Abstract
Introduction
Mammalian cells contain three distinct serine/threonine protein kinases with highly conserved catalytic domains, including aurora A and B kinases that are essential regulators of mitotic entry and progression. Overexpression of aurora A and/or B kinase is associated with high proliferation rates and poor prognosis, making them ideal targets for anti-cancer therapy. Disruption of mitotic machinery is a proven anti-cancer strategy employed by multiple chemotherapeutic agents. Numerous small molecule inhibitors of the aurora kinases have been discovered and tested in vivo and in vitro, with a few currently in phase II testing.
Areas covered
This review provides the reader with updated results from both preclinical and human studies for each of the aurora kinase inhibitors (AKI) that are currently being investigated. The paper also covers in detail the late breaking and phase I data presented for AKIs thereby allowing the reader to compare and contrast individual and classrelated effects of AKIs.
Expert opinion
While the successful development and approval of an AKI for anti-cancer therapy remains unresolved, pre-clinical identification of resistant mechanisms would help design better early phase clinical trials where relevant combinations may be evaluated prior to phase II testing. The authors believe that aurora kinases are important anti-cancer targets that operate in collaboration with other oncogenes intimately involved in uncontrolled tumor proliferation and by providing a unique, targeted and complimentary anti-cancer mechanism, expand the available armamentarium against cancer.
Keywords: Mitosis, Centrosome assembly, Aurora kinase inhibitor (AKI), Small molecule inhibitors, Targeted cancer therapy, Spindle assembly checkpoint, Aneuploidy
1.0 Introduction
The blight of cancer upon humanity is unparalleled, already attaining the distinction of being the leading cause of death and economic burden worldwide.1,2 Cancer is characterized by uncontrolled proliferation leading to a malignant phenotype. Mitosis is a critical step in the proliferation of cancer cells and involves many redundant and checkpoint systems controlling key steps of the process. The family of aurora kinases plays an important role in maintaining the fidelity of mitosis. This has fueled the theory that anticancer benefits may be derived from inhibition of aurora kinase activity and has led to the development of several aurora kinase inhibitors (AKI).
1.1 Aurora Kinases
The aurora kinases are a family of oncogenic serine/threonine kinases involved in the mitotic (M) phase of the cell cycle, acting to establish the mitotic spindle, bipolar spindle formation, alignment of centrosomes on mitotic spindle, centrosome separation, cytokinesis, and monitoring of the mitotic checkpoint.3,4,5,6 Aurora kinases are critical for accurate and organized chromosome division and allocation to each daughter cell. Furthermore, aurora kinases are often overexpressed in tumor cells, particularly those with high growth fractions.
There are three known aurora kinases (Aurora A, B, and C) in human neoplastic and non-neoplastic tissues. Aurora A and B kinases are expressed globally throughout all tissues, whereas aurora C kinase is primarily expressed in testes tissue to participate in meiosis. However recent research has linked Aurora C kinase activity with tumorigenesis in somatic tissue and may be a relevant cancer target.3,7,8 All three aurora kinases possess substantial sequence and structural homology and overlap in gene expression, catalytic domain, genomic length, and kinase activity, although the cellular functions and N-terminal portions of each differ.9,10 Inhibition of aurora kinase activity leads to catastrophic errors of mitosis, such as defective cytokinesis, misaligned centrosomes, and mitotic spindle malformation, culminating in apoptosis.10,11 Several compounds are being developed capitalizing on anticancer effect of inhibition of aurora kinase activity.
1.2 Relevance of Aurora A Kinase
Aurora A kinase is frequently amplified in many epithelial tumors, cancers of solid organs and hematological malignancies. Aurora A kinase has been implicated in causing and/or maintaining the malignant phenotype and resistance to microtubule-targeted chemotherapy, such as paclitaxel.5,12,13,14 Aurora A kinase controls many steps of mitosis, such as mitotic entry and exit and bipolar spindle assembly, becoming localized on the centrosome during early G2 phase.5,15 As such, inhibition of aurora A kinase activity has been shown to cause centrosome separation and maturation defects, spindle aberrations, cell cycle arrest, and apoptosis.16 Notably, aurora A kinase interacts with p53 at multiple levels, with evidence that p53 negative tumors are more sensitive to aurora A kinase inhibitors than p53 positive tumors.17
1.3 Relevance of Aurora B Kinase
High levels of aurora B kinase have been found in many tumor lineages, including hematologic neoplasms. Aurora B kinase overexpression, similar to aurora A kinase overexpression, has been linked with chromosome instability and aneuploidy.11,18 Aurora B kinases act as the catalytic component of the chromosomal passenger complex (CPC) and play a key role in chromosome orientation, chromosome condensation, spindle assembly and cytokinesis.4,6,16 Inhibition of aurora B kinase activity abrogates the spindle assembly checkpoint and causes premature mitotic exit without cytokinesis. This results in polyploid cells that eventually stop proliferation and/or undergo apoptosis, depending upon cell line. Neutropenia is a common consequence of aurora B kinase inhibition, whether singularly inhibited or as part of multi-aurora inhibition.19
1.4 Relevance of Aurora C Kinase
Relatively little is known about aurora C kinase, other than its role in testicular meiosis. Emerging data indicate potential role in tumorigenesis, possibly due to similar activity as aurora B kinase.8 The role in tumorigenesis remains controversial. Currently, there are no aurora C kinase-specific inhibitors in development, limiting elucidation of aurora C kinase-specific anticancer effects.
2.0 Principles and Therapeutic Targeting of Aurora Kinases
All AKIs currently in development for clinical use are small molecule inhibitors (SMI) designed to bind to the ATP-binding pocket via hydrogen bonding, hydrophobic, aromatic and van der Waals interactions. By definition, all ATP-binding AKIs are competitive and reversible. Many AKIs, including isoform-specific AKI, inhibit all three aurora kinases owing to the highly conserved catalytic site among the aurora kinases. However, SMIs inhibit aurora kinase isoforms with differential Ki values (Table 1), creating selective activity.
Table 1.
IC50 for selected Aurora kinase inhibitors
| IC50 values in nM | ||||
|---|---|---|---|---|
| Aurora A kinase | Aurora B kinase | Aurora C kinase | Reference | |
| ENMD-981693 | 25 | 700 | NA | 23 |
| ENMD-2076 | 14 | 350 | NA | 24 |
| MK-5108/VX-689 | 0.064 | 14 | 12 | 29 |
| MLN-8054 | 4 | 172 | NA | 32 |
| MLN-8237 | 1 | >200 | NA | 44 |
| XL-228 | 3 | NA | NA | 52 |
| KW-2449 | 48 | NA | NA | 55 |
| Hesperadin | NA | 50 | NA | 10 |
| BI-811283 | NA | 9 | NA | 57 |
| AZD-1152 | 1369 | 0.36 | 17 | 20 |
| GSK-1070916 | 490 | 0.38 | 1.5 | 81 |
| ZM-447439 | 100 | 100 | NA | 77 |
| JNJ-7706621 | 11 | 15 | NA | 89 |
| AT-9283 | 3 | 3 | NA | 90 |
| PF-03814735 | 5 | 0.8 | NA | 101 |
| VX-680/MK-0457 (Tozasertib) | 0.7 | 18 | 4.6 | 10 |
| PHA-680632 | 27 | 135 | 120 | 64 |
| PHA-739358 (Danusertib) | 13 | 79 | 61 | 125 |
| CYC-116 | 44 | 19 | 65 | 131 |
| SNS-314 | 9 | 31 | 3.4 | 136 |
| AMG-900 | 5 | 4 | 1 | 139 |
| VE-465 | 1 | 26 | 8.7 | 142 |
| AS-703569 (R-763) | 4 | 4.8 | 6.8 | 145 |
NA = Not available
Although specific inhibition of either aurora A kinase or aurora B kinase induces a different phenotype from each other, disagreement exists regarding therapeutic targeting of the aurora kinases. Initially, aurora A-specific targeting was considered a more therapeutically viable target given its role in tumorigenesis. Pre-clinical data determined that inhibition of aurora A and aurora B kinases simultaneously produced a biologic effect and phenotype similar to aurora B kinase inhibition alone.20 However, no clinical data in humans have shown specific AKIs to be more or less therapeutically valuable than multi- or pan-aurora inhibitors. Evidence of clinical activity of Aurora inhibitors by malignancy and study design are highlighted in Table 2. Emerging data indicate that combination with spindle poisons, such as taxanes or vinca alkaloids, with aurora A kinase inhibitors (Figure 1) may prove synergistic.14,21 Similarly, due to interaction of aurora B kinase with histone H3, combination with histone deacetylase inhibitors (HDACI) with AKIs inhibitors may prove synergistic.22 Therapeutic dosing of aurora kinase-specific agents may be difficult to elucidate as higher doses of AKIs may lead to a pan-aurora inhibitory effect.
Table 2.
Evidence of clinical activity of Aurora kinase inhibitors by malignancy and study design*
| Breast | Bladder | Head and Neck |
Lung | Prostate | Renal Cell |
Colorectal | Pancreatic | Hepatocellular | Melanoma | Glioma | Ovarian | Endometroid Carcinoma |
Cervix | Acute Myelogenous Leukemia |
Chronic Myelogenous Leukemia |
Acute Lymphoblastic Leukemia |
Multiple Myeloma |
Lymphomas | Mantle Cell Lymphoma |
Myeloproliferative Disorders |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ENMD-981693 | 1 | 1 | 1 | 1 | 1 | ||||||||||||||||
| ENMD-2076 | 1,2 | 1,4 | 1 | 4 | 1 | 1,2 | |||||||||||||||
| MK-5108/VX-689 | 1 | 1 | 1 | 1 | 1,2 | 1 | 1 | ||||||||||||||
| MLN-8054 | 1,6 | 6 | 6 | 1,6 | 1 | 1,6 | 6 | 6 | 6 | 1,6 | 1 | ||||||||||
| MLN-8237 | 1,2 | 4 | 1,4 | 2 | 1,4 | 4 | 1,2 | 1,2 | 1 | 1 | 1,2 | 1,2 | |||||||||
| XL-228 | 4 | 1,4 | 1,4 | ||||||||||||||||||
| KW-2449 | 1,4 | 1,4 | 1 | ||||||||||||||||||
| BI-811283 | 1 | 1 | |||||||||||||||||||
| AZD-1152 | 1 | 1 | 1,2 | 1 | 1 | 1 | 1 | 1,2 | 1 | 1,2 | 1 | 1,2 | |||||||||
| GSK-1070916 | 1,3 | 1 | 1 | 1 | 1 | ||||||||||||||||
| ZM-447439 | 1 | 1 | 1 | 1 | 1 | ||||||||||||||||
| JNJ-7706621 | 1 | 1 | 1 | 1 | 1 | 1 | |||||||||||||||
| AT-9283 | 1 | 1,4,6 | 1,6 | 1 | 1 | 1,4 | 1,4 | 1 | 1 | ||||||||||||
| PF-03814735 | 1,6 | 6 | 6 | 1,6 | 6 | 1,6 | 6 | 1 | 1 | ||||||||||||
| VX-680/MK-0457 (Tozasertib) | 1,2 | 6 | 2,6 | 1,2,6 | 1,6 | 1,6 | 1,6 | 1,2,6 | 1,2,4 | 1,2,4,5 | 1,2,4,5 | 1 | 1,2 | 1,4 | |||||||
| PHA-680632 | 1 | 1 | 1 | 1 | 1 | 1 | |||||||||||||||
| PHA-739358 (Danusertib) | 1,5,6,7 | 1,4 | 1,6 | 1,6 | 1,5,6 | 6,7 | 1 | 1,5,6,7 | 1 | 1 | 1,2,4 | 1,4 | |||||||||
| CYC-116 | 1 | 1 | 1 | 1 | |||||||||||||||||
| SNS-314 | 1 | 1 | 1 | 1,2 | |||||||||||||||||
| AMG-900 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||
| VE-465 | 1,2 | 1 | 1 | 1,2 | 1,2 | 1,2 | 1,2 | ||||||||||||||
| AS-703569/R-763 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1,4 | 1,4 | 1 | 1 |
Single-drug efficacy, Preclinical†;
Combination efficacy, Preclinical†;
No/minimal efficacy, Preclinical‡;
Single-drug efficacy, Phase I†;
Combination efficacy, Phase I†;
No/minimal efficacy, Phase I‡;
Single-drug efficacy, Phase II†
Where data available/reported;
Defined as objective response or partial/complete response;
Defined as stable disease or no objective response
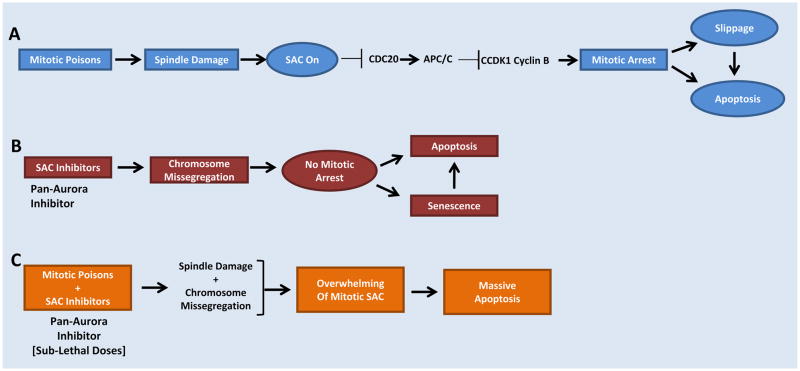
Figure 1.
Sensitizing Cancer Cells to SAC inhibition plus microtubule targeting agents to optimize enhanced Apoptosis
2.1 Selective Inhibitors of Aurora A Kinase
2.1.1 ENMD-981693 and ENMD-2076
The molecule initially described as ENMD-981693 was further developed into ENMD-2076, the L(+) tartrate salt of ENMD-981693.23 ENMD-2076 is more selective for aurora A kinase than ENMD-981693, with an IC50 value of 14 nM for aurora A kinase and 350 nM for aurora B kinase, respectively.24 Furthermore, ENMD-2076 also inhibits FGFR3, PDGFR, VEGFR1, and potently inhibits FLT3 with IC50 values ranging from 0.04 – 21 μM. Pre-clinical studies of ENMD-2076 in murine models have shown promise for multiple myeloma (single agent and in combination with lenalidomide), breast cancer, leukemia and colorectal cancer.24,25,26,27 Additionally, several phase I and II trials are currently ongoing in ovarian cancer, acute leukemia and multiple myeloma.28
ENMD-2076 displays favorable pharmacokinetic profile as it is approximately 90% protein bound, displays no significant inhibition of cytochrome P450 isoenzymes CYP1A2, 2A6, 2C19, or 3A4/5 and is orally bioavailable.25,26 The spectrum of antiproliferative, antiangiogenic and cell cycle effects, combined with favorable pharmacokinetic profile makes this agent appealing for investigation in a myriad of tumor types.
2.1.2 MK-5108
MK-5108, also known as VX-689, is a competitive inhibitor of the ATP-binding site of aurora A kinase. Pre-clinical studies show efficacy in a variety of breast, cervix, colorectal, ovary, and pancreas neoplasms. This antitumor effect was enhanced by the addition of docetaxel in vitro and in vivo a murine model with acceptable toxicity, irrespective of treatment sequence.29 The combination of MK-5108 and the HDACI, vorinostat, was investigated in multiple lymphoma cell lines.22 The addition of MK-5108 to vorinostat sensitized the cell lines to apoptosis, with inhibition of c-Myc playing a crucial role.
A phase 1 study in patients with advanced solid tumors investigated the toxicities of single-agent MK-5108 and MK-5108 in combination with docetaxel 60mg/m2 IV every 21 days.30 Febrile neutropenia and myelotoxicity was identified as the dose-limiting toxicity (DLT) in combination patients, but no DLT was identified in the monotherapy arm. Disease stabilization was seen in 11 of 34 (32%) patients from both arms, while partial response was seen in 2 of 17 (12%) patients in the combination arm and 0 of 17 (0%) in the monotherapy arm.
2.1.3 MLN8054
MLN8054 potently inhibits aurora A kinase by competitively blocking the ATP-binding pocket. Importantly, MLN8054 is structurally and functionally similar to benzodiazepines, leading to the DLT of somnolence at clinically-relevant doses.31,32 Preclinical studies in a several cell culture and murine xenograft models displayed potent antitumor activity as determined by direct tumor measurement and surrogate markers, consistent with aurora A kinase-specific inhibition.32,33,34,35 Furthermore, MLN8054 was able to induce senescence both in vitro and in vivo.36 This study was the first to link aurora A kinase inhibition and senescence, an effect classically seen with antimitotic agents. In murine models, dose-related and reversible somnolence and neutropenia were the DLTs.
A dose-finding study of MLN8054 was performed in 63 patients with advanced cancer (gastrointestinal, lung, genitourinary, sarcoma, and breast) utilizing once-daily doses of 5–40mg/day as a single dose or 25–80mg/day in four divided doses.37 Doses above 45mg/day were administered with methylphenidate to mitigate sedation. The maximum tolerated dose for once-daily administration was 30mg/day, 45mg/day if divided into 4 daily doses and 60mg/day if divided into 4 daily doses and used concomitantly with methylphenidate for 7–21 consecutive days of a 35-day cycle. Somnolence was the only DLT and no responses were seen with any dose level.
A second dose-finding study was performed in 43 patients with advanced tumors evaluating daily doses from 10mg to 80mg orally per day in divided doses.38 The DLTs identified were grade 3 reversible somnolence and liver function test elevations. It was evident that somnolence and liver toxicity limited dose escalations to level required to adequately inhibit aurora kinase A. Based upon these results, MLN8054 development was abandoned in favor of MLN8237.
2.1.4 MLN8237
MLN8237 shares structural homology to MLN8054, but has four-fold greater inhibitory potency for aurora A kinase and decreased tendency to cause somnolence. In vitro and in vivo testing using murine models investigated MLN8237 in a variety of malignancies common to pediatrics, both solid and hematologic.39,40 Further preclinical studies in models of lymphoma41,42, Philadelphia chromosome (Ph+) positive leukemias (including T315I BCR-Abl mutant )43, multiple myeloma44, acute myeloid leukemia (AML) as single agent and in combination45, breast and prostate cancer (in combination with docetaxel)46, have consistently shown anti-tumor effects by direct and surrogate marker evaluation. Importantly, in models of chronic myelogenous leukemia (CML) and Ph+ acute lymphoblastic leukemia (ALL), MLN8237 showed similar effects irrespective of p53 activity status.42
A phase I study of 43 patients with advanced tumors demonstrated antiproliferative effects at a dose level of 80mg/day orally and DLTs at 150mg/day orally for 7 consecutive days every 21 days.47 The side effect profile differed substantially from MLN8054 as only grade I somnolence, grade 3 neutropenia and mucositis were observed. Two similar phase I studies in advanced solid tumors determined MLN8237 50mg orally twice daily for 7 days every 21 days to be most promising regimen in adults, with DLT of febrile neutropenia and myelotoxicity.48,49 Other adverse events, such as mild somnolence, nausea, and diarrhea was dose-related and reversible. A secondary analysis of 117 patients enrolled in the phase I trials confirmed 50mg orally twice daily for 7 days every 21 days to produce steady-state average serum concentrations approximately 1.7μM, almost double the serum concentration determined in preclinical models to maximize anti-tumor effects.50 A phase I study in 37 pediatric patients found increased dose-related toxicities of myelosuppression and dermatologic toxicity with multiple daily dosing and determined a phase 2 dose in pediatric patients to be 80mg/m2/day orally.51 Based upon these results, numerous phase I and phase II studies are currently ongoing with MLN8237, both as single agent and in combination with other anti-cancer therapies.28
2.1.5 XL228
While XL228 is selective for aurora A kinase over aurora B or C kinases, it has very broad inhibitory effects of many other protein kinases, including FLT3, BCR-Abl (wild-type and T315I mutant), IGF-1R, ALK, SRC, and LYN, with IC50 values ranging from 1.4 – 6,912 μM.52 Although a paucity of data exists about XL228, one may consider the aurora A kinase inhibition effect an off-target effect. Pre-clinical data have focused on hematological malignancies, including CML (wild-type and imatinib resistant), Ph+ ALL, and MM.52
The first phase I study of XL228 studied 27 patients with Ph+ leukemias, including 20 patients (74%) with BCR-Abl mutations conferring clinical resistance to imatinib.53 XL228 was administered as a 1-hr intravenous infusion once or twice weekly. The maximum dose administered in once-weekly arm was 10.8mg/kg and twice weekly arm was 3.6mg/kg. The DLT observed in once-weekly arm was grade 3 syncope and hyperglycemia. The twice weekly arm has not reached DLT. Objective responses were observed in patients receiving at least 3.6mg/kg/dose.
A phase I study of XL228 administered as a 1-hr infusion weekly in 41 patients with solid tumors or multiple myeloma determined a DLT of 8mg/kg/dose due to grade 3 and 4 neutropenia.54 The MTD was determined to be 6.5mg/kg and expanded this cohort by adding 22 additional patients to study. The predominant response was stable disease, seen most often in non-small cell lung cancer patients (6 of 9 enrolled patients). Hypotension and hyperglycemia were commonly encountered and generally mild. Ongoing phase I trials are currently underway.28
2.1.6 KW-2449
KW-2449, like XL228, is an orally-administered multi-targeted agent primarily coveted for its ability to inhibit non-aurora kinases, including FLT3, FGFR1 and BCR-Abl (wild-type and T315I). However, it possesses potent aurora A kinase inhibition with an IC50 of 48nM/L with limited aurora B or C kinase inhibition.55 Preclinical data indicate efficacy in AML, myelodysplastic syndrome (MDS), CML, and ALL.55
A phase I study of 37 patients (31 AML, 5 CML, and 1ALL) were treated at 7 dose levels.56 Pharmacokinetic assessment of parent drug and metabolite revealed a short half-life of 2.4–4.9 hours. The effect of a given dose was evident 8 hours after ingestion of dose, but absent at 12 hours. Neutropenia, the DLT, occurred in 24% of cycles. Eight of 31 patients (26%) with AML exhibited >50% reduction in blasts, occurring in both FLT3 wild-type and FLT3-mutated patients. One patient with T315I BCR-Abl CML demonstrated complete clearance of mutant T315I clone. Authors conclude that KW-2449 is tolerable and produces objective responses, but needs three or four daily doses to maintain adequate plasma levels. Phase I trials in hematologic malignancies are currently underway.28
3.0 Aurora B Kinase-Specific Inhibitors
3.1 Hesperadin
Hesperadin is one of the first AKIs discovered and was instrumental in the understanding of the role of aurora B kinase and spindle assembly. Drug development was abandoned after it was discovered that cells exposed to hesperadin developed aberrant ploidy, but did not lose viability or undergo apoptosis. Currently, hesperadin is used as a laboratory tool to probe for aurora B kinase.
3.1.1 BI811283
A potent inhibitor of aurora B kinase, BI811283 has demonstrated antitumor activity in multiple murine xenograft models, including non-small cell lung cancer and colorectal cancer.57,58 The MTD in models was determined to be 20mg/kg via continuous infusion once weekly. Moreover, evidence of polyploidy and senescence was identified within 48 hrs and 96 hrs, respectively. Two dosing schemas were tested in concurrent phase I trials conducted in patients with advanced solid tumors.59,60 Administration of BI811283 by 24-hr continuous infusion on day 1 every 21 days yielded a MTD of 230mg with the DLT of neutropenia.59 Stable disease was the best response and seen in 19 of 57 (33%) of patients enrolled. Administration of BI-811283 via 24-hr infusion on days 1 and 15 of a 28-day treatment cycle determined 140mg as MTD.60 In this study of 52 patients neutropenia was the DLT with stable disease reported as the best response in 15 of 52 (29%) patients. While both schedules were not compared to each other, both schemas allowed a mean of 3 (range, 1–16+) cycles to be administered. Current phase I trials of both administration schedules are ongoing.28
3.1.2 AZD1152
AZD1152 is a very selective inhibitor for aurora B kinase while being devoid of aurora A kinase inhibition at clinically relevant doses. AZD1152 is a prodrug and is rapidly converted in plasma to the active moiety, AZD1152-HQPA, where it competitively blocks the ATP-binding pocket of aurora B kinase.
Pre-clinical studies of human tumor cultures and murine xenograft models using single-agent AZD1152 have been conducted in numerous tumor types, including breast61,62, pancreas62, colorectal62,63,64,65,66, non-small cell lung63,64, small cell lung67, hepatocellular carcinoma68, malignant mesothelioma69, AML62,70,71,72, and multiple myeloma (MM)73. AZD1152 is also a potent FLT3 inhibitor, potentially adding a dual mechanism to the antitumor effects in AML.74 The combination of AZD1152 with anticancer agents or ionizing radiation revealed enhanced antitumor effects versus AZD1152 alone.62,66,75,76 While preclinical data are promising, a signal emerged indicating that AZD1152-induced mitotic aberrations do not always lead to apoptosis in AML models.70,77 Nonetheless, preclinical data were compelling and led to phase I studies. Despite the myriad of preclinical studies with AZD1152, investigation in humans is still emerging. The first phase I study administered AZD1152 as a 2-hr infusion weekly in a dose escalation design to 13 patients with advanced, pretreated solid malignancies.78 DLT was grade 3 neutropenia at a dose of 450mg, with little other adverse effects noticed. In these patients, bone marrow recovery occurred approximately 14 days post-dose, which is similar to traditional anti-neoplastic agents. Three patients with 3 different solid malignancies (melanoma, nasopharyngeal carcinoma and adenoid cystic carcinoma) reported stable disease, which was the best response noted.
A phase I/II study evaluated the MTD of AZD1152 given as continuous 7-day infusion every 21 days in patients with advanced AML.79 This study enrolled 32 patients with de novo or secondary AML arising from antecedent MDS or chemotherapy exposure to the dose finding (part A) portion. The MTD was determined to be 1200mg due to DLTs of mucositis and stomatitis. Common adverse events were febrile neutropenia and nausea. Of the 32 patients, there were 16 (50%) deaths, but 14 were determined to be from progression of AML, and 7 (22%) with a clinical response. The clinical response was 1 (3%) with complete remission (CR) at 1200mg dose level, 2 (6%) complete remissions with incomplete blood count recovery (CRi) at the 400mg and 800mg cohorts, and 4 (13%) partial remissions (PR) (100mg – 1600mg cohorts). An additional 32 patients were enrolled into the efficacy (part B) portion of the trial whereby all patients received 1200mg as continuous 7-day infusion every 21 days. Demographics of patients in part B were similar to those in part A. Febrile neutropenia and stomatitis was identified as the most common adverse effects in 12 (38%) patients. In part B, there were 5 (16%) deaths, with 3 (9%) due to disease progression and 2 (6%) due to infectious complications. Eight (25%) patients had clinical response, with 2 (6%) CR, 3 (9%) CRi, and 3 (9%) PR. Neither of the studies evaluated AML cells after exposure to AZD1152-HQPA to correlate polyploidy with cell viability and should be the focus of future research. There are currently multiple phase I and II clinical trials ongoing evaluating AZD1152 in multiple solid and hematologic malignacies.28
Although the clinical relevance of this is unknown, resistance to AZD1152 has been induced in cell cultures of colorectal and pancreatic cancers.80 These cell cultures were purposefully incubated with sublethal doses of AZD1152 with the intent of causing resistance and elucidating the cause. This study determined that both cell lines upregulated the ABC transporter, MDR1, and BCRP, both of which are cellular efflux pumps for numerous pharmaceutical agents, leading to a >100-fold higher resistance to AZD1152 than wild-type cells. Furthermore, upregulation of MDR1 and BCRP by AZD1152 produced cross-resistance to the pan-aurora kinase inhibitor VX-680/MK-0457.80
3.1.3 GSK1070916
GSK1070916, discovered through cross-screening and structure-activity relationship refinement, competitively binds to aurora B and C kinases with far greater selectivity than aurora A.81 Of note is the extremely slow rate of dissociation, with dissociation half-life of >480 minutes for aurora B kinase, compared to dissociation half-life of AZD1152 of <30 minutes. Due to slow offset of activity, this compound may confer advantages in slower growing tumors and/or less frequent dosing. Preclinical studies in cell tissue cultures and murine models show efficacy (either tumor regression or stable disease) in tumors of breast, colon, non-small cell lung, CML, and AML.82 No human data are currently available, but a phase I trial in advanced solid tumors in underway in the United Kingdom administering GSK1070916 intravenously over 1 hour once-daily on days 1–5 every 21 days.28
4.0 Dual Aurora A and Aurora B Kinase Inhibitors
4.1 ZM447439
ZM447439 is one of the first AKIs to be developed and served as a template for AZD1152.83 Despite inhibiting aurora A and B equipotently, the phenotype induced in tumor cells following exposure to ZM447439 is more consistent with aurora B kinase inhibition.84 This incongruency may be due more selective in vivo aurora B kinase inhibition, though data are lacking. Early work with ZM447439 focused on elucidation of aurora kinase activity, rather than drug development. Preclinical studies with ZM447439 in cell lines of AML85, neuroendocrine tumor86, breast cancer87, and mesothelioma88 have led to understanding of importance of aurora kinase inhibition. ZM447439 is included in this review for historical context as the current use is restricted to exploratory laboratory studies.
4.2 JNJ-7706621
Also a potent inhibitor of the family of cyclin-dependent kinases CDK1, CDK2, and CDK3 (IC50 values ranging from 3 – 58nM, respectively), JNJ-7706621 displays high affinity for both aurora A and B kinases (IC50 values of 11 and 15nM, respectively), making it active from S through G2 phase of cell cycle.89 As seen with other members of the dual inhibitor class, exposure to JNJ-7706621 creates a phenotype more similar to aurora B kinase inhibition. Little is published in manuscript or abstract form about JNJ-7706621 and no clinical trials are currently open.28
4.3 AT9283
Discovered through fragment-based high throughput X-ray crystallography techniques, AT9283 is equally potent at inhibiting aurora A and B kinases, in addition to inhibiting JAK2, JAK3, STAT3, BCR-Abl (T315I), Tyk2 and VEGF, with IC50 values ranging from 1 – 30nM.90 Preclinical studies in human tumor cell lines and murine xenograft models of colorectal, ovarian, non-small cell lung, breast and pancreatic carcinomas determined potency across these tumor types with IC50 of AT9283 ranging from 7.7 – 20nM.91 Notably, the pro-apoptotic effects of AT9283 were maintained in cells irrespective of p53 status after one cell cycle, which differs from observed data indicating that p53-deficient cells are more susceptible to aurora B kinase inhibition.91 AT9283 has preclinical efficacy data in several hematologic neoplasms, such as JAK2-positive (both wild-type and mutant) myeloproliferative disorders92, CML (BCR-Abl T315I mutant)93, FLT3 or c-kit positive AML94, pediatric ALL95, and MM96.
AT9283 was administered as a 72-hr continuous infusion to 20 patients with refractory hematological malignancies at 6 different dose levels, ranging from 3–48mg/m2/day for 72 hrs in a standard 3+3 dose escalation phase I design.97 Nineteen of the 20 (95%) patients had AML, with 15 of 20 (75%) with high-risk cytogenetics. AT9283 was found to have non-linear pharmacokinetics with multiphasic elimination and terminal half-life of 6–13 hrs. No MTD was defined in this trial with 6 of 20 (30%) displaying antileukemic activity. Notably, all dose levels produced significant reductions in bone marrow blast cells. A follow-up phase I study administered AT9283 via 72-hr continuous infusion to 29 patients with refractory leukemia and high-risk MDS at 8 dose levels, ranging from 3–162mg/m2/day for 72 hrs in a standard 3+3 dose escalation phase I design.98 Correlative pharmacodynamic studies yielded significant reduction in histone H3 phosphorylation, indicative of aurora B inhibition. Elevation in liver function tests and myocardial infarction at dose level of 162mg/m2/day signified the DLT and established MTD as 108mg/m2/day as a 72-hr continuous infusion. Doses above 6mg/m2/day produced predictable and reversible neutropenia and alopecia. Approximately 33% of patients experienced hematological response, with CML patients benefiting the most.
AT9283 was administered to 22 patients with advanced solid tumors, including squamous cell carcinoma and colorectal adenocarcinoma, as a 72-hr continuous intravenous infusion over 5 doses levels, ranging from 1.5–12mg/m2/day, in a standard 3+3 dose escalation design.99 Aurora B kinase inhibition was seen across all dose levels, as evidenced by skin and serum samples. The MTD was determined to be 9mg/m2/day as a 72-hr continuous infusion with DLT of febrile neutropenia. The best response was stable disease achieved after at least 6 cycles. A second phase I study in 33 patients with refractory solid tumors administered AT9283 with administration parameters and same design as previously described.100 The MTD of 9mg/m2/day as a 72-hr continuous infusion with DLT of febrile neutropenia were replicated. Seven patients were administered a single oral dose of 0.9mg/m2 prior to starting IV, revealing an oral bioavailability of 27% (range, 17–45%). The best response was partial response in 1 (3%) patient with non-small cell lung cancer and stable disease in 4 other patients (12%) after receiving a minimum of 6 cycles.
4.4 PF-03814735
Preclinical studies of PF-03814735 displayed broad activity in cell lines and murine xenografts of breast, colorectal, lung, and promyelocytic leukemia.101 A single phase I study in 20 patients with varying refractory solid tumors was conducted using an accelerated dose-escalation scheme.102 After 20 patients received a median of 2 cycles ranging from 5–100mg/day orally × 5 days, the MTD was determined to be 80mg/day × 5 days with a DLT of febrile neutropenia. Other adverse effects include gastrointestinal toxicity and fatigue. No objective responses were reported in this study and no subsequent studies are currently ongoing.28
5.0 Pan-Aurora Kinase Inhibitors
5.1 VX-680/MK-0457 (Tozasertib)
Discovered through a molecular screening campaign, VX-680/MK-0457 also potently inhibits Src and GSK3β, Flt3, JAK2, BCR-Abl (wild-type) and BCR-Abl (T315I mutant) at nanomolar concentrations.103 The inhibition of a wide array of kinases stems from the ability to bind to non-aurora kinases in their inactive conformations and preventing activation.103 Many preclinical investigations with VX-680/MK-0457 were performed in cell lines and/or xenografts in animal models showing high degree of anti-tumor activity. The tumor types investigated as single-agent included ovarian104, renal cell carcinoma105, thyroid106, oral squamous cell107, CML (wild-type and mutant BCR-Abl)108,109,110, AML111, and MM112.
Phenotypic changes induced by VX-680/MK-0457 indicated that synergy may be obtained by combining VX-680/MK-0457 with HDACI. Vorinostat inhibits HDAC6 causing acetylation and disruption of heat shock protein 90 (hsp90). By inducing acetylation of hsp90, vorinostat inhibits the chaperone function of hsp90 leading to depleted aurora kinase levels in AML and CML cells.113 Several pre-clinical studies combining vorinostat with VX-680/MK-0457 demonstrated additive or synergistic activity in AML113,114, colorectal cancer114, pancreatic cancer114, CML (wild-type and mutant BCR-Abl)113,115, Ph+ ALL116, and breast cancer117. Synergy was also seen when VX-680/MK-0457 is combined with chemotherapy agents or erlotinib, an orally-available epidermal growth factor receptor antagonist, in preclinical studies of AML, CML, Ph+ ALL, and lung cancer.118,119,120 An early phase I/II study in humans attempted to study not only the inhibitor effect of aurora kinase, but also the anti-JAK2 effect by enrolling 15 patients including 6 with V617F-mutant JAK2 myeloproliferative disease (MPD).121 All patients received MK-0457 as a 5-day continuous infusion every 2–3 weeks on a dose escalation schedule. Clinical correlates of CD34+ and peripheral blood morphonuclear cells were described, as well. Results were mixed, with 5 of 6 MPD patients displaying limited apoptosis and slight decrease in JAK2 (V617F) transcripts. Three of 6 CML patients displayed no cytogenetic response and 3 exhibited a response. Notably, one of the 6 CML patients received MK-0457 while in lymphoid blast crisis and displayed substantial apoptosis. In the 15 patients enrolled, virtually all of the in vitro markers for cell death were evident, but did not translate to in vivo findings.
Another phase I study of 40 patients, including 16 CML patients (11 with T315I mutation), 2 Ph+ ALL (1 with T315I mutation), 13 with AML and 10 with rapidly progressing or transforming MPD evaluated dose-escalation of MK-0457 as 5-day continuous infusion.122 Still in progress at time of publication, authors note that MTD was not reached despite using 24mg/m2/day as a 5-day continuous infusion, with only grade 1 nausea and alopecia observed. These interim results note that all 11 T315I BCR-Abl CML patients and the T315I BCR-Abl Ph+ALL patient experienced objective response. Six of 8 evaluable MPD patients also experienced objective responses.
A subsequent phase I study in refractory CML and Ph+ ALL patients studied the effect of combining dasatinib, a second-generation BCR-Abl inhibitor, with MK-0457 in 3 patients (2 with Ph+ ALL and 1 with CML).123 All patients received dasatinib 70mg orally twice daily for 3 consecutive months. Patients who achieved major hematologic response (MHR) received MK-0457 dosed at 64mg/m2/hr for 6 hours twice weekly. Patients who did not achieve MHR after 3 months of dasatinib received MK-0457 at a dose of 240mg/m2/day as continuous infusion for 5 days administered every 4 weeks. Both Ph+ ALL patients received biweekly treatment with MK-0457 and maintained hematologic response with no hematologic toxicity. The CML patient who clinically failed dasatinib showed marked improvement after the first cycle of MK-0457. Due to serious cardiac events, including QTc prolongation, all further trials of VX-680/MK-0457 were terminated and drug development halted.28
5.2 PHA-739358 (Danusertib)
An analogue of PHA-680632 with enhanced inhibitory potency for all aurora kinases, danusertib potently inhibits all aurora kinases, BCR-Abl, FGFR-1 and FLT3, in addition to almost 30 other kinases at clinically-relevant doses.124,125 Notably, danusertib is a very potent inhibitor of VEGFR2/3 at doses used clinically. Preclinical activity from cell lines and xenograft models displayed high degree of activity in colorectal, breast, prostate, lung, ovary, and hepatocellular tumors, in addition to CML (wild-type and T315I mutant BCR-Abl).125,126,127
Based upon preclinical data, danusertib was studied as both bolus128 and continuous infusion administration129 in separate phase I studies. The bolus infusion study evaluated administration of 45mg/m2 intravenously over 6 hours and 250mg/m2 intravenously over 3 hours with standard dose escalation in a heterogeneous population of patients with solid tumors.128 Colorectal adenocarcinoma and sarcoma accounted for approximately 50% of patients. The 3-hour infusion schedule was determined after interim analysis of 6-hr infusion cohort. The DLT for 6-hr infusion was identified at 330mg/m2, but DLT for 3-hr infusion was not identified, as neutropenia was dose-limiting. PK and PD correlates favored 330mg/m2 intravenously as a 6-hr infusion. However, no complete or partial responses were observed in this cohort, with objective response observed in 6 of 30 evaluable patients. Authors recommend 330mg/m2 given over 6 hours on days 1, 8, 15 of a 28-day cycle should be used in phase II testing.
The phase I study of danusertib administered as continuous infusion included 56 patients with advanced solid tumors (colorectal adenocarcinoma accounted for 33% of patients).129 The initial cohort of 40 patients received escalating doses of danusertib without granulocyte colony-stimulating factor (G-CSF) and subsequent 16 patients received G-CSF support. The MTD was determined to be 500mg/m2 intravenously over 24 hours every 14 days with DLT being neutropenia. When danusertib was administered with G-CSF support, the MTD was determined to be 750mg/m2 intravenously over 24 hours every 14 days due to renal damage at the next-higher dose level. Non-hematologic adverse events were generally mild and reversible, with the exception of hypertension, which occurred in 12 patients and reversible reduction in left ventricular ejection fraction (LVEF) by approximately 10% from baseline in 2 (4%) cases. Pharmacodynamic correlates of skin biopsies revealed low-grade phenotypic changes consistent with aurora B kinase inhibition starting at 500mg/m2 cohort. Stable disease was most frequently detected, occurring in 18 of 42 (43%) patients, with durable stabilization of disease detected in 4 (10%) patients.
Twenty-three patients with CML (4 in accelerated phase and 8 in blast crisis) and Ph+ ALL were enrolled in a phase I study of danusertib administered via 3-hr infusion daily for 7 consecutive days every 14 days.130 Fifteen of 23 patients (65%) harbored T315I BCR-Abl mutation. The MTD was not determined at publication, but a single episode of syncope was observed at 90mg/m2 cohort. Three patients (13%) experienced cytogenic response and 5 (22%) demonstrated hematologic response. Phase II studies are currently ongoing in both solid and hematologic tumors using both 6-hr infusion and 24-hour continuous infusion schedule.28
5.3 CYC-116
CYC-116 is a potent, orally-administered inhibitor of all 3 aurora kinases, Flt3, and VEGFR-2.131,132 Preclinical models in both cell lines and murine xenografts indicate activity against leukemia, pancreatic, colorectal, prostate, glioma, thyroid, melanoma, breast, and non-small cell lung cancers, with inhibition of angiogenesis playing a distinct role in overall anti-tumor effect. Preclinical data have also demonstrated synergy with combining CYC-116 with chemotherapeutic agents or in combination with ionizing radiation.133,134 Of note, the preclinical study of CYC-116 with ionizing radiation demonstrated a distinctly potent anti-tumor effect in Ras-mutated colorectal adenocarcinoma cell lines over Ras-wild type cell lines.134 A phase I trial was completed in October 2009 in patients with advanced solid tumors with results forthcoming.28
5.4 SNS-314
SNS-314 displays high selectivity for aurora kinases, binding with high affinity. A unique feature to SNS-314 is lack of off-target inhibitory effects.135 Where many other AKIs co-inhibit BCR-Abl, FLT3, and VEGFR, none of these kinases are inhibited by SNS-314 at clinically-relevant doses. Preclinical studies of single-agent SNS-314 in cell lines and murine models show anti-tumor efficacy for tumors of colon, breast, prostate, lung, ovary and melanoma.136 Combination studies of SNS-314 with chemotherapy agents in colorectal adenocarcinoma cell lines displayed synergy, with antimicrotubule agents providing most substantial synergy.137 This study evaluated SNS-314 with various chemotherapeutic agents, either concurrently or in sequence. This model showed additive effect with many agents, except when SNS-314 was used concurrently with nucleoside antagonists or carboplatin. When used sequentially, agents that were antagonistic as concurrent therapy yielded additive effect. Furthermore, administration of SNS-314 prior to docetaxel was more efficacious than docetaxel prior to SNS-314. This innovative model has not been utilized with other AKIs and it remains to be seen if the effect on efficacy translates to humans.
A phase I study of 32 patients with advanced solid malignancies evaluated administration of SNS-314 by 3-hour infusion on days 1, 8, and 15 every 28 days.138 Neutropenia was determined to be DLT encountered at a dose of 1,440mg/m2 with skin biopsies showing phenotypic evidence of aurora B kinase inhibition at doses ≥240mg/m2. No MTD could be determined. Pharmacokinetic data determined a t1/2 of 10.4 hours and Vd approximating total body water. No objective responses were observed in any patient, but 6 patients (19%) experienced stable disease. No active clinical trials are currently registered in the United States.28
5.5 AMG-900
AMG-900 is an oral pan-aurora kinase inhibitor with extreme potency for all 3 aurora kinases, but little off-target inhibition.139 Preclinical investigation of single-agent AMG-900 demonstrated inhibition of proliferation in 26 tumor cell lines of both solid and hematologic malignancies, including cell lines resistant to paclitaxel and other AKIs (AZD-1152, tozasertib, danusertib).139 The first-in-human phase I study in advanced solid tumors is currently ongoing.28
5.6 VE-465
A pan-aurora kinase inhibitor related to MK0457, VE-465 inhibits a host of off-target kinases beyond aurora kinases at clinically-relevant doses.140 Preclinical tissue culture cells and murine xenograft models confirm activity in CML (wild-type and T315I mutant BCR-Abl) as single-agent and with imatinib140, multiple myeloma (as single-agent and with dexamethasone)141, hepatocellular carcinoma142, ovarian cancer (in combination with paclitaxel)143, and myeloid leukemia144. Currently, no studies in humans are ongoing.28
5.7 AS703569/R-763
Discovered through cell-based approach for drug design, AS703569 is an orally-available aurora kinase that exhibits potent off-target inhibition of FLT3, BCR-Abl, VEGFR-2, IGFR, Akt.145 Preclinical investigation in cell cultures and murine xenografts demonstrates anti-proliferative activity in solid organ and hematologic tumors including non-small cell lung, breast, pancreas adenocarcinoma, colorectal adenocarcinoma, prostate, cervix, ovary, osteogenic sarcoma, biphenotypic leukemia, acute promyelocytic leukemia, ALL, AML, CML, and MM.145,146,147
The first phase I study of AS703569 in humans was conducted using a two-arm, dose-escalation scheme in patients with advanced solid malignancies.148 The first arm administered AS703569 on days 1 and 8 every 21 days and the second arm administered AS-703569 on days 1, 2 and 3 every 21 days as a single oral dose. Fifteen patients were enrolled with the most common malignancies being uterine and breast carcinomas. At study publication, no DLT or MTD had been established and 1 patient (7%) experienced tumor progression while on study.
A second study also evaluated 2 different dosing schedules in patients with hematological malignancies.149 Forty-three total patients were assigned to receive AS703569 once daily on days 1–3 and 8–10 every 21 days (n=24) or once daily on days 1–6 ever 21 days (n=21). The majority of patients had de novo AML (n=20) or secondary AML (n=13). The MTD for both administration schedules was determined to be 37mg/m2/day, with mucositis and neutropenia serving as DLT. PK data determined a Tmax of 2–4 hours and t1/2 of 10–20 hours. Activity was modest with schedule of administration on days 1–3 and 8–10 demonstrating greater number of objective responses in this small cohort. Several clinical trials in both solid and hematologic malignancies, including combination studies with chemotherapy are either ongoing or recently completed.28
6.0 Conclusions
Aurora SMIs have been developed as anti-cancer therapies since they target aberrant centrosome amplification and/or a defective spindle assembly checkpoint associated with chromosomal instability in many human solid and hematologic malignancies. Approximately 15 distinct chemotypes reversibly targeting the ATP-binding site of Aurora A and/or B are in early clinical development as single agent (Table 1) or in combination with chemotherapy (Figure 1) or epigenetic therapy (e.g. HDAC inhibitors), but none has been approved by the US FDA. Clinical trial data emerging for the most advanced SMIs are promising (Table 2) and it is likely that proof-of-concept targeting will be achievable, and that AKIs will be part of combination treatment for solid and hematologic malignancies in the future. Important factors that are likely to drive progress for success of AKIs in the clinic are duration of enzyme inhibitory activity, schedule, routes of administration, predictive biomarker(s) [copy number by FISH], non-toxic mechanistic combinations with approved as well other targeted therapies, clinical development pathway(s), and enrichment of appropriate patient populations.
7.0 Expert Opinion
The succesful development and approval of an AKI for anti-cancer therapy remains unresolved. However, we believe that aurora kinases are important anti-cancer targets that operate in collaboration with other oncogenes intimately involved in uncontrolled tumor proliferation. Aurora inhibitors appear to have excellent activity in tumors with a high mitotic or proliferative index such as acute myeloid leukemia (AML), blast phase of chronic myeloid leukemia (CML), and certain aggressive B- and T-cell non-Hodgkin lymphomas.150 In acute leukemias, it is likely that off-target effects on several distinct oncogenic protein kinases contributes to efficacy, although further research is needed. However, resistance mechanisms are operant and pre-clinical identification of these would help design better early phase clinical trials where relevant combinations may be evaluated prior to phase II testing. A similar situation holds for AKI activity in chronic myeloproliferative diseases where these inhibitors are effective in blocking the T315I gate keeper mutation in BCR-ABL in CML and JAK-2 mutation in polycythemia vera and essential thrombocytosis in early investigations. In contrast, AKIs as single agents have shown modest clinical activity in soild tumor types. Various chemotherapy combinations are planned and/or ongoing to improve clinical activity of AKIs. One such combination is with microtubule targeting agents (Figure 1) that inhibits microtubule function and a defective spindle assembly checkpoint (SAC) simultaneously thereby enhancing apoptosis. However, despite ongoing apoptosis, some tumor cells may escape due to continuing unchecked proliferation. Therefore, additional agent(s) will be required that target proliferation most likely in the context of KRAS mutations and/or p53 loss, especially in solid tumor types.
In diffuse large B-cell lymphoma (DLBCL), several molecular abnormalities have been identified, such as c-Myc oncoprotein that enhances cell proliferation by regulating transcription of key cell cycle protein kinases including Aurora A and B. Both aurora kinases are over-expressed in c-Myc driven B-cell lymphomas which are resistant to standard R-CHOP chemotherapy. It has been demonstrated that induction of aurora A kinase by c-Myc is transcriptional and directly mediated via E-boxes, while aurora B kinase is indirectly regulated. Inhibition of aurora A and B kinases with a selective AKI triggered transient mitotic arrest, polyploidization, and apoptosis of c-Myc induced lymphomas. An aurora B kinase mutant resistant to AKI continues to have a phenotype of aurora B kinase activation demonstrating that the primary therapeutic target is aurora B kinase in the context of c-Myc mediated proliferation.151,152 Furthermore, apoptosis mediated by aurora kinase inhibition was p53 independent, indicating that pan-aurora kinase inhibitors will show efficacy in treating primary or relapsed malignancies with c-Myc involvement and/or loss of p53 function. Expression of c-Myc using immunohistochemistry or copy number by fluorescence in situ hybridization could be a useful biomarker of sensitivity for B-cell lymphoma inhibition of the chromosomal passenger protein complex(including aurora B kinase). Therefore, incorporation of a pan-aurora kinase inhibitor into standard R-CHOP or some components (R-VP) should be evaluated in phase II studies of c-Myc driven aggressive B- and T-cell lymphomas.
The major side-effects of aurora kinase inhibition are neutropenia, mucositis and alopecia which appear to mimick traditional chemotherapy agents. Therefore, dosing and scheduling without compromising efficacy are key to successful anti-cancer therapy. Agents that exquisitely synergize with aurora kinase inhibition without any additional adverse events are likely to move forward as effective therapies for many human malignancies.
Acknowledgments
We wish to thank Annette Krzysik for preparing Figure 1
Footnotes
Declaration of Interest
The authors declare financial support in the form of the Lymphoma SPORE grant (1P5O CA B080501A1) awarded by the National Institutes of Health and the National Cancer Institute. The authors declare that they have no further disclosures.
Annotated Bibliography
- 1. [accessed October 8, 2010];World cancer report. 2008 http://www.iarc.fr/en/publications/pdfs-online/wcr/2008/wcr_2008.pdf.
- 2. [accessed October 8, 2010];The global economic cost of cancer. http://www.cancer.org/acs/groups/content/@internationalaffairs/documents/document/acspc-026203.pdf.
- 3.Carmena M, Earnshaw WC. The cellular geography of aurora kinases. Nat Rev Mol Cell Biol. 2003;4:842–54. doi: 10.1038/nrm1245. [DOI] [PubMed] [Google Scholar]
- 4.Ducat D, Zheng Y. Aurora kinases in spindle assembly and chromosome segregation. Exp Cell Res. 2004;301:60–7. doi: 10.1016/j.yexcr.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 5.Marumoto T, Zhang D, Saya H. Aurora A, a guardian of poles. Nat Rev Cancer. 2005;5:42–50. doi: 10.1038/nrc1526. [DOI] [PubMed] [Google Scholar]
- 6.Fu J, Bian M, Jiang Q, Zhang C. Roles of Aurora kinases in mitosis and tumorigenesis. Mol Cancer Res. 2007;5:1–10. doi: 10.1158/1541-7786.MCR-06-0208. [DOI] [PubMed] [Google Scholar]
- 7•.Kobayashi M, Nakamura S, Ono T, et al. Analysis of aurora kinase expressions and cell cycle regulation by aurora C in leukemia cells. Blood (ASH Annual Meeting Abstracts) 2006;108 abstr 1366. Implicates overexpression of aurora C kinase in human leukemia, rather than restricted to testicular meiosis. [Google Scholar]
- 8.Slattery SD, Mancini MA, Brinkley BR, Hall RM. Aurora-C kinase supports mitotic progression in the absence of aurora-B. Cell Cycle. 2009;8:2986–97. [PubMed] [Google Scholar]
- 9.Zhang X. Aurora kinases. Curr Biol. 2008;18(4):R146–8. doi: 10.1016/j.cub.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Carvajal RD, Tse A, Schwartz GK. Aurora kinases: new targets for cancer therapy. Clin Cancer Res. 2006;12(23):6869–75. doi: 10.1158/1078-0432.CCR-06-1405. [DOI] [PubMed] [Google Scholar]
- 11.Girdler F, Gascoigne KE, Eyers PA, et al. Validating aurora B as an anti-cancer drug target. J Cell Sci. 2006;119(17):3664–75. doi: 10.1242/jcs.03145. [DOI] [PubMed] [Google Scholar]
- 12.Nishida N, Nagasaka T, Kashiwagi K, et al. High copy amplification of the aurora A gene is associated with chromosomal instability phenotype in human colorectal cancers. Cancer Biol Ther. 2007;6(4):525–33. doi: 10.4161/cbt.6.4.3817. [DOI] [PubMed] [Google Scholar]
- 13.Gritsko TM, Coppola D, Paciga JE, et al. Activation and overexpression of centrosome kinase BTAK/Aurora A in human ovarian cancer. Clin Cancer Res. 2003;9:1420–6. [PubMed] [Google Scholar]
- 14.Anand S, Penrhyn-Lowe S, Venkitaraman AR. Aurora-A amplification overrides the mitotic spindle assembly checkpoint, inducing resistance to Taxol. Cancer Cell. 2003;3(1):51–62. doi: 10.1016/s1535-6108(02)00235-0. [DOI] [PubMed] [Google Scholar]
- 15••.Karthigeyan D, Prasad SB, Shandilya J, et al. Biology of aurora A kinase: implications in cancer manifestation and therapy. Med Res Rev. 2010 doi: 10.1002/med.20203. published online 1 March 2010. In-depth review of aurora A kinase, covering structure, expression, transcription, protein interactions, signaling and therapeutic inhibition. [DOI] [PubMed] [Google Scholar]
- 16.Gautschi O, Heighway J, Mack PC, et al. Aurora kinases as anticancer drug targets. Clin Cancer Res. 2008;14(6):1639–48. doi: 10.1158/1078-0432.CCR-07-2179. [DOI] [PubMed] [Google Scholar]
- 17.Ujhazy P, Stewart D. DNA Repair. J Thorac Oncol. 2009;11(4 suppl 3):S1068–70. doi: 10.1097/01.JTO.0000361754.25037.2c. [DOI] [PubMed] [Google Scholar]
- 18.Smith SL, Bowers NL, Betticher DC, et al. Overexpression of aurora B kinase (AURBK) in primary non-small cell lung carcinoma is frequent, generally driven from one allele, and correlates with the level of genetic instability. Br J Cancer. 2005;93(6):719–29. doi: 10.1038/sj.bjc.6602779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keen M, Taylor S. Mitotic drivers – inhibitors of the aurora B kinase. Cancer Metastasis Rev. 2009;28:185–95. doi: 10.1007/s10555-009-9184-9. [DOI] [PubMed] [Google Scholar]
- 20.Mountzios G, Terpos E, Dimopoulos M-A. Aurora kinases as targets for cancer therapy. Cancer Treat Rev. 2008;34:175–82. doi: 10.1016/j.ctrv.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Mazumdar A, Henderson YC, et al. Aurora kinase A inhibition and paclitaxel as targeted combination therapy for head and neck squamous cell carcinoma. Head Neck. 2009;31(5):625–34. doi: 10.1002/hed.21007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kretzner L, Scuto A, Claudia K, et al. Combination therapy with the histone deacetylase inhibitor vorinostat plus the novel aurora kinase A inhibitor MK-5108 leads to enhanced lymphoma cell death due to acetylation of p53 and repression of c-Myc, hTERT, and miRNA levels. Blood (ASH Annual Meeting Abstracts) 2009;114 abstr 1690. [Google Scholar]
- 23.Bray MR, Fletcher GC, Denny TA, et al. ENMD-981693 is an orally-active kinase inhibitor with activity towards human hematologic cancers in vitro and in vivo. Blood (ASH Annual Meeting Abstracts) 2006;108 abstr 1377. [Google Scholar]
- 24.Tentler JJ, Bradshaw-Pierce EL, Serkova NJ, et al. Assessment of the in vivo antitumor effects of ENMD-2076, a novel multitargeted kinase inhibitor, against primary and cell line-derived human colorectal cancer xenograft models. Clin Cancer Res. 2010;16(11):2989–98. doi: 10.1158/1078-0432.CCR-10-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Sinn AL, Pollok K, et al. Preclinical activity of a novel multiple tyrosine kinase and aurora kinase inhibitor, ENMD-2076, against multiple myeloma. Br J Haematol. 2010;150:313–25. doi: 10.1111/j.1365-2141.2010.08248.x. [DOI] [PubMed] [Google Scholar]
- 26.Bastos BR, Diamond J, Hansen R, et al. An open-label, dose escalation, safety, and pharmacokinetic study of ENMD-2076 administered orally to patients with advanced cancer. J Clin Oncol. 2009;27(15s suppl) abstr 3520. [Google Scholar]
- 27.Wang X, Sinn AL, Suvannasankha A, et al. The novel aurora kinase inhibitor ENMD-2076 has potent single agent activity against multiple myeloma in vitro and in vivo, and shows synergistic activity in combination with lenalidomide. Blood (ASH Annual Meeting Abstracts) 2008;112 abstr 3660. [Google Scholar]
- 28.U.S. National Institutes of Health. [accessed October 9, 2010]; www.clinicaltrials.gov.
- 29.Shimomura T, Hasako S, Nakatsuru Y, et al. MK-5108, a highly selective aurora A kinase inhibitor, shows antitumor activity alone and in combination with docetaxel. Mol Cancer Ther. 2010;9(1):157–66. doi: 10.1158/1535-7163.MCT-09-0609. [DOI] [PubMed] [Google Scholar]
- 30.Minton SE, LoRusso P, Lockhart AC, et al. A phase I study of MK-5108, an oral aurora A kinase inhibitor, in both monotherapy and in combination with docetaxel in patients with advanced solid tumors. J Clin Oncol. 2010;28(15s suppl) doi: 10.1007/s10637-015-0306-7. abstr e13026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones SF, Cohen RB, Dees EC, et al. Phase I clinical trial of MLN8054, a selective inhibitor of aurora A kinase. J Clin Oncol. 2007;25(18s suppl) abstr 3577. [Google Scholar]
- 32.Manfredi MG, Ecsedy JA, Meetze KA, et al. Antitumor activity of MLN8054, an orally active small-molecule inhibitor of aurora A kinase. Proc Natl Acad Sci. 2007;104:4106–4111. doi: 10.1073/pnas.0608798104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoar K, Chakravarty A, Rabino C, et al. MLN8054, a small-molecule inhibitor of aurora A, causes spindle pole and chromosome congression defects leading to aneuploidy. Mol Cell Biol. 2007;27(12):4513–25. doi: 10.1128/MCB.02364-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galvin KM, Huck J, Burenkova O, et al. Preclinical pharmacodynamic studies of aurora A inhibition by MLN8054. J Clin Oncol. 2006;24(18s suppl) abstr 13059. [Google Scholar]
- 35.Huck J, Zhang M, Burenkova O, et al. Preclinical antitumor activity with MLN8054, a small molecule aurora A kinase inhibitor. Proc Amer Assoc Cancer Res. 2006;47 abstr 4698. [Google Scholar]
- 36.Huck J, Zhang M, McDonald A, et al. MLN8054, an inhibitor of aurora A kinase, induces senescence in human tumor cells both in vitro and in vivo. Mol Cancer Res. 2010;8(3):373–84. doi: 10.1158/1541-7786.MCR-09-0300. [DOI] [PubMed] [Google Scholar]
- 37.Dees E, Infante JR, Cohen RB, et al. Phase I and pharmacokinetic study of MLN8054, a selective inhibitor of aurora A kinase. Eur J Cancer Suppl. 2008;6(12):91. abstr 281. [Google Scholar]
- 38.Macarulla T, Cervantes A, Elez E, et al. Phase I study of the selective aurora A kinase inhibitor MLN8054 in patients with advanced solid tumors: safety, pharmacokinetics, and pharmacodynamics. Mol Cancer Ther. 2010;9(10):2844–52. doi: 10.1158/1535-7163.MCT-10-0299. [DOI] [PubMed] [Google Scholar]
- 39.Maris JM, Morton CL, Gorlick R, et al. Initial testing of the aurora kinase A inhibitor MLN8237 by the pediatric preclinical testing program (PPTP) Pediatr Blood Cancer. 2010;55:26–34. doi: 10.1002/pbc.22430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lipsitz EG, Nhuten V, Zhao H, et al. Modeling MLN8237, an aurora A kinase inhibitor, with irinotecan and temozolomide in neuroblastoma. J Clin Oncol. 2010;28(15s suppl) abstr 10593. [Google Scholar]
- 41.Huck JJ, Zhang M, Hyper ML, Manfredi MG. Anti-tumor activity of the aurora A inhibitor MLN8237 in diffuse large B-cell lymphoma preclinical model. Blood (ASH Annual Meeting Abstracts) 2008;112 abstr 1592. [Google Scholar]
- 42.Zhang M, Huck J, Sells T, et al. In vivo characterization of the aurora A kinase inhibitor MLN8237 in subcutaneous and disseminated models of human cancer. Proc Am Assoc Cancer Res. 2008;49 abstr 5646. [Google Scholar]
- 43.Nawrocki ST, Medina E, Smith S, et al. The aurora kinase inhibitor MLN8237 has potent anticancer activity in CML and Ph+ ALL models and significantly increases the efficacy of nilotinib. Blood (ASH Annual Meeting Abstracts) 2008;112 abstr 3198. [Google Scholar]
- 44.Gorgun G, Calabrese E, Hideshima T, et al. A novel aurora-A kinase inhibitor MLN-8237 induces cytotoxicity and cell-cycle arrest in multiple myeloma. Blood. 2010;115(25):5202–13. doi: 10.1182/blood-2009-12-259523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kelly KR, Swords RT, Mahalingam D, et al. The novel orally active aurora A kinase inhibitor MLN8237 is highly active in preclinical models of acute myeloid leukemia and significantly increases the efficacy of cytarabine. Blood (ASH Annual Meeting Abstracts) 2009;114 abstr 2087. [Google Scholar]
- 46.Huck J, Zhang M, Hyer M, et al. Antitumor activity of the aurora A inhibitor MLN8237 in combination with docetaxel in xenograft models of breast and prostate cancer. Proc Am Assoc Cancer Res. 2009;50 abstr 3724. [Google Scholar]
- 47.Infante J, Dees EC, Cohen RB, et al. Phase I study of the safety, pharmacokinetics (PK), and pharmacodynamics (PD) of MLN8237, a selective aurora A kinase inhibitor, in the United States. Eur J Cancer Suppl. 2008;6(12):90. abstr 280. [Google Scholar]
- 48.Cervantes-Ruiperez A, Elez ME, Rosello S, et al. Phase I pharmacokinetic and pharmacodynamic study of MLN8237, a novel selective aurora A kinase inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2009;27(15s suppl) abstr 2565. [Google Scholar]
- 49.Dees EC, Infante JR, Burris HA, et al. Phase I study of the investigational drug MLN8237, an aurora A kinase inhibitor, in patients with solid tumors. J Clin Oncol. 2010;28(15s suppl) abstr 3010. [Google Scholar]
- 50.Cervantes-Ruiperez, Burris HA, Cohen RB, et al. Pharmacokinetic and pharmacodynamic results from two phase I studies of the investigational selective aurora A kinase (AAK) inhibitor MLN8237: Exposure-dependent AAK inhibition in human tumors. J Clin Oncol. 2010;28(15s suppl) abstr 3031. [Google Scholar]
- 51.Mosse YP, Lipsitz EG, Maris JM, et al. A pediatric phase I trial and pharmacokinetic study of MLN8237, an oral selective small molecule inhibitor of aurora A kinase: A Children’s Oncology Group Phase I Consortium study. J Clin Oncol. 2010;28(15s suppl) doi: 10.1158/1078-0432.CCR-11-3251. abstr 9529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shah NP, Kasap C, Paquette R, et al. Targeting drug-resistant CML and Ph+ ALL with the spectrum selective protein kinase inhibitor XL-228. Blood (ASH Annual Meeting Abstracts) 2007;110 abstr 474. [Google Scholar]
- 53.Cortes J, Paquette R, Talpaz M, et al. Preliminary clinical activity in a phase I trial of the BCR-Abl/IGF-1R/aurora kinase inhibitor XL-228 in patients with Ph+ leukemias with either failure to multiple TKI therapies or with T315I mutations. Blood (ASH Annual Meeting Abstracts) 2008;112 abstr 3232. [Google Scholar]
- 54.Smith DC, Britten C, Garon EB, et al. A phase I study of XL228, a multitargeted protein kinase inhibitor, in patients with solid tumors or multiple myeloma. J Clin Oncol. 2010;28(15s suppl) abstr 3105. [Google Scholar]
- 55.Shiotsu Y, Kiyoi H, Ozeki K, et al. KW-2449, a novel multi-kinase inhibitor against FLT33, Abl, FGFR1 and aurora, suppresses the growth of AML both in vitro and in vivo. Blood (ASH Annual Meeting abstracts) 2007;110 abstr 1832. [Google Scholar]
- 56.Cortes J, Roboz GJ, Kantarjian HM, et al. A phase I dose escalation study of KW-2449, an oral multi-kinase inhibitor against FLT3, Abl, FGFR1 and aurora in patients with relapsed/refractory AML, ALL and MDS or resistant/intolerant CML. Blood (ASH Annual Meeting Abstracts) 2008;112 abstr 2967. [Google Scholar]
- 57.Gurtler U, Tontsch-Grunt U, Jarvis M, et al. Effect of BI-811283, a novel inhibitor of aurora B kinase, on tumor senescence and apoptosis. J Clin Oncol. 2010;28(15s suppl) abstr e13632. [Google Scholar]
- 58.Solca F, Rudolph D, Guertler U. Targeting the M-phase: Focus on PLK-1 and aurora B. J Thoracic Oncol. 2010;5(5 suppl) abstr 28IN (Abstracts of the European Lung Cancer Conference) [Google Scholar]
- 59.Mross KB, Scheulen ME, Frost A, et al. A phase I dose-escalation study of BI-811283, an aurora B inhibitor, administered every three weeks in patients with advanced solid tumors. J Clin Oncol. 2010;28(15s suppl) abstr 3011. [Google Scholar]
- 60.Scheulen ME, Mross KB, Richly H, et al. A phase I dose-escalation study of BI-811283, an aurora B inhibitor, administered days 1 and 15, every four weeks in patients with advanced solid tumors. J Clin Oncol. 2010;28(15s suppl) abstr e13065. [Google Scholar]
- 61.Gully CP, Zhang F, Chen J, et al. Antineoplastic effects of an aurora B kinase inhibitor in breast cancer. Mol Cancer. 2010;9:42–54. doi: 10.1186/1476-4598-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Azzariti A, Porcelli L, Simone G, et al. Aurora kinase inhibitor AZD1152 inhibits cell growth, induces apoptosis and enhances chemotherapeutics’ activity in various cancer in vitro models. Proc Am Assoc Cancer Res. 2007;48 abstr 4359. [Google Scholar]
- 63.Wilkinson RW, Keen N, Odedra R, et al. AZD1152: a highly potent and specific aurora kinase inhibitor. Proc Am Assoc Cancer Res. 2006;47 abstr 5673. [Google Scholar]
- 64.Wilkinson RW, Odedra R, Heaton SP, et al. AZD1152, a selective inhibitor of aurora B kinase, inhibits human tumor xenograft growth by inducing apoptosis. Clin Cancer Res. 2007;13(12):3682–8. doi: 10.1158/1078-0432.CCR-06-2979. [DOI] [PubMed] [Google Scholar]
- 65.Goodlad RA, Alferez D, Ryan A, et al. Targeting aurora B kinase activity with AZD1152 leads to antitumor effects in preclinical models of intestinal cancer. Proc Am Assoc Cancer Res. 2007;48 abstr 1640. [Google Scholar]
- 66.Nair J, Schwartz G. A selective aurora B kinase inhibitor with potent anticancer activity either as a single agent or in combination with irinotecan (CPT-11) in colon cancer cells. Proc Am Assoc Cancer Res. 2008;49 abstr 5645. [Google Scholar]
- 67.Helfrich B, Theodoro M, Varella-Garcia M, et al. The selective aurora B kinase inhibitor AZD1152-HQPA inhibits in vitro growth of small cell lung cancer cell lines. Proc Am Assoc Cancer Res. 2009;50 abstr 4775. [Google Scholar]
- 68.Aihara A, Tanaka S, Yasen M, et al. The selective aurora B kinase inhibitor AZD-1152 as a novel treatment for hepatocellular carcinoma. J Hepatol. 2010;52:63–71. doi: 10.1016/j.jhep.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 69.Betta P, Bensi T, Trincheri NF, et al. Aurora B kinase and malignant mesothelioma. J Clin Oncol. 2010;28(15s suppl) abstr e21021. [Google Scholar]
- 70.Oke A, Pearce D, Wilkinson RW, et al. AZD1152 rapidly and negatively affects the growth and survival of human acute myeloid leukemia cells in vitro and in vivo. Cancer Res. 2009;69(1):4150–8. doi: 10.1158/0008-5472.CAN-08-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Walsby E, Walsh V, Pepper C, et al. The aurora kinase inhibitor AZD1152 causes perturbation of cell cycle distribution in cell lines and primary AML samples. Blood (ASH Annual Meeting Abstracts) 2005;106 abstr 2759. [Google Scholar]
- 72.Pearce D, Odedra R, Wilkinson R, Bonnet D. The specific aurora kinase inhibitor AZD1152 significantly affects the growth of human leukaemic cells in an in vivo AML model. Blood (ASH Annual Meeting Abstracts) 2006;108 abstr 162. [Google Scholar]
- 73.Evans RP, Naber C, Steffler T, et al. The selective aurora B kinase inhibitor AZD1152 is a potential new treatment for multiple myeloma. Br J Haematol. 2007;140:295–302. doi: 10.1111/j.1365-2141.2007.06913.x. [DOI] [PubMed] [Google Scholar]
- 74.Grundy M, Seedhouse C, Shang S, et al. The FLT3 internal tandem duplication mutation is a secondary target of the aurora B kinase inhibitor AZD-1152-HQPA in acute myelogenous leukemia cells. Mol Cancer Ther. 2010;9(3):661–72. doi: 10.1158/1535-7163.MCT-09-1144. [DOI] [PubMed] [Google Scholar]
- 75.Yang J, Ikezoe R, Nichioka C, et al. AZD1152, a novel and selective aurora B kinase inhibitor, induces growth arrest, apoptosis, and sensitization for tubulin depolymerizing agent or topoisomerase II inhibitor in human acute leukemia cells in vitro and in vivo. Blood. 2007;110:2034–40. doi: 10.1182/blood-2007-02-073700. [DOI] [PubMed] [Google Scholar]
- 76.Tao Y, Leteur C, Calderaro J, et al. The aurora B kinase inhibitor AZD1152 sensitizes cancer cells to fractionated irradiation and induces mitotic catastrophe. Cell Cycle. 2009;8(19):3172–81. doi: 10.4161/cc.8.19.9729. [DOI] [PubMed] [Google Scholar]
- 77.Walsby E, Walsh V, Pepper C, et al. Effects of the aurora kinase inhibitors AZD1152-HQPA and ZM447439 on growth arrest and polyploidy in acute myeloid leukemia cell lines and primary blasts. Haematologica. 2008;93(5):662–9. doi: 10.3324/haematol.12148. [DOI] [PubMed] [Google Scholar]
- 78.Schellens JH, Boss D, Witteveen PO, et al. Phase I and pharmacological study of the novel aurora kinase inhibitor AZD1152. J Clin Oncol. 2006;24(18s suppl) abstr 3008. [Google Scholar]
- 79.Lowenberg B, Rousselot P, Martinelli G, et al. Phase I/II study to assess the safety and efficacy of the aurora B kinase inhibitor, AZD1152, in patients with advanced acute myeloid leukemia. Blood (ASH Annual Meeting Abstracts) 2009;114 abstr 2080. [Google Scholar]
- 80.Guo J, Anderson MG, Tapang P, et al. Identification of genes that confer tumor cell resistance to the aurora B kinase inhibitor, AZD-1152. Pharmacogenomics J. 2009;9:90–102. doi: 10.1038/tpj.2008.20. [DOI] [PubMed] [Google Scholar]
- 81.Anderson K, Lai Z, McDonald OB, et al. Biochemical characterization of GSK1070916, a potent and selective inhibitor of aurora B and aurora C kinases with an extremely long residence time. Biochem J. 2009;420:259–65. doi: 10.1042/BJ20090121. [DOI] [PubMed] [Google Scholar]
- 82.Hardwicke MA, Oleykowski CA, Plant R, et al. GSK1070916, a potent aurora B/C kinase inhibitor with broad antitumor activity in tissue culture cells and human tumor xenograft models. Mol Cancer Ther. 2009;8(7):1808–17. doi: 10.1158/1535-7163.MCT-09-0041. [DOI] [PubMed] [Google Scholar]
- 83.Mahadevan D, Beeck S. Aurora kinase targeted therapeutics in oncology: past, present and future. Expert Opin Drug Discov. 2007;2(7):1011–1026. doi: 10.1517/17460441.2.7.1011. [DOI] [PubMed] [Google Scholar]
- 84.Gadea BB, Ruderman JV. Aurora kinase inhibitor ZM447439 blocks chromosome induced spindle assembly, the completion of chromosome condensations, and the establishment of the spindle integrity checkpoint in Xenopus egg extracts. Mol Biol Cell. 2005;16:1305–18. doi: 10.1091/mbc.E04-10-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ikezoe T, Nichioka C, Tasaka T, et al. ZM447439, a novel aurora kinase inhibitor, induces growth arrest and apoptosis of human leukemia cells. Blood (ASH Annual Meeting Abstracts) 2006;108 abstr 1990. [Google Scholar]
- 86.Georgieva I, Koychev D, Wang Y, et al. ZM447439, a novel promising aurora kinase inhibitor, provokes antiproliferative and proapoptotic effects alone and in combination with bio- and chemotherapeutic agents in gastroenteropancreatic neuroendocrine tumor cell lines. Neuroendocrinology. 2010;91(2):121–30. doi: 10.1159/000258705. [DOI] [PubMed] [Google Scholar]
- 87.Vidarsdottir L, Bodvarsdottir S, Ogmundsdottir H, Eyfjord J. Targeting aurora kinases in BRCA2-mutated breast cell lines. Proc Am Assoc Cancer Res. 2008;49 abstr 2395. [Google Scholar]
- 88.Crispi S, Fagliarone C, Biroccio A, et al. Antiproliferative effect of aurora kinase targeting in mesothelioma. Lung Cancer. 2010 doi: 10.1016/j.lungcan.2010.03.2005. article in press. [DOI] [PubMed] [Google Scholar]
- 89.Emanuel S, Rugg CA, Gruninger RH, et al. The in vitro and in vivo effects of JNJ-7706621: A dual inhibitor of cyclin-dependent kinases and aurora kinases. Cancer Res. 2005;65(19):9038–46. doi: 10.1158/0008-5472.CAN-05-0882. [DOI] [PubMed] [Google Scholar]
- 90.Howard S, Berdini V, Boulstridge JA, et al. Fragment-based discovery of the pyrazol-4-yl urea (AT9283), a multitargeted kinase inhibitor with potent aurora kinase activity. J Med Chem. 2009;52:379–88. doi: 10.1021/jm800984v. [DOI] [PubMed] [Google Scholar]
- 91.Curry J, Angove H, Fazal L, et al. Aurora B kinase inhibition in mitosis: strategies for optimizing the use of aurora kinase inhibitors such as AT9283. Cell Cycle. 2009;8(12):1921–9. doi: 10.4161/cc.8.12.8741. [DOI] [PubMed] [Google Scholar]
- 92.Dawson MA, Curry JE, Barber K, et al. AT9283, a potent inhibitor of the aurora kinases and JAK2, has therapeutic potential in myeloproliferative disorders. Br J Haematol. 2010;150:46–57. doi: 10.1111/j.1365-2141.2010.08175.x. [DOI] [PubMed] [Google Scholar]
- 93.Squires M, Reule M, Curry J, et al. AT9283, a potent inhibitor of BCR-Abl T315I, is active in CML models. Proc Am Assoc Cancer Res. 2008;49 abstr 2820. [Google Scholar]
- 94.Goodall J, Squires MS, Lock V, et al. Outcome of aurora kinase inhibition of acute myeloid leukemia by AT9283 is dependent upon the presence or absence of mutations in type 1 oncogenic kinase signaling pathways. Blood (ASH Annual Meeting Abstracts) 2008;112 abstr 1613. [Google Scholar]
- 95.Lotfi S, Jayanthan A, Lewis VA, et al. AT9283, a novel aurora kinase/JAK2 inhibitor, demonstrates activity against refractory infant leukemia cells: studies on growth inhibition, biological correlates, drug synergy and effects on leukemia stem-like cells. Blood (ASH Annual Meeting Abstracts) 2009;114 abstr 3078. [Google Scholar]
- 96.Santo L, Hideshima T, Nelson EA, et al. AT9283, a small molecule multi-targeted kinase inhibitor induces antimyeloma activity via potent aurora kinase and STAT3 inhibition. Blood (ASH Annual Meeting Abstracts) 2009;114 abstr 3833. [Google Scholar]
- 97.Ravandi F, Foran J, Verstovsek S, et al. A phase I trial of AT9283, a multitargeted kinase inhibitor, in patients with refractory hematological malignancies. Blood (ASH Annual Meeting Abstracts) 2007;110 abstr 904. [Google Scholar]
- 98.Foran JM, Ravandi F, O’Brien SM, et al. Phase I and pharmacodynamic trial of AT9283, an aurora kinase inhibitor, in patients with refractory leukemia. J Clin Oncol. 2008;26(15s suppl) abstr 2518. [Google Scholar]
- 99.Plummer ER, Calvert H, Arkenau H, et al. A dose-escalation and pharmacodynamic study of AT9283 in patients with refractory solid tumours. J Clin Oncol. 2008;26(15s suppl) abstr 2519. [Google Scholar]
- 100.Kristeleit R, Calvert H, Arkenau H, et al. A phase I study of AT9283, an aurora kinase inhibitor, in patients with refractory solid tumors. J Clin Oncol. 2009;27(15s suppl) abstr 2566. [Google Scholar]
- 101.Jani JP, Arcari J, Bernardo V, et al. PF-03814735, an orally bioavailable small molecule aurora kinase inhibitor for cancer therapy. Mol Cancer Ther. 2010;9(4):883–94. doi: 10.1158/1535-7163.MCT-09-0915. [DOI] [PubMed] [Google Scholar]
- 102.Jones SF, Burris HA, III, Dumez H, et al. Phase I accelerated dose-escalation, pharmacokinetic and pharmacodynamic study of PF-03814735, an oral aurora kinase inhibitor, in patients with advance solid tumors: preliminary results. J Clin Oncol. 2008;26(15s suppl) abstr 2517. [Google Scholar]
- 103.Bebbington D, Binch H, Charrier J-D, et al. The discovery of the potent aurora inhibitor MK-0457 (VX-680) Bioorg Med Chem Lett. 2009;19:3586–92. doi: 10.1016/j.bmcl.2009.04.136. [DOI] [PubMed] [Google Scholar]
- 104.Lin YG, Immaneni A, Merritt WM, et al. Targeting aurora kinase with MK-0457 inhibits ovarian cancer growth. Clin Cancer Res. 2008;14(17):5437–446. doi: 10.1158/1078-0432.CCR-07-4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li Y, Zhang Z-F, Chen J, et al. VX680/MK-0457, a potent and selective aurora kinase inhibitor, targets both tumor and endothelial cells in clear cell renal cell carcinoma. Am J Transl Res. 2010;2(3):296–308. [PMC free article] [PubMed] [Google Scholar]
- 106.Arlot-Bonnemains Y, Baldini E, Martin B, et al. Effects of the aurora kinase inhibitor VX-680 on anaplastic thyroid cancer-derived cell lines. Endocrine Related Cancer. 2008;15:559–68. doi: 10.1677/ERC-08-0021. [DOI] [PubMed] [Google Scholar]
- 107.Pan C, Yan M, Yao J, et al. Aurora kinase small molecule inhibitor destroys mitotic spindle, suppresses cell growth, and induces apoptosis in oral squamous cancer cells. Oral Oncology. 2008;44:639–45. doi: 10.1016/j.oraloncology.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 108.Cheetham GMT, Charlton PA, Golec JMC, Pollard JR. Structural basis for potent inhibition of the aurora kinases and a T315I multi-drug resistant mutant form of Abl kinase by VX-680. Cancer Lett. 2007;251:323–9. doi: 10.1016/j.canlet.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 109.Donato NJ, Fang D, Sun H, et al. Targets and effectors of the cellular response to aurora kinase inhibitor MK-0457 (VX-680) in imatinib sensitive and resistant chronic myelogenous leukemia. Biochem Pharmacol. 2010;79:688–97. doi: 10.1016/j.bcp.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 110.Shah NP, Skaggs B, Branford S, et al. The most common dasatinib-resistant BCR-ABL kinase domain mutations in patients with chronic myeloid leukemia are sensitive to VX-680: rationale for early combination kinase inhibitor therapy. Blood (ASH Annual Meeting Abstracts) 2006;108 abstr 2175. [Google Scholar]
- 111.Huang X-F, Luo S-K, Xu J, et al. Aurora kinase inhibitory VX-680 increases Bax/Bcl-2 ratio and induces apoptosis in aurora-A-high acute myeloid leukemia. Blood. 2008;111:2854–65. doi: 10.1182/blood-2007-07-099325. [DOI] [PubMed] [Google Scholar]
- 112.Hose D, Reme T, Meissner T, et al. Inhibition of aurora kinases for tailored risk-adapted treatment of multiple myeloma. Blood. 2009;113:4331–40. doi: 10.1182/blood-2008-09-178350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fiskus W, Wang Y, Joshi R, et al. Cotreatment with vorinostat enhances activity of MK-0457 (VX-680) against acute and chronic myelogenous leukemia cells. Clin Cancer Res. 2008;14(19):6106–15. doi: 10.1158/1078-0432.CCR-08-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Harrington EA, Bebbington D, Moore J, et al. VX-680, a potent and selective small-molecule inhibitor of the aurora kinases, suppresses tumor growth in vivo. Nat Med. 2004;10(3):262–7. doi: 10.1038/nm1003. [DOI] [PubMed] [Google Scholar]
- 115.Dai Y, Chen S, Venditti CA, et al. Vorinostat synergistically potentiates MK-0457 lethality in chronic myelogenous leukemia cells sensitive and resistant to imatinib mesylate. Blood. 2008;112:793–804. doi: 10.1182/blood-2007-10-116376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Okabe S, Tauchi T, Ohyashiki K. Efficacy of MK-0457 and in combination with vorinostat against Philadelphia chromosome positive acute lymphoblastic leukemia cells. Ann Hematol. 2010;89:1081–7. doi: 10.1007/s00277-010-0998-x. [DOI] [PubMed] [Google Scholar]
- 117.Samuel TA, Fiskus W, Wang Y, et al. Novel aurora kinases-targeted combination therapy for breast cancers. J Clin Oncol. 2008;26(15s suppl) abstr 14569. [Google Scholar]
- 118.Hoover RR, Harding MW. Synergistic activity of the aurora kinase inhibitor MK-0457 (VX-680) with idarubicin, Ara-C, and inhibitors of BCR-Abl. Blood (ASH Annual Meeting Abstracts) 2006;108 abstr 1384. [Google Scholar]
- 119.Hoover RR, Harding MW. Activity of the aurora kinase inhibitor MK-0457 (VX-680) in combination with taxotere. J Clin Oncol. 2007;25(18s suppl) abstr 14069. [Google Scholar]
- 120.Hoover RR, Furey B, Pollard J, Harding M. Synergistic activity of the aurora kinase inhibitor MK-0457 (VX-680) with erlotinib. Proc Am Assoc Cancer Res. 2008;49 abstr 4016. [Google Scholar]
- 121.Tibes R, Giles F, McQueen T, et al. Translational in vivo and in vitro studies in patients with acute myeloid leukemia, chronic myeloid leukemia and myeloproliferative disease treated with MK-0457, a novel aurora kinase, FLT3, JAK2, and BCR-Abl inhibitor. Blood (ASH Annual Meeting Abstracts) 2006;108 abstr 1362. [Google Scholar]
- 122.Giles F, Cortes J, Bergstrom DA, et al. MK-0457, a novel multikinase inhibitor, is active in patients with chronic myeloid leukemia and acute lymphocytic leukemia with the T315I BCR-Abl resistance mutation and patients with refractory JAK2-positive myeloproliferative diseases. Blood (ASH Annual Meeting Abstracts) 2006;108 abstr 253. [Google Scholar]
- 123•.Papayannidis C, Iacobucci I, Soverini S, et al. Innovative phase I study of concomitant and consecutive treatment with dasatinib and MK-0457 in refractory Ph+ CML and ALL patients. J Clin Oncol. 2009;27(15s suppl) abstr 7080. Trial demonstrating effect of predominant BCR-Abl inhibitor and multitargeted kinase inhibitor when used in sequence. [Google Scholar]
- 124.Fancelli D, Moll J, Varasi M, et al. 1,4,5,6-tetrahydropyrrolo[3,4-c]pyrazoles: identification of a potent aurora kinase inhibitor with favorable antitumor kinase inhibition profile. J Med Chem. 2006;49:7247–51. doi: 10.1021/jm060897w. [DOI] [PubMed] [Google Scholar]
- 125.Carpinelli P, Ceruti R, Giorgini ML, et al. PHA-739358, a potent inhibitor of aurora kinases with a selective target inhibition profile relevant to cancer. Mol Cancer Ther. 2007;6(12):3158–68. doi: 10.1158/1535-7163.MCT-07-0444. [DOI] [PubMed] [Google Scholar]
- 126.Benten D, Keller G, Quaas A, et al. Aurora kinase inhibitor PHA-739358 suppresses growth of hepatocellular carcinoma in vitro and in a xenograft mouse model. Neoplasia. 2009;11(9):934–44. doi: 10.1593/neo.09664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gontarewicz A, Balabanov S, Keller G, et al. Simultaneous targeting of aurora kinases and BCR-Abl kinase by the small molecule inhibitor PHA-739358 is effective against imatinib-resistant BCR-Abl mutations including T315I. Blood. 2008;111:4355–64. doi: 10.1182/blood-2007-09-113175. [DOI] [PubMed] [Google Scholar]
- 128.Steeghs N, Eskens F, Gelderblom H, et al. Phase I pharmacokinetic and pharmacodynamic study of the aurora kinase inhibitor danusertib in patients with advanced or metastatic solid tumors. J Clin Oncol. 2009;27:5094–101. doi: 10.1200/JCO.2008.21.6655. [DOI] [PubMed] [Google Scholar]
- 129.Cohen RB, Jones SF, Aggarwal C, et al. A phase I dose-escalation study of danusertib (PHA-739358) administered as a 24-hour infusion with and without granulocyte-stimulating factor in a 14-day cycle in patients with advanced solid tumors. Clin Cancer Res. 2009;15(21):6694–701. doi: 10.1158/1078-0432.CCR-09-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cortes-Franco J, Dombret H, Schafhausen P, et al. Danusertib hydrochloride (PHA-739358), a multi-kinase aurora inhibitor, elicits clinical benefit in advanced chronic myeloid leukemia and Philadelphia chromosome positive acute lymphoblastic leukemia. Blood (ASH Annual Meeting Abstracts) 2009;114 abstr 864. [Google Scholar]
- 131.Hajduch M, Vydra D, Dzubak P, et al. In vivo mode of action of CYC116, a novel small molecule inhibitor of aurora kinases and VEGFR2. Proc Am Assoc Cancer Res. 2008;49 abstr 5645. [Google Scholar]
- 132.Griffiths G, Scaerou F, Midgley C, et al. Anti-tumor activity of CYC116, a novel small molecule inhibitor of aurora kinases and VEGFR2. Proc Am Assoc Cancer Res. 2008;49 abstr 5644. [Google Scholar]
- 133.Maccallum D, Melville J, Campbell K, Green S. Combination studies with the oral aurora kinase inhibitor CYC116 and chemotherapeutic agents. Proc Am Assoc Cancer Res. 2008;49 abstr 4015. [Google Scholar]
- 134•.Leteur C, Tao Y, Deutsch E, Bourhis J. Enhancement of radiation response by the aurora kinase inhibitor CYC116 in Ras mutant and p53-deficienct cancer cells. Proc Am Assoc Cancer Res. 2009;50 abstr 2481. Combination of pan-AKI with ionizing radiation demonstrated synergistic effect and different effect in colorectal tissue cultures depending upon status of Ras. [Google Scholar]
- 135.Oslob JD, Romanowski MJ, Allen DA, et al. Discovery of a potent and selective aurora kinase inhibitor. Bioorg Med Chem Lett. 2008;18:4880–4. doi: 10.1016/j.bmcl.2008.07.073. [DOI] [PubMed] [Google Scholar]
- 136.Arbitrario JP, Belmont BJ, Evanchik MJ, et al. SNS-314, a pan-aurora kinase inhibitor, shows potent anti-tumor activity and dosing flexibility in vivo. Cancer Chemother Pharmacol. 2010;65:707–17. doi: 10.1007/s00280-009-1076-8. [DOI] [PubMed] [Google Scholar]
- 137••.VanderPorten EC, Taverna P, Hogan JN, et al. The aurora kinase inhibitor SNS-314 shows broad therapeutic potential with chemotherapeutics and synergy with microtubule-targeted agents in a colon carcinoma model. Mol Cancer Ther. 2009;8(4):930–9. doi: 10.1158/1535-7163.MCT-08-0754. Innovative preclinical model of sequencing AKI with chemotherapy agents using differential qualitative doses of the AKI and chemotherapy agents. [DOI] [PubMed] [Google Scholar]
- 138.Robert F, Verschraegen C, Hurwitz H, et al. A phase I trial of SNS-314, a novel and selective pan-aurora kinase inhibitor, in advanced solid tumor patients. J Clin Oncol. 2009;27(15s suppl) abstr 2536. [Google Scholar]
- 139.Payton M, Bush TL, Chung G, et al. Preclinical evaluation of AMG 900, a novel potent and highly selective pan-aurora kinase inhibitor with activity in taxane-resistant tumor cell lines. Cancer Res. 2010;70(23):9846–54. doi: 10.1158/0008-5472.CAN-10-3001. [DOI] [PubMed] [Google Scholar]
- 140.Akahane D, Tauchi T, Okabe S, et al. Activity of a novel aurora kinase inhibitor against the T315I mutant form of BCR-Abl: in vitro and in vivo studies. Cancer Sci. 2008;99:1251–7. doi: 10.1111/j.1349-7006.2008.00810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Evans R, Naber C, Steffler R, et al. Aurora A kinase RNAi and small molecule inhibition of aurora kinases with VE-465 induce apoptotic death in multiple myeloma cells. Leuk Lymphoma. 2008;49(3):559–69. doi: 10.1080/10428190701824544. [DOI] [PubMed] [Google Scholar]
- 142.Lin Z-Z, Hsu H-C, Hsu C-H, et al. The aurora kinase inhibitor VE-465 has anticancer effects in preclinical studies of human hepatocellular carcinoma. J Hepatol. 2009;50:518–27. doi: 10.1016/j.jhep.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 143.Scharer CD, Laycock N, Osunkoya AO, et al. Aurora kinase inhibitors synergize with paclitaxel to induce apoptosis in ovarian cancer cells. J Transl Med. 2008;6:79–91. doi: 10.1186/1479-5876-6-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ohmine K, Nagai T, Yoshida K, et al. Vincristine potentiates the anti-leukemia effect of the aurora kinase inhibitor VE-465 by enhancing apoptosis in myeloid leukemia cells. Blood (ASH Annual Meeting Abstracts) 2009;114 abstr 2762. [Google Scholar]
- 145.McLaughlin J, Markovtsov V, Li H, et al. Preclinical characterization of the aurora kinase inhibitor R763/AS703569 identified through image-based phenotypic screen. J Cancer Res Clin Oncol. 2010;136:99–113. doi: 10.1007/s00432-009-0641-1. [DOI] [PubMed] [Google Scholar]
- 146.Sarno S, Shaw J, Spooner E, et al. The novel aurora kinase inhibitor AS703569 shows potent anti-tumor activity in acute myeloid leukemia. Blood (ASH Annual Meeting Abstracts) 2007;110 abstr 915. [Google Scholar]
- 147.Delmore J, Cervi DN, McMillin D, et al. The transcriptional signature of kinases inhibited by the multi-targeted kinase inhibitor AS703569 is associated with clinical outcome in multiple myeloma (MM): anti-MM activity of AS703569 in preclinical studies. Blood (ASH Annual Meeting Abstracts) 2009;114 abstr 730. [Google Scholar]
- 148.Renshaw JS, Patnaik A, Gordon M, et al. A phase I two arm trial of AS703569 (R763), an orally available aurora kinase inhibitor, in subjects with solid tumors: preliminary results. J Clin Oncol. 2007;25(18s suppl) abstr 14130. [Google Scholar]
- 149.Sonet A, Graux C, Maertens J, et al. Phase I, dose-escalation study of 2 dosing regimens of AS703569, an inhibitor of aurora and other kinases, administered orally in patients with advanced hematological malignancies. Blood (ASH Annual Meeting Abstracts) 2008;112 abstr 2963. [Google Scholar]
- 150.Mahadevan D, Qi W, Cooke L, et al. Targeting aurora kinase in aggressive B-cell non-Hodgkin’s lymphomas. Blood (ASH Annual Meeting Abstracts) 2009;114 abstr 284. [Google Scholar]
- 151.den Hollander J, Rimpi S, Doherty JR, et al. Aurora kinases A and B are up-regulated by Myc and are essential for maintenance of the malignant state. Blood. 2010;116(9):1498–505. doi: 10.1182/blood-2009-11-251074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yang D, Liu H, Goga A, et al. Therapeutic potential of a synthetic lethal interaction between the MYC proto-oncogene and inhibition of aurora-B kinase. Proc Natl Acad Sci U S A. 2010;107(31):13836–41. doi: 10.1073/pnas.1008366107. [DOI] [PMC free article] [PubMed] [Google Scholar]