Abstract
Experimental studies suggest that nitric oxide produced by endothelial nitric oxide synthase (NOS3) plays a role in maintaining cerebral blood flow (CBF) after traumatic brain injury (TBI). The purpose of this study was to determine if common variants of the NOS3 gene contribute to hypoperfusion after severe TBI. Fifty-one patients with severe TBI were studied. Cerebral hemodynamics, including global CBF by the stable xenon computed tomography (CT) technique, internal carotid artery flow volume (ICA-FVol), and flow velocity in intracranial vessels, were measured within 12 h of injury, and at 48 h after injury. A blood sample was collected for DNA analysis, and genotyping of the following variants of the NOS3 gene was performed: −786T>C, 894G>T, and 27bp VNTR. Cerebral hemodynamics were most closely related to the−786T>C genotype. CBF averaged 57.7±3.0 mL/100 g/min with the normal T/T genotype, 47.0±2.5 mL/100 g/min with the T/C, and 37.3±8.8 mL/100 g/min with the C/C genotype (p=0.0146). Cerebrovascular resistance followed an inverse pattern with the highest values occurring with the C/C genotype (p=0.0027). The lowest ICA-FVol of 124±43 mL/min was found at 12 h post-injury in the more injured hemisphere of the patients with the C/C genotype (p=0.0085). The mortality rate was 20% in patients with the T/T genotype and 17% with the T/C genotype. In contrast, both of the patients with the C/C genotype were dead at 6 months post-injury (p=0.022). The findings in this study support the importance of NO produced by NOS3 activity in maintaining CBF after TBI, since lower CBF values were found in patients having the −786C allele. The study suggests that a patient's individual genetic makeup may contribute to the brain's response to injury and determine the patient's chances of surviving the injury. The results here will need to be studied in a larger number of patients, but could explain some of the variability in outcome that occurs following severe TBI.
Key words: CBF autoregulation, cerebral vascular disease, head trauma, vascular reactivity, traumatic brain injury
Introduction
Endothelial nitric oxide synthase (NOS3) plays a role in the maintenance of basal cerebral blood flow (CBF), and may have some activity in CO2 reactivity and pressure autoregulation (Buchanan and Phyllis, 1993; Kobari et al., 1994; Reutens et al., 1997; White et al., 2000). Under pathologic conditions, reduction in nitric oxide (NO) produced by NOS3 could result in hypoperfusion of the injured brain. Following experimental traumatic brain injury (TBI), the concentration of NO is reduced in injured brain after cortical impact injury (Cherian et al., 2000), impact acceleration injury (Tuzgen et al., 2003), and fluid percussion injury (Ahn et al., 2004). Constitutive NOS activity is reduced between 1 and 7 days after injury (Wada et al., 1998). Rats treated post-injury with L-arginine, which is the substrate for NOS3, have partial recovery of cerebral blood flow (CBF) and reduced brain injury (Cherian and Robertson, 2003b; Cherian et al., 2003a). Transgenic mice that are deficient in NOS3 have lower CBF post-injury, and their CBF does not increase with infusion of L-arginine (Hlatky et al., 2003).
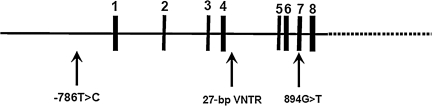
The human NOS3 gene is located at 7q35-36 and contains 26 exons that span 21 kb (Marsden et al., 1993). Several allelic variations of the gene have been identified and evaluated for possible links to cardiovascular and cerebrovascular disease. The following three variants are common in the general population: −786T>C in the 5′-flanking region, a 27-bp deletion(a)/insertion(b) in intron 4, and 894G>T in exon 7 (Fig. 1).
FIG. 1.
The nitric oxide synthase 3 (NOS3) gene consists of 26 exons (vertical lines) spanning 21 kb on the chromosome. The three polymorphisms that were studied are indicated by the arrows. The −786T>C location is in the promoter region. The 27-bp VNTR location is in intron 4, and the 894G>T location is in exon 7.
Variations in the NOS3 gene promoter region, such as −786T>C, have the potential to affect transcription, and therefore could alter enzyme levels directly. The −786C allele has been associated with some functional changes in endothelial cell responses. Decreased NOS3 mRNA in umbilical vein endothelial cells and lower serum nitrate/nitrite levels have been reported in normal subjects with the −786C allele (Miyamoto et al., 2000). Subjects who are homozygous C/C have been observed to have decreased maximal forearm blood flow response to acetylcholine, a response that suggests reduced capacity to produce NO (Rossi et al., 2003). In vitro studies in cultured endothelial cells demonstrate differences in protein expression response to shear stress, with homozygous C/C cells exhibiting a reduced NOS3 expression and a decreased NO synthesis capacity (Asif et al., 2009). The −786T>C variant has been associated with coronary artery vasospasm and cerebral vasospasm.
Variations in the NOS3 gene at the open reading frame at exon 7 alter the primary structure of the protein and therefore have the potential to alter one or more functional properties of the enzyme directly. Substitution of thymine for guanine at the 894 locus leads to an aspartate substitution for glutamate at position 298 in the protein. An association between the 894T allele and reduced NO synthesis and impaired endothelial function have been described (Leeson et al., 2002; Persu et al., 2002; Savvidou et al., 2001;Tesauro et al., 2000). However, a difference in enzymatic activity in endothelial cells measured in vitro has not been observed (McDonald et al., 2004). The 894G>T variant has been associated with coronary artery disease, myocardial infarction, essential hypertension, and carotid atherosclerosis.
Variations in NOS3 gene introns are the most common variants, but variation at this location would be expected to have minimal physiological effects. Although the RNA transcripts derived from such a variant and from normal NOS3 alleles would differ, the intron sequences would be excised during RNA processing. The NO produced by variant and normal NOS3 would be identical; however, the rates of transcription could be reduced. It is also possible that this variant is in linkage disequilibrium with other variants that do have functional consequences. The 27-bp VNTR variant has been associated with brain and aortic aneurysms, coronary artery disease, and acute myocardial infarction.
Limited work has been done relating NOS3 variants to cerebrovascular physiology. One study in normal subjects showed an association between the −786T>C variant and a reduced basal CBF in smokers, but not in nonsmokers (Nasreen et al., 2002). Field and associates (Field et al., 2001) observed an association between the −786T>C variant and the development of cerebral vasospasm after subarachnoid hemorrhage in a pilot study of 21 patients. Six of these 21 patients developed severe vasospasm. Patients who were homozygous C/C were significantly more likely to develop severe cerebral vasospasm compared to those who were homozygous T/T (odds ratio=14; 95% confidence interval [CI[ 1.05,2.00). Ko and colleagues (Ko et al., 2008) reported similar findings in a cohort study of 347 patients with subarachnoid hemorrhage. The C/C genotype compared with the T/C and T/T genotypes was associated with an increased risk of angiographic cerebral vasospasm (odds ratio 2.97, 95% CI 1.32,6.67, p=0.008). The risk of cerebral aneurysm rupture has also been associated with the 27-bp VNTR, −786T>C, and 894G>T variants of the NOS3 gene (Khurana et al., 2005). Subjects with the haplotype consisting of the variant allele for each locus (4a/-786C/894T) had an odds ratio of 11.4 (95% CI 1.8,41.3), and an increased risk of aneurysm rupture over subjects with normal alleles at each locus.
The role of NOS3 variants in acute stroke remains controversial, and most studies have concentrated on the susceptibility to developing a stroke rather than on the cerebrovascular response to stroke. Relatively small studies have shown variable associations between individual NOS3 variants and NOS3 haplotypes with the risk of stroke (Howard et al., 2005; Majumdar et al., 2010; Saidi et al., 2010; Song et al., 2010; Yemisci et al., 2009). A meta-analysis of case-controlled studies through 2008 examining the 894G>T and 27-bp VNTR variants of NOS3 in stroke suggested that the 4a allele was not associated with an increased risk, but that the 894T allele may have a marginally increased risk of stroke (odds ratio 1.14; 95% CI 0.99,1.31; Tao and Chen, 2009).
Since variants of NOS3 appear to have some association with other cerebrovascular disorders, and because experimental studies suggest a role for NOS3 in preserving CBF after TBI, it is reasonable to hypothesize that NOS3 variants might impair the ability of the injured brain to maintain an adequate CBF after TBI. The purpose of this study was to examine the effect of three common variants of the NOS3 gene on cerebral hemodynamics in patients with severe TBI.
Methods
Patient selection and clinical management
A total of 51 patients with severe TBI (motor component of the Glasgow Coma Scale score [GCS] ≤5) admitted to Ben Taub General Hospital in Houston, Texas were studied. Patients who arrived more than 12 h after injury, who were hypotensive after resuscitation, or who had unstable intracranial pressure (ICP) were excluded from the study. The study was approved by the Institutional Review Board of the Baylor College of Medicine, and informed consent was obtained from the patients' families.
Upon arrival in the emergency department, the patients immediately underwent intubation, hemodynamic stabilization, and resuscitation when necessary. Patients in whom an intracranial mass lesion was demonstrated on the initial computed tomography (CT) scan underwent a neurosurgical procedure to evacuate the hematoma. ICP, mean arterial blood pressure (MAP), end-tidal CO2 (ETCO2), arterial and jugular venous oxygen saturation (SaO2 and SjvO2), and brain tissue oxygen (PbtO2) were monitored during the acute post-injury period. The PbtO2 probe was positioned in peri-contusional brain tissue when possible. Otherwise the PbtO2 probe was placed in the white matter of the right frontal lobe. The goals of the early management were to keep ICP<20 mm Hg and cerebral perfusion pressure (CPP)>60 mm Hg, unless the SjvO2 and/or PbtO2 values indicated that a higher level of CPP was needed.
Study protocol
Baseline cerebral hemodynamic tests were performed at two different time periods: first at approximately 12 h post-injury and again at 48 h post-injury. All measurements were performed after resuscitation and surgical removal of mass lesions if needed. The 12-h time period was chosen as a time when the patient is usually well-resuscitated, but CBF is often still low, and the 48-h time period was chosen because CBF has usually recovered, but pressure autoregulation is typically at its nadir (Hlatky et al., 2002).
The following parameters were obtained at each time period: ICP, MAP, ETCO2, SaO2, and several indices of cerebral perfusion (SjvO2, PbtO2, internal carotid artery flow volume [ICA-FVol], flow velocity in the anterior cerebral artery [ACA-FV], middle cerebral artery [MCA-FV], posterior cerebral artery [PCA-FV], and internal carotid artery [ICA-FV], carbon dioxide reactivity [CO2R], and dynamic pressure autoregulation), as well as blood and cerebrospinal fluid (CSF) samples for nitrate/nitrite levels. For the flow velocity and flow volume measurements, CO2R, and dynamic pressure autoregulation parameters, both left and right sides were measured, and these were designated as the “better” or “worse” side, depending on the appearance of the injury on the admission CT scan in the individual patient. The hemisphere with the most severe TBI on the initial CT scan was designated the “worse” side.
ICA-FVol was measured using an angle-independent dual-beam Doppler ultrasound device. ICA-FVol measured with this device has good correlation with hemispheric cerebral blood flow measured using the 133Xenon clearance technique (Soustiel et al., 2003) The FV measurements were performed on a DWL MultiDop transcranial Doppler (Compumedics DWL, Singen, Germany). CO2R was calculated by the following formula: CO2R=(ΔMCA-FV * 100%/baseline MCA-FV) / ΔpCO2). Dynamic pressure autoregulation was assessed by two different methods: the cuff deflation technique and the carotid compression technique.
For the cuff deflation method, the autoregulation index (ARI) was calculated by the method of Aaslid and associates (Aaslid et al., 1989). Three determinations of ARI were averaged to give a single baseline value. Mean blood pressure and bilateral middle cerebral artery flow velocity were recorded continuously before and after a step drop in blood pressure. The thigh cuff deflation technique was used to induce the drop in blood pressure. Large bilateral thigh cuffs were inflated to 20 mm Hg above the systolic arterial pressure for a period of 2 min. A transient blood pressure drop was induced by rapid deflation of the thigh cuffs. A drop in blood pressure of at least 12 mm Hg was required to be considered a valid cuff deflation test (Hlatky et al., 2006).
An ARI ranging from 0 to 9 was calculated by the method proposed by Tiecks and associates (Tiecks et al., 1995), and implemented in the DWL MultiDop transcranial Doppler software (Compumedics DWL). Briefly, the recorded flow velocity response to the cuff deflation was compared with the 10 models constructed using the mean arterial pressure as the input function. Each model was generated from the arterial tracing by a specific combination of time constant, damping factor, and autoregulatory dynamic gain. The closest match was selected based on the highest correlation coefficient. Normal ARI using this method was 5.0±1.1 (Tiecks et al., 1995).
Dynamic pressure autoregulation was also assessed using carotid compression technique. The transient hyperemic response ratio (THRR) following release of unilateral carotid compression for 5 sec was calculated by the method of Smielewski and colleagues (Smielewski et al., 1996). Three determinations of THRR were averaged to give a single value. Normal values for THRR are mean 1.20 (95% CI 1.17,1.24; Smielewski et al., 1996).
Blood and CSF samples were obtained for measurement of nitrate and nitrite (NOx) levels. These samples were analyzed using a multiwell plate fluorometric assay in which nitrate was converted to nitrite using nitrate reductase, yielding total NOx, which converts 2,3-diaminonaphthalene to a measureable fluorescent product, 1(H)-naphthotriazide (Caymen Chemical Company, Ann Arbor, MI).
Genotyping
Genomic DNA was extracted from peripheral whole blood in each patient. Genotyping was performed using sense and anti-sense polymerase chain reaction using target-appropriate primers for the single nucleotide polymorphisms −786T>C and 894G>T with sequencing of the products (Applied Biosystems, Prism, now Life Technologies Inc., Carlsbad, CA). For the NOS3 −786T>C variant, the primer sequences used were 5′-GGG-ATGTCACCGTGTCTTGA-3′ (forward), and 5′-ATG-ACTCAAGTGGGGGACAC-3′ (reverse). For the NOS3 894G>T variant, the primers used were 5′-CTG-GAGATGAAGGCAGGAGAC-3′ (forward), and 5′-CTC-CATCCCACCCAGTCAATC-3′ (reverse). VNTRs were also performed using sense and anti-sense primers positioned on either side of the a 27-bp deletion(a)/insertion(b) in intron 4, followed by sequencing. For the NOS3 27-bp sequence repeat polymorphism, primers with the following sequences were used: 5′-CCT-GGTTATCAGGCCCTATG-3′ (forward), and 5′-GTTTCT-TAGGCTGCTCCTGCTACTGAC-3′ (reverse). If there was any ambiguity regarding the number of tandem repeats, confirmatory gel electrophoresis was performed.
Statistical analysis
Physiological parameters at 12 and 48 h after injury were analyzed using a general linear mixed model. The analysis method was chosen because the data were from multiple assessments over time. This model provides unbiased estimates of the random effect, flexibility in the choice of the variance-covariance structures of the model, and maximum likelihood estimation. There were two observations per day across 2 days. There was an effect of genotype and day, and for the TCD variables there was an effect of side. In addition, there was an interaction term that tested whether the variable varied significantly by genotype, by day, and by side (when appropriate). To correct for type 1 error rate, the Benjamini-Hochberg method was used after examining the results for normality using the Cramer von Mises test (Benjamini and Hochberg, 1995). This method orders the tests by significance, and tests against alpha/order of the significance. The smallest significance is tested against alpha, the second smallest is tested against alpha/2, etc. Summary data are expressed as mean±standard error.
Results
Patient characteristics
The distribution of the genotypes for the three NOS3 gene variants in the 51 TBI patients is summarized in Table 1. The distributions of genotypes for the three variants showed no deviation from the Hardy Weinberg equilibrium. The estimated haplotype frequencies are shown in Table 2. There were no differences in demographic characteristics, injury severity measures (including initial GCS and pupil reactivity, initial CT scan characteristics, presence of pre-hospital hypoxia and hypotension, initial hemoglobin, and glucose level), or predicted outcome among the different genotype groups (Table 3).
Table 1.
Genotype Distributions for the Three NOS3 Variants
| |
NOS3 variant |
||
|---|---|---|---|
| -786T>C | 894G>T | 27-bp VNTR | |
| Allele distribution: | T/T=25 (49.0%) | G/G=35 (68.6%) | 4a/4a=4 (7.8%) |
| T/C=24 (47.1%) | G/T=15 (29.4%) | 4a/4b=16 (31.4%) | |
| C/C=2 (3.9%) | T/T=1 (2.0%) | 4b/4b=31 (60.8%) | |
| HWE: X2 | 0.98 | 0.05 | 0.84 |
| p Value | NS | NS | NS |
| Allele frequency | |||
| p | 0.73 | 0.84 | 0.76 |
| q | 0.27 | 0.16 | 0.24 |
HWE, Hardy Weinberg Equilibrium; NOS, nitric oxide synthase.
Table 2.
Haplotype Frequencies
| Haplotype | Frequency |
|---|---|
| T-G-4b | 0.5215 |
| T-G-4a | 0.1140 |
| T-T-4b | 0.0755 |
| T-T-4a | 0.0144 |
| C-G-4b | 0.0909 |
| C-G-4a | 0.1069 |
| C-T-4b | 0.0768 |
| C-T-4a | 0.0000 |
Table 3.
Demographic, Injury Severity, and Mortality Rate by the Three NOS3 Variants
| |
-786T>C |
|
894G>T |
|
27-bp VNTR |
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T/T | T/C | C/C | p Value | G/G | G/T | T/T | p Value | 4a/4a | 4a/4b | 4b/4b | p Value | |
| Demographic characteristics | ||||||||||||
| Number | 25 | 24 | 2 | 35 | 15 | 1 | 4 | 16 | 31 | |||
| Age | 38.2±2.9 | 31.1±2.3 | 44.0±23.0 | 0.128 | 35.5±2.4 | 33.9±3.5 | 41 | 0.717 | 38.0±5.7 | 34.9±3.3 | 34.8±2.7 | 0.911 |
| Race: African-American | 9 (36%) | 8 (33%) | 1 (50%) | 0.635 | 14 (40%) | 4 (27%) | 0 | 0.433 | 1 (25%) | 10 (63%) | 7 (23%) | 0.093 |
| Asian | 2 (8%) | 0 | 0 | 2 (6%) | 0 | 0 | 0 | 0 | 2 (6%) | |||
| Caucasian | 8 (32%) | 11 (46%) | 0 | 13 (37%) | 6 (40%) | 0 | 0 | 3 (19%) | 9 (29%) | |||
| Hispanic | 6 (24%) | 5 (21%) | 1 (50%) | 6 (17%) | 5 (33%) | 1 (100%) | 3 (75%) | 3 (19%) | 13 (42%) | |||
| Admission injury severity characteristics | ||||||||||||
| Motor GCS | 3.2±0.4 | 3.3±0.4 | 3.5±2.5 | 0.947 | 3.2±0.3 | 3.3±0.4 | 5 | 0.864 | 2.8±0.9 | 3.5±0.5 | 3.2±0.3 | 0.751 |
| Reactive pupils: Both | 13 (52%) | 14 (58%) | 1 (50%) | .883 | 19 (54%) | 8 (53%) | 1 (100%) | .902 | 2 (50%) | 9 (56%) | 15 (48%) | 0.948 |
| Reactive pupils: One | 5 (20%) | 3 (13%) | 0 | 5 (14%) | 3 (20%) | 0 | 1 (25%) | 3 (19%) | 5 (16%) | |||
| Reactive pupils: None | 7 (28%) | 7 (29%) | 1 (50%) | 11 (31%) | 4 (27%) | 0 | 1 (25%) | 4 (25%) | 11 (35%) | |||
| CT code: D1/D2 | 6 (24%) | 9 (38%) | 0 | 0.455 | 11 (31%) | 3 (20%) | 1 (100%) | 0.073 | 1 (24%) | 5 (31%) | 12 (39%) | 0.882 |
| CT code: D3/D4 | 6 (24%) | 6 (25%) | 0 | 5 (14%) | 7 (47%) | 0 | 1 (24%) | 2 (13%) | 6 (19%) | |||
| CT code: EM | 13 (52%) | 9 (38%) | 2 (100%) | 19 (54%) | 5 (33%) | 0 | 2 (50%) | 9 (56%) | 13 (42%) | |||
| SAH, yes | 20 (80%) | 19 (79%) | 1 (50%) | 0.607 | 27 (77%) | 12 (80%) | 1 (100%) | 0.859 | 3 (75%) | 13 (81%) | 24 (77%) | 0.941 |
| SAH, no | 5 (20%) | 5 (21%) | 1 (50%) | 8 (23%) | 3 (20%) | 0 | 1 (25%) | 3 (19%) | 7 (23%) | |||
| EDH, yes | 5 (20%) | 5 (21%) | 0 | 0.774 | 7 (20%) | 3 (20%) | 0 | 0.883 | 0 | 4 (25%) | 2 (6%) | 0.130 |
| EDH, no | 20 (80%) | 19 (79%) | 2 (100%) | 28 (80%) | 12 (80%) | 1 (100%) | 4 (100%) | 12 (75%) | 29 (94%) | |||
| Hypoxia, yes | 4 (16%) | 4 (17%) | 0 | 0.822 | 7 (20%) | 1 (7%) | 0 | 0.449 | 2 (50%) | 2 (13%) | 4 (13%) | 0.145 |
| Hypoxia, no | 21 (84%) | 20 (83%) | 2 (100%) | 28 (80%) | 14 (93%) | 1 (100%) | 2 (50%) | 14 (87%) | 27 (87%) | |||
| Hypotension, yes | 3 (12%) | 2 (8%) | 0 | 0.814 | 7 (20%) | 1 (7%) | 0 | 0.449 | 1 (25%) | 3 (19%) | 3 (10%) | 0.549 |
| Hypotension, no | 22 (88%) | 22 (92%) | 2 (100%) | 28 (80%) | 14 (93%) | 1 (100%) | 3 (75%) | 13 (81%) | 28 (90%) | |||
| Hemoglobin (g/dL) | 9.5±1.7 | 12.7±1.0 | 13.0±1.0 | 0.290 | 13.4±0.4 | 13.4±0.4 | 13.5 | 0.961 | 12.1±1.7 | 13.8±0.5 | 13.3±0.4 | 0.389 |
| Glucose (mmol/L) | 9.6±0.7 | 9.7±1.0 | 8.7±1.0 | 0.935 | 9.1±0.4 | 9.8±0.8 | 5.9 | 0.523 | 10.8±2.6 | 8.0±0.4 | 9.7±0.5 | 0.109 |
| Injury Severity Score | 31.4±1.7 | 32.0±1.8 | 37.5±12.5 | 0.658 | 30.9±1.4 | 34.7±2.5 | 20 | 0.163 | 36.7±4.6 | 31.6±1.9 | 31.5±1.7 | 0.539 |
| Predicted outcome | ||||||||||||
| IMPACT risk of death | 0.36±0.04 | 0.29±0.05 | 0.37±0.18 | 0.519 | 0.34±0.04 | 0.33±0.05 | 0.10 | 0.948 | 0.48±0.11 | 0.28±0.04 | 0.34±0.04 | 0.254 |
| IMPACT risk of poor outcome | 0.54±0.05 | 0.43±0.06 | 0.55±0.25 | 0.331 | 0.50±0.05 | 0.49±0.07 | 0.20 | 0.955 | 0.68±0.10 | 0.44±0.07 | 0.49±0.05 | 0.272 |
| Alive at 6 months, yes | 20 (80%) | 20 (83%) | 0 | 0.022 | 30 (86%) | 10 (67%) | 1 (100%) | 0.264 | 3 (75%) | 13 (81%) | 25 (81%) | 0.960 |
| Alive at 6 months, no | 5 (20%) | 4 (17%) | 2 (100%) | 5 (14%) | 5 (33%) | 0 | 1 (25%) | 3 (19%) | 6 (19%) | |||
GCS, Glasgow Coma Scale; CT, computed tomography; D1/D2, diffuse injury 1 and 2 by Marshall CT category; D3/D4, diffuse injury 3 and 4 by Marshall CT category; EM, evacuated mass lesion by Marshall CT category; SAH, subarachnoid hemorrhage; EDH, epidural hematoma; IMPACT risk of death and risk of poor outcome, predicted outcome by IMPACT indices; NOS, nitric oxide synthase.
For the −786T>C variant, the mortality rate was significantly different among the genotypes (p=0.022). The two patients who were homozygous C/C both died by 6 months post-injury. In contrast, the mortality rate for patients who were heterozygous T/C was 17%, and for patients who were homozygous for the normal T/T was 20%. Mortality rates for the 894G>T and the 27-bp VNTR genotype groups were not significantly different.
Cerebral hemodynamics by −786T>C genotype
The cerebral hemodynamics are summarized in Table 4 for the patients with the different genotypes of the −786T>C variant. Patients who had the normal T/T genotype had the highest CBF and ICA-FVol, and the lowest CVR.
Table 4.
Cerebral Hemodynamic Variables by −786T>C Genotypes
| |
-786T>C genotype |
p Values for main effects |
|
||||
|---|---|---|---|---|---|---|---|
| T/T | T/C | C/C | Genotype | Side | Day | p Values for interactions | |
| Flow variables | |||||||
| Ave CBF | 57.7±3.0 | 47.0±25* | 37.3±8.8 | 0.0146† | 0.4468 | ||
| Ave CVR | 1.42±0.09 | 1.80±0.11* | 2.34±0.73* | 0.0027† | 0.0149† | ||
| ICA-FVol | 189.7±8.9 | 167.4±9.7 | 167.0±30.2 | 0.2294 | 0.8666 | 0.5182 | genotype×day×side; p=0.0085† |
| Flow velocity and reactivity variables | |||||||
| ICA-FV | 62.2±2.8 | 66.6±2.9 | 71.6±10.2 | 0.4275 | 0.2406 | 0.0002† | |
| ACA-FV | 52.3±1.0 | 57.0±1.0 | 58.6±1.1 | 0.3597 | 0.3309 | 0.0001† | |
| MCA-FV | 71.8±1.0 | 80.9±1.0* | 71.7±1.1 | 0.0456 | 0.3466 | <0.0001† | |
| PCA-FV | 56.8±1.0 | 65.1±1.0* | 61.2±1.1 | 0.2582 | 0.4295 | <0.0001† | |
| ARI | 2.29±1.09 | 1.61±0.10 | 2.22±1.32 | 0.1722 | 0.3909 | 0.2584 | |
| THRR | 1.18±0.03 | 1.17±0.02 | 0.99±0.08* | 0.0100† | 0.0283† | 0.7139 | |
| CO2R | 2.65±0.25 | 2.09±0.23 | 1.01±0.79 | 0.0758 | 0.7649 | 0.2131 | |
| Nitric oxide end-products | |||||||
| CSF NOx | 2.03±0.11 | 1.95±0.10 | 2.15±0.32 | 0.7428 | <0.0001† | ||
| Plasma NOx | 1.83±0.11 | 1.87±0.09 | 1.99±0.31 | 0.8821 | <0.0001† | ||
p value remained significant after correcting for type 1 error using the Benjamini-Hochberg method.
Significantly different from the normal T/T genotype.
Ave CBF, average cortical cerebral blood flow; Ave CVR, average cerebral vascular resistance; ICA-FVol, internal carotid artery flow volume; ICA-FV, internal carotid artery flow velocity; ACA-FV, anterior cerebral artery flow velocity; MCA-FV, middle cerebral artery flow velocity; PCA-FV, posterior cerebral artery flow velocity; ARI, autoregulatory index; THRR, transient hyperemic response ratio; CO2R, CO2 reactivity; CSF, cerebrospinal fluid; NOx, nitrate and nitrite.
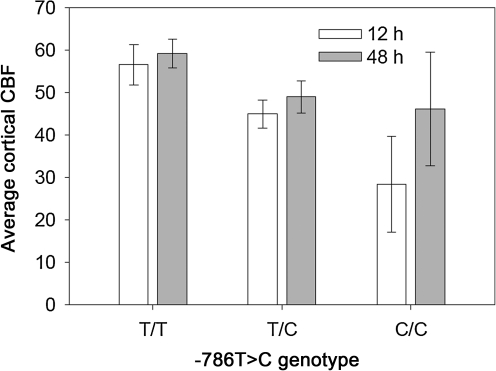
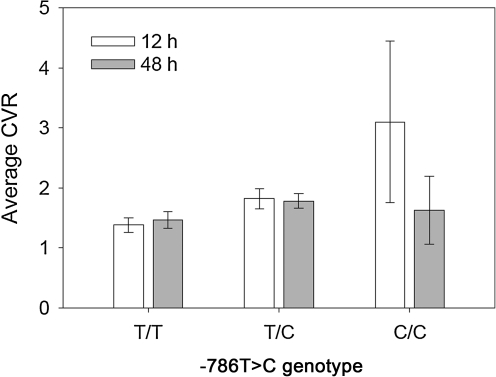
For global CBF, which is shown by −786T>C genotype and day in Figure 2, the values were highest in the patients with T/T genotype, intermediate with T/C genotype, and lowest with the C/C genotype at both 12 and 48 h after injury. For each genotype, the 48-h global CBF tended to be higher than the CBF at 12 h, but the time effect was not significant (p=0.4486). CVR, shown by the −786T>C genotype and day in Figure 3, followed an inverse pattern to CBF, but was also significantly higher at 12 h than at 48 h post-injury (time effect, p=0.0149).
FIG. 2.
Average cortical cerebral blood flow (CBF) by −786T>C genotype and by day (-786T>C genotype effect, p=0.0146).
FIG. 3.
Average cortical cerebral vascular resistance (CVR) by −786T>C genotype and by day (-786T>C genotype effect, p=0.0027; day effect, p=0.0149).
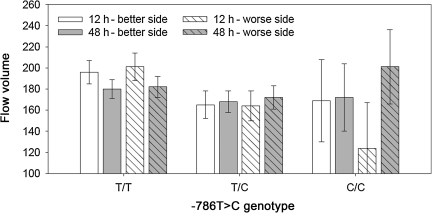
Although there was not a significant main effect of −786T>C genotype or of time for ICA-FVol, there was a significant genotype×day×side interaction (p=0.0085) that is illustrated in Figure 4. This interaction indicated that the change in ICA-FVol over time was different for the different genotypes. The lowest ICA-FVol (124±43 mL/min) was found at 12 h post-injury in the more injured hemisphere of the patients with the C/C genotype at the −786T>C locus.
FIG. 4.
Internal carotid artery flow volume by −786T>C genotype and side of brain and day (genotype×side×day interaction, p=0.0085).
FV in all of the intracranial vessels studied were significantly higher at 48 h than at 12 h post-injury (Table 4). In addition, MCA-FV and PCA-FV were significantly higher in the heterozygous T/C patients than in the patients who had the normal T/T genotype at −786T>C. Dynamic pressure autoregulation, assessed by ARI using the cuff deflation method, was not significantly different among the −786T>C genotype groups. With the carotid compression method for assessing dynamic pressure autoregulation, the THRR was lowest in patients with the C/C genotype (p=0.01), and CO2R was lowest in the C/C patients (p=0.0758).
Cerebral hemodynamics by 894G>T genotype
The cerebral hemodynamics for the patients with different 894G>T genotypes is summarized in Table 5. The flow variables were not significantly related to the 894G>T genotype. However, ICA-FV, ACA-FV, MCA-FV, and CO2R were all significantly higher in the patients with the homozygous T/T genotype.
Table 5.
Cerebral Hemodynamic Variables by 894G>T Genotypes
| |
894G>T Genotype |
p Values for main effects |
|
||||
|---|---|---|---|---|---|---|---|
| G/G | G/T | T/T | Genotype | Side | Day | p Values for interactions | |
| Flow variables | |||||||
| Ave CBF | 50.2±2.2 | 58.3±5.2 | 40.7±.05 | 0.3612 | 0.1586 | ||
| Ave CVR | 1.66±.10 | 1.51±.12 | 2.14±.13 | 0.5614 | 0.0970 | ||
| ICA-FVol | 179.5±1.03 | 179.5±1.0 | 0.9976 | 0.8042 | 0.5812 | ||
| Flow velocity and reactivity variables | |||||||
| ICA-FV | 61.1±1.0 | 70.4±1.0** | 99.3±1.1* | 0.0032† | 0.2472 | <0.0001† | |
| ACA-FV | 53.6±1.8 | 54.5±2.8* | 119.6±9.2* | <0.0001† | 0.0687 | 0.0003† | |
| MCA-FV | 72.7±2.4 | 78.4±4.0** | 168.3±13.2* | <0.0001† | 0.0464 | 0.2416 | Genotype×side×day; p=0.0005† |
| PCA-FV | 59.8±2.4 | 66.6±3.9 | 50.6±12.6 | 0.2281 | 0.6472 | <.0001† | |
| ARI | 1.83±0.21 | 1.94±0.32 | 3.03±1.01 | 0.5007 | 0.3813 | 0.2843 | |
| THRR | 1.17±0.02 | 1.18±0.03 | 1.04±0.11 | 0.4842 | 0.0303† | 0.6170 | |
| CO2R | 2.39±0.18 | 1.64±0.30** | 4.56±0.86* | 0.0053† | 0.7644 | 0.3596 | |
| Nitric oxide end-products | |||||||
| CSF NOx | 2.01±0.08 | 1.86±0.14 | 2.52±0.32 | 0.1652 | <0.0001† | ||
| Plasma NOx | 1.86±0.08 | 1.76±0.14 | 2.36±0.29 | 0.1919 | <0.0001† | ||
p value remained significant after correcting for type 1 error using the Benjamini-Hochberg method.
Significantly different from the normal G/G genotype.
Significantly differently from both G/G and T/T.
Ave CBF, average cortical cerebral blood flow; Ave CVR, average cerebral vascular resistance; ICA-FVol, internal carotid artery flow volume; ICA-FV internal carotid artery flow velocity; ACA-FV, anterior cerebral artery flow velocity; MCA-FV, middle cerebral artery flow velocity; PCA-FV, posterior cerebral artery flow velocity; ARI, autoregulatory index; THRR, transient hyperemic response ratio; CO2R, CO2 reactivity; CSF, cerebrospinal fluid; NOx, nitrate and nitrite.
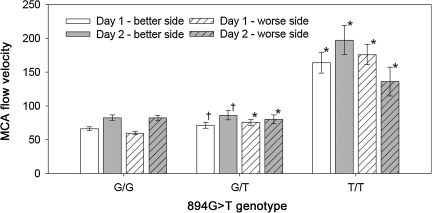
For the MCA-FV, there was also a significant genotype×day×side effect (p=0.0005), which is illustrated in Figure 5. The highest MCA-FV (197.0±21.6 cm/sec) occurred on the better side on day 1 in the patient with the variant homozygous T/T genotype. The lowest MCA-FV (59.9±2.6 cm/sec) occurred on the worse side on day 2 in the patients with the normal homozygous G/G genotype.
FIG. 5.
Middle cerebral artery flow velocity by 894G>T genotype and side of brain and day (genotype×side×day interaction, p=0.0005; †different from both G/G and T/T on same day and side [p<0.05]; *different from G/G on same side and day [p<0.05]).
Only one patient had the variant homozygous T/T genotype. This one patient had favorable injury severity measures with a motor GCS score of 5, bilaterally reactive pupils, and a diffuse injury without any signs of brain swelling for a predicted risk of death of only 10% by the IMPACT prediction model. Although these injury severity factors were not significantly different among the 894G>T genotype groups, the findings for the cerebral hemodynamics of this gene variant may reflect the less severe injury of this one patient rather than the effects of the genotype.
Cerebral hemodynamics by 27bp VNTR genotype
None of the cerebral hemodynamic parameters were significantly related to the different 27-bp VNTR genotypes.
Discussion
TBI and genetic variability
Although severe TBI is not a disease with “susceptibility genes” in the usual sense, the response to TBI is a complex interaction among the injury, the individual patient's genotype, and the treatment that is provided. Increasingly in critical care medicine, emphasis is being placed on understanding the role that an individual's genetic makeup plays in the response to critical illness and injury, and also in response to therapies that we use to treat these disorders.
Apolipoprotein E (APOE) is the most thoroughly studied gene that has been observed to be associated with the response to TBI. The gene for apoE is located on chromosome 19, and is highly polymorphic. The three most common alleles are e2, e3, and e4, which encode the main three isoforms of apoE, E2, E3, and E4. In several studies, patients with the E4 genotype have significantly worse outcomes than patients without the E4 genotype (Friedman et al., 1999; Graham et al., 1999; Liaquat et al., 2002; Liberman et al., 2002; Sabo et al., 2000). This relationship of E4 to poor outcome has not, however, been consistently replicated in later studies.
Other genes are also beginning to be examined for importance after TBI. Brain edema is a common pathophysiological feature of TBI. A mutation in the CACNA1A calcium channel subunit gene on chromosome 19 has been associated with delayed brain swelling and coma following minor TBI (Kors et al., 2001). Variants of the aquaporin 4 gene have been found to reduce cellular water permeability, which could have implications for cerebral edema formation after injury (Sorani et al., 2008). In a stroke population, severe cerebral edema was associated with one variant of aquaporin 4 (Kleffner et al., 2008).
A GT repeat variant of the neprilysin gene was examined in 81 fatal cases of TBI using available autopsy data. The TBI patients with longer GT repeats had an increased risk of Aβ plaques, suggesting that neprilysin polymorphism may cause TBI patients to be more vulnerable to plaque formation (Johnson et al., 2009).
A functional polymorphism of the p53 gene in codon 72, which alters the properties of the produced protein (Dumont et al., 2003), was studied in a group of 90 severely head-injured patients who were admitted to an intensive care unit (Martinez-Lucas et al., 2005). Patients with the Arg/Arg genotype were associated with unfavorable outcome at the time of discharge. However, 6 months later, no significant difference was found between the groups of patients with and without the Arg/Arg genotype.
The NOS3 gene was chosen as a candidate gene for these studies because experimental data in TBI models implicated the importance of NO produced by the endothelial isoform of NOS in determining the CBF in contused brain after cortical impact injury.
786T>C variant and cerebral hemodynamics
The promoter −786T>C variant genotype had a significant relationship to global CBF and CVR. These results are similar to the CBF findings in normal subjects who smoke (Nasreen et al., 2002), and are consistent with findings of an increased risk of cerebral vasospasm following subarachnoid hemorrhage with the C/C genotype (Field et al., 2001; Ko et al., 2008).
A reduced CBF in the patients with the −786C variant could have been due to the presence of a more severe neurological injury. However, a detailed examination of factors that predict a more severe injury, including older age, lower initial motor GCS and unreactive pupils, presence of pre-hospital hypoxia or hypotension, mass lesion or diffuse injury with basal cistern compression and/or midline shift, traumatic subarachnoid hemorrhage, lower admission hemoglobin, and higher admission blood glucose, did not reveal any significant differences in the frequency of these adverse features among the −786T>C genotype groups. In addition, the predicted outcome based on the IMPACT database algorithms (Steyerberg et al., 2008) was not significantly different among the −786T>C genotype groups.
Although there were no adverse clinical features in the 2 patients with the C/C genotype, one patient died prior to hospital discharge, and both of these patients were dead by 6 months post-injury. It is possible that a more severe injury could explain the lower CBF and also the worse outcome in these 2 patients. Regardless, there was still a significant difference in CBF between the patients with the normal T/T genotype and those who had the heterozygous T/C genotype. A more severe neurological injury could not explain this cerebrovascular difference.
Although global CBF was highest in patients with the normal T/T genotype, FV in the middle cerebral and posterior cerebral arteries was highest in the patients who were heterozygous T/C. This finding may seem to be contradictory; however, FV is not always directly related to CBF, and can be increased by either an increase in flow or a decrease in the diameter of the vessel. The increased FV in these vessels may represent a greater degree of vasoconstriction in the heterozygous T/C patients.
The population studied was racially diverse, and some racial differences in the distribution of NOS3 variants has been described (Casas et al., 2004; Tanus-Santos et al., 2001). In these previous studies, the 894T and the −786C alleles were significantly more common in Caucasians than in African-Americans or Asians. In contrast, the 4a allele for the 27-bp VNTR variant was more common in African-Americans than in Caucasians or Asians. Although no significant race/ethnicity differences in the distribution of the three NOS3 variants was seen in this study, the numbers were too small to adequately assess this issue.
Nitrate/nitrite levels in plasma and CSF were not reduced in patients with the −786C variant, even though CBF was lower. Since NO measured in plasma and CSF is not exclusively from the activity of NOS3, the lack of a difference in nitrate/nitrite concentrations could be due to contributions from other isoforms of NOS. In normal subjects, some studies have observed reduced plasma NO levels with the 4a variant of the 27-bp VNTR (Tsukada et al., 1998), and the −786C variant (Miyamoto et al., 2000). Other studies have not reported significant differences in plasma nitrate/nitrite levels by NOS3 variant groups.
Conclusions
The findings in this study support the importance of NO produced by NOS3 activity in maintaining CBF after a severe TBI, since lower CBF values were found in patients having the variant −786C allele. The study also suggests that a patient's individual genetic makeup may contribute to the brain's response to injury and may determine the patient's ability to survive the injury. These results will need to be studied in a larger number of patients. Future studies are also needed to genotype additional eNOS gene variants to have more confidence that the relationships identified are truly related to the −786C variant and are not simply due to linkage dysequilibrium to another variant. Nevertheless, the findings could explain some of the variability in outcome that occurs following severe TBI.
Acknowledgments
This work was funded by National Institutes of Health grant no. R01-NS048428.
Author Disclosure Statement
No competing financial interests exist.
References
- Aaslid R. Lindegaard K.F. Sorteberg W. Nornes H. Cerebral autoregulation dynamics in humans. Stroke. 1989;20:45–52. doi: 10.1161/01.str.20.1.45. [DOI] [PubMed] [Google Scholar]
- Ahn M.J. Sherwood E.R. Prough D.S. Lin C.Y. DeWitt D.S. The effects of traumatic brain injury on cerebral blood flow and brain tissue nitric oxide levels and cytokine expression. J. Neurotrauma. 2004;21:1431–1442. doi: 10.1089/neu.2004.21.1431. [DOI] [PubMed] [Google Scholar]
- Asif A.R. Oellerich M. Armstrong V.W. Hecker M. Cattaruzza M. T-786C polymorphism of the NOS-3 gene and the endothelial cell response to fluid shear stress-a proteome analysis. J. Proteome Res. 2009;8:3161–3168. doi: 10.1021/pr800998k. [DOI] [PubMed] [Google Scholar]
- Benjamini Y. Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. Royal Statistical Soc. 1995;57:289–300. [Google Scholar]
- Buchanan J.E. Phillis J.W. The role of nitric oxide in the regulation of cerebral blood flow. Brain Res. 1993;610:248–255. doi: 10.1016/0006-8993(93)91408-k. [DOI] [PubMed] [Google Scholar]
- Casas J.P. Bautista L.E. Humphries S.E. Hingorani A.D. Endothelial nitric oxide synthase genotype and ischemic heart disease: meta-analysis of 26 studies involving 23028 subjects. Circulation. 2004;109:1359–1365. doi: 10.1161/01.CIR.0000121357.76910.A3. [DOI] [PubMed] [Google Scholar]
- Cherian L. Robertson C.S. L-arginine and superoxide dismutase alter cerebral hemodynamics and reduction in nitric oxide concentration after controlled cortical impact injury. J. Neurotrauma. 2003b;20:77–85. doi: 10.1089/08977150360517209. [DOI] [PubMed] [Google Scholar]
- Cherian L. Chacko G. Goodman J.C. Robertson C.S. Neuroprotective effects of L-arginine administration after cortical impact injury in rats: dose response and time window. J. Pharmacol. Exp. Ther. 2003a;304:617–623. doi: 10.1124/jpet.102.043430. [DOI] [PubMed] [Google Scholar]
- Cherian L. Goodman J.C. Robertson C.S. Brain nitric oxide changes after controlled cortical impact injury in rats. J. Neurophysiol. 2000;83:2171–2178. doi: 10.1152/jn.2000.83.4.2171. [DOI] [PubMed] [Google Scholar]
- Dumont P. Leu J.I. Della P.A., III George D.L. Murphy M. The codon 72 polymorphic variants of p53 have markedly different apoptotic potential. Nat. Genet. 2003;33:357–365. doi: 10.1038/ng1093. [DOI] [PubMed] [Google Scholar]
- Field M. Peters D. Kassam A. Seever G. Chang Y.F. Horowitz M. Yonas H. A potential association between the endothelial nitric oxide synthase gene promoter and the development of severe cerebral vasospasm in humans. American Association of Neurological Surgeons. 2001 Abstracts. [Google Scholar]
- Friedman G. Froom P. Sazbon L. Grinblatt I. Shochina M. Tsenter J. Babaey S. Yehuda B. Groswasser Z. Apolipoprotein E-epsilon4 genotype predicts a poor outcome in survivors of traumatic brain injury. Neurology. 1999;52:244–248. doi: 10.1212/wnl.52.2.244. [DOI] [PubMed] [Google Scholar]
- Graham D.I. Horsburgh K. Nicoll J.A. Teasdale G.M. Apolipoprotein E and the response of the brain to injury. Acta Neurochir. 1999;73(Suppl (Wien.)):89–92. doi: 10.1007/978-3-7091-6391-7_15. [DOI] [PubMed] [Google Scholar]
- Hlatky R. Furuya Y. Valadka A.B. Gonzalez J. Chacko A. Mizutani Y. Contant C.F. Robertson C.S. Dynamic autoregulatory response after severe head injury. J. Neurosurg. 2002;97:1054–1061. doi: 10.3171/jns.2002.97.5.1054. [DOI] [PubMed] [Google Scholar]
- Hlatky R. Liu H. Cherian L. Goodman J.C. O'Brien J.C. Contant C.F. Robertson C.S. The role of endothelial nitric oxide synthase in the cerebral hemodynamics after controlled cortical impact injury in mice. J. Neurotrauma. 2003;20:995–1006. doi: 10.1089/089771503770195849. [DOI] [PubMed] [Google Scholar]
- Hlatky R. Valadka A.B. Robertson C.S. Analysis of dynamic autoregulation assessed by the cuff deflation method. Neurocrit. Care. 2006;4:127–132. doi: 10.1385/NCC:4:2:127. [DOI] [PubMed] [Google Scholar]
- Howard T.D. Giles W.H. Xu J. Wozniak M.A. Malarcher A.M. Lange L.A. Macko R.F. Basehore M.J. Meyers D.A. Cole J.W. Kettner S.J. Promoter polymorphisms in the nitric oxide synthase 3 gene are associated with ischemic stroke susceptibility in young black women. Stroke. 2005;36:1848–1851. doi: 10.1161/01.STR.0000177978.97428.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson V.E. Stewart W. Stewart J.E. Graham D.I. Praestgaard A.H. Smith D.H. A neprilysin polymorphism and amyloid-beta plaques following traumatic brain injury. J. Neurotrauma. 2009;26:1197–1202. doi: 10.1089/neu.2008.0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana V.G. Meissner I. Sohni Y.R. Bamlet W.R. McClelland R.L. Cunningham J.M. Meyer F.B. The presence of tandem endothelial nitric oxide synthase gene polymorphisms identifying brain aneurysms more prone to rupture. J. Neurosurg. 2005;102:526–531. doi: 10.3171/jns.2005.102.3.0526. [DOI] [PubMed] [Google Scholar]
- Kleffner I. Bungeroth M. Schiffbauer H. Schabitz W.R. Ringelstein E.B. Kuhlenbaumer G. The role of aquaporin-4 polymorphisms in the development of brain edema after middle cerebral artery occlusion. Stroke. 2008;39:1333–1335. doi: 10.1161/STROKEAHA.107.500785. [DOI] [PubMed] [Google Scholar]
- Kobari M. Fukuuchi Y. Tomita M. Tanahashi N. Takeda H. Role of nitric oxide in regulation of cerebral microvascular tone and autoregulation of cerebral blood flow in cats. Brain Res. 1994;667:255–262. doi: 10.1016/0006-8993(94)91503-2. [DOI] [PubMed] [Google Scholar]
- Ko N.U. Rajendran P. Kim H. Rutkowski J. Pawlikowska L. Kwok P.Y. Higashida R.T. Lawton M.T. Smith W.S. Zaroff J.G. Young W.L. Endothelial nitric oxide synthase polymorphism (-786T->C) and increased risk of angiographic vasospasm after aneurysmal subarachnoid hemorrhage. Stroke. 2008;39:1103–1108. doi: 10.1161/STROKEAHA.107.496596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kors E.E. Terwindt G.M. Vermeulen F.L. Fitzsimons R.B. Jardine P.E. Heywood P. Love S. van den Maagdeberg A.M. Haan J. Frants R.R. Ferrari M.D. Delayed cerebral edema and fatal coma after minor head trauma: role of the CACNA1A calcium channel subunit gene and relationship with familial hemiplegic migraine. Ann. Neurol. 2001;49:753–760. doi: 10.1002/ana.1031. [DOI] [PubMed] [Google Scholar]
- Leeson C.P. Hingorani A.D. Mullen M.J. Jeerooburkhan N. Kattenhorn M. Cole T.J. Muller D.P. Lucas A. Humphries S.E. Deanfield J.E. Glu298Asp endothelial nitric oxide synthase gene polymorphism interacts with environmental and dietary factors to influence endothelial function. Circ. Res. 2002;90:1153–1158. doi: 10.1161/01.res.0000020562.07492.d4. [DOI] [PubMed] [Google Scholar]
- Liaquat I. Dunn L.T. Nicoll J.A. Teasdale G.M. Norrie J.D. Effect of apolipoprotein E genotype on hematoma volume after trauma. J. Neurosurg. 2002;96:90–96. doi: 10.3171/jns.2002.96.1.0090. [DOI] [PubMed] [Google Scholar]
- Liberman J.N. Stewart W.F. Wesnes K. Troncoso J. Apolipoprotein E epsilon 4 and short-term recovery from predominantly mild brain injury. Neurology. 2002;58:1038–1044. doi: 10.1212/wnl.58.7.1038. [DOI] [PubMed] [Google Scholar]
- Majumdar V. Nagaraja D. Karthik N. Christopher R. Association of endothelial nitric oxide synthase gene polymorphisms with early-onset ischemic stroke in South Indians. J. Atheroscler. Thromb. 2010;17:45–53. doi: 10.5551/jat.1560. [DOI] [PubMed] [Google Scholar]
- Marsden P.A. Heng H.H. Scherer S.W. Stewart R.J. Hall A.V. Shi X.M. Tsui L.C. Schappert K.T. Structure and chromosomal localization of the human constitutive endothelial nitric oxide synthase gene. J. Biol. Chem. 1993;268:17478–17488. [PubMed] [Google Scholar]
- Marshall L.F. Marshall S.B. Klauber M.R. van Berkum Clark M. Eisenberg H.M. Jane J.A. Manmaron A. Foulkes M.A. A new classification of head injury based on computerized tomography. J. Neurosurg. 1991;75:S14–S20. [Google Scholar]
- Martinez-Lucas P. Moreno-Cuesta J. Garcia-Olmo D.C. Sanchez-Sanchez F. Excribano-Martinez J. del Pozo A.C. Lizan-Garcia M. Garcia-Olmo D. Relationship between the Arg72Pro polymorphism of p53 and outcome for patients with traumatic brain injury. Intensive Care Med. 2005;31:1168–1173. doi: 10.1007/s00134-005-2715-0. [DOI] [PubMed] [Google Scholar]
- McDonald D.M. Alp N.J. Channon K.M. Functional comparison of the endothelial nitric oxide synthase Glu298Asp polymorphic variants in human endothelial cells. Pharmacogenetics. 2004;14:831–839. doi: 10.1097/00008571-200412000-00006. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y. Saito Y. Nakayama M. Shimasaki Y. Yoshimura T. Yoshimura M. Harada M. Kajiyama N. Kishimoto I. Kuwahara K. Hino J. Ogawa E. Hamanaka I. Kamitani S. Takahashi N. Kawakami R. Kangawa K. Yasue H. Nakao K. Replication protein A1 reduces transcription of the endothelial nitric oxide synthase gene containing a −786T–>C mutation associated with coronary spastic angina. Hum. Mol. Genet. 2000;9:2629–2637. doi: 10.1093/hmg/9.18.2629. [DOI] [PubMed] [Google Scholar]
- Nasreen S. Nabika T. Shibata H. Moriyama H. Yamashita K. Masuda J. Kobayashi S. T-786C polymorphism in endothelial NO synthase gene affects cerebral circulation in smokers: possible gene-environmental interaction. Arterioscler. Thromb. Vasc. Biol. 2002;22:605–610. doi: 10.1161/01.atv.0000013286.60021.fe. [DOI] [PubMed] [Google Scholar]
- Persu A. Stoenoiu M.S. Messiaen T. Davila S. Robino C. El-Khattabi O. Mourad M. Horie S. Feron O. Balligand J.L. Wattiez R. Pirson Y. Chauveau D. Lens X.M. Devuyst O. Modifier effect of ENOS in autosomal dominant polycystic kidney disease. Hum. Mol. Genet. 2002;11:229–241. doi: 10.1093/hmg/11.3.229. [DOI] [PubMed] [Google Scholar]
- Reutens D.C. McHugh M.D. Toussaint P.J. Evans A.C. Gjedde A. Meyer E. Stewart D.J. L-arginine infusion increases basal but not activated cerebral blood flow in humans. J. Cereb. Blood Flow Metab. 1997;17:309–315. doi: 10.1097/00004647-199703000-00008. [DOI] [PubMed] [Google Scholar]
- Rossi G.P. Taddei S. Virdis A. Cavallin M. Ghiadoni L. Favilla S. Versari D. Sudano I. Pessina A.C. Salvetti A. The T-786C and Glu298Asp polymorphisms of the endothelial nitric oxide gene affect the forearm blood flow responses of Caucasian hypertensive patients. J. Am. Coll. Cardiol. 2003;41:938–945. doi: 10.1016/s0735-1097(02)03011-5. [DOI] [PubMed] [Google Scholar]
- Sabo T. Lomnitski L. Nyska A. Beni S. Maronpot R.R. Shohami E. Roses A.D. Michaelson D.M. Susceptibility of transgenic mice expressing human apolipoprotein E to closed head injury: the allele E3 is neuroprotective whereas E4 increases fatalities. Neuroscience. 2000;101:879–884. doi: 10.1016/s0306-4522(00)00438-3. [DOI] [PubMed] [Google Scholar]
- Saidi S. Mallat S.G. Almawi W.Y. Mahjoub T. Endothelial nitric oxide synthase Glu298Asp, 4b/a, and −786T>C gene polymorphisms and the risk of ischemic stroke. Acta Neurol. Scand. 2010;121:114–119. doi: 10.1111/j.1600-0404.2009.01192.x. [DOI] [PubMed] [Google Scholar]
- Savvidou M.D. Vallance P.J. Nicolaides K.H. Hingorani A.D. Endothelial nitric oxide synthase gene polymorphism and maternal vascular adaptation to pregnancy. Hypertension. 2001;38:1289–1293. doi: 10.1161/hy1201.097305. [DOI] [PubMed] [Google Scholar]
- Smielewski P. Czosnyka M. Kirkpatrick P. McEroy H. Rutkowska H. Pickard J.D. Assessment of cerebral autoregulation using carotid artery compression. Stroke. 1996;27:2197–2203. doi: 10.1161/01.str.27.12.2197. [DOI] [PubMed] [Google Scholar]
- Song J. Kim O.J. Kim H.S. Bae S.J. Hong S.P. Oh D. Kim N.K. Endothelial nitric oxide synthase gene polymorphisms and the risk of silent brain infarction. Int. J. Mol. Med. 2010;25:819–823. doi: 10.3892/ijmm_00000410. [DOI] [PubMed] [Google Scholar]
- Sorani M.D. Manley G.T. Giacomini K.M. Genetic variation in human aquaporins and effects on phenotypes of water homeostasis. Hum. Mutat. 2008;29:1108–1117. doi: 10.1002/humu.20762. [DOI] [PubMed] [Google Scholar]
- Soustiel J.F. Glenn T.C. Vespa P. Rinsky B. Hanuscin C. Martin N.A. Assessment of cerebral blood flow by means of blood-flow-volume measurement in the internal carotid artery: comparative study with a 133Xenon clearance technique. Stroke. 2003;34:1876–1880. doi: 10.1161/01.STR.0000080942.32331.39. [DOI] [PubMed] [Google Scholar]
- Steyerberg E.W. Mushkudiani N. Perel P. Butcher I. Lu J. McHugh G.S. Murray G.D. Marmarou A. Roberts I. Habbema J.D. Maas A.I. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med. 2008;5:e165. doi: 10.1371/journal.pmed.0050165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanus-Santos J.E. Desai M. Flockhart D.A. Effects of ethnicity on the distribution of clinically relevant endothelial nitric oxide variants. Pharmacogenetics. 2001;11:719–725. doi: 10.1097/00008571-200111000-00011. [DOI] [PubMed] [Google Scholar]
- Tao H.M. Chen G.Z. Endothelial NO synthase gene polymorphisms and risk of ischemic stroke: a meta-analysis. Neurosci. Res. 2009;64:311–316. doi: 10.1016/j.neures.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Tesauro M. Thompson W.C. Rogliani P. Qi L. Chaudhary P.P. Moss J. Intracellular processing of endothelial nitric oxide synthase isoforms associated with differences in severity of cardiopulmonary diseases: cleavage of proteins with aspartate vs. glutamate at position 298. Proc. Natl. Acad. Sci. USA. 2000;97:2832–2835. doi: 10.1073/pnas.97.6.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiecks F.P. Lam A.M. Aaslid R. Newell D.W. Comparison of static and dynamic cerebral autoregulation measurements. Stroke. 1995;26:1014–1019. doi: 10.1161/01.str.26.6.1014. [DOI] [PubMed] [Google Scholar]
- Tsukada T. Yokoyama K. Arai T. Takemoto F. Hara S. Yamada A. Kawaguchi Y. Hosoya T. Igari J. Evidence of association of the ecNOS gene polymorphism with plasma NO metabolite levels in humans. Biochem. Biophys. Res. Commun. 1998;245:190–193. doi: 10.1006/bbrc.1998.8267. [DOI] [PubMed] [Google Scholar]
- Tuzgen S. Tanriover N. Uzan M. Tureci E. Tanriverdi T. Gumustas K. Kuday C. Nitric oxide levels in rat cortex, hippocampus, cerebellum, and brainstem after impact acceleration head injury. Neurol. Res. 2003;25:31–34. doi: 10.1179/016164103101201085. [DOI] [PubMed] [Google Scholar]
- Wada K. Chatzipanteli K. Busto R. Dietrich W.D. Role of nitric oxide in traumatic brain injury in the rat. J. Neurosurg. 1998;89:807–818. doi: 10.3171/jns.1998.89.5.0807. [DOI] [PubMed] [Google Scholar]
- White R.P. Vallance P. Markus H.S. Effect of inhibition of nitric oxide synthase on dynamic cerebral autoregulation in humans. Clin. Sci. 2000;99:555–560. [PubMed] [Google Scholar]
- Yemisci M. Sinici I. Ozkara H.A. Hayran M. Ay H. Celtikci B. Onder E. Buyukserbetci G. Kaya E.B. Tokgozoglu L. Dalkara T. Protective role of 27bp repeat polymorphism in intron 4 of eNOS gene in lacunar infarction. Free Radic. Res. 2009;43:272–279. doi: 10.1080/10715760802691489. [DOI] [PubMed] [Google Scholar]