Abstract
Oligosaccharide (OS)-protein conjugates are promising candidate vaccinesagainst encapsulated bacteria, such as Haemophilus influenzae, Neisseria meningitidis, and Streptococcus pneumoniae. Although the effects of several variables such as OS chain length and protein carrier have been studied, little is known about the influence of adjuvants on the immunogenicity of OS-protein conjugates. In this study, a minimal protective trisaccharide epitope of Streptococcus pneumoniae type 3 conjugated to the cross-reacting material of diphtheria toxin was used for immunization of BALB/c mice in the presence of different adjuvants. Subsequently, half of the mice received a booster immunization with conjugate alone. Independent of the use and type of adjuvant, all mice produced long-lasting anti-polysaccharide type 3 (PS3) antibody levels, which provided full protection against challenge with pneumococcal type 3 bacteria. All adjuvants tested increased the anti-PS3 antibody levels and opsonic capacities as measured by an enzyme-linked immunosorbent assay and an in vitro phagocytosis assay. The use of QuilA or a combination of the adjuvants CpG and dimethyl dioctadecyl ammonium bromide resulted in the highest phagocytic capacities and the highest levels of Th1-related immunoglobulin G (IgG) subclasses. Phagocytic capacity correlated strongly with Th1-associated IgG2a and IgG2b levels, to a lesser extent with Th2-associated IgG1 levels, and weakly with thiocyanate elution as a measure of avidity. Thus, the improved immunogenicity of OS-protein conjugates was most pronounced for Th1-directing adjuvants.
Vaccination with polysaccharide-protein conjugates effectively reduces mortality and morbidity due to infections with encapsulated bacteria, such as Streptococcus pneumoniae (6). In the murine model, addition of adjuvants such as QuilA (2) and monophosphoryl lipid A (MPL) (24) to experimental polysaccharide-protein conjugates enhances the polysaccharide-specific immune response. Adjuvants in combination vaccines can be used to reduce the immunization dose and number of injections, thereby decreasing undesired side effects (8). Adjuvants potentiate or modulate the immune response of a particular antigen by creating a depot effect, targeting immune cells, or increasing the production of certain cytokines (19, 12). Adjuvants can induce changes in the Th1-Th2 balance and thus in the antibody subclass generated. In mice, immunoglobulin G1 (IgG1) is associated with a Th2-like response, while a Th1 response is associated with the induction of IgG2a, IgG2b, and IgG3 antibodies (10). Each IgG subclass can contribute to the clearance of encapsulated bacteria by different mechanisms. IgG2a and IgG2b exhibit strongest binding to Fc receptors (22) and together with IgG3 can fix complement better than IgG1 can (20); IgG3 can cooperatively bind to bacteria (11) and provides protection (7), as does IgG1 (18). Therefore, an immune response with a broad subclass distribution may be beneficial against encapsulated bacteria.
Recently, it has been shown that oligosaccharide (OS)-protein conjugates containing short-chain synthetic carbohydrates related to S. pneumoniae types 3 (5) and 6 (16), without the use of adjuvants, can induce full protection in mice against challenge with homologous bacteria. In the present study, we investigated whether addition of different adjuvants would allow for a lower vaccination dose and lead to antibodies with broader subclass distributions and increased functional activity. To this end, mice were immunized once with a low dose (0.5 μg of saccharide) of the cross-reacting material-trisaccharide (CRM-Tri) conjugate in the presence of different adjuvants known to stimulate the immune response in either the Th1 or the Th2 direction (see Table 1) and were then given booster immunizations with conjugate alone. A CRM-Tri conjugate consisting of the minimal carbohydrate epitope that afforded full protection against S. pneumoniae type 3, i.e., the synthetic trisaccharide β-d-Glcp-(1→3)-β-d-GlcpA-(1→4)-β-d-Glcpcoupled via a spacer to the CRM of diphtheria toxin (CRM197) was used.
TABLE 1.
Immunization doses and Th1 versus Th2 profiles of the adjuvants used
All vaccinated mice were protected against challenge with pneumococcal type 3 bacteria (S3). However, sera with highest phagocytic capacities were generated by adjuvants inducing the most pronounced Th1 profile, i.e., QuilA or a combination of dimethyl dioctadecyl ammonium bromide (DDA) and CpG. Thus, Th1-directing adjuvants were most potent in improving the immunogenicity of low-dose S3 OS-protein vaccines and are to be considered for future conjugate vaccine formulations.
MATERIALS AND METHODS
Adjuvants and conjugates.
The oligodeoxynucleotides ODN1826 (CpG, TCCATGACGTTCCTGACGTT), containing CpG motifs, and ODN1982 (non-CpG, TCCAGGACTTCTCTCAGGTT) were from Eurogentec (Seraing, Belgium), MPL was from Ribi ImmunoChem Research (Hamilton, Mont.), QuilA was from Superfos Biosector (Vedaek, Denmark), aluminum hydroxide [Al(OH)3] was from the Dutch National Health Institute (Bilthoven, The Netherlands), and DDA was purchased from Eastman Kodak (Rochester, N.Y.) (Table 1). CRM-polysaccharide conjugates, designated CRM-PS3, CRM-PS6A, and CRM-PS6B, were obtained from Wyeth-Lederle Vaccines (West Henrietta, N.Y.).
Immunization studies.
For the immunization studies, inbred female BALB/c mice of 8 to 10 weeks of age were obtained from the Animal Laboratory of Utrecht University. The Ethics Committee on Animal Experimentation of the University Medical Center (Utrecht, The Netherlands) approved the animal experiments described in this article. The CRM-Tri conjugate (17) consists of the synthetic trisaccharide β-d-Glcp-(1→3)-β-d-GlcpA-(1→4)-β-d-Glcp representing part of the capsule polysaccharide of S. pneumoniae type 3 coupled via a 3-aminopropyl spacer to CRM197. Eight mice per group were immunized subcutaneously with CRM-Tri at four sites (0.5 μg of saccharide per mouse) in combination with different adjuvants (see Fig. 1 and 2). In each group, four mice received a second immunization with CRM-Tri (0.5 μg of saccharide per mouse) without adjuvant after 5 weeks. Control mice were injected with either CRM-PS6A, CRM-PS6B, or phosphate-buffered saline (PBS) and received booster injections with the homologous conjugate or PBS. Blood samples were taken at weeks 5, 7, and 66 for the groups receiving booster injections and at weeks 5, 10, and 32 after primary immunization for the groups not receiving booster injections. Mice were challenged intraperitoneally 2 weeks after the last blood sampling with a 20× lethal dose (400 CFU) of S. pneumoniae type 3 (ATTC 6303; American Type Culture Collection, Rockville, Md.) or S. pneumoniae type 6A or 6B as a control. Survival of mice was recorded daily for 15 days.
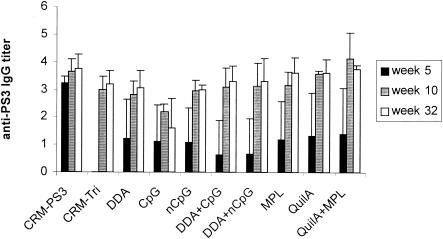
FIG. 1.
PS3-specific IgG titers (mean ± standard deviation [SD] [n = 4]) in mice receiving a single injection of CRM-Tri (0.5 μg) combined with the indicated adjuvants. nCpG, non-CpG.
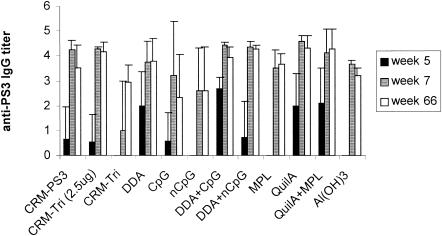
FIG. 2.
PS3-specific IgG titers (mean ± SD [n = 4]) in mice primed with CRM-Tri (0.5 μg) combined with the indicated adjuvants and given booster immunizations (week 7) with CRM-Tri (0.5 μg) alone. nCpG, non-CpG.
Measurement of PS3-specific antibodies by ELISA.
Levels of antibodies binding to polysaccharide type 3 (PS3) were measured by enzyme-linked immunosorbent assay (ELISA) as described previously (1). Maxisorb 96-well plates (Nunc Laboratories, Roskilde, Denmark) were coated with PS3 (1 μg/ml in PBS) by drying overnight at 37°C. After 1 h of blocking with 3% gelatin in PBS, serum dilutions in 0.05% Tween 20-3% Protifar (Nutricia, Zoetermeer, The Netherlands) in PBS were transferred to the coated plates and incubated for 1 h at 37°C. Horseradish peroxidase-conjugated goat anti-mouse IgM and IgG subclasses (Nordic Immunological Laboratories, Tilburg, The Netherlands) diluted 1:4,000 in the same diluent were incubated for 1 h at 37°C. The amount of bound peroxidase was visualized by incubation with 3,3′,5,5′-tetramethylbenzidine (Sigma Chemical Co., St. Louis, Mo.). After 5 to 15 min, the reaction was stopped with 0.1 M H2SO4 and A450 was measured with a microplate reader (model 3550; Bio-Rad). Antibody titers were defined as the log10 of the dilution giving twice the absorbance value for control mice (immunized with the buffer). If twice the absorbance value for control mice surpassed 0.2, the analysis was repeated.
Measurement of avidity.
Avidities of the sera were measured by a thiocyanate elution ELISA as described before (21). Briefly, after blocking, the PS3-coated plates were incubated with serum at a concentration corresponding to an A450 of about 1.0. Then the plates were washed and incubated with sodium isothiocyanate at a range of concentrations (0 to 1.0 M) in PBS for 15 min at 37°C. After the plates were washed, the amount of antibodies left was detected as described above. The concentration of sodium isothiocyanate needed to reduce the A450 by 50% was taken as the avidity index (AI). Results from experiments in which A450 did not drop below 50% or stayed below 0.4 in the absence of sodium isothiocyanate were excluded.
Measurement of phagocytosis titers.
The opsonic activity of the mouse antisera was determined as described previously (2). The mouse macrophage cell line J774 was cultured in Iscove’s modified Dulbecco’s medium supplemented with 5% fetal calf serum. Before use, cells were washed twice with 1% bovine serum albumin-Hanks balanced salt solution (BSA-HBSS) and homogenized. Dilutions of individual mouse sera in cold 1% BSA-HBSS were made in round-bottomed microtiter plates, and samples of 2.5 × 106 fluorescein isothiocyanate-labeled bacteria were added to each well. Opsonization was allowed to proceed in a volume of 50 μl at 37°C with shaking. After incubation for 30 min, the plates were placed on ice and 2.5 × 105 J774 cells in 50 μl of cold 1% BSA-HBSS were added to each well. After 30 min of shaking at 37°C, plates were placed on ice. Cells were spun down and washed twice with ice-cold 1% BSA-HBSS and resuspended in 150 μl of 1% BSA-HBSS. PBS-2% paraformaldehyde (100 μl) was added to each well, and the suspensions were transferred to tubes for analysis in a FACScan flow cytometer (Becton Dickinson, Mountain View, Calif.). Phagocytosis titers are expressed as the log10 of the serum dilution during opsonization resulting in 25% of J774 cells' being positive for fluorescein isothiocyanate.
Statistical methods.
Statistical significance of differences in antibody levels was analyzed with a two-tailed Student t test. Correlations between parameters were assayed using Pearson's correlation analysis and multiple linear regression by using the statistics program SPSS (n = 40). In all analyses, the log10 of concentrations was used.
RESULTS
PS3-specific antibody levels in mice receiving booster immunizations and in those not receiving booster immunizations.
Groups of four mice were immunized subcutaneously with the CRM-Tri conjugate (0.5 μg of saccharide per mouse) in the absence or presence of adjuvants. As positive controls, mice were immunized with the CRM-PS3 and CRM-Tri conjugates at the dose used in previous experiments (2.5 μg) (5), and CRM-PS6A-, CRM-PS6B-, or PBS-immunized mice were used as negative controls.
All the groups receiving a single immunization with the CRM-Tri conjugate in the presence of different adjuvants produced similar anti-PS3 antibody levels after 32 weeks. The main effect of the different adjuvants occurred in the earlier phase of the immune response: at week 5, only groups receiving adjuvants produced detectable anti-PS3 antibody levels (Fig. 1). In the groups receiving booster immunizations, similar effects were observed. At week 5, anti-PS3 antibody levels were produced only in the presence of adjuvants, whereas at week 66, long-lasting antibody levels were detected in all groups (Fig. 2). The booster injection with the CRM-Tri conjugate increased total IgG antibody titers in most groups. Negative controls showed no detectable PS3-specific antibody levels.
Antibody avidities of sera from mice receiving booster injections.
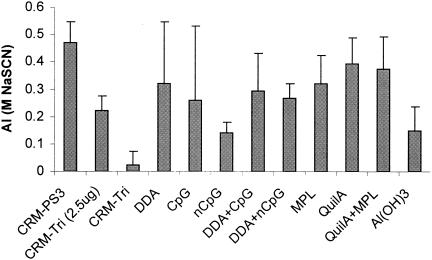
As a measure of the anti-PS3 antibody avidities of the sera obtained 2 weeks after the booster immunizations, the thiocyanate elution ELISA was used (Fig. 3).
FIG. 3.
AIs (mean ± SD [n = 4]) of sera obtained 2 weeks after the booster immunizations with CRM-Tri (0.5 μg). Data are expressed as molar concentrations of sodium isothiocyanate (NaSCN) needed for elution of 50% of bound antibodies. Individual sera were analyzed five times. nCpG, non-CpG.
Anti-PS3 antibody avidities of CRM-PS3 (P < 0.001) and QuilA, either alone (P < 0.02) or in combination with MPL (P < 0.05), were significantly higher than the avidities detected in the groups immunized with CRM-Tri without adjuvants. In general, all groups immunized with adjuvant showed significantly higher avidities as measured by this method (P < 0.05) than those immunized with CRM-Tri (0.5 μg) alone, except for the groups immunized with CpG or DDA.
PS3-specific IgG subclass distributions in sera from mice receiving booster immunizations.
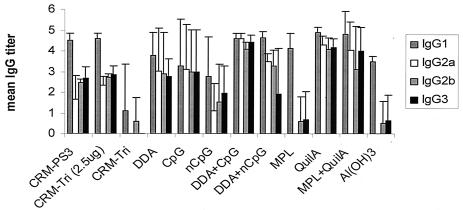
As measured by ELISA 2 weeks after the booster immunizations, the adjuvants DDA-CpG and QuilA induced a significant increase (P < 0.01) in the levels of Th1-related PS3-specific subclasses IgG2a, IgG2b, and IgG3 compared to the levels in groups immunized with CRM-Tri and CRM-PS3 without adjuvants (Fig. 4). In contrast, in all groups of mice receiving only one immunization, similar Th2-like PS3-specific IgG subclass distributions were found irrespective of the use of adjuvant (data not shown).
FIG. 4.
PS3-specific IgG subclass distributions in mouse sera obtained 2 weeks after the booster immunizations with CRM-Tri (0.5 μg).
Phagocytic capacities of sera from mice receiving booster immunizations and mouse survival after an S3 pneumococcal challenge.
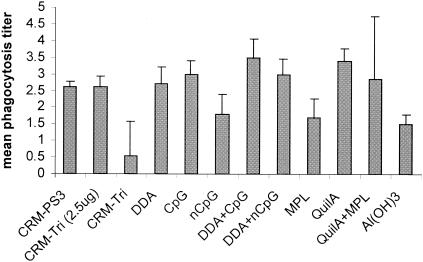
To analyze the phagocytic capacities of the sera from groups receiving different adjuvants, individual mouse sera obtained 2 weeks after the booster immunizations (week 7) were tested in a phagocytosis assay using type 3 pneumococci (Fig. 5).
FIG. 5.
Average phagocytosis titers (mean ± SD [n = 4]) in mouse sera obtained 2 weeks after the booster immunizations with CRM-Tri (0.5 μg). nCpG, non-CpG.
The groups receiving adjuvants DDA-CpG and QuilA showed increased phagocytosis titers (P < 0.05) compared to the groups immunized with CRM-Tri and CRM-PS3 without adjuvants.
Two weeks after the last blood sampling, all mice were challenged with an otherwise lethal dose of S. pneumoniae type 3. All negative controls (n = 16) died within 2 to 4 days, whereas 98% (n = 82) of the mice with detectable PS3-specific antibodies (84 of 87 mice) survived the challenge. Mice immunized with control conjugates CRM-PS6A or CRM-PS6B survived challenge with the homologous bacteria S6A or S6B but died from challenge with S3.
Correlations of phagocytic capacity, PS3-specific antibody titer, and average avidity.
To determine the predictive value of both avidity and PS3-specific IgG subclass distribution for the phagocytic capacities of the sera, a Pearson correlation analysis was performed (Table 2). Both total IgG levels and subclass titers showed strong correlation with phagocytic capacity. Among levels of all subclasses, Th1-related IgG2a and IgG2b levels showed the strongest correlation with phagocytosis, whereas the AI as measured by the thiocyanate elution ELISA showed only weak correlation.
TABLE 2.
Correlation (R2) of phagocytosis titers and AIs with total IgG levels and individual IgG subclasses
| Parameter | Correlation with phagocytosis titer | Correlation with AI |
|---|---|---|
| AI | 0.18a | 1.0 |
| Total IgG | 0.67a | 0.34b |
| IgG1 | 0.53a | 0.50a |
| IgG2a | 0.72a | 0.39b |
| IgG2b | 0.71a | 0.37b |
| IgG3 | 0.58a | 0.32 |
P < 0.01.
P < 0.05.
To determine the individual contribution of both avidity and IgG subclass levels to the phagocytic capacities of the sera, the individual parameters were analyzed in a multiple linear regression model. Combination of Th2-related IgG1 levels with either IgG2a or IgG2b levels (R2 of 0.82 or 0.81, respectively) gave the highest predictive value (R2) for phagocytosis. Addition of avidity to the individual subclass levels only marginally increased R2, however; only for IgG3 was a significant contribution observed.
DISCUSSION
In general, development of different bacterial vaccines follows similar tracks, starting with large, rather crude vaccines and progressing towards smaller, better-defined subunit vaccines. Ultimately, this track leads to the development and evaluation of minimal, highly defined subunit vaccines based on a collection of single protective epitopes. Our group aims at the identification of minimal protective epitopes in pneumococcal conjugate vaccines. In this study, we further explored the minimal requirements for protective immunity by testing the influence of adjuvants on the magnitude, time course, IgG subclass distribution, and functional activity of the antibody response upon vaccination with a low dose of an experimental pneumococcal conjugate vaccine. To this end, mice were immunized with an S3-CRM vaccine (0.5 μg of saccharide) comprised of the minimal epitope, and adjuvants were used only in the first immunization. All vaccine formulations provided protective immunity to mice. However, Th1-directing adjuvants, especially CpG-DDA and QuilA, most potently stimulated the generation of an anti-S3 immune response. This was evident from the induction of a broad anti-PS3 IgG subclass distribution and high phagocytic capacity.
All adjuvants potentiated the antibody response in the early phase after immunization. However, no differences in PS3-specific antibody titers were found after a prolonged period of time, and thus, long-term effects of adjuvants, such as slow release of antigens (depot effect), were not apparent and were not needed for the induction of long-lasting antibody levels.
Except for MPL, the adjuvants used in this study behaved according to their previously reported Th1-Th2 profiles as measured by the IgG subclasses produced. In contrast to the reported Th1 effect of MPL on polysaccharide-protein conjugates (24), we found increased antibody titers associated with a Th2 profile when MPL in saline was used.
Since the 50% lethal dose of serotype 3 pneumococci in mice and corresponding protective antibody cutoff levels are rather low, the level of S3 challenge could not be used to discriminate between the protective capacities of the different vaccine formulations. Therefore, we chose in vitro phagocytic capacities of antisera as the ultimate in vitro correlate of protection against pneumococcal infections (3). The increased phagocytosis titers observed when the adjuvants CpG and DDA or QuilA were used strongly suggest an improved protective potential of these vaccine formulations compared to that of the formulations used for the control groups. The contribution to phagocytosis of antibody isotypes and subclass levels as well as antibody avidity was investigated. From our results, it is clear that a broad subclass distribution causes highest phagocytosis titers. IgG2a and IgG2b levels correlated best with opsonophagocytosis. Multiple linear regression analysis showed that addition of IgG1 to either IgG2a or IgG2b significantly increased the correlation between antibody levels and phagocytosis levels. Apparently, both types of subclasses are important in phagocytosis and thus protection against S. pneumoniae. Conflicting results have appeared on the role of antibody avidity in host defense. Anttila et al. (3) found no correlation between avidity and opsonophagocytosis in sera from polysaccharide-protein-immunized humans. However, Schlesinger et al. (23) reported a positive correlation between avidity and serum bactericidal activity in infant sera. Our results from the thiocyanate elution ELISA, which gives an indication for affinity, suggest a minor role for antibody avidity in phagocytosis of and host protection against S. pneumoniae as measured by this assay.
Comparison of mice receiving booster immunizations with those not receiving booster immunizations showed a remarkable difference in Th1-Th2 balances. After a booster immunization with conjugate only, a clear shift towards Th1 was visible. However, after a single injection in the presence of adjuvants, a predominant Th2 profile was detected. Since the booster immunization did not contain adjuvants, the immunological environment for the response to the booster immunization must already have been established after the primary immunization. As no antibody-producing cells with Th1 specificity were present after priming, it is likely that Th1 memory cells or memory B cells predestined to produce Th1-related antibodies were produced. The immunological mechanism of this effect is not clear and requires further investigation.
In conclusion, all adjuvants used in this study were able to improve the immunogenicity, as measured by antibody avidity, subclass distribution, and phagocytic capacity, of a CRM-Tri conjugate. Importantly, the adjuvants that shifted the immune response in a Th1 direction, especially the combination of CpG and DDA or QuilA alone, produced the largest increase in immunogenicity of the CRM-Tri conjugate. Compared to the control group that did not receive adjuvants, mice receiving these adjuvants showed an earlier induction of PS3-specific antibodies with significantly higher avidities, Th1-related IgG subclass levels, and phagocytic capacities. Taken together with the strong correlation observed between the Th1-related IgG2a and IgG2b titers and phagocytosis, these data indicate that Th1-directing adjuvants are strong enhancers of immunogenicity of OS-protein conjugate vaccines and should be considered for inclusion in future vaccine formulations.
Acknowledgments
We thank David van de Vijver for help with statistical analyses and Dace Madore (Wyeth-Lederle Vaccines) for generously providing CRM-polysaccharide conjugates.
Editor: J. N. Weiser
REFERENCES
- 1.Alonso de Velasco, E., A. F. Verheul, A. M. van Steijn, H. A. Dekker, R. G. Feldman, I. M. Fernandez, J. P. Kamerling, J. F. G. Vliegenthart, J. Verhoef, and H. Snippe. 1994. Epitope specificity of rabbit immunoglobulin G (IgG) elicited by pneumococcal type 23F synthetic oligosaccharide- and native polysaccharide-protein conjugate vaccines: comparison with human anti-polysaccharide 23F IgG. Infect. Immun. 62:799-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alonso de Velasco, E., H. A. T. Dekker, P. Antal, K. P. Jalink, J. A. G. van Strijp, A. F. M. Verheul, J. Verhoef, and H. Snippe. 1994. The adjuvant Quil A improves protection in mice and enhances the opsonic capacity of antisera induced by pneumococcal polysaccharide conjugates. Vaccine 12:1419-1422. [DOI] [PubMed] [Google Scholar]
- 3.Anttila, M., J. Eskola, H. Ahman, and H. Kayhty. 1999. Contribution of serotype specific IgG concentration, IgG subclasses, and relative antibody avidity to opsonophagocytic activity against Streptococcus pneumoniae. Clin. Exp. Immunol. 118:402-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker, P. J., J. R. Hiernaux, M. B. Fauntleroy, P. W. Stashak, B. Prescott, J. L. Cantrell, and J. A. Rudbach. 1988. Ability of monophosphoryl lipid A to augment the antibody response of young mice. Infect. Immun. 56:3064-3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benaissa-Trouw, B., D. J. Lefeber, J. P. Kamerling, J. F. G. Vliegenthart, H. Snippe, and K. Kraaijeveld. 2001. Synthetic di-, tri-, and tetrasaccharide-CRM197 conjugates induce protection against Streptococcus pneumoniae type 3 in mice. Infect. Immun. 69:4698-4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Black, S., H. Shinefield, B. Fireman, E. Lewis, P. Ray, J. R. Hansen, L. Elvin, K. M. Ensor, J. Hackell, G. Siber, F. Malinoski, D. Madore, I. Chang, R. Kohberger, W. Watson, R. Austrian, K. Edwards, et al. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr. Infect. Dis. J. 19:187-195. [DOI] [PubMed] [Google Scholar]
- 7.Briles, D. E., J. L. Claflin, K. Schroeor, and C. Forman. 1981. Mouse IgG3 antibodies are highly protective against infection with Streptococcus pneumoniae. Nature 294:88-90. [DOI] [PubMed] [Google Scholar]
- 8.Dagan, R., J. Eskola, C. Leclerc, and O. Leroy. 1998. Reduced response to multiple vaccines sharing common protein epitopes that are administered simultaneously to infants. Infect. Immun. 66:2093-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis, H. L., R. Weeratna, T. J. Waldschmidt, L. Tygrett, J. Schorr, A. M. Krieg, and R. Weeranta. 1998. CpG DNA is a potent enhancer of specific immunity in mice immunized with recombinant hepatitis B surface antigen. J. Immunol. 160:870-876. [PubMed] [Google Scholar]
- 10.Germann, T., M. Bongartz, H. Dlugonska, H. Hess, E. Schmitt, L. Kolbe, E. Kölsch, F. J. Podlaski, M. K. Gately, and E. Rüde. 1995. Interleukin-12 profoundly up-regulates the synthesis of antigen-specific complement-fixing IgG2a, IgG2b and IgG3 antibody subclasses in vivo. Eur. J. Immunol. 25:823-829. [DOI] [PubMed] [Google Scholar]
- 11.Greenspan, N. S., and L. J. N. Cooper. 1992. Intermolecular cooperativity: a clue to why mice have IgG3? Immunol. Today 13:164-168. [DOI] [PubMed] [Google Scholar]
- 12.Gupta, R. K., and G. R. Siber. 1995. Adjuvants for human vaccines—current status, problems and future prospects. Vaccine 13:1263-1276. [DOI] [PubMed] [Google Scholar]
- 13.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740-745. [DOI] [PubMed] [Google Scholar]
- 14.Hilgers, L. A., and H. Snippe. 1992. DDA as an immunological adjuvant. Res. Immunol. 143:494-503. [DOI] [PubMed] [Google Scholar]
- 15.Jakob, T., P. S. Walker, A. M. Krieg, E. von Stebut, M. C. Udey, and J. C. Vogel. 1999. Bacterial DNA and CpG-containing oligodeoxynucleotides activate cutaneous dendritic cells and induce IL-12 production: implications for the augmentation of Th1 responses. Int. Arch. Allergy Immunol. 118:457-461. [DOI] [PubMed] [Google Scholar]
- 16.Jansen, W. T. M., S. Hogenboom, M. J. L. Thijssen, J. P. Kamerling, J. F. G. Vliegenthart, J. Verhoef, H. Snippe, and A. F. M. Verheul. 2001. Synthetic 6B di-, tri-, and tetrasaccharide-protein conjugates contain pneumococcal type 6A and 6B common and 6B-specific epitopes that elicit protective antibodies in mice. Infect. Immun. 69:787-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lefeber, D. J., J. P. Kamerling, and J. F. G. Vliegenthart. 2001. Synthesis of Streptococcus pneumoniae type 3 neoglycoproteins varying in oligosaccharide chain length, loading and carrier protein. Chemistry 7:4411-4421. [DOI] [PubMed] [Google Scholar]
- 18.McLay, J., E. Leonard, S. Petersen, D. Shapiro, N. S. Greenspan, and J. R. Schreiber. 2002. γ3 gene-disrupted mice selectively deficient in the dominant IgG subclass made to bacterial polysaccharides. II. Increased susceptibility to fatal pneumococcal sepsis due to absence of anti-polysaccharide IgG3 is corrected by induction of anti-polysaccharide IgG1. J. Immunol. 168:3437-3443. [DOI] [PubMed] [Google Scholar]
- 19.Moingeon, P., J. Haensler, and A. Lindeberg. 2001. Towards the rational design of Th1 adjuvants. Vaccine 19:4363-4372. [DOI] [PubMed] [Google Scholar]
- 20.Neuberger, M. S., and K. Rajewski. 1981. Activation of mouse complement by monoclonal mouse antibodies. Eur. J. Immunol. 11:1012-1016. [DOI] [PubMed] [Google Scholar]
- 21.Pullen, G. R., M. G. Fitzgerald, and C. S. Hosking. 1986. Antibody avidity determination by ELISA using thiocyanate elution. J. Immunol. Methods 86:83-87. [DOI] [PubMed] [Google Scholar]
- 22.Ravetch, J. V., and J.-P. Kinet. 1991. Fc receptors. Annu. Rev. Immunol. 9:457-492. [DOI] [PubMed] [Google Scholar]
- 23.Schlesinger, Y., D. M. Granoff, et al. 1992. Avidity and bactericidal activity of antibodies elicited by different Haemophilus influenzae type b conjugate vaccines. JAMA 267:1489-1494. [PubMed] [Google Scholar]
- 24.Schneerson, R., A. Fattom, S. C. Szu, D. Bryla, J. T. Ulrich, J. A. Rudbach, G. Schiffman, and J. B. Robbins. 1991. Evaluation of monophosphoryl lipid A (MPL) as an adjuvant. Enhancement of the serum antibody response in mice to polysaccharide-protein conjugates by concurrent injection with MPL. J. Immunol. 147:2136-2140. [PubMed] [Google Scholar]
- 25.Van de Wijgert, J. H., A. F. Verheul, H. Snippe, I. J. Check, and R. L. Hunter. 1991. Immunogenicity of Streptococcus pneumoniae type 14 capsular polysaccharide: influence of carriers and adjuvants on isotype distribution. Infect. Immun. 59:2750-2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wuorimaa, T., H. Kayhty, O. Leroy, and J. Eskola. 2001. Tolerability and immunogenicity of an 11-valent pneumococcal conjugate vaccine in adults. Vaccine 19:1863-1869. [DOI] [PubMed] [Google Scholar]