidase and their roles in the antimicrobial mechanism against Salmonella typhimurium infection in mice. Infect. Immun. 65:2932-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Essen, D., P. Dullforce, and D. Gray. 2000. Role of B cells in maintaining helper T-cell memory. Philos. Trans. R. Soc. Lond. B 355:351-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vidal, S., M. L. Tremblay, G. Govoni, S. Gauthier, G. Sebastiani, D. Malo, E. Skamene, M. Olivier, S. Jothy, and P. Gros. 1995. The Ity/Lsh/Bcg locus: natural resistance to infection with intracellular parasites is abrogated by disruption of the Nramp1 gene. J. Exp. Med. 182:655-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wolf, S. D., B. N. Dittel, F. Hardardottir, and C. A. Janeway, Jr. 1996. Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J. Exp. Med. 184:2271-2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang, X., and R. C. Brunham. 1998. Gene knockout B cell-deficient mice demonstrate that B cells play an important role in the initiation of T cell responses to Chlamydia trachomatis (mouse pneumonitis) lung infection. J. Immunol. 161:1439-1446. [PubMed] [Google Scholar]
- 70.Yrlid, U., and M. J. Wick. 2000. Salmonella-induced apoptosis of infected macrophages results in presentation of a bacteria-encoded antigen after uptake by bystander dendritic cells. J. Exp. Med. 191:613-624. [DOI] [PMC free article] [PubMed] [Google Scholar]