Abstract
Human monocytes can be divided into two major subpopulations, CD14++ CD16− and CD14+ CD16+ cells, which are suggested to play different roles in antimicrobial responses. In neonates, characteristics and functional responses of monocyte subsets have not previously been explored, and might contribute to the qualitative difference between neonatal and adult cytokine profiles. We report that at baseline, monocyte subsets in cord blood and adult peripheral blood are present in similar frequencies, and show similar expression of CD11c, CD80/CD86, CD163 and HLA-DR. In response to the bacterial ligand peptidoglycan, cord blood monocytes had high inherent capacity for production of the early-response cytokines with levels of tumour necrosis factor and interleukin-12p70 exceeding adult levels, and also a higher phosphorylation of p38-mitogen-activated protein kinase. The CD14+ CD16+ cells expressed more interleukin-12p70 than CD14++ CD16− cells and were present in a higher frequency in peptidoglycan-stimulated cord blood mononuclear cell cultures. Together, the behaviour of cord blood CD14+ CD16+ cells following peptidoglycan stimulation might indicate a qualitative difference between the neonatal antimicrobial response and that of the adult. In addition we found that serum factors in cord blood and adult sera affected cytokine production similarly, with the exception of tumour necrosis factor, regardless of the source of serum or cells. Overall, our data provide new insights into monocyte heterogeneity in cord blood and monocyte subset responses to a bacterial ligand at birth.
Keywords: CD14++ CD16− cells, CD14+ CD16+ cells, interleukin-12p70, neonatal immunity, tumour necrosis factor
Introduction
Innate cells are important for early-life immune responses because adaptive immunity in the newborn is suboptimal.1 Monocytes are an important source of early cytokines in microbial infections and express a range of pattern recognition receptors, including high levels of toll-like receptor 2 (TLR2) and TLR4.2 Triggering of TLRs on monocytes by microbial products activates common mediators such as MyD88, mitogen-activated protein kinases (MAPKs) and nuclear factor-κB, leading to cytokine production.
There are two major monocyte subpopulations, distinguished by the presence or absence of CD16 (FCγRIII).3 The two subsets display distinct expression patterns of chemokine, adhesion and scavenger receptors and are thought to play different roles in antimicrobial responses.4,5 Furthermore, they may differentiate into macrophage and dendritic cell (DC) subpopulations with distinct phenotypes.6–8
The ‘classical’ CD14++ CD16− subpopulation constitutes 80–90% of total monocytes in comparison with the minor subset of ‘inflammatory’ CD14+ CD16+ monocytes.3 The latter subset produces higher levels of pro-inflammatory cytokines, has increased potency for antigen presentation, increased expression of genes involved in Fcγ receptor-mediated phagocytosis and higher levels of TLR2 and TLR4.9–11 Furthermore, the frequency of CD14+ CD16+ monocytes is increased in adult and neonatal sepsis12,13 as well as in a number of other inflammatory conditions.14,15
The neonatal innate immune response differs qualitatively from that of the adult and seems to preferentially favour the T helper type 2 (Th2) and Th17 types of immunity over Th1-type of immunity.16–18 Although neonatal monocytes are commonly considered to be impaired in the production of Th1-promoting cytokines when assayed in whole blood, there are also reports describing robust production of tumour necrosis factor (TNF) and interleukin-12 (IL-12) from mononuclear cells.16,19 One study found that elevated adenosine levels in cord blood (CB) serum enhanced cAMP content in mononuclear cells, which conferred suppression of TLR-induced TNF production whereas IL-6 production was not affected.20 Furthermore, the specific CB response seems to depend on the TLR agonist, for instance CB monocyte TNF production was found to be deficient in response to lipopolysaccharide (LPS) and bacterial lipopeptides but was preserved to R-848.21 Taken together these findings illustrate that the neonatal innate response is defined by both extrinsic and intrinsic cell factors.
Given the importance of the innate branch of immunity for early-life protection, the definition of functional responses and characteristics of cells such as monocytes, and their subpopulations, in newborns is of particular interest. We hypothesized that if the CB monocyte compartment differed in characteristics or composition from that of the adult, this could potentially contribute to the observed differences in cytokine production profile and antimicrobial responses.
To test this hypothesis we stimulated cells with peptidoglycan (PGN), which is an essential part of the cell wall of nearly all bacteria and a strong target for immune recognition.22 The PGN activates TLR2/nucleotide-binding oligomerization domain (Nod)-like receptors 1/2, which are highly expressed by monocytes2,23 and so we could model potent monocyte activation in response to a general microbial stimulus. We measured cytokine production and activation of the PGN-induced signalling intermediates extracellular signal-regulated kinase (ERK) 1/2 and p38-MAPK that has previously shown good correlation to cytokine output.24 Furthermore, to clearly separate cell-inherent cytokine production capacity from the effect of soluble factors we investigated the effect of CB versus adult serum on cytokine production.
Here, we demonstrate similar monocyte expression of activation-related surface receptors and frequencies of monocyte populations in CB and adults. The CB monocyte subsets showed potent production of TNF and IL-12p70 in response to the bacterial ligand PGN, together indicating that monocyte subset responses to bacteria at birth are not deficient.
Materials and methods
Blood sample processing
Cord blood was collected from 20 healthy pregnancies, 10 vaginal deliveries and 10 elective caesarean sections, at the Karolinska University Hospital, Stockholm, Sweden. In addition, peripheral blood (PB) was obtained from 10 healthy non-pregnant adult volunteers. Venous blood (from the umbilical cord immediately after delivery or from PB) was collected into heparinized tubes for cell separation or into serum tubes. Mononuclear cells (MC) were isolated within 24 hr using Ficoll–Paque (Pharmacia–Upjohn, Uppsala, Sweden) gradient centrifugation and cryopreserved in liquid nitrogen. Serum was separated by centrifugation, frozen and stored at −85° until use. All mothers and healthy volunteers gave their informed consent and the Regional Ethics Committee in Stockholm approved the study.
Culture of mononuclear cells
Both CBMC and PBMC were thawed, washed three times and then re-suspended in RPMI-1640 supplemented with 10% heat-inactivated fetal calf serum (FCS; Hyclone Laboratories Inc., Logan, UT), l-glutamine (2 mm), penicillin G sodium (100 units/ml) and streptomycin sulphate (100 μg/ml; Merck, Darmstadt, Germany) to a concentration of 106 cells/ml and cultured at 37° and 5% CO2. To analyse surface receptors, cells were cultured for 3 hr before staining. For the phosphorylation assay, cells were cultured for 3 hr, followed by 30 min of stimulation with either medium control or PGN (10 μg/ml; Staphylococcus aureus; Fluka; Sigma-Aldrich, Stockholm, Sweden). For detection of secreted and intracellular cytokine production, cells were cultured for 24 hr with medium control or with PGN (1 μg/ml). Supernatants were recovered and stored at −85° until analysis.
Serum substitution experiments
For analysis of serum effects on cytokine production, four different CBMC or PBMC were cultured for 24 hr with PGN stimuli as described above. However, 10% FCS in the culture medium was substituted with either 10% autologous serum (n = 4 for CBMC and PBMC) or serum from four different adult donors (n = 4 × 4 combinations for CBMC) or four different cord bloods (n = 4 × 4 combinations for PBMC). Also, four different PBMC were cultured in medium with either 10% autologous (n = 4 for PBMC) or heterologous serum from four different adult donors (n = 4 × 4 combinations for PBMC). Data are presented as modulation index from the value 1, which represents data from FCS cultures. The index was calculated as follows (cytokine values from serum substitution experiments)/(cytokine values from FCS cultures).
Flow cytometry
Monoclonal antibodies were conjugated to fluorescein isothiocyanate (CD14, CD16) phycoerythrin (CD14, CD163, IL-12p70), phycoerythrin-Cy7 (CD16), allophycocyanin (HLA-DR, CD11c, TNF), Alexa Fluor 488 (p-p38-MAPK) or Alexa Fluor 647 (p-ERK1/2) and used for cell labelling according to standard procedures. The biotinylated primary antibodies for CD80 and CD86 were used in combination and detected with streptavidin–peridinin chlorophyll protein conjugate. All conjugates obtained from BD Biosciences (San Diego, CA). To detect intracellular IL-12p70 and TNF, GolgiStop (BD Biosciences) was added to cultures at the start of incubation. After surface labelling, cells were fixed, permeabilized and stained for IL-12p70 and TNF. For detection of phosphorylated p38-MAPK and ERK1/2 in monocytes, BD Phosflow Phosphorylation State Analysis (BD Biosciences Pharmingen) was performed according to the manufacturer's instructions for human PBMC protocol 1. In brief, cells were fixed (Fix Buffer 1, BD Biosciences Pharmingen) immediately after stimulation and were then pelleted by centrifugation and supernatants were discarded. Cells were resuspended and washed three times with permeabilization/washing buffer (Perm/Wash buffer 1, BD Biosciences Pharmingen) before being labelled with antibodies against phosphorylated epitopes on p38-MAPK (pT180/pY182) and ERK1/2 (pT202/pY204), for 30 min at room temperature in the dark. Data were acquired by FACSCalibur flow cytometer (BD Biosciences) and analysed by FlowJo software 8.8.6 (TreeStar, Ashland, OR). Gating was performed on live CD14+ monocytes. For stimulation experiments, quadrants were set with a maximum of 2·5% positive cells in unstimulated cultures and were then transferred to stimulated samples from which statistical data were acquired. Data are presented as geometrical mean fluorescence intensity (GeoMFI) values or in Fig. 3 as % CD14+ CD16+ cells of total CD14+ cells.
Figure 3.
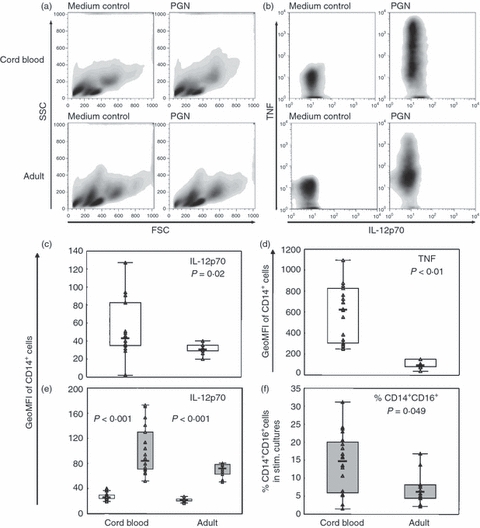
Peptidoglycan (PGN) -stimulated intracellular interleukin-12p70 (IL-12p70) and tumour necrosis factor (TNF) is higher in cord blood than in adult monocytes. Cord blood (CBMC; n = 20) or peripheral blood (PBMC; n = 10) mononuclear cells were stimulated for 24 hr with 1 μg/ml PGN in the presence of GolgiStop and CD14+ cells were analysed by flow cytometry for intracellular expression of IL-12p70 and TNF. (a) Representative forward and side scatter characteristics of CBMC (upper panel) or PBMC (lower panel). (b) Representative cytokine responses of cord blood (upper panel) or adult monocytes (lower panel). In (c) and (d), respectively, IL-12p70 and TNF levels in cord blood versus adult monocytes are shown. (e) IL-12p70 levels in cord blood and adult CD14++ CD16− (□) and CD14+ CD16+ monocytes ( ). Data are shown as geometric mean fluorescence intensity (GeoMFI) or in (f) as % CD14+ CD16+ cells of total CD14+ cells in PGN-stimulated cultures. Boxes cover 50% of the values between the 25th and the 75th percentiles, and whiskers extend to the 10th and 90th percentiles. Raw data are shown excluding outliers and extremes. The Mann–Whitney U-test was used to evaluate differences between groups.
). Data are shown as geometric mean fluorescence intensity (GeoMFI) or in (f) as % CD14+ CD16+ cells of total CD14+ cells in PGN-stimulated cultures. Boxes cover 50% of the values between the 25th and the 75th percentiles, and whiskers extend to the 10th and 90th percentiles. Raw data are shown excluding outliers and extremes. The Mann–Whitney U-test was used to evaluate differences between groups.
Secreted cytokine measurements
Levels of IL-1β, IL-6, IL-8, IL-10, IL-12p70 and TNF were measured in culture supernatants using the Cytometric Bead Array (CBA; BD Biosciences Pharmingen). Briefly, standards and samples were mixed with phycoerythrin-labelled beads and incubated for 3 hr. After incubation, the samples and standards were washed to remove unbound material and analysed using the FCAP Array software. Calibration of the flow cytometer was performed using BD FACSComp™ and BD CaliBRITE™ Beads (BD Biosciences Pharmingen). Levels of IL-12p70 were too low for reliable detection and were not included in further analyses.
Statistical analyses
Statistical differences between groups were evaluated by the Mann–Whitney U non-parametric test and correlations by the Spearman rank correlation test, both performed with Statistica (statistica Statsoft Software Inc., Tulsa, OK). All box plots display the following information: the box covers 50% of the values between the 25th and the 75th percentiles and the whiskers extend to the 10th and 90th percentiles. Raw data are displayed, outliers and extremes are included in the statistical analyses but not shown in the figures. Data in the text indicate median (number in parentheses, range). Statistical significance was assumed when P < 0·05.
Results
Cord blood and adult monocyte subsets have similar frequencies and expression of activation-related surface receptors
The neonatal monocyte population is thought to be immature but little is known regarding the monocyte subpopulations. We examined the frequencies of the monocyte subsets and their expression of activation-related surface markers at baseline in CB and compared this with adults. The percentages of CD14++ CD16− [94% (60–98) for CBMC and 92% (85–96) for PBMC] and CD14+ CD16+ cells [6% (40–2) for CBMC and 8% (15–4) for PBMC] were similar in CB and adults. In both groups CD14+ CD16+ cells expressed significantly higher levels of CD163, CD80/CD86 and CD11c; adult CD14+ CD16+ cells also expressed higher levels of HLA-DR compared with CD14++ CD16− cells (Fig. 1). No differences were seen in levels of surface markers on the monocyte population as a whole between CB and adults (data not shown).
Figure 1.
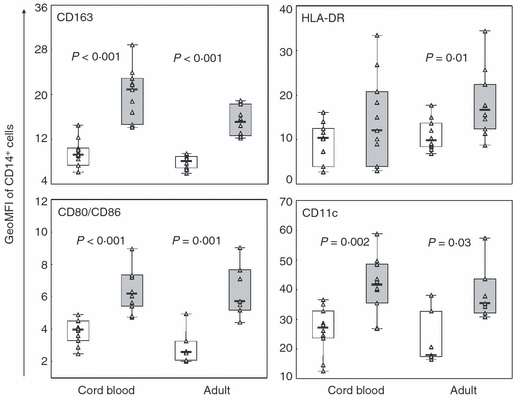
Similar expression of activation-related surface receptors on monocyte subsets in cord blood and adults. CD14++ CD16− (□) and CD14+ CD16+ monocytes ( ) from cord blood (n = 10) and adults (n = 10) were assayed after 3 hr rest in culture by flow cytometry for expression of CD163, HLA-DR, CD80/CD86 and CD11c. Data are presented as geometric mean fluorescence intensity (GeoMFI), boxes cover 50% of the values between the 25th and the 75th percentile and whiskers extend to the 10th and 90th percentile. Raw data are shown excluding outliers and extremes. The Mann–Whitney U-test was used to evaluate differences between CD14++ CD16− and CD14+ CD16+ cells.
) from cord blood (n = 10) and adults (n = 10) were assayed after 3 hr rest in culture by flow cytometry for expression of CD163, HLA-DR, CD80/CD86 and CD11c. Data are presented as geometric mean fluorescence intensity (GeoMFI), boxes cover 50% of the values between the 25th and the 75th percentile and whiskers extend to the 10th and 90th percentile. Raw data are shown excluding outliers and extremes. The Mann–Whitney U-test was used to evaluate differences between CD14++ CD16− and CD14+ CD16+ cells.
Potent peptidoglycan-stimulated cytokine production from cord blood monocyte subsets
Results from previous studies of cytokine production by neonatal cells diverge depending on the method and mode of stimulation. To examine cell-inherent cytokine production capacity of CB monocytes and their subsets in response to a bacterial ligand, we stimulated cells with PGN in cultures with FCS as the source of serum. Levels of IL-6, IL-8 and IL-10 from CBMC cultures equalled those from PBMC (Fig. 2a) whereas TNF levels were significantly higher and IL-1β levels were borderline significant (Fig. 2b). Furthermore, intracellular IL-12p70 and TNF production in monocytes and their subpopulations was assessed after PGN stimulation (Fig. 3). Representative examples of forward and side scatter characteristics of CBMC and PBMC are shown in Fig. 3(a), and those of TNF and IL-12p70 production of CB or adult monocytes are shown in Fig. 3(b). The IL-12p70 response to stimulation was low but detectable in both CB and adults. When quantified, intracellular levels of IL-12p70 and TNF were significantly higher in CB than in adult monocytes (measured as GeoMFI in Fig. 3c,d). The CD14+ CD16+ cells had higher levels of IL-12p70 compared with the CD14++ CD16− cells in both CB and adults (Fig. 3e) whereas for TNF no difference was seen between the subsets in either group (data not shown). The percentage of CD14+ CD16+ cells in unstimulated cultures did not differ between CB and adults, but after stimulation the percentage of CD14+ CD16+ cells decreased in adult cell cultures whereas there was no change in CB cultures. This led to a significantly higher percentage of CD14+ CD16+ cells in stimulated CB cultures compared with adult (Fig. 3f). Additionally, in accordance with several studies19,21 there was no difference in cytokine production, secreted or intracellular, between CBMC acquired from vaginal deliveries or caesarean sections (data not shown).
Figure 2.
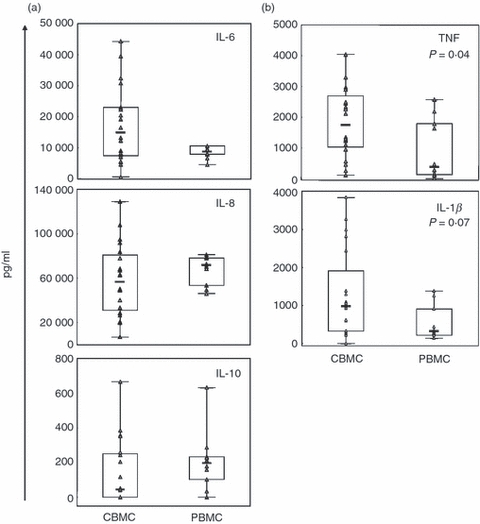
Potent peptidoglycan (PGN) -stimulated production of early-response cytokines from cord blood mononuclear cells (CBMC). The CBMC (n = 20) or peripheral blood mononuclear cells (PBMC; n = 10) were stimulated with 1 μg/ml PGN for 24 hr and culture supernatants were analysed by cytometric bead array for (a) interleukin-6 (IL-6), IL-8 and IL-10, and (b) tumour necrosis factor (TNF) and IL-1β. Cytokine levels are shown in pg/ml, boxes cover 50% of the values between the 25th and the 75th percentiles, and whiskers extend to the 10th and 90th percentiles. Raw data are shown excluding outliers and extremes. The Mann–Whitney U-test was used to evaluate differences between groups.
High phosphorylation of p38-MAPK in cord blood monocytes
As MAPKs are activated following PGN recognition we examined whether the observed potent cytokine production from CB monocytes was associated with high phosphorylation of p38-MAPK or ERK1/2. Following PGN stimulation, CB monocytes had a significantly higher level of p-p38-MAPK (GeoMFI in Fig. 4) than adult monocytes, which correlated with levels of intracellular IL-12p70 production (rs = 0·54, P = 0·046). ERK1/2 was also phosphorylated in response to PGN but no difference was noted between groups (data not shown).
Figure 4.
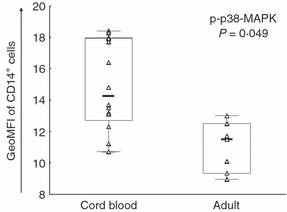
Phosphorylation of p38-mitogen-activated protein kinase (MAPK) in response to peptidoglycan (PGN) is higher in cord blood than in adult monocytes. Cord blood (CBMC; n = 16) or peripheral blood (PBMC; n = 7) mononuclear cells were stimulated with 10 μg/ml PGN for 30 min and subsequently CD14+ cells were analysed by flow cytometry for p-p38-MAPK. Data are shown as geometric mean fluorescence intensity (GeoMFI). Boxes cover 50% of the values between the 25th and the 75th percentiles, and whiskers extend to the 10th and 90th percentiles. Raw data are shown excluding outliers and extremes. The Mann–Whitney U-test was used to evaluate differences between groups.
Cytokine release from cord blood and adult mononuclear cells is similarly affected by serum factors
As soluble factors in serum have been shown to affect cytokine production,20,21 we investigated how the substitution of FCS in culture medium with CB or adult sera affected cytokine production from both CBMC and PBMC. For the purposes of this comparison, data are presented as modulation index from baseline 1, which represents cytokine values obtained from FCS cultures. We found that IL-6 and IL-8 levels in culture supernatants were increased by 150–400% regardless of cell origin when FCS was replaced with CB or adult sera (Fig. 5a). In contrast, IL-10 and TNF levels were markedly reduced (Fig. 5b). When cells were cultured with adult sera IL-10 levels were consistently reduced by 70–90% but the reduction by CB sera was quite variable among subjects. Production of TNF from CBMC was reduced by approximately 50–60% by both types of sera. In contrast, there was a significant difference in TNF production from PBMC depending on which serum was used. Cord blood sera decreased TNF levels by 70–80% whereas adult sera only conferred a 25% decrease compared with FCS (P < 0·001). Interleukin-1β production from PBMC increased slightly with serum substitution whereas no consistent pattern was seen in CBMC cultures (Fig. 5c).
Figure 5.
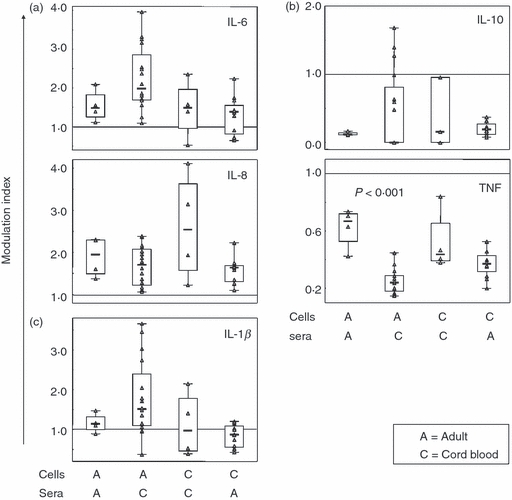
Effect of soluble factors in serum on cytokine release. Cord blood (CBMC; n = 4) or peripheral blood (PBMC; n = 4) mononuclear cells were cultured for 24 hr with 1 μg/ml peptidoglycan (PGN) with either 10% autologous serum in culture media, or 10% sera from four different adult donors (for CBMC n = 4 × 4 combinations) or four different cord bloods (for PBMC n = 4 × 4 combinations). Supernatants were subsequently analysed by cytometric bead array for (a) interleukin-6 (IL-6) and IL-8, (b) IL-10 and tumour necrosis factor (TNF) and (c) IL-1β. For the purposes of comparison, data are shown as modulation index with the horizontal line at 1 representing cytokine levels in cultures with 10% fetal calf serum as serum source. Boxes cover 50% of the values between the 25th and the 75th percentiles, and whiskers extend to the 10th and 90th percentiles. Raw data are shown excluding outliers and extremes. The Mann–Whitney U-test was used to evaluate differences between groups.
To elucidate if autologous and heterologous sera affect cytokine production differently, PBMC were cultured with adult sera from the two different sources. The observed differences compared with FCS were consistent, and overall PGN-induced cytokine production from PBMC did not differ with autologous or heterologous serum. Only IL-8 levels were further increased with heterologous serum (data not shown).
Discussion
Monocytes are a heterogeneous cell population that is important for early-life host defence. Despite the apparent diversity of the monocyte subsets and their suggested differential contribution to antimicrobial responses, their characteristics and cytokine response capacity have not previously been investigated in neonates. Here we found that already in CB, CD14++ CD16− and CD14+ CD16+ cells were present at frequencies similar to those in adults. High expression of the adhesion receptor CD11c, the co-stimulatory molecules CD80/CD86 and the haemoglobin scavenger receptor CD163 distinguished CD14+ CD16+ cells (Fig. 1), as previously shown for adults.4,25 Interestingly, CD163 has recently been implicated as an innate sensor for bacteria26 in line with strong expression of TLRs on CD14+ CD16+ cells.9,10 Noticeably, both CB monocyte subsets had similar expression of HLA-DR whereas in adults CD14+ CD16+ cells had higher levels (Fig. 1).4 The neonatal monocyte population as a whole has been reported to have decreased HLA-DR expression;27 however, that was not observed here.
Preferential hyperproduction of certain cytokines, including the pro-inflammatory, from neonatal mononuclear cells has been reported in studies using a variety of TLR stimuli.16,18,19,27 In line with these studies we found that CBMC had similar capacity to PBMC to produce the early-response cytokines IL-6, IL-8 and IL-10 and a tendency for even higher production of IL-1β (Fig. 2) when stimulated with PGN – a TLR2/nucleotide-binding oligomerization domain (Nod)-like receptor 1/2 ligand. TNF production on the other hand appears to differ substantially depending on ligand specificity and experimental setup.16,21,28 Here we found that inherent TNF production by CBMC in response to PGN was potent and even exceeded that of PBMC, both secreted and intracellular TNF (Figs 2b and 3d). The CD14+ CD16+ cells did not exhibit higher intracellular levels of TNF in contrast to previous findings.9 However, as TNF production from monocytes appears to be dependent on the stimulus used, PGN might induce an optimal response from both CD14+ CD16+ and CD14++ CD16− cells. In addition, CD14+ CD16+ cells can be further subdivided into CD14highCD16+ and CD14dimCD16+ cells, where the latter seem to have a role in sensing viral ligands by way of intracellular TLR7/8, rather than responding through cell-surface TLRs.29 Accordingly, they produce lower amounts of TLR2-induced TNF than CD14high CD16+ cells,29 which could confound the data regarding CD16+ monocytes as a whole.
Interleukin-12p70 was undetectable in culture supernatants but both CB and adult monocytes expressed intracellular IL-12p70 upon PGN stimulation, with higher levels in CB monocytes (Fig. 3c). In contrast, following LPS stimulation CBMC are consistently reported to show decreased IL-12p70 production,28,30,31 which has been connected to decreased stability of mRNA for the IL-12 subunit p40.32 The defective IL-12p70 response to LPS seems to originate from neonatal monocyte-derived DCs30 and so does not preclude the potent monocyte production capacity to another bacterial ligand found here. The highest levels of IL-12p70 were found in CD14+ CD16+ cells from both CB and adults (Fig. 3e). This has previously been indicated for adults33 and our previous study provided indirect evidence to support this finding as CD14+ CD16+ cells induced higher natural killer cell interferon-γ production than CD14++ CD16− cells.34 The increased capacity to respond to microbial stimulation is concurrent with higher expression of TLR26,9 and CD163 noted on both CB and adult CD14+ CD16+ cells (Fig. 1). However, only IL-12p70 production was augmented in these cells indicating a differential regulation of TNF and IL-12p70 responses downstream of PGN recognition.
Differences in regulation and activation level of mediators involved in the PGN recognition pathway could affect production of the early-response cytokines. As TLR expression is at an adult level, activation of MAPK in CB monocytes has been investigated with varying results depending on experimental conditions.21,35,36 Here phosphorylation of p38-MAPK in CB monocytes in response to PGN stimulation was higher than in adult monocytes (Fig. 4), which also correlated with higher levels of intracellular IL-12p70. It would have been interesting to elucidate p38-MAPK activation in the monocyte subsets and relate this to cytokine production but unfortunately this was not possible because of poor resolution of the CD16 antibody in the phosphorylation assay. In addition, although ERK1/2 was activated in response to PGN, no connection to cytokine production was observed. However, ERK1/2 and p38-MAPK appear to differentially regulate antigen-presenting cell maturation pathways. Whereas p38-MAPK activation has been shown to have a crucial role in driving Th1 responses because blocking p38-MAPK inhibits LPS-mediated IL-12 production in DCs,37 inhibition of ERK1/2 seems to have only partial or negative effects on DC maturation.38,39
In a recent study by Kollmann et al.16 cytokine assays in whole blood, in contrast to studies of MCs, yielded more marked differences between CB and adults. Assaying cytokines in whole blood has been proposed to represent a minimally perturbed system. However, CB contains a larger amount of leucocytes per millilitre than adult peripheral blood,40 which could complicate comparisons of cytokine secretion between neonates and adults in whole blood. Also, inconsistencies between studies using different cell sources can in part be explained by the presence of regulatory factors in serum capable of altering the cytokine response.20,21 Here we found that cytokine release in response to PGN was affected by serum factors with IL-6 and IL-8 following the same pattern (increased) as opposed to TNF and IL-10 (decreased), relative to production in medium containing FCS (Fig. 5). The changes, as compared with FCS, were quite dramatic in some cases and have implications for the choice of serum for culture of human cells, at least when attempting to quantitatively determine cytokine release. For the majority of the cytokines the effects were similar regardless of the origin of human serum or cells, notably with the exception of TNF. When PBMC were cultured with CB serum TNF levels were significantly lower than when adult serum was used. This is in line with the study from Levy et al. indicating that CB serum contains factors, e.g. adenosine, that preferentially suppress TNF production20 presumably mediated by cAMP in monocytes as shown by others.41 However, although both types of human serum suppressed TNF production from CBMC in comparison with FCS, no difference was seen between the two serum sources, suggesting that in our study the PBMC were more susceptible to serum factors, which is in contrast to previous reports.20
As PBMC were cultured with CB serum and vice versa, cells were also subjected to autologous or heterologous serum conditions. To rule out that this affected cytokine production and hence influenced our results, PBMC were cultured with autologous or heterologous adult serum, but showed no major difference in production of most of the PGN-induced cytokines measured here (data not shown). However, IL-8 levels were further increased with heterologous serum. As IL-8 levels were in fact slightly lowered from PBMC with CB (heterologous) serum as compared with adult (autologous) serum (Fig. 5), there seems to be no reason to believe that it is the difference between autologous and heterologous sera and not that between CB and adult that we have measured.
The rationale for control of neonatal TNF responses has been discussed extensively. Considering that the CB TNF response to bacterial ligands could be as potent as observed here and that TNF has been associated with premature birth and spontaneous abortion,42 this regulation seems apt. Presumably, controlling the TNF response would also be important in relation to TNFs’ well-known tissue damaging effects that could adversely affect fetal development. The basis for a strong suppressive effect of sera on IL-10 production is less clear. Noticeably the spread among subjects in the CB group was larger suggesting that in some neonates an IL-10 bias (over TNF) could contribute to an overall dampening of the inflammatory response. How long after birth regulatory factors in serum are present is currently unknown, but some studies describe slow age-dependent maturation of Th1-polarizing responses in early life,19,27,30,40 and one study showed a maintained pattern of decreased TNF to IL-6 ratio in sera of children in the first week of life.18
A limited amount of cord blood cells necessitated the choice of one stimulus for functional studies. We examined monocyte and monocyte subset cytokine responses following stimulation with PGN to model strong monocyte activation to a microbial stimulus. Also, previous assays of mononuclear cells in our laboratory have not shown major differences between LPS-stimulated and PGN-stimulated cells in quantity or kinetics of cytokine production.24,43 Other TLR ligands, such as viral components, may elicit a different response from CB monocyte subsets, which remains to be defined. The time-point 24 hr was chosen to ensure maximum production of most of the assayed cytokines, and in particular of IL-12p70. However, TNF production peaks early, at around 4–6 hr, and then declines21 and we cannot exclude the possibility that TNF measurement at an earlier time-point would have generated a different result with higher adult TNF production. On the other hand, the intracellular assay shows cumulative cytokine production and clearly shows that inherent CB monocyte TNF production is not deficient in response to PGN.
In this study we did not find support for an immature neonatal monocyte antibacterial response; however, newborns do exhibit a heightened susceptibility to microbial infection. This might be related to a delayed DC maturation or to altered DC function and a failure in development of subsequent adaptive immune responses.44 Interestingly, we found that the CD14+ CD16+ cells were more abundant in stimulated CBMC cultures than in adult. As CD14+ CD16+ cells have a more DC-like and macrophage-like transcription programme6,11 they may represent a more mature subtype of monocyte displaying specific differentiation in response to environmental cues. Different behaviour of this subset in neonates, perhaps with reduced capacity to further differentiate following bacterial stimulation, could potentially also affect the quality of the neonatal antimicrobial response. Naturally one has to consider the use of an in vitro system and CBMC to mimic early-life immunity. Maturation of immune responses takes place following birth and in contact with environmental microbes and therefore CB rather represents the inherent capacity of newborn immune cells rather than immune responses throughout the newborn/infant period.
In summary, we have characterized for first time the human CB monocyte subpopulations, using the same individuals for both phenotypic and functional assays, and show similar baseline properties of CB and adult monocytes. The data presented here are novel regarding CB monocyte heterogeneity and prompt future investigation into the function and further maturation of neonatal CD14+ CD16+ cells.
Acknowledgments
The authors are grateful to all midwifes and nurses, especially to Mari Jonsson, at the delivery ward and to Agneta Nordwall at the Department of Cardiology, both at the Karolinska University Hospital, Solna for their kind assistance with sample collection. This work was supported by grants from Swedish Research Council Grants 57X-15160-05-2 and 57X-15160-07-3; the Swedish Medical Society and the Golden Jubilee Memorial, Crown-Princess Lovisa and Axel Tielman, Groschinsky and Hesselman Foundations.
Glossary
Abbreviations
- CBMC
cord blood mononuclear cells
- DC
dendritic cell
- ERK
extracellular signal-regulated kinase
- FCS
fetal calf serum
- GeoMFI
geometric mean fluorescence intensity
- IL-12
interleukin-12
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- PBMC
peripheral blood mononuclear cells
- PGN
peptidoglycan
- Th1
T helper type 1
- TLR
toll-like receptor
- TNF
tumour necrosis factor
Disclosures
The authors declare no competing interests.
Author contributions
E.S., S.S-H., K.B. and E.S-E. participated in research design, data analysis and interpretation of the results; E.S. and S.S-H. performed the experiments and E.S. wrote the manuscript together with S.S-H., K.B. and E.S-E.
References
- 1.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4:553–64. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 2.Kadowaki N, Ho S, Antonenko S, Malefyt RW, Kastelein RA, Bazan F, Liu YJ. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863–9. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Passlick B, Flieger D, Ziegler-Heitbrock HW. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood. 1989;74:2527–34. [PubMed] [Google Scholar]
- 4.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–64. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 5.Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol. 2007;81:584–92. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- 6.Ancuta P, Liu KY, Misra V, Wacleche VS, Gosselin A, Zhou X, Gabuzda D. Transcriptional profiling reveals developmental relationship and distinct biological functions of CD16+ and CD16– monocyte subsets. BMC Genomics. 2009;10:403. doi: 10.1186/1471-2164-10-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krutzik SR, Tan B, Li H, et al. TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nat Med. 2005;11:653–60. doi: 10.1038/nm1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanchez-Torres C, Garcia-Romo GS, Cornejo-Cortes MA, Rivas-Carvalho A, Sanchez-Schmitz G. CD16+ and CD16– human blood monocyte subsets differentiate in vitro to dendritic cells with different abilities to stimulate CD4+ T cells. Int Immunol. 2001;13:1571–81. doi: 10.1093/intimm/13.12.1571. [DOI] [PubMed] [Google Scholar]
- 9.Belge KU, Dayyani F, Horelt A, Siedlar M, Frankenberger M, Frankenberger B, Espevik T, Ziegler-Heitbrock L. The proinflammatory CD14+ CD16+ DR++ monocytes are a major source of TNF. J Immunol. 2002;168:3536–42. doi: 10.4049/jimmunol.168.7.3536. [DOI] [PubMed] [Google Scholar]
- 10.Amoudruz P, Holmlund U, Saghafian-Hedengren S, Nilsson C, Sverremark-Ekstrom E. Impaired toll-like receptor 2 signalling in monocytes from 5-year-old allergic children. Clin Exp Immunol. 2009;155:387–94. doi: 10.1111/j.1365-2249.2008.03792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao C, Zhang H, Wong WC, et al. Identification of novel functional differences in monocyte subsets using proteomic and transcriptomic methods. J Proteome Res. 2009;8:4028–38. doi: 10.1021/pr900364p. [DOI] [PubMed] [Google Scholar]
- 12.Fingerle G, Pforte A, Passlick B, Blumenstein M, Strobel M, Ziegler-Heitbrock HW. The novel subset of CD14+/CD16+ blood monocytes is expanded in sepsis patients. Blood. 1993;82:3170–6. [PubMed] [Google Scholar]
- 13.Skrzeczynska J, Kobylarz K, Hartwich Z, Zembala M, Pryjma J. CD14+CD16+ monocytes in the course of sepsis in neonates and small children: monitoring and functional studies. Scand J Immunol. 2002;55:629–38. doi: 10.1046/j.1365-3083.2002.01092.x. [DOI] [PubMed] [Google Scholar]
- 14.Iwahashi M, Yamamura M, Aita T, et al. Expression of toll-like receptor 2 on CD16+ blood monocytes and synovial tissue macrophages in rheumatoid arthritis. Arthritis Rheum. 2004;50:1457–67. doi: 10.1002/art.20219. [DOI] [PubMed] [Google Scholar]
- 15.Novak N, Allam P, Geiger E, Bieber T. Characterization of monocyte subtypes in the allergic form of atopic eczema/dermatitis syndrome. Allergy. 2002;57:931–5. doi: 10.1034/j.1398-9995.2002.23737.x. [DOI] [PubMed] [Google Scholar]
- 16.Kollmann TR, Crabtree J, Rein-Weston A, et al. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol. 2009;183:7150–60. doi: 10.4049/jimmunol.0901481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vanden Eijnden S, Goriely S, De Wit D, Goldman M, Willems F. Preferential production of the IL-12(p40)/IL-23(p19) heterodimer by dendritic cells from human newborns. Eur J Immunol. 2006;36:21–6. doi: 10.1002/eji.200535467. [DOI] [PubMed] [Google Scholar]
- 18.Angelone DF, Wessels MR, Coughlin M, Suter EE, Valentini P, Kalish LA, Levy O. Innate immunity of the human newborn is polarized toward a high ratio of IL-6/TNF-alpha production in vitro and in vivo. Pediatr Res. 2006;60:205–9. doi: 10.1203/01.pdr.0000228319.10481.ea. [DOI] [PubMed] [Google Scholar]
- 19.Yerkovich ST, Wikstrom ME, Suriyaarachchi D, Prescott SL, Upham JW, Holt PG. Postnatal development of monocyte cytokine responses to bacterial lipopolysaccharide. Pediatr Res. 2007;62:547–52. doi: 10.1203/PDR.0b013e3181568105. [DOI] [PubMed] [Google Scholar]
- 20.Levy O, Coughlin M, Cronstein BN, Roy RM, Desai A, Wessels MR. The adenosine system selectively inhibits TLR-mediated TNF-alpha production in the human newborn. J Immunol. 2006;177:1956–66. doi: 10.4049/jimmunol.177.3.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levy O, Zarember KA, Roy RM, Cywes C, Godowski PJ, Wessels MR. Selective impairment of TLR-mediated innate immunity in human newborns: neonatal blood plasma reduces monocyte TNF-alpha induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod, but preserves the response to R-848. J Immunol. 2004;173:4627–34. doi: 10.4049/jimmunol.173.7.4627. [DOI] [PubMed] [Google Scholar]
- 22.Royet J, Dziarski R. Peptidoglycan recognition proteins: pleiotropic sensors and effectors of antimicrobial defences. Nat Rev Microbiol. 2007;5:264–77. doi: 10.1038/nrmicro1620. [DOI] [PubMed] [Google Scholar]
- 23.Ogura Y, Inohara N, Benito A, Chen FF, Yamaoka S, Nunez G. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J Biol Chem. 2001;276:4812–8. doi: 10.1074/jbc.M008072200. [DOI] [PubMed] [Google Scholar]
- 24.Saghafian-Hedengren S, Holmlund U, Amoudruz P, Nilsson C, Sverremark-Ekstrom E. Maternal allergy influences p38-mitogen-activated protein kinase activity upon microbial challenge in CD14+ monocytes from 2-year-old children. Clin Exp Allergy. 2008;38:449–57. doi: 10.1111/j.1365-2222.2007.02917.x. [DOI] [PubMed] [Google Scholar]
- 25.Buechler C, Ritter M, Orso E, Langmann T, Klucken J, Schmitz G. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. J Leukoc Biol. 2000;67:97–103. [PubMed] [Google Scholar]
- 26.Fabriek BO, van Bruggen R, Deng DM, et al. The macrophage scavenger receptor CD163 functions as an innate immune sensor for bacteria. Blood. 2009;113:887–92. doi: 10.1182/blood-2008-07-167064. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen M, Leuridan E, Zhang T, De Wit D, Willems F, Van Damme P, Goldman M, Goriely S. Acquisition of adult-like TLR4 and TLR9 responses during the first year of life. PLoS ONE. 2010;5:e10407. doi: 10.1371/journal.pone.0010407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Wit D, Tonon S, Olislagers V, Goriely S, Boutriaux M, Goldman M, Willems F. Impaired responses to toll-like receptor 4 and toll-like receptor 3 ligands in human cord blood. J Autoimmun. 2003;21:277–81. doi: 10.1016/j.jaut.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Cros J, Cagnard N, Woollard K, et al. Human CD14(dim) monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010 doi: 10.1016/j.immuni.2010.08.012. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Upham JW, Lee PT, Holt BJ, Heaton T, Prescott SL, Sharp MJ, Sly PD, Holt PG. Development of interleukin-12-producing capacity throughout childhood. Infect Immun. 2002;70:6583–8. doi: 10.1128/IAI.70.12.6583-6588.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aksoy E, Albarani V, Nguyen M, et al. Interferon regulatory factor 3-dependent responses to lipopolysaccharide are selectively blunted in cord blood cells. Blood. 2007;109:2887–93. doi: 10.1182/blood-2006-06-027862. [DOI] [PubMed] [Google Scholar]
- 32.Lee SM, Suen Y, Chang L, et al. Decreased interleukin-12 (IL-12) from activated cord versus adult peripheral blood mononuclear cells and upregulation of interferon-gamma, natural killer, and lymphokine-activated killer activity by IL-12 in cord blood mononuclear cells. Blood. 1996;88:945–54. [PubMed] [Google Scholar]
- 33.Grage-Griebenow E, Flad HD, Ernst M. Heterogeneity of human peripheral blood monocyte subsets. J Leukoc Biol. 2001;69:11–20. [PubMed] [Google Scholar]
- 34.Saghafian-Hedengren S, Sundstrom Y, Sohlberg E, Nilsson C, Linde A, Troye-Blomberg M, Berg L, Sverremark-Ekstrom E. Herpesvirus seropositivity in childhood associates with decreased monocyte-induced NK cell IFN-gamma production. J Immunol. 2009;182:2511–7. doi: 10.4049/jimmunol.0801699. [DOI] [PubMed] [Google Scholar]
- 35.Yan SR, Qing G, Byers DM, Stadnyk AW, Al-Hertani W, Bortolussi R. Role of MyD88 in diminished tumor necrosis factor alpha production by newborn mononuclear cells in response to lipopolysaccharide. Infect Immun. 2004;72:1223–9. doi: 10.1128/IAI.72.3.1223-1229.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sadeghi K, Berger A, Langgartner M, et al. Immaturity of infection control in preterm and term newborns is associated with impaired toll-like receptor signaling. J Infect Dis. 2007;195:296–302. doi: 10.1086/509892. [DOI] [PubMed] [Google Scholar]
- 37.Bohnenkamp HR, Papazisis KT, Burchell JM, Taylor-Papadimitriou J. Synergism of toll-like receptor-induced interleukin-12p70 secretion by monocyte-derived dendritic cells is mediated through p38 MAPK and lowers the threshold of T-helper cell type 1 responses. Cell Immunol. 2007;247:72–84. doi: 10.1016/j.cellimm.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 38.Rescigno M, Martino M, Sutherland CL, Gold MR, Ricciardi-Castagnoli P. Dendritic cell survival and maturation are regulated by different signaling pathways. J Exp Med. 1998;188:2175–80. doi: 10.1084/jem.188.11.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Puig-Kroger A, Relloso M, Fernandez-Capetillo O, Zubiaga A, Silva A, Bernabeu C, Corbi AL. Extracellular signal-regulated protein kinase signaling pathway negatively regulates the phenotypic and functional maturation of monocyte-derived human dendritic cells. Blood. 2001;98:2175–82. doi: 10.1182/blood.v98.7.2175. [DOI] [PubMed] [Google Scholar]
- 40.Belderbos ME, van Bleek GM, Levy O, Blanken MO, Houben ML, Schuijff L, Kimpen JL, Bont L. Skewed pattern of toll-like receptor 4-mediated cytokine production in human neonatal blood: low LPS-induced IL-12p70 and high IL-10 persist throughout the first month of life. Clin Immunol. 2009;133:228–37. doi: 10.1016/j.clim.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shames BD, McIntyre RC, Jr, Bensard DD, Pulido EJ, Selzman CH, Reznikov LL, Harken AH, Meng X. Suppression of tumor necrosis factor alpha production by cAMP in human monocytes: dissociation with mRNA level and independent of interleukin-10. J Surg Res. 2001;99:187–93. doi: 10.1006/jsre.2001.6178. [DOI] [PubMed] [Google Scholar]
- 42.Vitoratos N, Papadias C, Economou E, Makrakis E, Panoulis C, Creatsas G. Elevated circulating IL-1beta and TNF-alpha, and unaltered IL-6 in first-trimester pregnancies complicated by threatened abortion with an adverse outcome. Mediators Inflamm. 2006;2006:30485. doi: 10.1155/MI/2006/30485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amoudruz P, Holmlund U, Malmstrom V, Trollmo C, Bremme K, Scheynius A, Sverremark-Ekstrom E. Neonatal immune responses to microbial stimuli: is there an influence of maternal allergy? J Allergy Clin Immunol. 2005;115:1304–10. doi: 10.1016/j.jaci.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 44.Willems F, Vollstedt S, Suter M. Phenotype and function of neonatal DC. Eur J Immunol. 2009;39:26–35. doi: 10.1002/eji.200838391. [DOI] [PubMed] [Google Scholar]


