Abstract
The primary cutaneous T-cell lymphomas (CTCL) represent a clonal T-lymphocyte proliferation infiltrating the skin. CD30+T-cell lymphomas present clinically as nodules with a diameter between 1 and 15 cm, mostly in elderly patients. The role of the CD30 molecule in patients suffering from T-cell lymphomas is not completely clear yet. The signal transduction pathway which includes CD30 seems to play a key role in tumor progression. In certain forms of T-cellular lymphomas, the interaction between CD30/CD30-ligand is able to provoke apoptosis of the “tumor lymphocytes”. The modern conceptions of the pathogenesis of T-cell lymphomas include disorders in the pathways involved in programmed cellular death and disregulation in the expression of certain of its regulatory molecules. We are presenting an unusual case of a female patient with a primary cutaneous form of CD30+/ALK− anaplastic large T-cell lymphoma. Upon the introduction of systemic PUVA, (psoralen plus ultraviolet light radiation) combined with beam therapy, a complete remission could be noticed. Eight months later, we observed a local recurrence, which was overcome by CHOP chemotherapy (Cyclophosphamide, Hydroxydaunorubicin (Doxorubicin), Vincristin (Oncovin®), Predniso(lo)n). Six months later, new cutaneous lesions had been noticed again. A new therapeutic hope for the patients with anaplastic large CTCL is actually based on the influence of the activity of the different apoptotic pathways. Death ligands, including tumor necrosis factor (TNF)-α, CD95L/FasL, and TRAIL, mediate also some important safeguard mechanisms against tumor growth in patients with CD30+ cutaneous anaplastic large T-cell lymphomas and critically contribute to lymphocyte homeostasis.
Keywords: T-cell lymphoma, CD30+, ALK-, apoptosis, CHOP, methotrexate
Introduction
Cutaneous lymphomas are the second most common lymphoma form within the group of extra-nodal, non-Hodgkin lymphomas.[1,2] Primary cutaneous lymphomas are located in the skin and as a rule remain there for at least 6 months, while the secondary cutaneous lymphomas represent the cutaneous manifestation of primarily disseminated nodal or extranodal lymphomas.[1,2]
The primary cutaneous lymphomas are of heterogeneous nature both clinically and histologically. T-cell lymphomas are found in around 65% of the cases, whereas in 25% of the cases lymphomas of a B-cell nature can be found.[1] The remaining 10% can be hardly distinguished or have a partially combined nature.[1,3]
The role of the CD30 molecule [tumor necrosis factor (TNF) receptor] in patients suffering from T-cell lymphomas is not completely clear yet.[4,5] In general, the prognosis of cutaneous CD30+ cutaneous T-cell lymphomas (CTCL) is more favorable than that of their CD30− counterpart.[1–4] A spontaneous regression is observed in up to 25% of the cases of primary cutaneous anaplastic CD30+large T-cell lymphoma.[6,7] A transition from cutaneous to single or multiple extracutaneous manifestation, in lymph nodes for instance, is also possible. This transformation must not be always interpreted as a negative prognostic factor.[6,7]
Cell death ligands, including TNF-α, CD95L/FasL, and TRAIL, mediate protective mechanisms against tumor growth in patients with cutaneous anaplastic large CD30+ T-cell lymphoma and critically contribute to lymphocyte homeostasis.
Case Report
Anamnesis
A 86-year-old lady was suffering from suddenly appearing skin alterations in the areas of her forehead, back and left breast for 6 months [Figures 1 and 2], without any pain, paresthesias or pruritus. A hospitalization was considered for clarification and scheduling of therapy.
Figure 1.
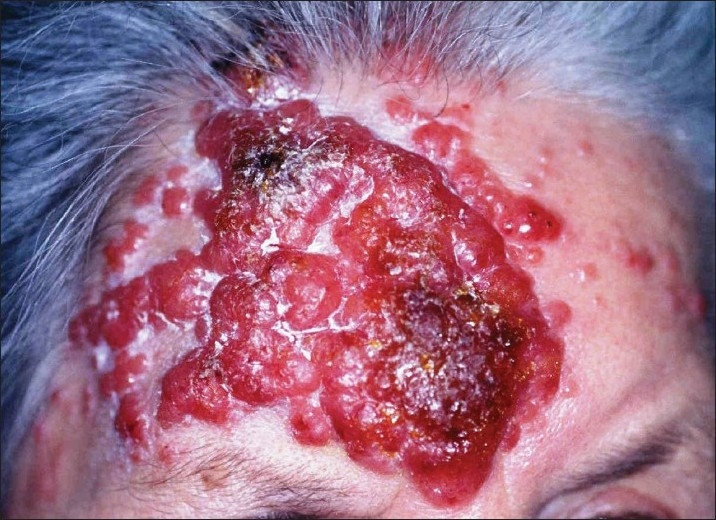
Disseminated red, semicircular, partially confluent plaques with infiltrating endophytic/exophytic growth, located in the areas of the forehead and body
Figure 2.
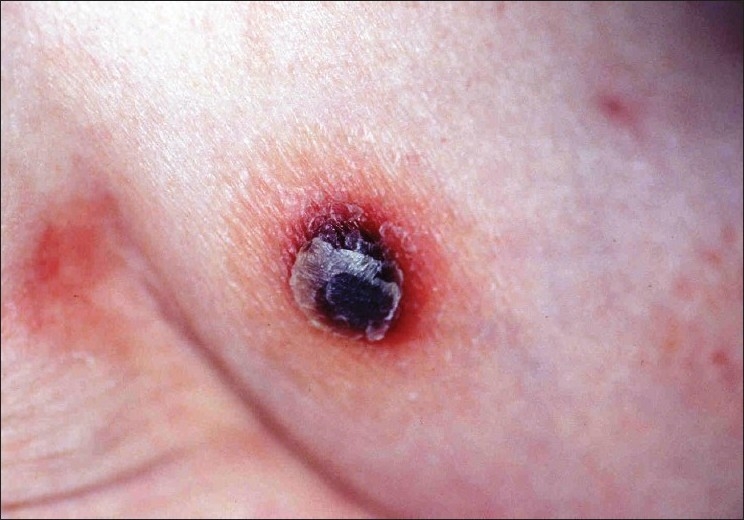
Close-up of a lesion showing infiltration and necrosis
Dermatologic status
Disseminated red, semicircular, partially confluent plaques with infiltrating endophytic/exophytic growth, located in the areas of the forehead and body (left breast, paravertebrally right) were found [Figures 1 and 2]. Clinically, the lesions on the forehead had a crateriform structure of size 7 Χ 7 cm, showing a trend to ulceration, covered by exudate [Figure 1].
Laboratory findings
The diagnosis of CTCL was histologically made [Figure 3]. In the infiltrates, a strong expression of CD3 and CD30 was found by immunohistology. Simultaneously, no CD20, ALK, CD79a or cytokeratins’ expression could be proven. The following lymphocyte subpopulations were assessed in the blood serum: total T lymphocytes 94%, total B lymphocytes 1%, T suppressive cells 45%, T and total natural killer (NK) cells 4%. Erythrocyte sedimentation, C-reactive protein and other laboratory parameters were within the normal range. Epstein-Barr-Virus (EBV) serology was negative.
Figure 3.
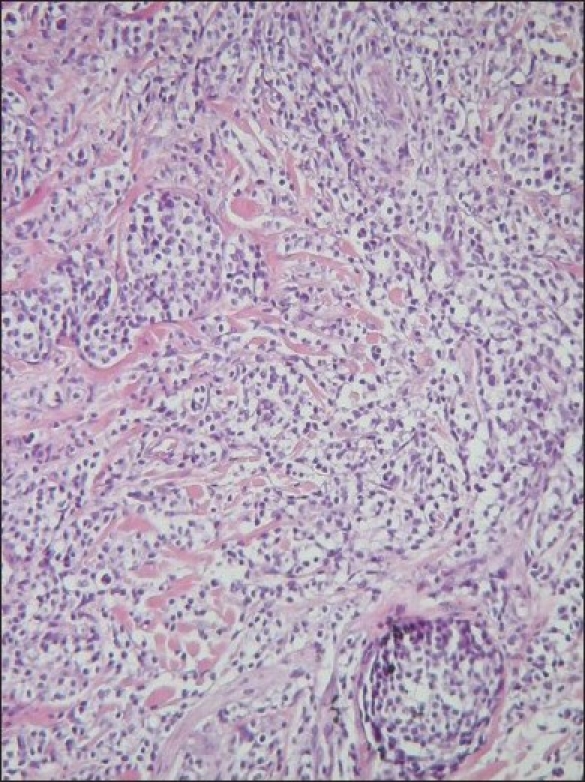
Dermo-epidermally located dense T-lymphocyte infiltrates in cutaneous CD30+ and ALK− T-cell lymphoma (H&E stain, 400X)
Immunophenotyping of a biopsy specimen and medulla cytology excluded the possibility of systemic lymphoma with a secondary cutaneous manifestation.
The histologic record of the biopsy specimen showed a hypercellular, regenerative medulla with an absolute sideropenia and dismegakariopoiesis as a possible initial form of myelodisplastic syndrome. T-cell infiltrates in the histologic preparation were missing. Ultrasonography of the inguinal, axillary lymph nodes and the abdominal area, as well as the computed tomography of the abdominal and thoracic areas, did not show any pathologic changes.
Therapy and course of the disease
Complete remission was achieved by systemic PUVA therapy (psoralen plus ultraviolet light radiation) for 2 months combined with beam therapy of 2.0 Gy of single dose and the total dose was 44.0 Gy [Figure 4]. However, the lymphoma relapsed at the places of initial presentation after 8 months [Figure 5]. The extracutaneous manifestation of the T-cellular lymphoma was ruled.
Figure 4.
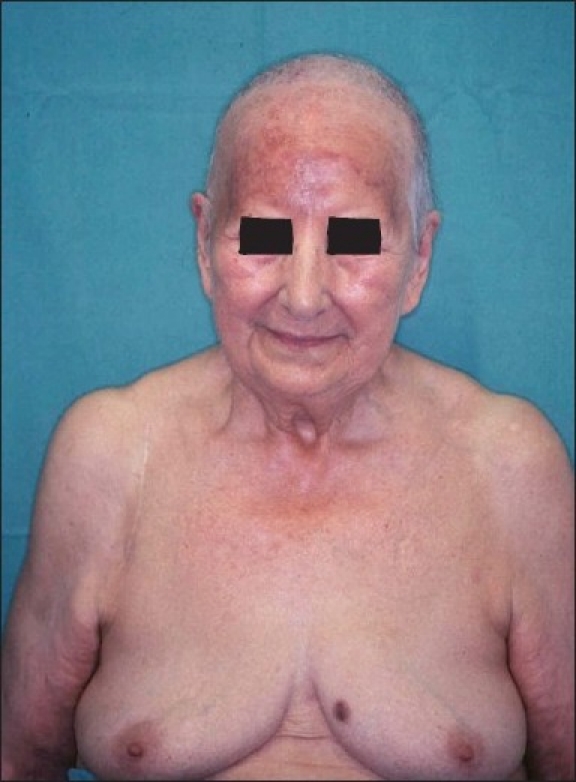
Complete remission achieved with systemic PUVA therapy(psoralen plus ultraviolet light radiation) in combination with beam therapy
Figure 5.
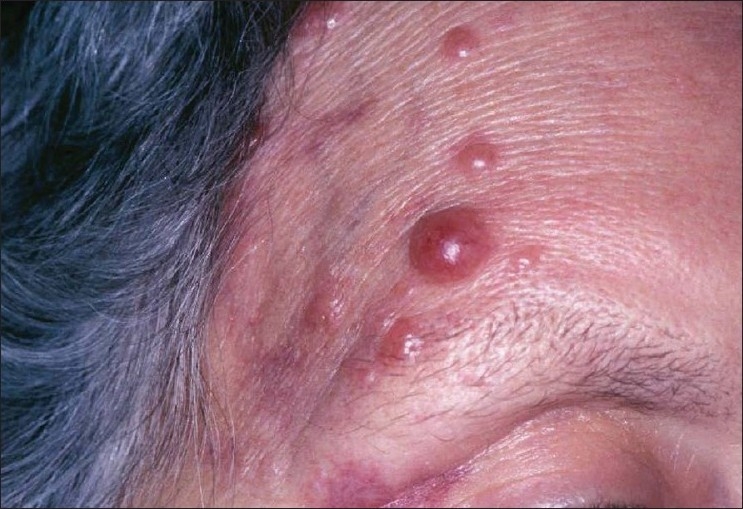
Relapse of cutaneous CD30+ and ALK− T-cell lymphoma
The applied CHOP chemotherapy (three cycles) (Cyclophosphamide, Hydroxydaunorubicin (Doxorubicin), Vincristin (Oncovin;), Predniso(lo)n) resulted in a second complete remission followed by a new relapse within 6 months. The relapse had a nuchal, occipital and abdominal skin localization. Repeated beam therapy combined with a systemic interferon, Bexaroten and PUVA was initiated.
Discussion
Only recommendations, but no defined therapeutic protocols, for the treatment of patients with CD30+T-cell lymphomas are available [Tables 1 and 2]. The therapy at the early stages is a challenge [Table 1and Table 2]. The systemic or local PUVA therapy in combination with retinoids is a possible alternative. If no clinical improvement is achieved, radiotherapy by fast electrons as well as a local application of Carmustine (BCNU) is also possible [Table 2]. Systemic application of Bexaroten (Targretin®) in IB stage is considered a second-line therapy. Local therapy includes also the application of glucocorticoids, class III-IV [Tables 1 and 2].
Table 1.
Recommendations for treatment of cutaneous CD30+ T-cell lymphoma

Table 2.
Recommendations for treatment of CTCL – Mycosis fungoides and its subtypes
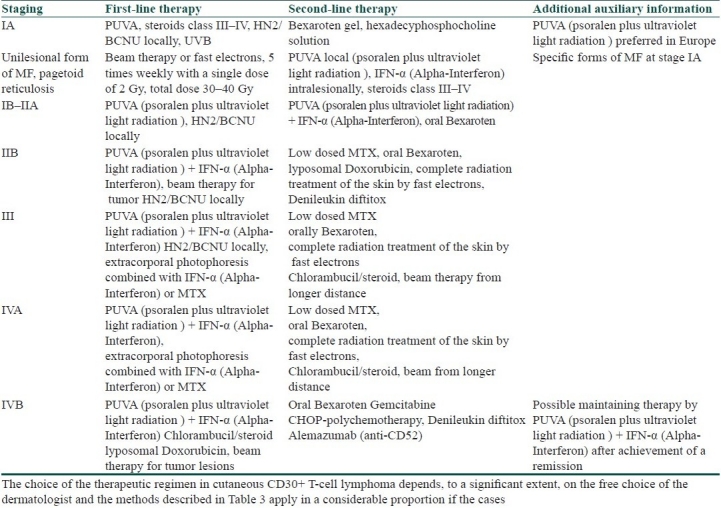
Therapies for the advanced stages include high-dose therapeutic regimens, which must be adapted not only to the patient's general physical status, but also to the individual tolerance of the preparations [Tables 1 and 2]. The combination of recombinant interferon α with retinoids and systemic PUVA (psoralen plus ultraviolet light radiation) is promising, but does not exclude the possibility of relapses [Table 2].[8,9] In the case of poor tolerance of the systemic PUVA (psoralen plus ultraviolet light radiation), remissions, although partial, are frequently achieved by extracorporal photophoresis combined with interferon-α and Acitretin (Table 2). Great expectations are laid on the new retinoidal remedies such as Bexaroten (Targretin). In the generalized forms of T-cellular lymphoma, the palliative chemotherapy with CHOP protocol [Table 2], KNOSPE protocol (Chlorambucil, Prednisolon) and COP protocol (Cyclophosphamide, Vincristin, Prednisolon) frequently provides good results. Methotrexate (MTX) may also be used[10] [Tables 1 and 2].
Detailed data regarding the prognosis and therapy of ALK- and CD30+ cutaneous lymphomas in larger patient cohorts are missing. The ALK expression is a direct result of t(2;5) translocation and correlates with the expression of other markers such as EMA and the cytotoxic phenotype. At the same time, this expression is incidental to or characteristic of younger patients.[4] ALK expression correlates with a lower international prognostic index (IPI) and is a more favorable prognosis for the patients.[11] The lack of this expression in the present case is one of the possible reasons for the bad prognosis and the described relapses.
Tumor formations often relapse without a clear reason. A new therapeutic hope is attributed to the influence on the activity of the different apoptotic pathways; the expression and interactions between Fas and FasL in the different T lymphocyte clones, of the TGF-β receptor gene mutations and the interaction between the T cells themselves, probably play a key role in the determination of the behavior of the so-called metastatic phenotype.[12]
Whereas CD95 stimulation strongly induced apoptosis in cutaneous anaplastic large T lymphoma cells, the extrinsic pro-apoptotic pathways of TNF-α and TRAIL could be completely blocked at early stages.[13] TRAIL resistance may be explained by the consistent over-expression of cellular flice inhibitory protein (c-FLIP) and frequent loss of the pro-apoptotic Bcl-2 protein Bid.[13] CD30/CD95 crosstalk experiments have shown that CD30 ligation leads to NF-kappa B-mediated c-FLIP up-regulation in cALCL cells, which in turn conferred enhanced resistance to CD95-mediated apoptosis. Knockdown of c-FLIP by a lentiviral approach enhanced basic apoptosis rates in cALCL cells and diminished the CD30-mediated suppression of apoptosis, thus proving the significance of c-FLIP in this context.[13]
These in vitro findings may be indicative of cALCL. Further clarifications of the apoptosis pathway defects in the cutaneous lymphomas may lead to improved or novel therapies for these disorders.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Willemze R, Kerl H, Sterry W, Berti E, Cerroni L, Chimenti S, et al. EORTC classification for primary cutaneous lymphomas: a proposal from the Cutaneous Lymphoma Study Group of the European Organization for Research and Treatment of Cancer. Blood. 1997;90:354–71. [PubMed] [Google Scholar]
- 2.Marcus Muche J, Sterry W. Vakzinationstherapie kutaner T-Zell-Lymphome. Z Hautkr. 2002;77:679–84. [Google Scholar]
- 3.Burg G, Dummer R, Kerl H. Classification of cutaneous lymphomas. Derm Clinics. 1994;12:213–17. [PubMed] [Google Scholar]
- 4.Ten Berge RL, Oudejans JJ, Ossenkoppele GJ, Meijer CJ. ALK-negative systemic anaplastic large cell lymphoma: differential diagnostic and prognostic aspects- a review. J Pathol. 2003;200:4–15. doi: 10.1002/path.1331. [DOI] [PubMed] [Google Scholar]
- 5.Bekkenk MW, Geelen FA, van Voorst Vader PC, Heule F, Geerts ML, Van Vloten WA, et al. Primary and secondary cutaneous CD30+ lymphoproliferative disorders: a report from the Dutch Cutaneous Lymphoma Group on the long -term follow-up data of 219 patients and guidelines for diagnosis and treatment. Blood. 2000;95:3653–61. [PubMed] [Google Scholar]
- 6.Kempf W, Dummer R, Burg G. Klinische und therapeutische Besonderheiten kutaner Lymphome. Dt Ärztebl. 2001;98:697–703. [Google Scholar]
- 7.Kerl H, Sterry W. Classification and staging. In: Burg G, Sterry W, editors. EORTC/BMFT Cutaneous Lymphoma Project Group: Recommendations for staging and therapy of cutaneous lymphomas. 1987. pp. 1–10. [Google Scholar]
- 8.Dummer R, Kempf W, Hess Schmid M, Haffner A, Burg G. Therapy of cutaneous lymphoma-current practice and future developments. Onkologie. 2003;26:366–72. doi: 10.1159/000072098. [DOI] [PubMed] [Google Scholar]
- 9.Duvic M, Martin AG, Kim Y, Olsen E, Wood GS, Crowley CA, et al. Phase 2 and 3 clinical trial of oral Bexaroten (Targretin capsules) for the treatment of refractory or persistent early-stage cutaneous T-cell lymphoma. Arch Dermatol. 2001;137:581–93. [PubMed] [Google Scholar]
- 10.Vonderheid EC, Sajjadian A, Kadin ME. Methotrexate is effective therapy for lymphomatoid papulosis and other primary cutaneous CD-30 positive lymphoproliferative disorders. J Am Acad Dermatol. 1996;34:470–81. doi: 10.1016/s0190-9622(96)90442-9. [DOI] [PubMed] [Google Scholar]
- 11.Falini B, Bigerna B, Fizzoti M, Pulford K, Pileri SA, Delso IG, et al. ALK expression defines a distinct group of T/null lymphomas (“ALK lymphomas”) with a wide morphological spectrum. Am J Pathol. 1998;153:875–86. doi: 10.1016/S0002-9440(10)65629-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knaus PI, Lindemann D, DeCoteau JF, Perlman R, Yankelev H, Hille M, et al. A dominant inhibitory mutant of the type II transforming growth factor beta receptor in the malignant progression of a cutaneous T-cell lymphoma. Mol Cell Biol. 1996;16:3480–9. doi: 10.1128/mcb.16.7.3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braun FK, Hirsch B, Al-Yacoub N, Dürkop H, Assaf C, Kadin ME, et al. Resistance of cutaneous anaplastic large-cell lymphoma cells to apoptosis by death ligands is enhanced by CD30-Mediated Overexpression of c-FLIP. J Invest Dermatol. 2010;130:826–40. doi: 10.1038/jid.2009.299. [DOI] [PubMed] [Google Scholar]


