Abstract
Natural killer T (NKT) cells are known to be specifically activated by α-galactosylceramide (α-GalCer) via their interaction with CD1d. At that time, NKT cells mediate autoreactivity and eventually induce hepatic injury. As these immune responses resemble acute autoimmune hepatitis, it was examined whether autoantibody production and the activation of autoantibody-producing B-1 cells were accompanied by this phenomenon. Autoantibodies against Hep-2 cells and double-stranded DNA were detected in sera as early as day 3 (showing a peak at day 14) when mice were treated with α-GalCer. On day 3, B220low cells appeared in the liver. These B220low cells were CD5− (i.e. B-1b cells) and CD69+ (an activation marker). Primarily, such B220low cells were present in the peritoneal cavity, but the proportion of B220low cells increased with the administration of α-GalCer even at this site. In parallel with the appearance of B220low cells in the liver, hepatic lymphocytes acquired the potential to produce autoantibodies in in vitro cell culture in the presence of lipopolysaccharide. These results suggested that hepatic injury induced by α-GalCer administration resembled acute autoimmune hepatitis and that the major effector lymphocytes were NKT cells with autoreactivity and autoantibody-producing B-1 cells.
Keywords: autoantibody, B-1 cells, hepatic injury, natural killer T cells, α-galactosylceramide
Introduction
It has been well established that the liver is an important immune organ and that it contains interesting lymphocyte subsets, including natural killer T (NKT) cells [NK1.1+ T-cell receptor intermediate (TCRint)] and NK1.1− TCRint cells.1–5 The NKT cells are activated by α-galactosylceramide (α-GalCer) via CD1d6–9 and their precursor cells are present in the thymus at the neonatal stage.10 However, thymectomy at the young adult stage, but not at the neonatal stage, does not change the population size of NKT cells in the liver of adult mice.11 In other words, the precursors migrate to the liver after the neonatal stage and their maturation from the migrated precursors occurs there.
On the other hand, NK1.1− TCRint cells and their precursors seem to be of extrathymic origin.12 In a series of our recent studies, NK1.1− TCRint cells have been revealed to have autoreactivity and the activation of NK1.1− TCRint cells has been found to be responsible for various autoimmune diseases and similar states.13–16 Such diseased conditions accompany autoantibody production and the appearance of B-1 cells. For example, at the onset of autoimmune disease in NZB/W F1 mice17 or in mice with chronic graft-versus-host disease,18 NK1.1− TCRint cells and autoantibody-producing B-1 cells are simultaneously activated in the liver. A similar phenomenon appears in mice with malarial infection19,20 and in aging mice.21
In light of these findings, in this study we investigated whether the activation of NKT cells accompanied autoantibody production. As NKT cells are known to be activated by the administration of α-GalCer,6–9 we applied this model for activation of NKT cells and report that the activation of NKT cells, but not of NK1.1− TCRint cells, also accompanied the activation of autoantibody-producing B-1 cells during the course of hepatic injury.
Materials and methods
Mice and administration of α-GalCer
C57BL/6J mice at the age of 8–12 weeks were used in this study. These female mice were kept under specific pathogen-free conditions in the animal facility of Niigata University. α-GalCer (KRN7000) was purchased from Funakoshi Co. (Tokyo, Japan). This reagent was dissolved in 0·5% polysorbate 20 at a concentration of 200 μg/ml and was then further diluted with physiological saline up to 10 μg/ml of α-GalCer.22 Then, 0·2 ml α-GalCer solution (i.e. 2 μg α-GalCer/mouse) was administered intravenously.
Cell preparation
Hepatic lymphocytes were isolated by a method described elsewhere.23 Briefly, mice anaesthetized with ether were killed by exsanguination via a cardiac puncture. To obtain lymphocytes, the liver was removed, pressed through a 200-gauge stainless steel mesh, and then suspended in Eagle's minimum essential medium supplemented with 5 mm HEPES (Nissui Pharmaceutical Co., Tokyo, Japan) and 2% heat-inactivated newborn calf serum. After being washed with the medium once, the cell pellet was resuspended in the medium. Lymphocytes were isolated from parenchymal hepatocytes, nuclei of hepatocytes and Kupffer cells by the Percoll (35% Percoll containing 100 U/ml heparin) gradient method.
Splenocytes were obtained by pressing the spleen through a 200-gauge stainless steel mesh; erythrocytes in the spleen were lysed with 0·83% NH4Cl–Tris buffer, pH 7·6. Lymphocytes were also isolated from the peritoneal cavity. They were aspirated using a syringe after the injection of 5 ml physiological saline.
Immunofluorescence assays
The surface phenotype of cells was identified by two-colour immunofluorescence.24 Fluorescein isothiocyanate-, phycoerythrin- or biotin-conjugated monoclonal antibodies (mAbs) were used and biotin-conjugated reagents were developed with TRIcolor-conjugated streptavidin (Caltag Laboratories, San Francisco, CA). The mAbs used here included anti-CD3 (145-2C11), anti-NK1.1 (PK136), anti-B220 (RA3-6B2), anti-CD5 (53-7.3) and anti-CD69 (H1.2F3) mAbs (PharMingen Co., San Diego, CA). The NKT cells were identified by two different stainings, including two-colour staining for CD3 and NK1.1, and staining for the CD1d tetramer.25 Phycoerythrin-conjugated CD1d tetrameter was purchased from Funakoshi Co. (the original was derived from ProImmune Ltd, Oxford, UK). Cells were analysed by FACScan (Becton Dickinson Co., Mountain View, CA). To prevent non-specific binding of mAb, CD16/32 (2.4G2) was added before staining with labelled mAb. Dead cells were excluded by forward scatter, side scatter and propidium iodide gating.
Identification of autoantibodies
The activity of such anti-double-stranded DNA (dsDNA) antibodies was detected in the sera and the supernatant by using an anti-dsDNA mouse ELISA kit (Shibayagi, Gunma, Japan). Autoantibodies were also detected using a Hep-2 cell line in conjunction with an immunofluorescence test.26 The FITC-conjugated anti-mouse immunoglobulin (Dako, Glostrup, Denmark) was used as a secondary antibody for detection.
Measurement of transaminase
Liver injury was estimated by measurement of alanine aminotransferase (ALT) activity in sera. Serum ALT was quantified with a Transferase CII-Test kit (Wako Pure Industries, Osaka, Japan).
Lymphocyte culture
Lymphocytes in the liver and peritoneal cavity were used in in vitro culture. For assay of lymphocyte responsiveness, cells (2 × 106/ml) were cultured in complete RPMI-1640 medium containing 10% fetal calf serum in the presence of 10 μg/ml lipopolysaccharide (LPS; Sigma, St Louis, MO) for 5 days in a 96-well microculture plate. The level of autoantibodies in the culture supernatant was determined using Hep-2.
Statistical analysis
The statistical significance of difference was determined by one-way analysis of variance, paired t-test.
Results
Time-kinetics in the number of lymphocytes during α-GalCer-mediated hepatic injury
When mice were given α-GalCer, many lymphocytes were activated. The number of lymphocytes was enumerated in the liver, spleen and peritoneal cavity (Fig. 1a). In all the organs tested, the number of lymphocytes increased, showing a peak 3 days after administration. The number of lymphocytes then returned to normal levels up to 14 days in all these organs.
Figure 1.
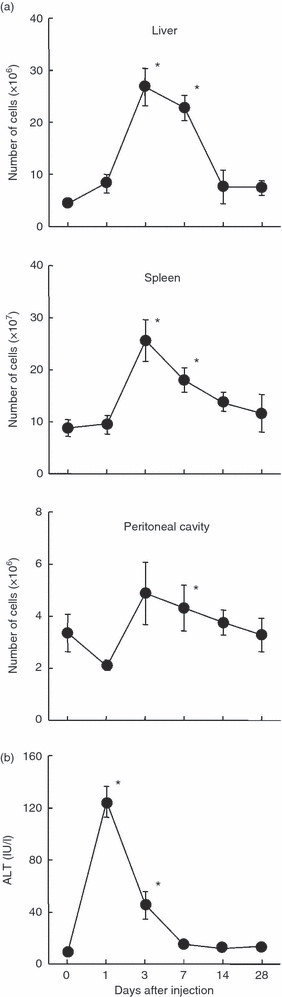
Time-kinetics in the number of lymphocytes yielded by various organs (a) and in the level of alanine aminotransferase (ALT) (b) after α-galactosylceramide (α-GalCer) administration. The mean and one SD were produced from four mice. *P < 0.05.
The level of hepatic injury was examined using an indicator of ALT (Fig. 1b). The elevation of ALT was seen as early as 1 day after α-GalCer administration.
Identification of autoantibodies in sera
α-GalCer-mediated hepatitis is known as a model of acute autoimmune hepatitis in mice,22 so it was investigated whether autoantibodies were produced during hepatic injury (Fig. 2). Such autoantibodies were first examined using Hep-2 cells in an immunofluorescence test (Fig. 2a). From days 3 to 14, prominent positive stainings were detected in both the nucleus and cytoplasm of Hep-2 cells. The perinuclear area was highly positive. The isotype of autoantibodies was then determined (Fig. 2b). The isotype was mainly IgG type and its staining was seen in the nucleus. The titre of autoantibody against dsDNA was examined using ELISA (Fig. 2c). The titre was elevated from day 1 and reached its highest level on day 14 after α-GalCer administration.
Figure 2.
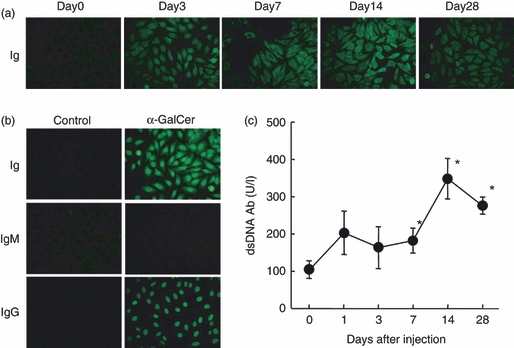
Identification of autoantibodies in sera of mice administered with α-galactosylceramide (α-GalCer). (a) Autoantibodies against Hep-2, (b) determination of isotype, (c) autoantibody against double-stranded DNA. Autoantibodies against Hep-2 cells were identified by immunofluorescence tests while autoantibodies against dsDNA were identified by ELISA method. *P < 0.05.
Determination of the activation of NKT cells and autoantibody-producing B cells
A major effector lymphocyte in α-GalCer-mediated hepatic injury is known to be NKT cells in the liver.22 In this regard, two-colour staining for CD3 and NK1.1 was conducted (Fig. 3a). The NKT cells were identified as NK1.1+ CD3int cells (17·2%) as shown on day 0. On day 3 after α-GalCer administration, NK1.1+ CD3int cells almost disappeared. In other words, NK1.1+ CD3int cells were down-regulated by α-GalCer stimulation. This situation was confirmed by the staining for CD1d tetramer (i.e. NKT cells). In parallel with this staining, two-colour staining for CD3 and B220 was conducted to identify B220low B cells (Fig. 3b). On day 3, the proportion of CD3− B220+ cells was found to have increased (from 48·0 to 70·7%). Some B220low cells newly appeared in CD3− B220+ fraction.
Figure 3.
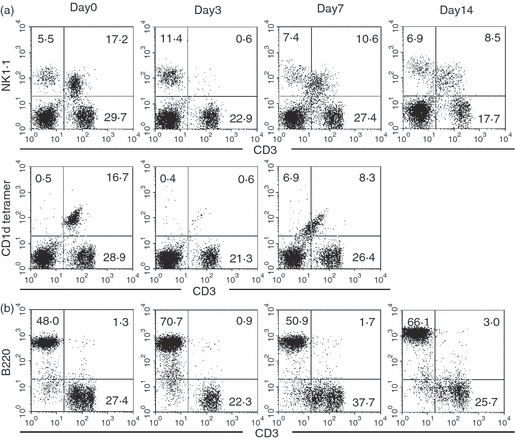
Phenotypic characterization of hepatic lymphocytes. (a) Two-colour staining for CD3 and NK1.1, and the staining for CD1d tetramer, (b) Two-colour staining for CD3 and B220. Numbers in the figure represent the percentages of fluorescence-positive cells in the corresponding area. Representative data from three experiments are depicted.
Further phenotypic characterization of lymphocyte subsets
Autoantibody-producing B220low cells consist of B-1a cells and B-1b cells. The B-1a cells are CD5+ and B-1b cells are CD5−.17 The expression of CD5 on B220low cells was examined in the liver (Fig. 4a). On day 3 after α-GalCer administration, some B220low cells appeared, but they were CD5−. The CD69 antigens are known to be one of the activation markers so the expression of CD69 was then examined. As shown at the bottom of this figure (Fig. 4a), some B220low cells seemed to express CD69.
Figure 4.
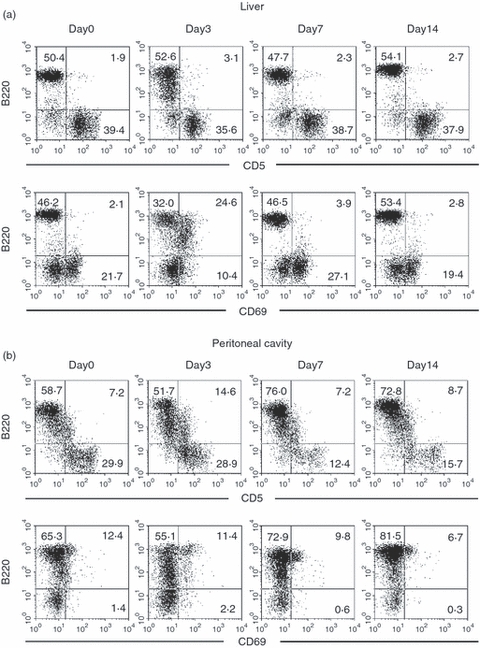
Further characterization of B-1 cells in the liver and peritoneal cavity. (a) Liver, (b) peritoneal cavity. Two-colour staining for CD5 (or CD69) and B220 cells were conducted. Numbers in the figure represent the percentages of fluorescence-positive cells in the corresponding area. Representative data from three experiments are depicted.
Since autoantibody-producing B cells are known to be abundant in the peritoneal cavity, similar experiments were conducted using lymphocytes isolated from this site (Fig. 4b). There were some CD5+ B220low cells in the peritoneal cavity, irrespective of the days examined. This was also true for the expression of CD69. Some CD69+ B cells were consistently present in the peritoneal cavity.
Expansion of B220low B cells both in the liver and peritoneal cavity
We have previously reported that B220low B cells eventually become autoantibody-producing B cells in mice with autoimmune diseases and with malarial infection.17–20 However, B220very low cells are present and they are activated NK cells. In this regard, we gated only B220high cells and B220low cells in various organs of mice with α-GalCer injection (Fig. 5a). By this gating, B220low cells were found to appear on day 3 after α-GalCer administration in the liver, spleen and peritoneal cavity.
Figure 5.
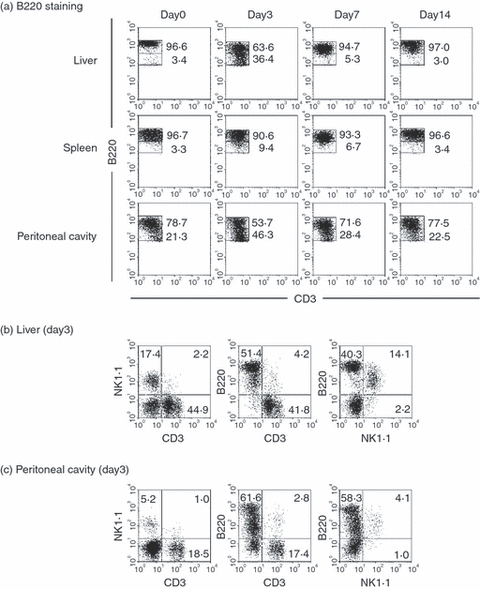
Identification of B220high and B220low cells and their phenotypic characterization. (a) B220 staining, (b) liver, (c) peritoneal cavity. By gated analysis, the ratio of B220high and B220low cells was identified. Lymphocytes were isolated from the liver and peritoneal cavity on day 3 and further phenotypic characterization was conducted.
It was also confirmed that B220very low cells were NK cells. As shown in Fig. 5(b), there were many NK cells (17·4%) and these NK cells were CD3− and NK1.1+ in the liver. In the peritoneal cavity, NK cells were sparse (5·2%) and they were also B220very low. All these experiments were conducted on day 3 after α-GalCer administration.
Actual autoantibody production in cell culture
We have previously demonstrated that B220low cells are capable of producing autoantibodies in in vitro culture.17–20 For this, LPS stimulation is required. In a final set of present experiments, it was examined whether lymphocytes isolated from various organs produced autoantibodies against Hep-2 cells in culture in vitro (Fig. 6). Lymphocytes isolated from the liver did not produce autoantibodies in the absence of LPS in normal mice or in mice with α-GalCer. However, lymphocytes isolated from the liver of mice with α-GalCer produced autoantibodies in culture supernatants by LPS stimulation (Fig. 6a). Such production was not detected in splenic lymphocytes even in the presence of LPS (Fig. 6b). This was also true in the bone marrow (Fig. 6c). In sharp contrast, lymphocytes isolated from the peritoneal cavity had the potential to produce autoantibodies against Hep-2 in control mice and in mice with α-GalCer, in the presence of LPS.
Figure 6.
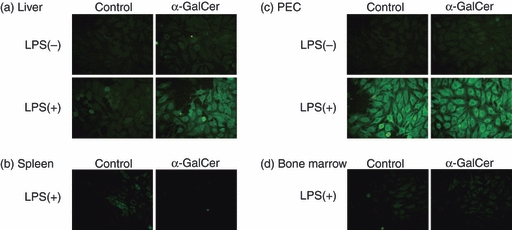
Determination of autoantibodies in the supernatants of cell culture. (a) Liver, (b) spleen, (c) peritoneal cavity exudate cells (PEC), (d) Bone marrow. Lymphocytes were cultured for 5 days in the absence or presence of lipopolysaccharide (LPS). The autoantibodies in supernatants were analysed using Hep-2.
Discussion
In the present study, we demonstrated that α-GalCer-mediated hepatic injury accompanied autoantibody production in parallel with the appearance of B-1 cells in the liver. α-GalCer is known to be recognized by invariant NKT cells via their CD1d (monomorphic MHC class I-like molecule).6–9 These activated NKT cells have the potential to exert cytotoxicity against autologous cells. Concerning this autoreactivity by activated NKT cells and the concomitant autoantibody production, α-GalCer-mediated hepatic injury might be estimated as acute autoimmune hepatitis. The appearance of B220low B cells in the liver after α-GalCer administration supports this speculation.
The most prominent changes in the number of lymphocytes and in hepatic damage were seen on day 3 after α-GalCer administration. At this time (day 3), autoantibodies were detected in sera by two methods, autoantibodies against Hep-2 cells in immunofluorescence tests and autoantibody against dsDNA by the ELISA. It should be mentioned that the highest titres of these autoantibodies showed a peak at day 14. The major isotype of autoantibodies against Hep-2 cells was found to be IgG.
The NKT cells activated by α-GalCer are known to down-regulate their expression of NK1.1 antigens.27 This was confirmed in the liver on day 3, and thereafter the expression of NK1.1 was gradually restored. On the other hand, B220low cells appeared in the liver on day 3. Among these cells, B220very low cells did not belong to B-1 cells but were activated NK cells (see Figs 3 and 5b). The time-kinetic study revealed that the most prominent appearance of B220low B-1 cells was seen on day 3 after α-GalCer administration (see Fig. 5a). The lymphocytes were found to produce autoantibodies in in vitro culture in the presence of LPS.
On the whole, B220low B-1 cells are rare in the liver but such B-1 cells are always present in the peritoneal cavity.17 However, the administration of α-GalCer had the potential to increase the proportion of B-1 cells in the peritoneal cavity, especially on day 3. With or without α-GalCer stimulation, lymphocytes isolated from the peritoneal cavity produced autoantibodies in culture in the presence of LPS. It is speculated that autoantibodies detected in sera might be derived from B-1 cells in both the liver and peritoneal cavity. A restricted production of the autoantibodies from purified B220low cells (B-1 cells) was confirmed by sorting experiments.21,28
In a series of recent studies, we emphasized that extrathymic T cells (NK1.1− TCRint cells) with autoreactivity and autoantibody-producing B-1 cells are simultaneously activated, especially in the liver.17–20 At that time, thymic atrophy or involution always accompanies such activation. In other words, autoimmune states might be considered not to be a failure of T-cell differentiation in the thymus and not to be a failure of B-2-cell differentiation in the immune organs (e.g. spleen, lymph nodes and bone marrow). It is speculated that autoimmune diseases or states are events involving the activation of fundamental lymphocyte subsets of extrathymic T cells and autoantibody-producing B-1 cells, possibly in the liver, intestine and peritoneal cavity.
A simultaneous activation of extrathymic T cells (NK1.1− TCRint) and autoantibody-producing B-1 cells was first found in the liver of mice with malarial infection.19,20 In the case of malaria, the malaria protozoa are present in the cytoplasm of hepatocytes and erythrocytes. Under these conditions, conventional T and B cells (B-2 cells) could not attack the protozoa directly. It is possible that extrathymic T cells with autoreactivity and autoantibodies from B-1 cells attack autologous protozoa-infected hepatocytes or erythrocytes.
The simultaneous activation of NK1.1− TCRint cells and B-1 cells was also seen in mice with autoimmune diseases. Such diseases include autoimmune states in NZB/W F1 autoimmune-prone mice17 and in mice with chronic graft-versus-host disease.18 Autoimmune states occurred in physiological events, namely, with aging.21 Aged mice show a high titre of autoantibodies in sera and the simultaneous activation of NK1.1− TCRint cells and B-1 cells.
Similar to α-GalCer-mediated hepatic injury, concanavalin A-induced hepatic injury accompanied autoantibody production in sera.27 These last two cases including α-GalCer-mediated and concanavalin A-induced hepatic injuries showed the simultaneous activation of NKT cells (NK1.1+ TCRint) and B-1 (CD5− B220low) cells. Indeed, both α-GalCer-induced hepatic injury29–31 and concanavalin A-induced hepatic injury32–34 are known to be acute autoimmune-like hepatitis. In any case, it was revealed in this study that autoantibody production by B-1 cells always occurred in parallel with the activation of unconventional T cells (NK1.1− TCRint or NK1.1+ TCRint cells), mainly in the liver.
We have revealed an intimate relationship between innate-like T cells (i.e. extrathymic T cells and invariant NKT cells) and innate-like B cells (i.e. B-1 cells) in various autoimmune diseases. The similar relationship was also reported in contact and delayed hypersensitivity by Askenase and colleagues.35–39 Briefly, contact sensitization triggered invariant NKT cells and then such NKT cells coactivated innate-like B-1 cells via IL-4. We emphasize that the function of unconventional T and B cells is important for understanding the immune mechanisms on the onset of autoimmune diseases and allergic diseases.
Acknowledgments
This work was supported by grants-in-aid for scientific research from the Ministry of Education, Science and Culture, Japan and Tsukada Medical Foundation. The authors thank Mrs Yuko Kaneko for preparation of the manuscript.
Glossary
Abbreviations
- ALT
alanine aminotransferase
- dsDNA
double-stranded DNA
- α-GalCer
α-galactosylceramide
- LPS
lipopolysaccharide
- mAb
monoclonal antibody
- NKT
natural killer T
- TCRint
intermediate T-cell receptor
Disclosures
The authors have no financial conflict of interest.
References
- 1.Sato K, Ohtsuka K, Hasegawa K, Yamagiwa S, Watanabe H, Asakura H, Abo T. Evidence for extrathymic generation of intermediate T cell receptor cells in the liver revealed in thymectomized, irradiated mice subjected to bone marrow transplantation. J Exp Med. 1995;182:759–67. doi: 10.1084/jem.182.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watanabe H, Miyaji C, Kawachi Y, Iiai T, Ohtsuka K, Iwanaga T, Takahashi-Iwanaga H, Abo T. Relationships between intermediate TCR cells and NK1.1+ T cells in various immune organs. NK1.1+ T cells are present within a population of intermediate TCR cells. J Immunol. 1995;155:2972–83. [PubMed] [Google Scholar]
- 3.Kawachi Y, Arai K, Moroda T, et al. Supportive cellular elements for hepatic T cell differentiation: T cells expressing intermediate levels of the T cell receptor are cytotoxic against syngeneic hepatoma, and are lost after hepatocyte damage. Eur J Immunol. 1995;25:3452–9. doi: 10.1002/eji.1830251237. [DOI] [PubMed] [Google Scholar]
- 4.Abo T, Watanabe H, Sato K, Iiai T, Moroda T, Takeda K, Seki S. Extrathymic T cells stand at an intermediate phylogenetic position between natural killer cells and thymus-derived T cells. Nat Immun. 1995;14:173–87. [PubMed] [Google Scholar]
- 5.Watanabe H, Miyaji C, Seki S, Abo T. c-kit+ stem cells and thymocyte precursors in the livers of adult mice. J Exp Med. 1996;184:687–93. doi: 10.1084/jem.184.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawano T, Junqing C, Koezuka Y, et al. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science. 1997;278:1626–9. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 7.Burdin N, Brossay L, Koezuka Y, et al. Selective ability of mouse CD1 to present glycolipids: α-Galactosylceramide specifically stimulates Vα14+NK T lymphocytes. J Immunol. 1998;161:3271–81. [PubMed] [Google Scholar]
- 8.Brossary L, Naidenko O, Burdin N, Matsuda J, Sakai T, Kronenberg M. Structural requirements for galactosylceramide recognition by CD1-restricted NK T cells. J Immunol. 1998;161:5124–8. [PubMed] [Google Scholar]
- 9.Burdin N, Brossary L, Kronenberg M. Immunization with α-galactosylceramide polarizes CD1-reactive NK T cells towards Th2 cytokines synthesis. Eur J Immunol. 1999;29:2014–25. doi: 10.1002/(SICI)1521-4141(199906)29:06<2014::AID-IMMU2014>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 10.Hammond K, Cain W, van Driel I, Godfrey D. Three day neonatal thymectomy selectively depletes NK1.1+ T cells. Int Immunol. 1998;10:1491–9. doi: 10.1093/intimm/10.10.1491. [DOI] [PubMed] [Google Scholar]
- 11.Kameyama H, Kawamura T, Naito T, Bannai M, Shimamura K, Hatakeyama K, Abo T. Size of the population of CD4+ natural killer T cells in the liver is maintained without supply by the thymus during adult life. Immunology. 2001;104:135–41. doi: 10.1046/j.0019-2805.2001.01289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki S, Sugahara S, Shimizu T, et al. Low level of mixing of partner cells seen in extrathymic T cells in the liver and intestine of parabiotic mice: its biological implication. Eur J Immunol. 1998;28:3719–29. doi: 10.1002/(SICI)1521-4141(199811)28:11<3719::AID-IMMU3719>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 13.Kawamura T, Kawachi Y, Moroda T, et al. Cytotoxic activity against tumour cells mediated by intermediate TCR cells in the liver and spleen. Immunology. 1996;89:68–75. doi: 10.1046/j.1365-2567.1996.d01-719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moroda T, Iiai T, Kawachi Y, Kawamura T, Hatakeyama K, Abo T. Restricted appearance of self-reactive clones into intermediate T cell receptor cells in neonatally thymectomized mice with autoimmune disease. Eur J Immunol. 1996;26:3084–91. doi: 10.1002/eji.1830261239. [DOI] [PubMed] [Google Scholar]
- 15.Moroda T, Kawachi Y, Iiai T, et al. Self-reactive forbidden clones are confined to pathways of intermediate T cell receptor cell differentiation even under immunosuppressive conditions. Immunology. 1997;91:88–94. doi: 10.1046/j.1365-2567.1997.00222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moroda T, Iiai T, Suzuki S, et al. Autologous killing by a population of intermediate TCR cells and its NK1.1+ and NK1.1− subsets, using Fas ligand/Fas molecules. Immunology. 1997;91:219–26. doi: 10.1046/j.1365-2567.1997.00240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morshed SRM, Mannoor K, Halder RC, Kawamura H, Bannai M, Sekikawa H, Watanabe H, Abo T. Tissue-specific expansion of NKT and CD5+B cells at the onset of autoimmune disease in (NZB×NZW)F1 mice. Eur J Immunol. 2002;32:2551–61. doi: 10.1002/1521-4141(200209)32:9<2551::AID-IMMU2551>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 18.Miyakawa R, Miyaji C, Watanabe H, Yokoyama H, Tsukada C, Asakura H, Abo T. Unconventional NK1.1− intermediate TCR cells as major T lymphocytes expanding in chronic graft-versus-host disease. Eur J Immunol. 2002;32:2521–31. doi: 10.1002/1521-4141(200209)32:9<2521::AID-IMMU2521>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 19.Mannoor MK, Halder RC, Morshed SRM, et al. Essential role of extrathymic T cells in protection against malaria. J Immunol. 2002;169:301–6. doi: 10.4049/jimmunol.169.1.301. [DOI] [PubMed] [Google Scholar]
- 20.Kanda Y, Kawamura H, Matsumoto H, Kobayashi T, Kawamura T, Abo T. Identification and characterization of autoantibody-producing B220low B (B-1) cells appearing in malarial infection. Cell Immunol. 2010;263:49–54. doi: 10.1016/j.cellimm.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 21.Tachikawa S, Kawamura T, Kawamura H, Kanda Y, Fujii Y, Matsumoto H, Abo T. Appearance of B220low autoantibody-producing B-1 cells at neonatal and older stages in mice. Clin Exp Immunol. 2008;153:448–55. doi: 10.1111/j.1365-2249.2008.03709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osman Y, Kawamura T, Naito T, Takeda K, van Kaer L, Okumura K, Abo T. Activation of hepatic NKT cells and subsequent liver injury following administration of α-galactosylceramide. Eur J Immunol. 2000;30:1919–28. doi: 10.1002/1521-4141(200007)30:7<1919::AID-IMMU1919>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 23.Kawabe S, Abe T, Kawamura H, Gejyo F, Abo T. Generation of B220low B cells and production of autoantibodies in mice with experimental amyloidosis: association of primordial T cells with this phenomenon. Clin Exp Immunol. 2004;135:200–8. doi: 10.1111/j.1365-2249.2003.02361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimamura K, Kawamura H, Nagura T, Kato T, Naito T, Kameyama H, Hatakeyama K, Abo T. Association of NKT cells and granulocytes with liver injury after reperfusion of the portal vein. Cell Immunol. 2005;234:31–8. doi: 10.1016/j.cellimm.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 25.Stanic AK, De Silva AD, Park J-J, et al. Defective presentation of the CD1d1-restricted natural Va14Ja18 NKT lymphocyte antigen caused by β-d-glucosylceramide synthase deficiency. PNAS. 2003;100:1849–54. doi: 10.1073/pnas.0430327100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naito T, Kawamura T, Bannai M, et al. Simultaneous activation of natural killer T cells and autoantibody production in mice injected with denatured syngeneic liver tissue. Clin Exp Immunol. 2002;129:397–404. doi: 10.1046/j.1365-2249.2002.01910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson MT, Johansson C, Olivares-Villagómez D, et al. The response of natural killer T cells to glycolipid antigens is characterized by surface receptor down-modulation and expansion. ProcNatl Acad Sci U S A. 2003;100:10913–8. doi: 10.1073/pnas.1833166100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujii Y, Kawamura H, Kawamura T, et al. Co-appearance of autoantibody-producing B220low B cells with NKT cells in the course of hepatic injury. Cell Immunol. 2010;260:105–12. doi: 10.1016/j.cellimm.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Cao Z, Dhupar R, Cai C, Li P, Billiar TR, Geller DA. A critical role for IFN regulatory factor 1 in NKT cell-mediated liver injury induced by alpha-galactosylceramide. J Immunol. 2010;185:2536–43. doi: 10.4049/jimmunol.1000092. [DOI] [PubMed] [Google Scholar]
- 30.Lappas CM, Day YJ, Marshall MA, Engelhard VH, Linden J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med. 2006;203:2639–48. doi: 10.1084/jem.20061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biburger M, Tiegs G. Alpha-galactosylceramide-induced liver injury in mice is mediated by TNF-α but independent of Kupffer cells. J Immunol. 2005;175:1540–50. doi: 10.4049/jimmunol.175.3.1540. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe S, Ohnuki K, Hara Y, et al. Suppression of Con A-induced hepatitis induction in ICOS-deficient mice. Immunol Lett. 2010;128:51–8. doi: 10.1016/j.imlet.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Kawamura T, Takeda K, Kaneda H, et al. NKG2A inhibits invariant NKT cell activation in hepatic injury. J Immunol. 2009;182:250–8. doi: 10.4049/jimmunol.182.1.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakashima H, Kinoshita M, Nakashima M, Habu Y, Shono S, Uchida T, Shinomiya N, Seki S. Superoxide produced by Kupffer cells is an essential effector in concanavalin A-induced hepatitis in mice. Hepatology. 2008;48:1979–88. doi: 10.1002/hep.22561. [DOI] [PubMed] [Google Scholar]
- 35.Askenase PW, Szczepanik M, Itakura A, Kiener C, Campos RA. Extravascular T cell recruitment requires initiation begun by Vα14+ NKT cells and B-1 B cells. Trends Immunol. 2004;25:441–9. doi: 10.1016/j.it.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Herzog WR, Ferreri NR, Ptak W, Askenase PW. The antigen-specific DTH-initiating Thy-1+ cell is double negative (CD4−, CD8−) and CD3 negative; and expresses IL-3 receptors, but no IL-2 receptors. J Immunol. 1989;143:3125–33. [PubMed] [Google Scholar]
- 37.Compos RA, Szczepanik M, Itakura A, Akahira-Azuma M, Sidobre S, Kronenberg M, Askenase PW. Cutaneous immunization rapidly activates liver invariant Vα14 NKT cells stimulating B-1 B cells to initiate T cell recruitment for elicitation of contact sensitivity. J Exp Med. 2003;198:1785–96. doi: 10.1084/jem.20021562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Compos RA, Szczepanik M, Itakura A, Lisbonne M, Dey N, Leite-de-Moraes MC, Askenase PW. IL-4 dependent innate collaboration between iNKT cells and B-1 B cells controls adaptive contact sensitivity. Immunology. 2006;117:536–47. doi: 10.1111/j.1365-2567.2006.02330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Compos RA, Szczepanik M, Lisbonne M, Itakura A, Leite-de-Moraes M, Askenase PW. Invariant NKT cells rapidly activated via immunization with diverse contact antigens collaborate in vitro with B-1 cells to initiate contact sensitivity. J Immunol. 2006;177:3686–94. doi: 10.4049/jimmunol.177.6.3686. [DOI] [PubMed] [Google Scholar]


