Abstract
Background:
Hair loss is seen as an irreversible process. Most research concentrates on how to elongate the anagen, reduce the negative factors of obstructing hair growth and improve the hair number and size.
Aim:
In our experiment, we tried to prove that the cow placenta extract can promote hair growth by elongating hair shaft and increasing hair follicle number.
Materials and Methods:
Cow placenta extract (CPE), water and minoxidil applied separately on the back of depilated B57CL/6 mice for the case, negative and positive control respectively. We checked the proliferation of cells which are resident in hair sheath, and the expression of a few growth factors which stimulate hair growth.
Results:
Result shows that placenta extract more efficiently accelerates cell division and growth factor expression, by raising the insulin-like growth factor (IGF-1) mRNA and protein level to increase HF size and hair length.
Conclusions:
The extract is not a purified product; so, it is less effective than minoxidil, which is approved by the US FDA for the treatment of male pattern baldness. If refinement is done, the placenta extract would be a good candidate medicine for hair loss.
Keywords: Cell proliferation, cow placenta extract, hair follicle, minoxidil
Introduction
The hair growth cycle in mammals is composed of three phases as follows. a) Anagen: Hair follicles (HFs) grow from the termination of the quiescent phase to the beginning of the regressing phase. During this phase, hair synthesis takes place and skin thickness increases. b) Catagen: This is the phase of apoptosis-driven regression. During this phase, follicles involute and skin thickness decreases. c) Telogen: During this phase, both follicles and skin are at rest. This cycle is regulated by a variety of mediators, including several members of the insulin-like growth factor family.[1–3]
Insulin-like growth factor (IGF-1) is a peptide hormone that promotes growth, survival, and differentiation of cells in various organs and tissues, including skin.[4] The functions of IGF-1 signaling in the skin is proved by the original studies with IGF-1 receptor null (Igf-1r-/-) mice, which have reduced number and size of the HFs. Also, deficiency in human growth hormone or its target IGF-1 is associated with decreased epidermal thickness and sparse hair growth.[5] IGF-1 has been reported to be exclusively expressed by mesenchymal cells of the dermis and the dermal papilla.[6] Another study reported that IGF-1 has a positive remarkable promotion on HF growth, and was potent in preventing HFs from entering into a catagen-like state.[7] In vitro, IGF-1 prevented HF entering into a catagen-like state.[7]
Placenta extracts have been used as Chinese folk medicines to accelerate wound healing. However, the molecular mechanism of placenta extracts on wound healing has not been identified.[6] It is known that placenta is an extremely rich reservoir of bioactive molecules.[8] Placenta extract contains abundant growth factors and hormones such as vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), transforming growth factor-β (TGF-β), IGF, adrenocorticotropic hormone (ACTH), etc.[6,9] Some gradients of curd protein extract, such as VEGF and IGF-1, can accelerate cell proliferation of HF and interfollicle cells.
Materials and Methods
Main experimental procedures
As there are many kinds of growth factors and hormones in placenta, crude extract of cow placenta was used in this experiment and Bradford method was used to detect the protein concentration. Then, the crude protein concentration was adjusted to 1 mg/ml. We applied the cow placenta extract (CPE) on the back of depilated B57CL/6 mice once every day. The 5-bromodeoxyuridine (BrdU) immunohistochemistry (IHC) was used for detecting cell proliferation. This method is proved efficient in identifying S-phase cells.[10] A certain time after BrdU injection, we checked the number of labeled cells, and calculated the number of labeled cells.
Hematoxylin and eosin (H and E) staining was used to observe the change, and the average diameter and number of HFs in certain area of each section were calculated. Then, we tried to find the reasons of hair promotion by placenta extract, and polymerase chain reaction (PCR) and western blot were performed to detecting the protein and gene of IGF-1.[11]
Sample treatment
The B57CL/6 mice were purchased from Danhan Biolink Inc. (South Korea) to be used in this experiment. They were 55 days of age and were divided into three groups: the first group (n = 9) was treated with minoxidil (Mandi minoxidil solution 2% hair regrowth treatment, produced by Wanma Group, China) as a topical application, which was 10 times diluted by distilled water; the, second (n = 9) group had topical CPE application and the last group (n = 9) received topical water application. Depilation was done with depilatory Bikiro cream (Tai Guk Pharm. Co. Ltd., South Korea), five times diluted in distilled water, at the first telogen. Each group was applied with its reagent once per day for 2 weeks, each time the amount of solution was 0.5 ml per mouse.
Two weeks later, we captured the picture of hair growth situation for each group, and 5 weeks later, samples were isolated from full-thickness back skin samples of mice, harvesting the same part on the back. The mice were sacrificed and the skin samples were fixed with 0.4% formalin and 80% ethanol dissolved in distilled water, at 4°C overnight, and then with 90% ethanol for 3 hours, and finally, the samples were marinated into 100% ethanol for 3 hours. They were put into half ethanol and half xylene (v/v), half xylene and half paraffin (v/v), paraffin in turns, with 6 hours for each step, and embedded in paraffin (Sigma Company, Korea).
Detection of the crude protein concentration
We used Bradford method to obtain the protein extract concentration.
Hematoxylin and eosin staining
The sections were washed with distilled water, dissolved with the hematoxylin solution (Sigma, USA), rinsed in distilled water, differentiated with 0.1% hydrochloric acid, rinsed in distilled water, dipped into saturated Li2 CO3 for 30 seconds, washed in running tap water, rinsed in 95% alcohol and counter-stained with eosin for 1 minute.
Incorporating 5-bromodeoxyuridine and immunohistochemistry
We injected the mice subcutaneously with BrdU (50 μg/g body weight) beginning at 24 hours before sacrificing.[12] IHC step is the same as mentioned above.
Polymerase chain reaction
Messenger ribonucleic acid (mRNA) extraction and PCR analysis of IGF-1 and Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were performed. We selected the skin samples as mentioned above, and the total mRNA was extracted. The homogenization procedure was performed using a modification of the trizol reagent method. Measured and adjusted samples′ mRNA concentration into same, and mRNA was transcribed to cDNA. Reverse transcription polymerase chain reaction was carried out. A 10-fold diluted solution of original cDNA was used for the transcription. PCR pre-mixed reaction solution was made in iNtRON (Seoul, Korea). Primers used were as follows.[3] For IGF-1, 5´- TCA ACA AGC CCA CAG GGT AT; 3´-ACT CGT GCA GAG CAA AGG AT;[13] denaturing of each cycle done at 94°C for 1 minute, annealing done at 60°C for 45 seconds, and extension at 72°C for 45 seconds. Products were analyzed using 8% agarose gel (Sigma, USA) electrophoresis.
Western blot
Western blot was performed to verify the IGF-1. Tissue samples were homogenized and lysed in protein extraction buffer containing 1 mM PMSF , 50 mM Tris-base, 1 mM EDTA, 5 mM dithiothreitol, NaCl 150 mM, 0.1% SDS, 1% Trionx-100 at room temperature for 3 hours. The samples were centrifuged at 3000 g for 10 minutes and the insoluble tissue discarded. The protein concentration was measured using Bradford (BioRad, Boston, MA, USA) assay; equal amounts of protein (20 mg/sample) were separated by 15% SDS-PAGE electrophoresis (Mini Protean II; BioRad) and then transferred to a nitrocellulose membrane (Millipore, Billerica, MA, USA) prewetted with transfer buffer at 2 mA/cm2 for 0.5 hour. The membrane was blocked for 12 hours at 4°C with 5% skimmed milk (Uppsala, Sweden) in 1Χ TBBS (10 mM Tris pH 7.5, 100 mM NaCl and 0.5% Tween 20). Immunodetection was performed by incubation of IGF-1 goat polyclonal IgG (Santa Cruz Biotechnology, CA, USA) at 1:500 dilution at 4°C overnight. Then samples were incubated with ECL anti-rabbit IgG, horseradish peroxidase linked with whole antibody (Amersham, Biosciences, Uppsala, Sweden) at a 1:5000 dilution in blocking solution for 1 hour at room temperature. Detected by chemiluminescence (Santa Cruz Biotechnology western blotting detection kit).[14]
Results and Discussion
Effect of different treatments on prompting hair growth
The efficacy of 1.0 mg/ml placenta extract was evaluated with respect to the promotion of hair growth. Water and minoxidil were topically applied separately as the negative and positive control. Mice of different treatment groups are shown in Figure 1, and their skin histology slices are exhibited in Figure 2. New hair came out more early in minoxidil treatment group than the other groups. In 1988, Food and Drug Administration (FDA) approved minoxidil for hair loss treatment, and some research elucidated that it shortened telogen and induced resting HFs into anagen. Some evidence of the stimulatory effect of minoxidil on hair growth has been documented. Firstly, minoxidil sulfate opens potassium channels, stimulates the cell proliferation and induces VEGF.[15]
Figure 1.
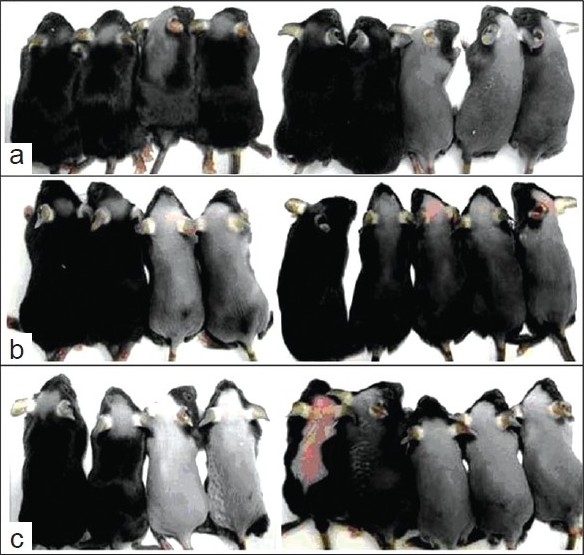
Effect of different treatments on prompting hair growth. Minoxidil topical application has the best effect on hair growth (a) There are many negative effect growth factors in CPE; (b) group B has better influence on hair growth than water topical application (c) topical water application group had least growth of hairs, nine experimental animals.
Figure 2.
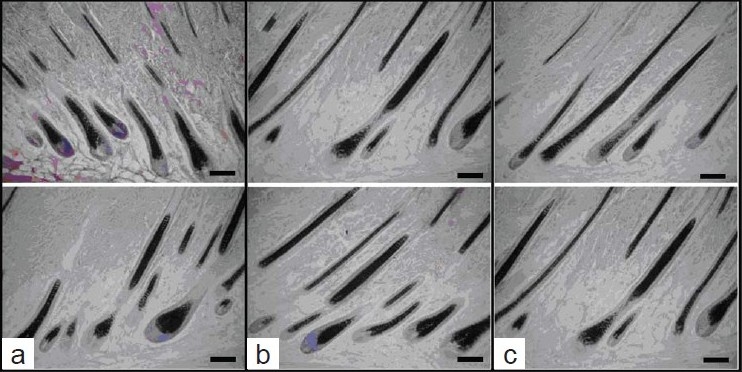
Histologic slices of each treatment group; (a) Minoxidil topical application; (b) topical application of CPE; (c) water topical application group; bar = 50 µm
Figure 1 demonstrates hair regrowth at 21 days for the negative control group C (water) and positive control group A (0.2% minoxidil solution). In minoxidil treatment group, mature hair was full on the back of six mice; hair of other three mice had fully regrown to the original status.[16] CPE treatment group (B) had hair similar to that of minoxidil treatment group. In the negative control group, more mice showed only a faint hair appearance than the other two groups after treatment for 21 days. Histologic studies [Figure 2] showed that minoxidil treatment (A) and CPE treatment (B) markedly increased the depth and size of HFs as compared with that of control (C).
Before sacrificing the mice, we plucked hair from five different parts of mouse and measured the length. The results are shown in Figure 3. Topical minoxidil influences superficial hair length more obviously than control and CPE treatment groups.
Figure 3.
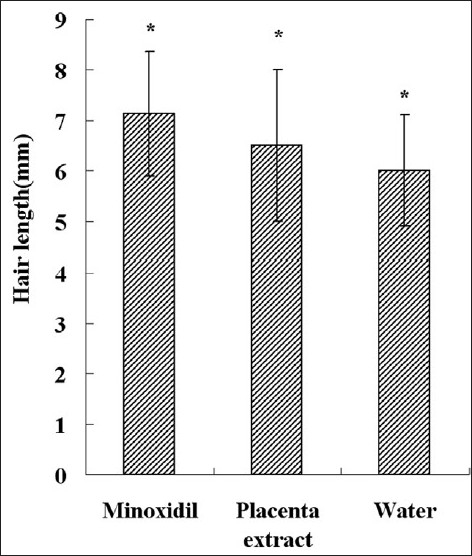
Average length of hair for each group. Each bar represents mean ± SE from nine animal experiments. *P < 0.05 vs. water topical application group
Average number and diameter of hair follicles
Ten pictures which were taken along the hair shaft from each treatment group, were selected, and average HF number per 780 μm [Figure 4] and average diameter [Figure 5] and hair length [Figure 3] were calculated. We compared the HF number of experiment groups with that of normal mouse in full anagen phase. Results showed that minoxidil and placenta extract cannot increase the HF number, for statistically placenta extract and minoxidil had no effect on hair density. In both minoxidil and placenta extract groups, hair length was longer and HF diameter was larger than that of control group.
Figure 4.
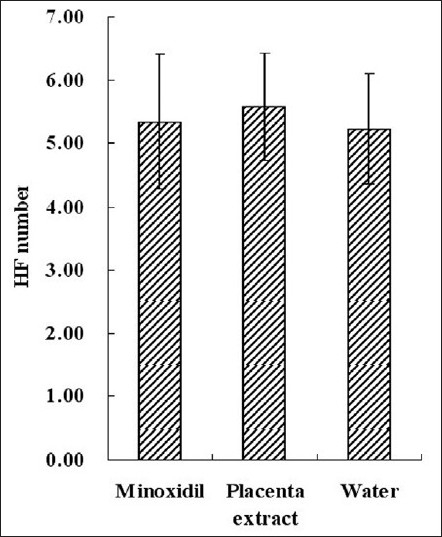
Average HF number per 780 µm length sample skin. This figure gives us the HF density. Each bar represents mean ± SE from nine animal experiments
Figure 5.
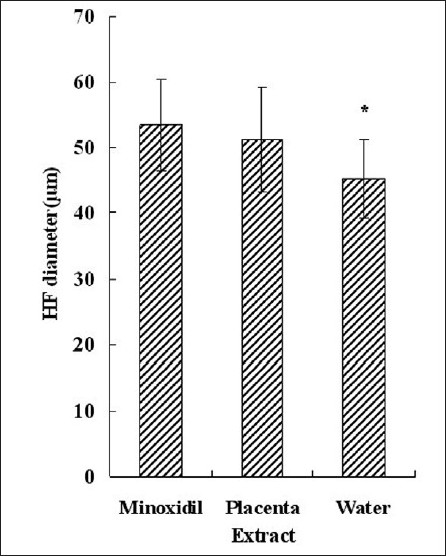
Average HF diameter. Each bar represents mean ± SE from nine animal experiments. *P < 0.05 vs. water topical application group
Immunohistochemistry
From IHC, it is seen that IGF-1 was strongly expressed in the outer root sheath of HFs, epidermis and subcutaneous layer, and slightly expressed in dermis layer in control group [Figure 6a]. In contrast, in the minoxidil treatment [Figure 6b] and CPE treatment [Figure 6c] groups, brown color appeared not only in subcutaneous layer and epidermis layer, but also in dermis layer. Most part of hair buried in skin was in dermis layer. Strong IGF-1 expression in dermis layer influenced hair growth. From IHC result, it is seen that minoxidil and CPE improved hair growth, stimulating the expression of IGF-1. IGF-1 is critically involved in promoting hair growth by regulating cellular proliferation and migration during the development and growth cycles of HFs.[17,18] So we detected the cell proliferation by BrdU incorporation.
Figure 6.

IHC results shown for groups A, B and C. (a) is control group, it has light brown coloration in dermis; (b and c) are CPE and minoxidil treatment groups; they have deeper color than control. Lesser labeled cells are found in water topical treatment group; (d) and the CPE treatment group; (e) has more labeled cells than water application group, (f), minoxidil topical treatment group had more brown cells than other two groups. Scale bar = 50 µm
Mice were injected with 2 mg/ml BrdU, 24 hours before sacrifice. The results obtained after IHC staining are shown in Figure 6d–f. Minoxidil had the optimal effect on hair growth; it can accelerate DNA synthesis through enhancing the expression of same growth factors.[19] BrdU incorporated cell number per HF was calculated [Figure 7]; the higher proliferated skin had more labeled cells. Placenta extract application had an apparent effect on stimulating cell proliferation, and minoxidil was the best reagent.
Figure 7.
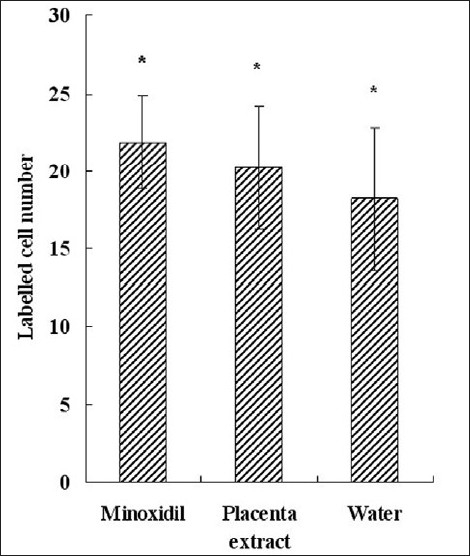
Calculation of the number of labeled cells in different treatment groups. Each bar represents mean ± SE from nine animal experiments. *P < 0.05 vs. water topical application group (control)
Western blot and polymerase chain reaction
IGF-1 is a multifunctional growth factor. It plays an important role in HF cycling and development, and it also specifies cell proliferation. Some researchers demonstrated that IGF-1 affects follicular proliferation, tissue remodeling, and the hair growth cycle. Also, for the transgenic mouse skin, the hair had an abnormal shape and growth phase.[20] From IHC results, it is seen that minoxidil and CPE enhance the expression of IGF-1 protein, Thus, we investigated the effect of minoxidil and CPE on the expression of IGF-1, which was implicated in the regulation of hair growth. To this end, we performed PCR and western blot analysis. As shown in Figure 8, minoxidil and CPE induced mRNA levels of IGF-1.
Figure 8.
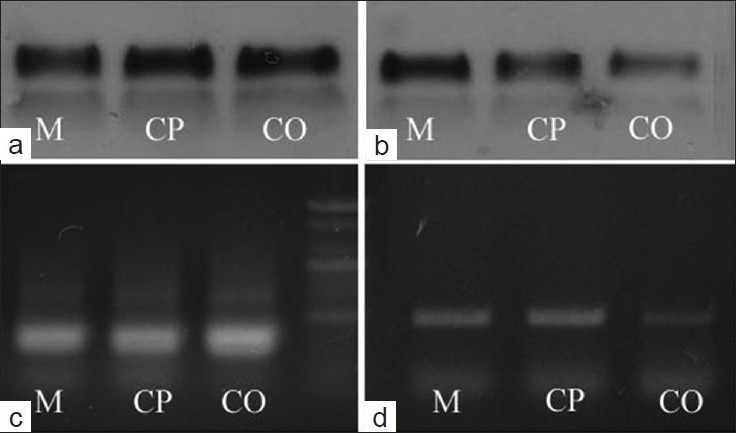
Effect of minoxidil and CPE on the mRNA level of IGF-1 in skin by PCR and western blot analysis; (a and b) Western blot analysis; (c and d) PCR; (a and c) are GAPDH; (b and d) are IGF. M means minoxidil treatment group, CP means CPE treatment group, CO means control group (mean ± SE, P < 0.05)
Conclusion
Cells, besides HF, in minoxidil and placenta applied groups grow faster than those of the control group. Mean minoxidil and placenta extract can accelerate cell growth and improve hair growth by raising the IGF-1 mRNA level to increase HF size and hair length.[21] The effect of minoxidil in improving hair growth has been reported by many authors. Mechanism of improving hair growth by placenta extract may be through enhancing the VEGF and IGF expression. Placenta extract and minoxidil can accelerate cell proliferation; it coincides with the elongation of hair length. More HFs came out in placenta extract and minoxidil treatment groups, but the hair number in these two groups was lesser than normal skin. This situation can be explained based on the finding that the minoxidil and placenta extract may shorten the hair growth cycle, especially, the telogen phase. But they can not induce the HF regrowth, which was destroyed by plucking. Further research about stem cells should be done to discover the mechanism.
Placenta contains many kinds of hormones and cytokines such as EGF, fibroblast growth factor (FGF), tumor necrosis factor (TNF), corticotropin-releasing hormone (CRH), etc. These factors contribute to the properties of placenta which is used for cosmetology and whitening. Segregation of the effective constituent from placenta should be done by further research. Purified or partly purified product would be more effective in promoting hair growth or other function.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Ota Y, Saitoh Y, Suzuki S, Ozawa K, Kawano M, Imamura T. Fibroblast growth factor 5 inhibits hair growth by blocking dermal papilla cell activation. Biochem Biophys Res Commun. 2002;290:169–76. doi: 10.1006/bbrc.2001.6140. [DOI] [PubMed] [Google Scholar]
- 2.Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;811:449–94. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- 3.Botchkarev VA, Botchkareva NV, Nakamura M, Huber O, Funa K, Lauster R, et al. Noggin is required for induction of the hair follicle growth phase in postnatal skin. Faseb J. 2001;15:2205–14. doi: 10.1096/fj.01-0207com. [DOI] [PubMed] [Google Scholar]
- 4.Yamauchi K, Kurosaka A. Inhibition of glycogen synthase kinase-3 enhances the expression of alkaline phosphatase and insulin-like growth factor-1 in human primary dermal papilla cell culture and maintains mouse hair bulbs in organ culture. Arch Dermatol Res. 2009;301:357–65. doi: 10.1007/s00403-009-0929-7. [DOI] [PubMed] [Google Scholar]
- 5.Semenova E, Koegel H, Hasse S, Klatte JE, Slonimsky E, Bilbao D, et al. Overexpression of mIGF-1 in keratinocytes improves wound healing and accelerates hair follicle formation and cycling in mice. Am J Pathol. 2008;173:1295–310. doi: 10.2353/ajpath.2008.071177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu CH, Chang GY, Chang WC, Hsu CT, Chen RS. Wound healing effects of porcine placental extracts on rats with thermal injury. Br J Dermatol. 2003;148:236–45. doi: 10.1046/j.1365-2133.2003.05164.x. [DOI] [PubMed] [Google Scholar]
- 7.Philpott MP, Sanders DA, Kealey T. Effects of Insulin and Insulin-Like Growth Factors on Cultured Human Hair Follicles: IGF-I at Physiologic Concentrations Is an Important Regulator of Hair Follicle Growth In vitro. J Investig Dermatol. 1994;102:857–61. doi: 10.1111/1523-1747.ep12382494. [DOI] [PubMed] [Google Scholar]
- 8.Pal P, Mallick S, Mandal SK, Das M, Dutta AK, Datta PK, et al. A human placental extract: in vivo and in vitro assessments of its melanocyte growth and pigment-inducing activities. Int J Dermatol. 2002;411:760–7. doi: 10.1046/j.1365-4362.2002.01524.x. [DOI] [PubMed] [Google Scholar]
- 9.Odorisio T, Cianfarani F, Failla CM, Zambruno G. The placenta growth factor in skin angiogenesis. J Dermatol Sci. 2006;41:11–9. doi: 10.1016/j.jdermsci.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Muskhelishvili L, Latendresse JR, Kodell RL, Henderson EB. Evaluation of cell proliferation in rat tissues with BrdU, PCNA, Ki-67(MIB-5) immunohistochemistry and in situ hybridization for histone mRNA. J Histochem Cytochem. 2003;51:1681–8. doi: 10.1177/002215540305101212. [DOI] [PubMed] [Google Scholar]
- 11.Tsuji Y, Denda S, Soma T, Raftery L, Momoi T, Hibino T. A potential suppressor of TGF-beta delays catagen progression in hair follicles. J Investig Dermatol Symp Proc. 2003;8:65–8. doi: 10.1046/j.1523-1747.2003.12173.x. [DOI] [PubMed] [Google Scholar]
- 12.Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell. 2000;102:451–61. doi: 10.1016/s0092-8674(00)00050-7. [DOI] [PubMed] [Google Scholar]
- 13.Roh SS, Kim CD, Lee MH, Hwang SL, Rang MJ, Yoon YK. The hair growth promoting effect of Sophora flavescens extract and its molecular regulation. J Dermatol Sci. 2000;30:43–9. doi: 10.1016/s0923-1811(02)00060-9. [DOI] [PubMed] [Google Scholar]
- 14.Gu L, Mo E, Yang Z, Zhu X, Fang Z, Sun B, et al. Expression and localization of insulin-like growth factor-I in four parts of the red deer antler. Growth Factors. 2007;25:264–79. doi: 10.1080/08977190701773187. [DOI] [PubMed] [Google Scholar]
- 15.Messenger AG, Rundegren J. Minoxidil: Mechanisms of action on hair growth. Bri J Dermatol. 2004;150:186–94. doi: 10.1111/j.1365-2133.2004.05785.x. [DOI] [PubMed] [Google Scholar]
- 16.Chen CH, Sheu MT, Wu AB, Lin KP, Ho HO. Simultaneous effects of tocopheryl polyethylene glycol succinate (TPGS) on local hair growth promotion and systemic absorption of topically applied minoxidil in a mouse model. Int J Pharm. 2005;306:91–8. doi: 10.1016/j.ijpharm.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Su HY, Hickford JG, Bickerstaffe R, Palmer BR. Insulin-like growth factor 1 and hair growth. Dermatol Online J. 1999;5:1. [PubMed] [Google Scholar]
- 18.Harada N, Okajima K, Narimatsu N, Kurihara H, Nakagata N. Effect of topical application of raspberry ketone on dermal production of insulin-like growth factor-I in mice and on hair growth and skin elasticity in humans. Growth Hormone Igf Res. 2008;18:335–44. doi: 10.1016/j.ghir.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Malhi H, Irani AN, Rajvanshi P, Suadicani SO, Spray DC, McDonald TV, et al. K-ATP channels regulate mitogenically induced proliferation in primary rat hepatocytes and human liver cell lines - Implications for liver growth control and potential therapeutic targeting. J Biol Chem. 2000;275:26050–7. doi: 10.1074/jbc.M001576200. [DOI] [PubMed] [Google Scholar]
- 20.Weger N, Schlake T. Igf-I signalling controls the hair growth cycle and the differentiation of hair shafts. J Invest Dermatol. 2005;125:873–82. doi: 10.1111/j.0022-202X.2005.23946.x. [DOI] [PubMed] [Google Scholar]
- 21.Jimenez JJ, Roberts SM, Mejia J, Mauro LM, Munson JW, Elgart GW, et al. Prevention of chemotherapy-induced alopecia in rodent models. Cell Stress Chaperones. 2008;13:31–8. doi: 10.1007/s12192-007-0005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


