Abstract
Bacterial DNA and unmethylated CpG oligodeoxynucleotides (CpG ODN) are known to be potent stimulators of the innate immune system in vitro and in vivo. We therefore investigated if oral administration of CpG ODN could enhance innate immunity in the gastric mucosa and control the extent of Helicobacter pylori infection in mice. Intragastric administration of a single dose of CpG ODN significantly increased local production of the CC chemokines macrophage inflammatory protein 1α (MIP-1α), MIP-1β, and RANTES and the CXC chemokine gamma interferon-inducible protein 10 in the stomach and/or the small intestine. Importantly, intragastric administration of CpG ODN to mice with an already established H. pylori infection, in the absence of any coadministered antigen, was found to reduce the bacterial load in the stomach compared to the load in H. pylori-infected control mice, while similar administration of non-CpG ODN had no effect on the bacterial load. The reduction in the bacterial numbers in the stomachs of mice treated with CpG ODN was associated with enhanced infiltration of immune cells and increased RANTES production in the gastric mucosa compared to the infiltration of immune cells and RANTES production in H. pylori-infected control animals. These findings suggest that intragastric administration of CpG ODN without antigen codelivery may represent a valuable strategy for induction of innate immunity against H. pylori infection.
Helicobacter pylori is a gram-negative bacterium that colonizes the gastric mucosa and duodenal mucosa in humans and is the etiological agent of chronic gastritis and peptic ulcers, as well as a risk factor for gastric cancer (4). The standard treatment for H. pylori infections has depended on antibiotics in combination with proton pump inhibitors (3). Despite the efficacy of such treatment for the resolution of gastritis and a decrease in the relapse rate of duodenal ulcers, the resistance of H. pylori to antibiotics is increasing (5). Therefore, there is a need for development of new strategies to control H. pylori infection and disease. Over the past decade, significant efforts have been made to develop vaccines for inducing H. pylori-specific acquired protective immunity. Thus, per oral immunization of mice with H. pylori candidate antigens together with a mucosal adjuvant conferred various degrees of protection against H. pylori infection in mice (7, 8, 23, 24, 30, 31). Recent studies have shown that T cells and Th1-mediated immune responses are important in the induction of vaccine-induced protective immunity against H. pylori infection (1).
While the vast majority of the efforts to understand the mechanism of protective immunity and to develop effective immunotherapeutic agents and vaccines for use against H. pylori infection have focused on the effector phase of the antigen-specific adaptive immune response, there is a lack of information concerning the effect of stimulating innate immunity on H. pylori infection. In this study, we examined the potential of stimulating the innate immune response in the gastrointestinal tract mucosa to induce immunity against H. pylori infection.
Innate immunity against pathogens is in part orchestrated by the ordered release of different chemokines that function as chemoattractants and activators of various immune cells, a property that enables immune cells to serve as the first line of cell-mediated host defense against infections. The chemokine superfamily is divided into four subfamilies, CXC, CC, CX3C, and C, based on the number and relative position of conserved cysteine residues (39). Of the different chemokines studied to date, members of the CXC and CC chemokine subfamilies appeared to have the greatest effects on the recruitment of various immune cells, such as natural killer (NK) cells, polymorphonuclear cells, dendritic cells, macrophages, and lymphocytes, to the site of infection. Thus, the CXC chemokine gamma interferon-inducible protein 10 (IP-10), which is produced by a variety of cell types, including fibroblasts, endothelial cells, and mononuclear cells, through binding to its specific receptor, CXCR3, chemoattracts T cells to the site of infection (21). The CC chemokine RANTES (regulated on activation, normal T-cell-expressed and secreted) is produced mainly by epithelial cells, endothelial cells, fibroblasts, and T lymphocytes and by binding to CCR1, CCR3, or CCR5 and chemoattracts T cells, monocytes, NK cells, and dendritic cells (25). Recent studies have shown that RANTES is an important factor involved in the induction and development of mucosal immune responses (18). Other members of the CC chemokine subfamily are macrophage inflammatory protein 1α (MIP-1α) and MIP-1β, which are produced by leukocytes in response to proinflammatory cytokines or endotoxin and can induce the influx of NK cells, macrophages, and immature dendritic cells (21).
The genomic DNAs of bacteria and vertebrates differ in the frequency and methylation of CpG dinucleotides, which are relatively common and unmethylated in bacterial DNA but are underrepresented and methylated in vertebrate genomes (14). Vertebrate immune systems appear to have evolved a specific Toll-like receptor, TLR9, that distinguishes bacterial DNA from vertebrate DNA. Interactions between CpG in bacterial DNA and TLR9 rapidly activate the antigen-presenting cells through the Toll-interleukin-1 (IL-1) receptor signaling pathway to up-regulate costimulatory molecules and to produce Th1-polarizing cytokines (11, 34). Bacterial DNA or synthetic oligodeoxynucleotides (ODN) containing CpG with appropriate flanking regions (CpG motif) have been found to activate macrophages, dendritic cells, and B cells to secrete a variety of immunomodulatory cytokines, such as IL-6, IL-12, IL-18, and gamma interferon (15, 20). Moreover, CpG DNA activates macrophages and NK cells (28, 35), dendritic cells (27), and B cells (14) to up-regulate major histocompatibility complex classes I and II, as well as costimulatory molecules, such as CD80 and CD86.
Recent studies have shown that systemic administration of CpG ODN without antigen codelivery can induce nonspecific Th1-like innate immune responses of a protective nature. Thus, systemic pretreatment of mice with CpG ODN was shown to elicit protective immunity against infection with Plasmodium yoelii (9), Listeria monocytogenes (6, 13), or Leishmania major (38). Despite the documented effects of CpG ODN on systemic immunity, little is known about the impact of CpG ODN on mucosal immunity. However, it was recently demonstrated in our laboratory that vaginal-mucosal administration of CpG ODN in the absence of any antigen elicits strong innate immune responses in the female genital tract mucosa that confer protection against genital herpes infection and disease (10).
The present study was undertaken to investigate the effects of intragastric-mucosal administration of CpG ODN on induction of innate immune responses in the gastrointestinal tract mucosa and to examine the impact of CpG ODN treatment on control of bacterial colonization in an already established H. pylori infection. We show here for the first time that intragastric administration of CpG ODN in mice rapidly elicits production of the CC chemokines RANTES and MIP-1α and the CXC chemokine IP-10 in the murine gastric mucosa. Furthermore, we show that intragastric mucosal treatment of mice with CpG ODN, in the absence of any bacterial antigen, reduces bacterial colonization in mice with an already established H. pylori infection that is concomitant with both an increase in local RANTES production and rapid influx of immune cells into the gastric mucosa.
MATERIALS AND METHODS
Animals.
Six- to eight-week-old female C57BL/6 mice were purchased from M&B (Denmark) and were housed in microisolators under specific-pathogen-free conditions at the Laboratory for Experimental Biomedicine, Göteborg University, during the study. All experiments were performed with approval from the Ethical Committee of the National Board for Laboratory Animals (ethical permit 291/99).
Bacteria and culture conditions for infection.
Mouse-adapted H. pylori strain SS1 (17), stored at −70°C and kept in Luria-Bertani medium containing 20% glycerol, was used as the stock culture for all experiments. The bacteria were cultured and used for infection as previously described (24). Each mouse received an infectious dose of approximately 3 × 108 CFU of H. pylori.
Synthetic ODN.
The ODN were purchased from Cybergene AB (Novum Research Park, Sweden). The CpG ODN used in this study was 1826 (TCC ATG ACG TTC CTG ACG TT), a 20-mer which has a nuclease-resistant phosphorothioate backbone and which contains two copies of a CpG motif. The control ODN was TCC AGG ACT TCT CTC AGG TT, a 20-mer that has a nuclease-resistant phosphorothioate backbone but contains no CpG motif. The ODN were tested for endotoxin by using the Limulus amebocyte lysate assay (Biowhitaker). All dilutions were made with pyrogen-free reagents.
H. pylori infection and administration of CpG ODN.
To study the effect of CpG ODN administration on production of chemokines, mice were given 30 μg of CpG ODN intragastrically and sacrificed at various times after CpG ODN administration. In another set of experiments, mice were initially infected with H. pylori SS1; then 2 weeks later (i.e., after establishment of infection), the infected mice were given 30 μg of either CpG ODN or non-CpG ODN intragastrically twice with a 1-week interval between the doses, or they were left untreated.
Quantification of H. pylori SS1 in the stomachs of H. pylori-infected mice.
Mice infected with H. pylori SS1 were sacrificed 3 days after administration of either the first dose or the second dose of ODN, and the number of bacteria in each stomach was quantified as previously described (24). Untreated mice infected for the same period were used as controls.
In vitro antibacterial analysis of CpG ODN.
H. pylori SS1 was grown to confluence on horse blood agar plates with disks containing either phosphate-buffered saline (PBS), gentamicin (50 μg), CpG ODN (20 μg), or non-CpG ODN (20 μg). Two days later the zone of inhibition was measured on the lawn of bacterial growth on each plate. In another set of experiments, H. pylori SS1 was cultured in liquid culture containing either CpG ODN or non-CpG ODN, incubated for 3 h under microaerophilic conditions, and subsequently plated on horse blood agar plates. Two days later, when well-defined colonies were visualized, the numbers of CFU were recorded.
Extraction of chemokines from tissues.
Extraction of MIP-1α, MIP-1β, RANTES, and IP-10 from the stomachs, small intestines, and mesenteric lymph nodes (MLNs) was performed by using a modified version of a PERFEXT method (12). Briefly, mice were sacrificed at various times after CpG ODN treatment, and the organs were excised and weighed before storage at −70°C in a PBS solution containing 2 mM phenylmethylsulfonyl fluoride, 0.1 mg of soybean trypsin inhibitor (Sigma) per ml, and 0.05 M EDTA. The tissue samples were thawed and then permeabilized with saponin (Sigma) at a final concentration of 2% (wt/vol) in PBS at 4°C overnight. The tissue samples were then centrifuged at 16,000 × g for 5 min, and the supernatants were analyzed for chemokine contents by an enzyme-linked immunosorbent assay (ELISA).
Chemokine quantification.
Concentrations of MIP-1α, MIP-1β, RANTES, and IP-10 in the tissue extracts and serum samples were determined by using Duoset chemokine ELISA kits from R&D Systems (Abingdon, United Kingdom) according to the manufacturer's recommendations.
Histology.
Strips of the entire longer curvature of the stomach were cut and fixed in 4% phosphate-buffered formalin and then embedded in paraffin. Sections 5 μm thick were cut and stained with hematoxylin and eosin. The slides were then examined by light microscopy (magnification, ×100), and the extent of immune cell infiltration was graded on a scale from 1 to 4 as previously described (29).
Statistical analysis.
The Mann-Whitney test was used for comparisons between groups with the help of the Graphpad Prism software system (GraphPad Software Inc., San Diego, Calif.).
RESULTS
Local production of the CXC chemokine IP-10 after CpG ODN administration.
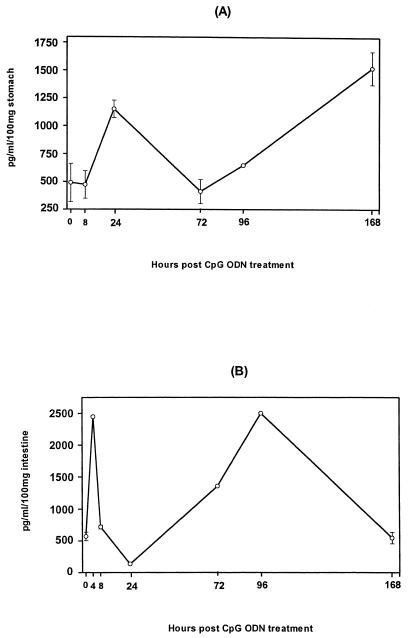
Since IP-10 plays an important role in protection against a variety of bacterial and viral infections (16, 26), we investigated whether intragastric CpG ODN administration induces IP-10 production in the gastrointestinal tract mucosa. Mice were given CpG ODN intragastrically, and the concentrations of IP-10 were determined in the saponin extracts of the stomach and small intestine at various times after CpG ODN delivery. A basal level of IP-10 was detected in the stomachs of naïve mice. The level of IP-10 more than doubled within 24 h after CpG ODN administration and waned by day 3; this was followed by a second wave of IP-10 production that resulted in a threefold increase in the IP-10 level by day 7 (Fig. 1A). A rapid and transient increase in the IP-10 level was also observed in the small intestine 4 h after CpG ODN administration, which waned within 24 h; this was followed by a second peak (fourfold increase) at 96 h (Fig. 1B). However, 7 days after administration of CpG ODN the level of IP-10 had returned to the background level (Fig. 1B). Thus, intragastric CpG ODN administration in mice resulted in strong biphasic induction of IP-10 both in the stomach and in the small intestine.
FIG. 1.
Time course of CXC chemokine IP-10 production after administration of CpG ODN. Mice were given 30 μg of CpG ODN intragastrically and sacrificed at various times after CpG ODN administration. Stomachs (A) and small intestines (B) were excised and after saponin extraction were subjected to ELISA.
Local production of the CC chemokines MIP-1α and MIP-1β in the gastrointestinal mucosa after CpG ODN treatment.
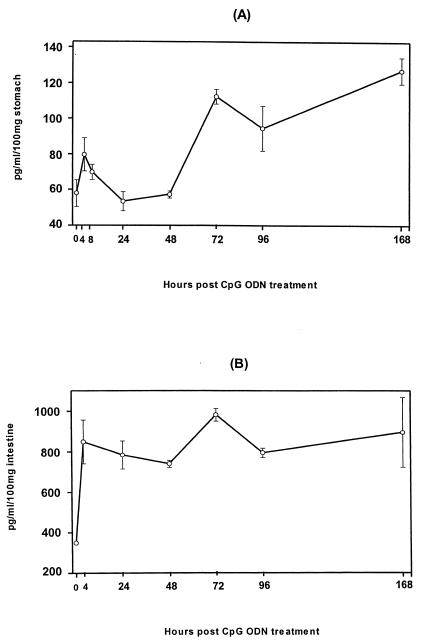
To examine the effect of intragastric administration of CpG ODN on local mucosal production of the CC chemokines MIP-1α and MIP-1β, we determined the levels of MIP-1α and MIP-1β in the stomachs, small intestines, MLNs, and sera of mice at various times after CpG ODN administration. As shown in Fig. 2A, the level of MIP-1α in the stomach increased by more than 100% within 4 h after CpG ODN treatment, declined to the basal level within 24 h, and rose again 72 h after CpG ODN treatment (Fig. 2B). The basal levels of MIP-1α in the small intestines, MLNs, and sera remained virtually unchanged throughout the test period (data not shown). Induction of a MIP-1β response, on the other hand, was seen only in the small intestines of CpG ODN-treated mice. A rapid peak of MIP-1β level was seen 6 h after CpG ODN administration, and the level had returned to the background level at 24 h after administration. A second peak was seen at 72 h, and high levels were maintained even 96 h after CpG ODN administration. Thus, intragastric administration of CpG ODN resulted in differential induction of MIP-1α and MIP-1β in the stomach and the small intestine.
FIG. 2.
Kinetics of MIP-1α and MIP-1β chemokine expression in the gastrointestinal mucosa after intragastric administration of CpG ODN: levels of MIP-1α in the stomach (A) and levels of MIP-1β in the intestine (B) after administration of CpG ODN. Mice were given 30 μg of CpG ODN intragastrically and sacrificed at various times after CpG ODN administration, and stomachs and small intestines were excised and after saponin extraction were subjected to ELISA.
Local production of RANTES after CpG ODN administration.
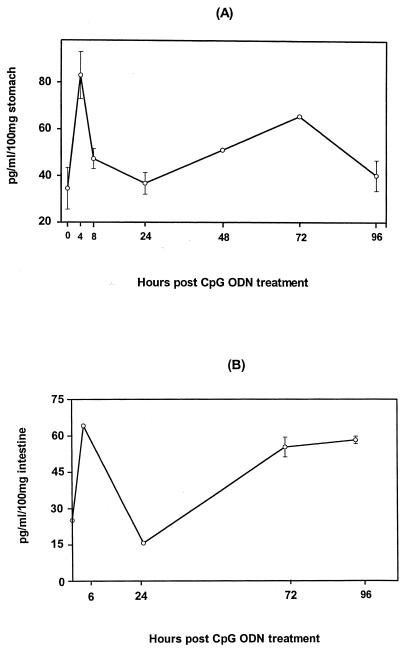
RANTES has been shown to be an important chemokine involved in the development of mucosal immune responses (18). We therefore examined the levels of RANTES in stomachs, small intestines, MLNs, and sera of mice at different times following intragastric administration of CpG ODN. As shown in Fig. 3A, the gastric level of RANTES increased within 4 h after CpG ODN administration and waned within 48 h; this was followed by a second wave of RANTES production on day 3, and the level remained markedly elevated for 7 days (the last time point examined). The level of RANTES in small intestine extracts dramatically increased within 4 h of CpG ODN treatment and stayed high for 7 days (the last time point examined) (Fig. 3B); however, the basal levels of RANTES in the MLNs and sera remained virtually unchanged throughout the test period (data not shown). Thus, CpG ODN stimulated rapid and sustained local RANTES production in the gastrointestinal tract mucosa.
FIG. 3.
Time course of RANTES production in the gastrointestinal tract after administration of CpG ODN. Mice were given 30 μg of CpG ODN intragastrically and sacrificed at various times after CpG ODN administration. Stomachs (A) and small intestines (B) were excised and after saponin extraction were subjected to ELISA.
H. pylori colonization in the stomachs of mice is reduced after CpG ODN administration.
To study the impact of CpG ODN administration on the course of an established H. pylori infection, groups of mice were infected with H. pylori SS1, and 2 weeks later (i.e., at the time when the infection was established) the animals were given either CpG ODN or non-CpG ODN intragastrically twice with a 1-week interval between the treatments. Three days after each dose of ODN, ODN-treated and untreated H. pylori-infected mice were sacrificed, and the bacterial loads in the stomachs were quantified. As shown in Table 1, the level of H. pylori in the infected control mice remained unchanged at the two observation times. After the first dose of CpG ODN, the level of bacterial colonization decreased by 40%, and this was followed by a further decrease 3 days after the second dose of CpG ODN when the data were compared to the data for control infected animals (Table 1) (P < 0.01). The numbers of bacteria in the stomachs of mice given non-CpG ODN were not reduced, and in some mice the numbers of bacteria were higher than the numbers in the control infected mice (Table 1). These data clearly demonstrate that intragastric administration of CpG ODN to H. pylori-infected mice results in suppression of bacterial colonization and that this effect of CpG ODN specifically depends upon the presence of the CpG motif.
TABLE 1.
Number of CFU after intragastric administration of CpG ODN or non-CpG ODN
| Dosea | CFU
(106)b
|
||
|---|---|---|---|
| Control | CpG ODN | non-CpG ODN | |
| First | 2.5 ± 0.8 | 1.5 ± 0.1 | NDc |
| Second | 2.5 ± 0.08 | 1.2 ± 0.04d | 3.3 ± 0.05 |
Mice were infected with H. pylori SS1, and 2 weeks later were given CpG ODN or non-CpG ODN or were left untreated (control). Three days after each dose mice were sacrificed, and the bacterial loads were quantified as described in Materials and Methods.
The data are geometric means ± standard errors of the means for each treatment group containing eight or nine mice.
ND, not determined.
P < 0.01 compared to control untreated mice.
CpG ODN has no direct antibacterial effect on H. pylori growth in vitro.
We then examined the possible direct antibacterial effect of CpG ODN on H. pylori replication using a disk diffusion test, as well as direct cultivation of bacteria in a liquid culture containing CpG ODN or non-CpG ODN. In the disk diffusion test neither CpG ODN nor non-CpG ODN had any effect on H. pylori growth (Table 2), while an expected broad zone of inhibition was observed around the positive control, the gentamicin disk. Similar results were obtained when we added CpG ODN or non-CpG ODN directly to bacteria growing in liquid cultures, and similar numbers of bacteria were recovered in control untreated, CpG ODN-treated, and non-CpG ODN-treated cultures (Table 2). Thus, CpG ODN does not directly inhibit multiplication of H. pylori. A slight insignificant reduction in the number of recovered bacteria was observed when a bacterial culture in liquid medium was treated with a high dose (100 or 50 μg/ml) of either ODN independent of the presence of the CpG motif (data not shown).
TABLE 2.
Effect of CpG ODN or non-CpG ODN ODN on the growth of H. pylori in vitroa
| Treatment | Disk
diffusion test (mm)
|
Liquid culture (CFU)
|
||
|---|---|---|---|---|
| 20 μg/disk | 5 μg/disk | 25 μg/ml | 5 μg/ml | |
| Gentamicin | 150 | 130 | 0 ± 0 | 0 ± 0 |
| CpG ODN | 1 | 1 | 1,666 ± 37 | 1,592 ± 188 |
| Non-CpG ODN | 1 | 0 | 1,661 ± 176 | 1,752 ± 67 |
| PBS | 1 | 1,800 ± 56 | ||
The values for the disk diffusion test are the diameters of the zones of inhibition. The values for the liquid culture assay are the numbers of CFU of H. pylori (mean ± standard deviation).
CpG ODN treatment elicits local production of RANTES and induces rapid influx of immune cells to the gastric mucosa of H. pylori-infected mice.
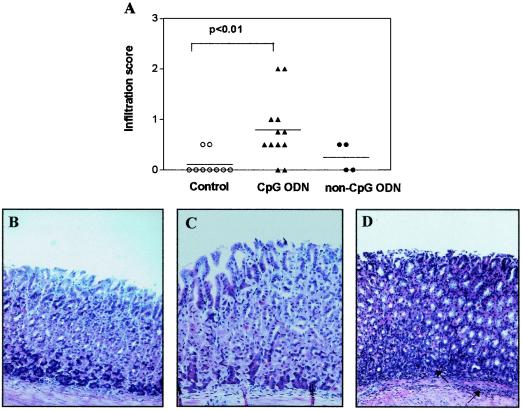
We next examined the levels of RANTES in the gastric mucosa of H. pylori-infected mice and H. pylori-infected, CpG ODN-treated mice. Groups of mice were infected with H. pylori SS1, and 2 weeks later the animals were given CpG ODN or were left untreated. Three days after the CpG ODN treatment, CpG ODN-treated mice, as well as untreated H. pylori-infected mice, were sacrificed, and the RANTES levels in their stomachs were quantified. H. pylori-infected mice did not have elevated levels of RANTES in their gastric mucosa compared to the levels found in naïve mice (60 ± 5 pg/ml/100 mg of tissue in naïve mice compared with 38 ± 12 pg/ml/100 mg of tissue in infected mice). In contrast, CpG ODN-treated, H. pylori-infected mice had increased levels of RANTES in their gastric mucosa (138 ± 54 pg/ml/100 mg of tissue) that were comparable to the levels seen in the gastric mucosa of uninfected mice 3 days after CpG ODN administration (Fig. 3). Given the importance of RANTES in the recruitment of immune cells to the gastric mucosa, we investigated the extent of immune cell infiltration in the gastric mucosa of infected mice treated with either CpG ODN or non-CpG ODN. Infected control mice had a mild acute influx of cells 2 weeks after infection (data not shown) that resolved 10 days later (Fig. 4A and B). Similar to the gastric mucosa of the infected control mice, the gastric mucosa of non-CpG ODN-treated H. pylori-infected mice had signs of only mild infiltration (Fig. 4A and C). Treatment of the infected mice with CpG ODN, on the other hand, led to a significant increase in the influx of immune cells to the gastric mucosa and submucosa that was more pronounced after the second dose of CpG ODN (P < 0.01) (Fig. 4A and D).
FIG. 4.
(A) Infiltration in the mucosa of H. pylori-infected mice after two doses of CpG ODN. Symbols: ▴, CpG ODN; •, non-CpG ODN; ○, untreated control. The horizontal lines indicate the means. (B to D) Histology of corpus mucosa of H. pylori-infected mice (B), H. pylori-infected, CpG ODN-treated mice (C), and H. pylori-infected, non-CpG ODN-treated mice (D). The mucosa of untreated H. pylori-infected mice or H. pylori-infected, non-CpG ODN-treated mice was normal, with minimal infiltration. The mucosa of CpG ODN-treated mice had infiltration of immune cells in both the lamina propria and the submucosa.
DISCUSSION
In the present study, we found that intragastrically administered immunostimulatory CpG ODN can strongly promote innate immunity in the murine gastrointestinal tract mucosa. Thus, intragastric administration of CpG ODN elicits strong local mucosal production of CC and CXC chemokines. Importantly, intragastric administration of CpG ODN, but not administration of non-CpG ODN, without any bacterial antigen codelivery suppressed bacterial colonization in the stomachs of mice with previously established H. pylori infections, which was concomitant with rapid recruitment of the immune cells to the gastric mucosa.
Immunostimulatory CpG DNA has been examined in a variety of systems for its ability to potentiate vaccine activity, but to date it has not been investigated directly for efficacy in induction of innate immunity in the gastric mucosa and in the control of H. pylori infection. The CpG motif evaluated in this report, 5′GACGTT3′, has been shown to be optimal for stimulation of the murine immune system when it is given systemically (37). Furthermore, it has recently been shown that CpG ODN 1826 containing two copies of this motif is capable of activating innate immunity in the murine female genital mucosa (10). Following intragastric-mucosal delivery of CpG ODN, we observed rapid and transient induction of IP-10 production both in the stomach and in the small intestine. Since the IP-10 receptor is mainly expressed on Th1 cells, it seems reasonable to speculate that IP-10 preferentially recruits Th1 cells to the gastric mucosa, which may in turn contribute to induction of protective immunity against H. pylori infection (1, 8). In addition to IP-10, we found that intragastric administration of CpG ODN in mice triggers local production of the CC chemokines RANTES, MIP-1α, and MIP-1β. These CC chemokines can be secreted by a variety of cell types, including macrophages, activated NK cells, and T cells (2). The common receptor for all three CC chemokines, CCR5 (22), is expressed on macrophages, dendritic cells, NK cells, and activated Th1 cells and could thus recruit these immunocompetent cells into the gastrointestinal mucosa of mice that received CpG ODN (32, 33). Recently, it was shown that a single vaginal-mucosal dose of CpG ODN elicits rapid Th1-like cytokine and chemokine responses in the female genital tract mucosa (10). Thus, it appears that CpG DNA is capable of inducing strong innate immunity at mucosal surfaces.
Using an immunohistochemistry technique, we documented the presence of macrophages in both the lamina propria and the epithelium of naïve mice, as well as mice infected with H. pylori (data not shown), proving that cells responding to CpG ODN are present in the gastric mucosa. Thus, it is tempting to speculate that within hours after intragastric CpG ODN administration, macrophages present in the gastric epithelium and lamina propria come into contact with CpG ODN and through TLR9 recognize CpG ODN, thus triggering a TLR9 downstream signaling pathway that eventually leads to production of CC and CXC chemokines either directly by macrophages in the tissue or indirectly through activation of epithelial and endothelial cells by CpG ODN-induced cytokines. This may explain the biphasic nature of the chemokine responses observed in the gastrointestinal mucosa after CpG ODN administration, in which the primary peak or phase of the chemokine responses is due to macrophages and epithelial cells in the tissue and the secondary peak or phase of the chemokine responses is due to recruited immune cells.Further studies to characterize the immune cells that infiltrate the gastric mucosa after CpG ODN administration would provide a better understanding of the mechanisms through which CpG ODN induces mucosal immunity at the gastric mucosa.
Interestingly, we found that intragastric administration of CpG ODN without bacterial antigen codelivery resulted in a consistent reduction in the bacterial load in the stomachs of mice with established H. pylori infection. The ODN lacking CpG motifs had absolutely no protective efficacy, supporting the conclusion that the effects observed were mediated solely by the contextual CpG motif. Moreover, our in vitro studies indicated that CpG ODN has no direct antibacterial effect. These data are in line with the recent observations made in our laboratory that a single mucosal-vaginal administration of CpG ODN (but not administration of non-CpG ODN) without viral antigen codelivery confers protective immunity against genital herpes simplex virus infection and disease (10). The present results are also in agreement with recent reports for other infectious disease models, in which systemic administration of CpG ODN, in the absence of any exogenous antigen, gave rise to protective innate immune responses against P. yoelii (9), L. monocytogenes (6, 13), and L. major (38). The observed reduction in the bacterial load of H. pylori-infected, CpG ODN-treated mice occurred concomitant with both up-regulation of RANTES production in the stomach and rapid recruitment of the immune cells to the gastric mucosa. It has been shown recently that H. pylori per se cannot up-regulate RANTES production in H. pylori-infected individuals (19, 36). Thus, the RANTES production induced by CpG ODN might be fundamentally important in rapid recruitment of mononuclear and polymorphonuclear cells to the site of infection, which eventually contributes to the control of bacterial infection. However, the effects of immunostimulatory CpG ODN on H. pylori infection could be multifaceted, involving different arms of both innate immunity and adaptive immunity. Immunostimulatory CpG ODN have been demonstrated to stimulate some of the effector arms of the immune system involved in immunity against H. pylori infection, including induction of Th1-associated immune responses (1). The positive effects of the CpG ODN on suppression of H. pylori colonization warrant continued evaluation, and we are currently expanding our studies to evaluate alternate formulations and delivery times to optimize the protection afforded by oral-mucosal CpG ODN administration.
In conclusion, we report in this study that oral-mucosal administration of CpG ODN induces local production of the CC chemokines MIP-1α and RANTES and the CXC chemokine IP-10 in the gastric mucosa. Importantly, intragastric administration of CpG ODN without bacterial antigen codelivery resulted in a significant reduction in the bacterial loads in the stomachs of mice with established H. pylori infections that occurred concomitant with recruitment of the immune cells to the stomach. To our knowledge, this is the first report of efficacy for oral-mucosal delivery of immunostimulatorty CpG ODN and induction of innate immunity against H. pylori infection in the gastric mucosa. These findings suggest that oral-mucosal delivery of CpG ODN may be a valuable supplement to the standard therapy for control of an ongoing H. pylori infection and in addition may also foster the development of subsequent adaptive antigen-specific immunity against H. pylori reinfection.
Acknowledgments
We thank the Swedish Medical Research Council (projects 6x-3382 and 6x-9084) and The Knut and Alice Wallenberg Foundation for financial support to GUVAX.
We thank Bing Ling for excellent technical assistance with the immunohistochemistry.
Editor: J. T. Barbieri
REFERENCES
- 1.Akhiani, A. A., J. Pappo, Z. Kabok, K. Schon, W. Gao, L. E. Franzen, and N. Lycke. 2002. Protection against Helicobacter pylori infection following immunization is IL-12-dependent and mediated by Th1 cells. J. Immunol. 169:6977-6984. [DOI] [PubMed] [Google Scholar]
- 2.Baggiolini, M., B. Dewald, and B. Moser. 1994. Interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Adv. Immunol. 55:97-179. [PubMed] [Google Scholar]
- 3.Bazzoli, F., P. Pozzato, and T. Rokkas. 2002. Helicobacter pylori: the challenge in therapy. Helicobacter 7(Suppl. 1):43-49. [DOI] [PubMed] [Google Scholar]
- 4.Blaser, M. J. 1990. Helicobacter pylori and the pathogenesis of gastroduodenal inflammation. J. Infect. Dis. 161:626-633. [DOI] [PubMed] [Google Scholar]
- 5.Boyanova, L., A. Mentis, M. Gubina, E. Rozynek, G. Gosciniak, S. Kalenic, V. Goral, L. Kupcinskas, B. Kantarceken, A. Aydin, A. Archimandritis, D. Dzierzanowska, A. Vcev, K. Ivanova, M. Marina, I. Mitov, P. Petrov, A. Ozden, and M. Popova. 2002. The status of antimicrobial resistance of Helicobacter pylori in eastern Europe. Clin. Microbiol. Infect. 8:388-396. [DOI] [PubMed] [Google Scholar]
- 6.Elkins, K., T. Rhinehart-Jones, S. Stibitz, J. Conover, and D. Klinman. 1999. Bacterial DNA containing CpG motifs stimulates lymphocyte-dependent protection of mice against lethal infection with intracellular bacteria. J. Immunol. 162:2291-2298. [PubMed] [Google Scholar]
- 7.Ermak, T. H., P. J. Giannasca, R. Nichols, G. A. Myers, J. Nedrud, R. Weltzin, C. K. Lee, H. Kleanthous, and T. P. Monath. 1998. Immunization of mice with urease vaccine affords protection against Helicobacter pylori infection in the absence of antibodies and is mediated by MHC class II-restricted responses. J. Exp Med. 188:2277-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garhart, C. A., R. W. Redline, J. G. Nedrud, and S. J. Czinn. 2002. Clearance of Helicobacter pylori infection and resolution of postimmunization gastritis in a kinetic study of prophylactically immunized mice. Infect. Immun. 70:3529-3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gramzinski, R., D. Doolan, M. Sedegah, H. Davis, A. Krieg, and S. Hoffman. 2001. Interleukin-12- and gamma interferon-dependent protection against malaria conferred by CpG oligodeoxynucleotide in mice. Infect. Immun. 69:1643-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harandi, A. M., K. Eriksson, and J. Holmgren. 2003. A protective role of locally administered immunostimulatory CpG oligodeoxynucleotide in a mouse model of genital herpes infection. J. Virol. 77:953-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740-745. [DOI] [PubMed] [Google Scholar]
- 12.Johansson, E. L., C. Rask, M. Fredriksson, K. Eriksson, C. Czerkinsky, and J. Holmgren. 1998. Antibodies and antibody-secreting cells in the female genital tract after vaginal or intranasal immunization with cholera toxin B subunit or conjugates. Infect. Immun. 66:514-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krieg, A., L. Love-Homan, A. Yi, and J. Harty. 1998. CpG DNA induces sustained IL-12 expression in vivo and resistance to Listeria monocytogenes challenge. J. Immunol. 161:2428-2434. [PubMed] [Google Scholar]
- 14.Krieg, A., A. Yi, S. Matson, T. Waldschmidt, G. Bishop, R. Teasdale, G. Koretzky, and D. Klinman. 1995. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 374:546-549. [DOI] [PubMed] [Google Scholar]
- 15.Krieg, A. M. 2002. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 20:709-760. [DOI] [PubMed] [Google Scholar]
- 16.Lauw, F. N., A. J. H. Simpson, J. M. Prins, S. J. H. Van Deventer, W. Chaowagul, N. J. White, and T. Van der Pol. 2000. The CXC chemokines gamma interferon (IFN-γ)-inducible protein 10 and monokine induced by IFN-γ are released during severe melioidosis. Infect. Immun. 68:3888-3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee, A., J. O'Rourke, M. C. De Ungria, B. Robertson, G. Daskalopoulos, and M. F. Dixon. 1997. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology 112:1386-1397. [DOI] [PubMed] [Google Scholar]
- 18.Lillard, J. W., P. N. Boyaka, D. D. Taub, and J. R. McGhee. 2001. RANTES potentiates antigen-specific mucosal immune responses. J. Immunol. 166:162-169. [DOI] [PubMed] [Google Scholar]
- 19.Lindholm, C., M. Quiding-Jarbrink, H. Lonroth, and A. M. Svennerholm. 2001. Induction of chemokine and cytokine responses by Helicobacter pylori in human stomach explants. Scand J. Gastroenterol. 36:1022-1029. [DOI] [PubMed] [Google Scholar]
- 20.Lipford, G., T. Sparwasser, M. Bauer, S. Zimmermann, E. Koch, K. Heeg, and H. Wagner. 1997. Immunostimulatory DNA: sequence-dependent production of potentially harmful or useful cytokines. Eur. J. Immunol. 27:3420-3426. [DOI] [PubMed] [Google Scholar]
- 21.Luster, A. D. 2002. The role of chemokines in linking innate and adaptive immunity. Curr. Opin. Immunol. 14:129-135. [DOI] [PubMed] [Google Scholar]
- 22.Moser, B., and P. Loetscher. 2001. Lymphocyte traffic control by chemokines. Nat. Immunol. 2:123-128. [DOI] [PubMed] [Google Scholar]
- 23.Pappo, J., D. Torrey, L. Castriotta, A. Savinainen, Z. Kabok, and A. Ibraghimov. 1999. Helicobacter pylori infection in immunized mice lacking major histocompatibility complex class I and class II functions. Infect. Immun. 67:337-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raghavan, S., A. M. Svennerholm, and J. Holmgren. 2002. Effects of oral vaccination and immunomodulation by cholera toxin on experimental Helicobacter pylori infection, reinfection, and gastritis. Infect. Immun. 70:4621-4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rollins, B. J. 1997. Chemokines. Blood 90:909-928. [PubMed] [Google Scholar]
- 26.Salazar-Mather, T. P., T. A. Hamilton, and C. A. Biron. 2000. A chemokine-to-cytokine-to-chemokine cascade critical in antiviral defense. J. Clin. Investig. 105:985-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sparwasser, T., E. S. Koch, R. M. Vabulas, K. Heeg, G. B. Lipford, G. B. Ellwart, and H. Wagner. 1998. Bacterial DNA and immunostimulatory CpG oligonucleotides trigger maturation and activation of murine dendritic cells. Eur. J. Immunol. 28:2045-2054. [DOI] [PubMed] [Google Scholar]
- 28.Sparwasser, T., T. Miethke, G. Lipford, A. Erdmann, H. Hacker, K. Heeg, and H. Wagner. 1997. Macrophages sense pathogens via DNA motifs: induction of tumor necrosis factor-alpha-mediated shock. Eur. J. Immunol. 27:1671-1679. [DOI] [PubMed] [Google Scholar]
- 29.Suri-Payer, E., and H. Cantor. 2001. Differential cytokine requirements for regulation of autoimmune gastritis and colitis by CD4+ CD25+ T cells. J. Autoimmun. 16:115-123. [DOI] [PubMed] [Google Scholar]
- 30.Sutton, P., S. J. Danon, M. Walker, L. J. Thompson, J. Wilson, T. Kosaka, and A. Lee. 2001. Post-immunisation gastritis and Helicobacter infection in the mouse: a long term study. Gut 49:467-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sutton, P., J. Wilson, and A. Lee. 2000. Further development of the Helicobacter pylori mouse vaccination model. Vaccine 18:2677-2685. [DOI] [PubMed] [Google Scholar]
- 32.Taub, D. D., K. Conlon, A. R. Lloyd, J. J. Oppenheim, and D. J. Kelvin. 1993. Preferential migration of activated CD4+ and CD8+ T cells in response to MIP-1 alpha and MIP-1 beta. Science 260:355-358. [DOI] [PubMed] [Google Scholar]
- 33.Uguccioni, M., M. D'Apuzzo, M. Loetscher, B. Dewald, and M. Baggiolini. 1995. Actions of the chemotactic cytokines MCP-1, MCP-2, MCP-3, RANTES, MIP-1 alpha and MIP-1 beta on human monocytes. Eur. J. Immunol. 25:64-68. [DOI] [PubMed] [Google Scholar]
- 34.Wagner, H. 2002. Interactions between bacterial CpG-DNA and TLR9 bridge innate and adaptive immunity. Curr. Opin. Immunol. 5:62-69. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto, S., T. Yamamoto, T. Kataoka, E. Kuramoto, O. Yano, and T. Tokunaga. 1992. Unique palindromic sequences in synthetic oligonucleotides are required to induce IFN-gamma and augment IFN-gamma-mediated natural killer activity. J. Immunol. 148:4072-4076. [PubMed] [Google Scholar]
- 36.Yamaoka, Y., M. Kita, T. Kodama, N. Sawai, T. Tanahashi, K. Kashima, and J. Imanishi. 1998. Chemokines in the gastric mucosa in Helicobacter pylori infection. Gut 42:609-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yi, A.-K., M. Chang, D. Peckham, A. Krieg, and R. Ashman. 1998. CpG oligodeoxyribonucleotides rescue mature spleen B cells from spontaneous apoptosis and promote cell cycle entry. J. Immunol. 160:5898-5906. [PubMed] [Google Scholar]
- 38.Zimmermann, S., O. Egeter, S. Hausmann, G. Lipford, M. Rocken, H. Wagner, and K. Heeg. 1998. CpG oligodeoxynucleotides trigger protective and curative Th1 responses in lethal murine leishmaniasis. J. Immunol. 160:3627-3630. [PubMed] [Google Scholar]
- 39.Zlotnick, A., and O. Yoshie. 2000. Chemokines: a new classification system and their role in immunity. Immunity 12:121-127. [DOI] [PubMed] [Google Scholar]