Abstract
Several classes and multiple subclasses of immunoglobulins are produced towards protein and polysaccharide antigens in response to Salmonella infection and play a key role in protection against systemic disease. The targeting of Salmonella to Fc receptors (FcR) on phagocytes is a key step in the antibody-mediated antibacterial functions of host cells. We wished to compare the relative efficiency of different human IgG subclasses, which targeted the Salmonella enterica OmpA surface protein in modulating the interaction of bacteria with human phagocytes. To this end, we developed a novel system by tagging OmpA with a foreign CD52 mimotope (TSSPSAD) and opsonizing the bacteria with a panel of humanized CD52 antibodies that share the same antigen-binding V-region, but have constant regions of different subclasses. Our data revealed that opsonization with all the IgG subclasses increases Salmonella uptake by human phagocytes. IgG3 resulted in the highest level of bacterial uptake and the highest average bacterial load per infected cell, which was closely followed by IgG1, then IgG4 and lastly IgG2. Phagocytosis mediated by IgG1, IgG3 and IgG4 had a higher dependency on FcγRI than FcγRIIA, whereas IgG2-mediated phagocytosis required FcγRIIA more than FcγRI. The results show that IgG binding to OmpA increases the uptake of Salmonella by human phagocytic cells and that the efficiency of this process depends both on the subclass of the IgG and the type of FcR that is available for antibody binding.
Keywords: antibody, Fc receptors, IgG subclasses, opsonization, phagocytosis
Introduction
Salmonella enterica threatens public health by causing a spectrum of diseases such as typhoid and paratyphoid fever, gastroenteritis in humans and similar diseases in other animals.1Salmonella enterica serovar Typhimurium (S. Typhimurium) usually causes gastroenteritis in humans, but is also a common cause of bacteraemia and sepsis in immunocompromised individuals (such as those with malaria and HIV-infected patients) and children, especially in developing countries.2–6 Increased drug resistance and emergence of new multi-drug-resistant S. enterica strains has rendered many antibiotics less effective against the bacteria, resulting in increased morbidity and mortality in humans.6–10 Hence, vaccines are a desirable and effective medical intervention for protection against salmonellosis. However, current vaccine development has been impeded by the lack of understanding of the qualitative requirements for a protective immune response against S. enterica.
Salmonella enterica is a facultative intracellular pathogen whose ability to grow and persist within phagocytes is a key determinant for virulence.11–16 Although phagocytes provide an intracellular niche for the bacteria, they also form a crucial component of the host immune response and mediate bacterial killing through reactive oxygen intermediates, and reactive nitrogen intermediates, phagolysosome fusion and antimicrobial proteins (defensins).17–23
Immunoglobulin G antibodies, in addition to T-cell receptor-αβ+ CD4+ and CD8+ T cells, are essential for resistance against enteric and septicaemic Salmonella diseases in humans and animals.24 The requirement for systemic antibody responses against such a facultative intracellular pathogen can be explained considering that the release of S. enterica from infected cells is necessary for the bacteria to spread and distribute to other areas to establish new sites of infection.13,25 In enteric infections, antibodies bind to bacteria in their transient extracellular phase and enhance the antibacterial functions of phagocytes when the micro-organisms are recaptured by these cells.
Bacteraemia can be a very serious consequence of Salmonella infections with fatal outcomes especially in young and immunocompromised individuals. Evidence from laboratory models and from epidemiological observations in humans and other animals indicates a crucial role for antibodies in protection against lethal septicaemic infections.26,27 For example, circulating immunoglobulin reduces or abrogates bacteraemia in animals by greatly accelerating the clearance of the bacteria from the blood.28 Rapidly evolving, often fatal, Salmonella septicaemias are prevalent in individuals with deficiencies in humoral immunity and in younger children with a peak of incidence between 6 and 24 months of age,27 which is typical of those infections where immunoglobulins play a prominent role in protection. It is likely that antibodies contribute to the control of S. Typhimurium bacteraemia in humans as indicated by the importance of antibody and complement for oxidative burst and blood cell killing of invasive non-typhoidal Salmonella in Africans.29
Immunoglobulin G molecules are key players in the anti-Salmonella antibody response; IgG is the most abundant antibody class in human serum, and is also the dominant antibody class in human immune serum from patients in areas of endemic typhoid fever.30 Human IgG antibodies have been shown to offer protection against an otherwise lethal S. enterica infection.31 Furthermore, natural infection or vaccination in mice induces mainly IgG production, which contributes to protection against secondary virulent infections.24,32 Although the crucial role of antibodies in protection against S. enterica infections has been well documented, the presence of antibodies does not always correlate well with protection.33 It is therefore reasonable to postulate that differences in the qualitative profile of the anti-Salmonella humoral response might influence its efficacy.
Factors that determine the antimicrobial activities of antibodies include the antibody isotype profile and, consequently, the efficiency of their interactions with Fc receptors (FcRs) on innate immune effector cells. We have shown that in the mouse typhoid model FcRs play a crucial role in host resistance and that IgG2a binding to FcγRI is the main mediator of the antimicrobial functions of phagocytes via the enhancement in the production of reactive oxygen intermediates.34,35 In humans, the main activating FcγRs are FcγRI, FcγRIIA and FcγRIII. Multiple IgG molecules that are bound to an antigenic surface and to activating FcγRs through their Fc regions cause cross-linking of the receptors, which triggers downstream signalling, leading to increased phagocytosis and pro-inflammatory cellular responses.36
Little is known about the relative contribution of the different human IgG subclasses to protection against S. enterica infections in humans. Uptake of the bacteria is the first key step in the antimicrobial activity of phagocytic cells and is essential in the control and termination of bacteraemia in septicaemic infections. In this study, we therefore wished to examine the relative efficiency of different IgG subclasses of anti-Salmonella antibodies in the enhancement of the phagocytic activity of the human monocytic cell line THP-1.
In the present study we used the surface abundant OmpA protein as our target antigen. The Omp proteins are highly immunogenic and have been highlighted as candidate targets for vaccine development against S. enterica infections37–39 but the functional consequences of targeting Omp with antibodies on the interaction of S. enterica with phagocytes is not clear. We developed a novel system by tagging OmpA with a foreign CD52 mimotope (TSSPSAD) and opsonizing the bacteria with a panel of humanized CD52 antibodies that share the same antigen-binding V-region, but have constant regions of different subclasses. This experimental system allowed us to examine the outcome of targeting OmpA with specific antibodies and also to study how each human IgG subclass and activating FcγR affects bacterial phagocytosis.
This study provides essential information on the relative efficacy of different IgG subclasses in enhancing the first key step of anti-S. enterica phagocyte functions and contributes to the overall understanding of the requirements for a protective immune response to S. enterica.
Materials and methods
Reagents and media
All reagents and media were obtained from Sigma-Aldrich, Poole, UK unless stated otherwise.
Bacterial strains and opsonization
Salmonella enterica serovar Typhimurium SL3261 is an attenuated aroA derivative of the wild-type SL1344 strain.40 To generate green fluorescent protein (GFP) -expressing SL3261, a DNA fragment that consists of the gfp gene from Aequoria victoria and a chloramphenicol resistance cassette41 were inserted between pseudogenes malX and malY on the chromosome by oligonucleotide-directed mutagenesis, where PCR was used to amplify the gfp gene and the chloramphenicol resistance cassette41 with 5′ and 3′ arms homologous to the DNA flanking the pseudogenes. Using the same technique, a short peptide (TSSPSAD), which is a mimotope of the human CD52 antigen, was inserted between S136 and T137 in the third surface exposed loop of OmpA. The insertion of the gfp gene between malX and malY and the insertion of the TSSPSAD mimotope-coding sequence in the ompA gene were confirmed by sequencing. Expression of GFP and the mimotope were verified by immunofluoresence.
Bacteria were grown overnight at 37° in Luria–Bertani broth and bacterial opsonization was performed by incubation in either the humanized anti-TSSPSAD antibodies or the non-specific control antibody at 37° with shaking for 30 min. The humanized anti-TSSPSAD antibodies share the same variable regions (CAMPATH-142) that recognize the human CD52 mimotope, but are of different human antibody subclasses (either IgG143, IgG243, IgG344, or IgG443). The non-specific control antibody used in this study is the recombinant human Fog-1 IgG1 antibody,44 which recognizes the human RhD antigen. The dilution of anti-TSSPSAD antibodies (either IgG1, IgG2, IgG3, or IgG4) for opsonizing bacteria was determined as the lowest dilution that does not cause bacterial agglutination, which corresponded to 25 μg/ml. As a control, the concentration of the recombinant human Fog-1 IgG1 antibody used was also 25 μg/ml. Unbound antibody was removed by extensive washing. Effective opsonization was visualized with fluorescence microscopy using Alexa Fluor 568-conjugated goat anti-human IgG (Invitrogen, Paisley, UK). We also tested the level of spontaneous release of the bound antibody in tissue culture medium. As determined by ELISA, the amount of antibody bound to the bacteria was < 2 ng per 106 bacteria (which is the number of bacteria added per well in the in vitro infection experiments). Less than 1% (< 20 pg) of the total antibody that was bound to the opsonized bacteria is released spontaneously within 45 min of incubation. Furthermore, we have confirmed that exogenous addition of this very low amount of released antibody does not affect the uptake of wild-type (non-CD52 expressing) bacteria by THP-1 cells.
Cell culture
The human monocyte cell line THP-1 was grown in RPMI-1640 supplemented with 10% fetal calf serum, 2 mm l-glutamine, 0·05 mm 2-mercaptoethanol at 37°. Before bacterial infection, THP-1 cells were grown in RPMI-1640 supplemented with 10% Nu serum (VWR International Ltd, Leicestershire, UK), 2 mm l-glutamine, 0·05 mm 2-mercaptoethanol for 22 days, followed by an incubation with 100 U/ml recombinant interferon-γ for 48 hr.45
THP-1 cells mainly express FcγRI and FcγRIIA whereas FcγRIII is only expressed at very low levels.45 For FcγR-blocking experiments, THP-1 cells were pre-incubated with either anti-FcγRI (10 μg/ml clone 10.1; AbD Serotec, Oxford, UK), anti-FcγRIIA (5 μg/ml clone IV.3; Stem Cell Technologies, Grenoble, France), anti-FcγRIII (5 μg/ml clone 3G8; Santa Cruz Biotechnology Inc, Heidelberg, Germany) antibodies or combinations of the three anti-FcγR antibodies for 30 min before bacterial infection. These antibody clones have been reported to block the respective FcγRs in various studies, where blocking the individual FcγRI, FcγRIIA and FcγRIII with clone 10.1, IV.3 and 3G8, respectively, has been shown to decrease phagocytosis.46–48 Each anti-FcγR antibody was used at the lowest concentration that gave the maximal inhibition of binding of monomeric or complexed anti-TSSPSAD antibodies to THP-1 cells as determined by flow cytometry. This concentration was determined by pre-incubating the THP-1 cells with increasing concentrations of the FcγR antibodies before exposure to each of the anti-TSSPSAD IgG subclasses.
Infection of THP-1 cells with S. Typhimurium
Opsonized bacteria were added to THP-1 cells at a multiplicity of infection of 10 : 1 (bacteria : THP-1 cells). After 45 min at 37°, the infected cells were washed three times in PBS and incubated with fresh culture medium containing 100 μg/ml gentamicin at 37° for 1 hr to kill any remaining extracellular bacteria. After three further washes the cells were processed for immunofluorescence (to determine the number of visible intracellular viable bacteria) or for the determination of intracellular viable bacterial counts.
Determination of the number of visible intracellular viable bacteria
THP-1 cells were plated onto poly-l-lysine-treated coverslips (Fisher Scientific) 12 hr before infection. After bacterial infection, THP-1 cells were fixed with 4% paraformaldehyde for 15 min, followed by incubation with mouse monoclonal anti-O4 antibodies (Abcam, Cambridge, UK) and secondary goat anti-mouse Alexa Fluor 405 antibody (Invitrogen). Each antibody reagent was diluted 1 : 1000 in 10% normal goat serum (Dako, Cambridge, UK). Following incubation with the secondary antibodies, the coverslips were mounted onto Vecta bond-treated glass slides (Vector Laboratories Ltd, Peterborough, UK) with mounting medium (Vectashield; Vector Laboratories) and viewed using a Leica DM6000B fluorescence microscope. Intracellular bacteria were discriminated from extracellular bacteria by the presence of GFP and the absence of labelling by the mouse monoclonal anti-O4 antibodies.
Determination of intracellular bacterial viable counts
The THP-1 cells were lysed with 0·1% Triton X-100 for 15 min. To determine the bacterial colony-forming units (CFU) in the lysates, viable bacteria were counted by the pour plate technique using Luria–Bertani agar.
Statistical analysis
For experiments exploring the percentage of infected cells and intracellular bacterial load distributions, 450 cells were counted for each of three repeats. Comparisons between means were conducted using the paired Student's t-test. To explore changes in the intracellular bacterial load distributions, pairwise Mann–Whitney U-tests were used to provide evidence of shifts in the bacterial distributions across the comparison groups. We considered every possible paired combination of groups and controlled for the family-wise error rate using a Holm–Bonferroni correction. It is worth noting here that the standard errors associated with these measurements are small because of the large sample size. As a result, we chose to focus on the size of the difference in the text, but, for comparison, have reported the P-values associated with these results in the figure legends. For brevity, we report only the least statistically significant P-value across the pairs of combinations analysed. For FcγR-blocking experiments, all possible paired combinations of FcγR blocking were considered, with the exception of FcγRIII blocking because the parameters studied in the presence of FcγRIII blocking were similar to those observed with no FcγR blocking. Error bars in figures indicate standard deviations.
Results
Relative importance of individual human IgG isotypes in phagocytosis of S. Typhimurium
We used a novel system that combines a recombinant S. Typhimurium SL3261-GFP strain where the CD52 mimotope TSSPSAD was inserted into OmpA and a panel of humanized anti-TSSPSAD antibodies43,44 with identical antigen-binding V-regions and different IgG constant regions (IgG1, IgG2, IgG3 and IgG4). This system allowed us to examine how different IgG subclasses affect the uptake of the bacteria by the human phagocyte cell line THP-1.
Salmonella Typhimurium was opsonized with either the anti-TSSPSAD antibodies or the human Fog-1 antibody, which recognizes the human RhD antigen, as a negative control. After a 45 min exposure to the opsonized bacteria, THP-1 cells were incubated for 1 hr with culture medium containing 100 μg/ml gentamicin. Then the cells were processed for visual detection of the bacteria by immunofluorescence to determine the percentage of infected cells in each culture and the number of visible intracellular bacteria per infected cell (bacterial load). Extracellular bacteria were identified by immunolabelling with mouse monoclonal anti-O4 antibodies followed by a goat anti-mouse antibody conjugated to Alexa Fluor 405, whereas intracellular bacteria were identified by the presence of GFP and the absence of the mouse monoclonal anti-O4 antibodies labelling.
Opsonization with each of the IgG subclasses resulted in a higher percentage of infected cells than when using the non-specific control antibody (Fig. 1). However, small but significant differences were noted when testing the different IgG subclasses individually. Opsonization with IgG3 resulted in the highest percentage of infected cells (mean ± SD: 23·73 ± 0·21%), followed closely by IgG1 (22·08 ± 0·15%), IgG4 (20·5 ± 0·18%), IgG2 (18·55 ± 0·18%), and lastly, the non-specific control (13·27 ± 0·16%). Opsonization resulted also in increases in the average bacterial loads per phagocyte (Fig. 2). The proportion of infected cells containing more than three bacteria was the highest with IgG3 antibody opsonization (48%), followed by IgG1 (38%), IgG4 (32%), IgG2 (24%) and lastly, the non-specific control (4%).
Figure 1.
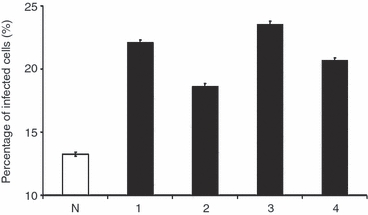
Percentage of infected cells after phagocytosis of Salmonella Typhimurium opsonized with different human IgG subclasses. THP-1 cells were infected with S. Typhimurium opsonized with anti-TSSPSAD antibodies or with bacteria exposed to control IgG. The anti-TSSPSAD antibodies of different subclasses, either IgG1, IgG2, IgG3 or IgG4, are labelled as 1, 2, 3, and 4, respectively and the non-specific control antibody is labelled as N; 450 cells were counted for each of the three experimental repeats. The graph shows the percentages of cells harbouring visible intracellular bacteria which are expressed as the mean percentage of infected cells from three experimental repeats. The error bars represent standard deviations. Every possible paired combination of groups was considered for comparison (i.e. N versus 1, N versus 2, N versus 3, N versus 4, 1 versus 2, 1 versus 3, 1 versus 4, 2 versus 3, 2 versus 4, 3 versus 4) and all P values are < 0·01.
Figure 2.
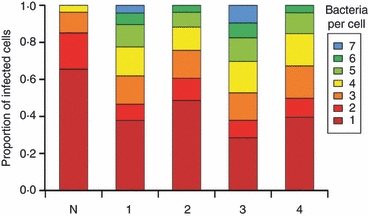
Number of intracellular bacteria per infected cell after phagocytosis of Salmonella Typhimurium opsonized with different human IgG subclasses. THP-1 cells were infected as described in Fig. 1; 450 cells were counted for each of the three experimental repeats. The graph shows the distributions of visible intracellular bacteria per infected cell, which are expressed as the mean distributions from three experimental repeats. Every possible paired combination of groups was considered for comparison (See Fig. 1 legend) and all P values are < 0·0013.
Next, we evaluated the effect of opsonization with different IgG subclasses on the numbers of viable intracellular bacteria. Infected THP-1 cells were lysed in 0·1% Triton X-100 and intracellular viable bacteria in the lysates were then counted by plating on agar. Opsonization with each of the IgG subclasses resulted in a higher number of recovered intracellular viable bacteria than when using the non-specific control antibody. However, small, but significant differences were noted when testing the different IgG subclasses individually. Opsonization with IgG3 resulted in the highest number of recovered intracellular viable bacteria (5·38 ± 0·009 log10 CFU), followed closely by IgG1 (5·29 ± 0·012 log10 CFU), IgG4 (5·17 ± 0·017 log10 CFU) and IgG2 (5·02 ± 0·011 log10 CFU). The number of recovered intracellular viable bacteria was lowest when the bacteria were incubated with the non-specific control antibody (4·53 ± 0·014 log10 CFU) (Fig. 3).
Figure 3.
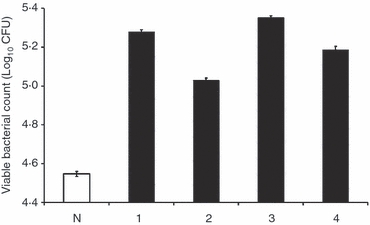
Viability of intracellular bacteria after phagocytosis of Salmonella Typhimurium opsonized with different human IgG subclasses. THP-1 cells were infected as described in Fig. 1. The graph shows the number of culturable intracellular bacteria (viable count) recovered from THP-1 cells. Data are expressed as the mean of three experimental repeats and the error bars represent standard deviations. Every possible paired combination of groups was considered for comparison (See Fig. 1 legend) and all P-values are < 0·0034.
Taken together, the data show that all IgG subclasses targeted to OmpA through the TSSPSAD mimotope enhance S. enterica uptake by phagocytes and affect the overall percentage of infected cells. Different IgG subclasses have different efficiencies and IgG3 is the most efficient antibody subclass in mediating this process, followed by IgG1, IgG4 and IgG2.
Relative importance of Fcγ receptors in antibody-mediated phagocytosis
We evaluated the involvement of the individual activating FcγRs in phagocytosis with respect to each of the different antibody subclasses. We pre-incubated the THP-1 cells with either anti-FcγRI, anti-FcγRIIA, anti-FcγRIII or various combinations of the three anti-FcγR antibodies before bacterial infection. After a 45-min exposure to the opsonized bacteria, THP-1 cells were incubated for 1 hr with culture medium containing 100 μg/ml gentamicin, then washed and either processed for immunofluorescence studies to determine the percentage of infected cells and the number of bacteria per infected cell (bacterial load) or lysed to harvest and count the intracellular bacteria by plating on agar.
The percentage of infected cells observed when the activating FcγRs were blocked, either individually or in combination, was compared with the percentage of infected cells observed when none of the FcγRs were blocked. When FcγRIII was blocked, the percentage of infected cells was similar to that observed when none of the FcγRs were blocked, regardless of the opsonizing antibody subclass (Fig. 4). This is probably the result of the low expression level of FcγRIII in THP1 cells.45 When other FcγRs were blocked, there were reductions in the percentage of infected cells. When THP-1 cells were infected with IgG1-, IgG3- and IgG4-opsonized bacteria the reduction in the percentage of infected cells was greatest with a combination of FcγRI and FcγRIIA blocking (5·86 ± 0·06%, 6·47 ± 0·04% and 4·9 ± 0·07% for IgG1, IgG3 and IgG4, respectively), followed by FcγRI blocking (3·9 ± 0·09%, 4·1 ± 0·06% and 3·85 ± 0·05% for IgG1, IgG3 and IgG4, respectively), and lastly, by FcγRIIA blocking (1·92 ± 0·07%, 1·61 ± 0·17% and 0·98 ± 0·05% for IgG1, IgG3 and IgG4, respectively) (Fig. 4a,c,d). When IgG2-opsonized bacteria were used (Fig. 4b), the reduction in the percentage of infected cells was greatest with a combination of FcγRI and FcγRIIA blocking (4·38 ± 0·04%), followed by FcγRIIA blocking (3·3 ± 0·06%). With FcγRI blocking, the reduction in the percentage of infected cells was lowest (0·77 ± 0·05%) compared with other combinations of FcγR blocking.
Figure 4.
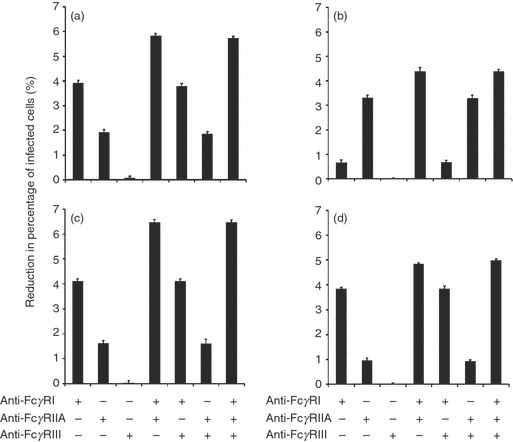
Importance of Fcγ receptors (FcγR) in mediating the percentage of infected cells. THP-1 cells were pre-incubated with anti-FcγR antibodies before being exposed to IgG1- (a), IgG2- (b), IgG3- (c), IgG4- (d) opsonized bacteria. The percentage of infected cells was determined by fluorescence microscopy; 450 cells were counted for each of the three experiment repeats. Data are expressed as the mean percentage of infected cells from three experimental repeats and the error bars represent standard deviations. The percentage of infected cells observed when the activating FcγRs were blocked either individually or in combination was compared with the percentage of infected cells observed when none of the FcγRs were blocked. For each figure panel (a, b, c or d), all possible paired combinations of FcγR blocking, with the exception of FcγRIII blocking (alone and in combination), were considered for comparison. FcγRIII blocking was excluded as there was negligible effect of FcγRIII blocking on the percentage of infected cells. For the combinations considered (which exclude those combinations involving FcγRIII), the P-values are < 0·015 for IgG1 (a), < 0·048 for IgG2 (b), < 0·026 for IgG3 (c) and < 0·03 for IgG4 (d).
We also examined the effect of FcγR blocking on the numbers of visible intracellular bacteria (bacterial load) within each infected phagocyte. For all antibody subclasses, the bacterial load was highest in the absence of the specific FcγR blocking (with the exception of FcγRIII blocking that did not show a significant effect). However, when other FcγRs were blocked, a decrease in the bacterial load was observed. When THP-1 cells were infected with IgG1-, IgG3- and IgG4-opsonized bacteria, the bacterial load was lowest in the presence of both FcγRI and FcγRIIA blocking, which was followed by FcγRI blocking, and lastly, by FcγRIIA blocking. More specifically, the proportion of infected cells containing more than three bacteria was lowest in the presence of both FcγRI and FcγRIIA blocking (12%, 14% and 4% for IgG1, IgG3 and IgG4, respectively), followed by FcγRI blocking (19%, 23% and 7% for IgG1, IgG3 and IgG4, respectively), and lastly, by FcγRIIA blocking (36%, 38% and 27% for IgG1, IgG3 and IgG4, respectively) (Fig. 5a,c,d). However, when the cells were infected with IgG2-opsonized bacteria, the bacterial load was lowest in the presence of both FcγRI and FcγRIIA blocking, which was followed by FcγRIIA blocking, and lastly, by FcγRI blocking (Fig. 5b). More specifically, the proportion of infected cells containing more than three bacteria was least in the presence of both FcγRI and FcγRIIA blocking (3%), followed by FcγRIIA blocking (9%), and lastly, by FcγRI blocking (21%).
Figure 5.
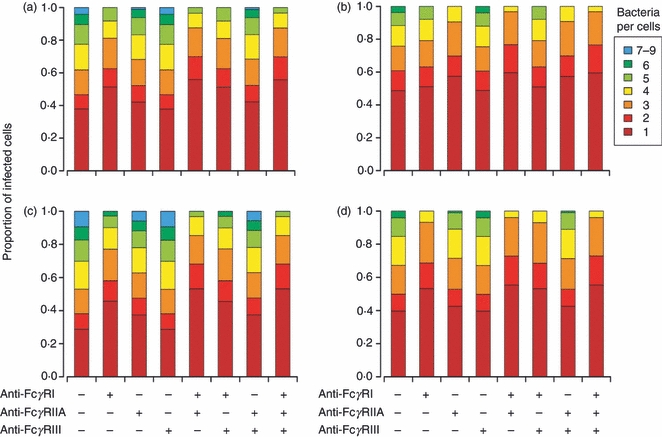
Importance of Fcγ receptors (FcγR) in affecting the bacterial load of infected cells. Cells were pre-incubated with anti-FcγR antibodies before being exposed to IgG1- (a), IgG2- (b), IgG3- (c), IgG4- (d) opsonized bacteria. The bacterial load (number of visible intracellular bacteria) per infected cell was determined by microscopy; 450 cells were counted for each of the three experiment repeats. Data were expressed as the mean distributions of visible intracellular bacteria per infected cell from three experimental repeats. The distributions of visible intracellular bacteria observed when the activating FcγRs were blocked either individually or in combination was compared with the distribution of visible intracellular bacteria observed when none of the FcγRs was blocked. For each figure panel (a, b, c or d), all possible paired combinations of FcγR blocking, with the exception of FcγRIII blocking (alone and in combination), were considered for comparison (See Fig. 4 legend). FcγRIII blocking was excluded as there was negligible effect of FcγRIII blocking on the bacterial load of infected cells. The P-values were < 0·0013 for IgG1 (a), 0·05 for IgG2 (b), 9·1 × 10−4 for IgG3 (c) and < 0·051 for IgG4 (d).
Next, we investigated the number of viable intracellular bacteria when FcγRs were blocked before infection. When FcγRIII was blocked, the reduction in the intracellular viable bacterial counts was negligible for all antibody subclasses (Fig. 6). However, when other FcγRs were blocked, there were reductions in the intracellular viable bacterial count. When THP-1 cells were infected with IgG1-, IgG3- and IgG4-opsonized bacteria, the reduction in the intracellular viable bacterial count was greatest with a combination of FcγRI and FcγRIIA blocking (0·42 ± 0·011 log10 CFU, 0·45 ± 0·005 log10 CFU and 0·37 ± 0·009 log10 CFU for IgG1, IgG3 and IgG4, respectively), followed by FcγRI blocking (0·22 ± 0·008 log10 CFU, 0·24 ± 0·006 log10 CFU and 0·24 ± 0·01 log10 CFU for IgG1, IgG3 and IgG4, respectively), and lastly, by FcγRIIA blocking (0·09 ± 0·007 log10 CFU, 0·1 ± 0·009 log10 CFU and 0·05 ± 0·007 log10 CFU for IgG1, IgG3 and IgG4, respectively) (Fig 6a,c,d). In contrast, when THP-1 cells were infected with IgG2-opsonized bacteria, the reduction in the intracellular viable bacterial count was greatest in the presence of both FcγRI and FcγRIIA blocking (0·27 ± 0·01 log10 CFU), followed by FcγRIIA blocking (0·19 ± 0·009 log10 CFU), and lastly, by FcγRI blocking (0·04 ± 0·008 log10 CFU) (Fig. 6b).
Figure 6.
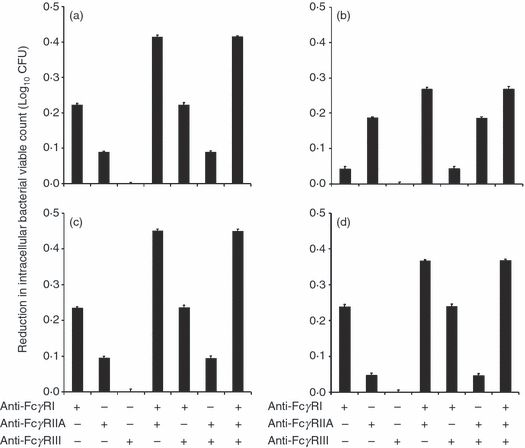
Importance of Fcγ receptors (FcγR) in affecting the intracellular viable bacterial count. Cells were pre-incubated with anti-FcγR antibodies and then exposed to IgG1- (a), IgG2- (b), IgG3- (c), IgG4- (d) opsonized bacteria. The intracellular viable bacteria were determined by pour plates. Data are expressed as the mean of three experimental repeats and the error bars represent standard deviations. The intracellular viable bacterial count observed when the activating FcγRs were blocked either individually or in combination was compared with the percentage of infected cells observed when none of the FcγRs was blocked. For each figure panel (a, b, c or d), every possible paired combination of FcγR blocking was considered, with the exception of FcγRIII blocking (alone and in combination). FcγRIII blocking was excluded as there was negligible effect of FcγRIII blocking on the intracellular viable bacterial count. The P-values are < 0·0049 for IgG1 (a), < 0·026 IgG2 (b), < 0·014I for gG3 (c) and < 0·02 for IgG4 (d).
Our data show that when THP-1 cells were exposed to IgG1-, IgG3- and IgG4-opsonized bacteria, the percentage of infected cells, bacterial load per infected cell and intracellular viable bacterial counts were highest in the presence of FcγRIIA blocking, followed by FcγRI blocking, and lastly, by a combination of FcγRI and FcγRIIA blocking. However, when THP-1 cells were exposed to IgG2-opsonized bacteria, the percentage of infected cells, bacterial load and the intracellular viable bacterial counts were highest in the presence of FcγRI blocking, followed by FcγRIIA blocking, and lastly, by a combination of FcγRI and FcγRIIA blocking. Taken together, this indicated that the involvement of the individual activating FcγRs in bacterial uptake is different with different antibody subclasses. The bacterial uptake mediated by IgG1, IgG3 and IgG4 has a higher dependency on FcγRI over FcγRIIA, whereas bacterial uptake mediated by IgG2 has a higher dependency on FcγRIIA over FcγRI. It is worth noting that the dependency on FcγRI over FcγRIIA in IgG4-mediated bacterial uptake is higher than in IgG1- and IgG3-mediated bacterial uptake.
Discussion
Using human anti-TSSPSAD IgG to target a mimotope-tagged OmpA on the bacterial surface results in enhanced bacterial uptake by human phagocytes, as indicated by increases in the percentage of infected cells and by higher intracellular bacterial loads. This work therefore indicates that surface outer membrane proteins can be targeted efficiently with antibodies resulting in the enhancement of phagocyte functions (i.e. phagocytosis) associated with immunoglobulin-mediated protection.
Exploiting a panel of human anti-TSSPSAD antibodies that share the same antigen-binding V-region, but have constant regions of different IgG subclasses, we were able to assess the relative potency of each IgG subclass in modulating the interactions between S. Typhimurium and human phagocytic cells. All subclasses enhanced bacterial uptake but there were functional differences between the individual IgG subclasses. The greatest enhancement of bacteria uptake was seen when S. Typhimurium was opsonized with IgG3, followed by IgG1, IgG4 and lastly, IgG2. These observations correlated with intracellular viable bacterial counts recovered from infected cells shortly after the phagocytosis event, where we observed a similar pattern between the IgG subclasses. In line with the above, IgG3 has been shown to be more efficient in mediating phagocytosis of antibody-sensitized red blood cells than IgG1 in human monocytes.49 Similarly, IgG3 is highly efficient in mediating phagocytosis of Neisseria meningitidis when the target cells were opsonized with antibody and neutrophils were used as effector cells, whereas IgG1 has an intermediate efficiency, and IgG2 and IgG4 have low efficiency.50
IgG3 antibodies are therefore more efficient than other subclasses in activating the observed functions of human THP-1 cells. The differences between subclasses were in some cases small in these in vitro assays. However, these differences were highly reproducible and were equally detectable both when evaluating total numbers of intracellular bacterial particles and numbers of viable intracellular bacteria. One possible reason for the different relative potencies of IgG sublasses for enhancing the uptake of S. enterica may be differences in the molecular flexibility. In line with the lengths of their hinge regions, IgG3 is the most flexible of the IgG subclasses, followed by IgG1, IgG4 and lastly, IgG2.51 More specifically, IgG3 has the greatest Fab-Fab and Fab-Fc flexibility, which could be important in FcγR binding.51,52 It has been reported that a hinge deletion mutant of IgG3 has reduced ability in FcγRI and FcγIIA binding.43 Hence, the enhanced efficiency in THP-1 phagocyte function mediated by IgG3 is likely to be the result of greater flexibility, leading to possibly a better association with activating FcγRs such as FcγRI and FcγRIIA. Furthermore, IgG3 has been shown to be more advantageous than the other IgG subclasses in protection. Plasmodium falciparum infections induce an IgG3-dominated antibody response against merozoite surface protein 2 (MSP2) in adults, and the presence of MSP2-specific IgG3 is negatively associated with risk of clinical malaria.53 In addition, when using the same variable regions against HIV antigen gp120, an IgG3 antibody was 100 times more capable than IgG1 in HIV neutralization.54
Hence, it is likely that the differences between the IgG subclasses in mediating phagocyte functions could have a biological significance in the protection against S. enterica infections, although this effect would need to be further examined. In fact, the greater enhancement of phagocytosis might have an impact on the bacterial spread within the host. One of the ways that S. enterica spreads within the host is through the bloodstream.12,25,55,56 Improvements in bacterial uptake could reduce the levels of bacteria in the circulating blood and hence possibly limit the spread of the bacteria in vivo and prevent or stop bacteraemia in septicaemic infections.
Studies on the distribution of IgG antibody subclasses in human immune serum in areas of endemic typhoid fever have shown that the dominating IgG antibody subclass is IgG2.30 However, IgG2 is the least efficient subclass in mediating THP-1 phagocyte function in our study. Remarkably, despite being unable to induce either high levels of protective antibodies in young children or a booster response, the Vi polysaccharide vaccine induces substantial, though not optimal, protection against typhoid fever.57–59 Interestingly, the predominant IgG subclass in immune serum from individuals vaccinated with Vi polysaccharides is IgG1,60 which was one of the more efficient subclasses in mediating phagocyte function in our study. It would be desirable to adopt vaccines and delivery systems that are able to evoke antibody responses with a predominant abundance of efficient subclasses such as IgG3 and IgG1. A serotype B meningococcal outer membrane vesicle delivered intramuscularly has been shown to increase IgG3 titres, suggesting that some delivery systems through appropriate routes of administration can deliver IgG3-biased responses.61
The work also revealed that the relative efficiency of FcγRI and FcγRIIA in the enhanced bacterial uptake mediated by specific antibodies is dependent on the subclass of the opsonizing antibody. The greater dependency on FcγRI over FcγRIIA in both IgG3- and IgG1-mediated bacterial uptake indicated that FcγRI has a more important role in the phagocytosis of bacteria opsonized with these isotypes.
Each of the activating FcγRs, FcγRI, FcγRIIA and FcγRIII, has a different antibody binding profile. FcγRI has a binding preference for IgG3, followed by IgG1, followed by IgG4 and lastly, IgG2; FcγRIIA has a binding preference for IgG3, followed by IgG1, IgG2 and lastly, IgG4; FcγRIII has a binding preference for IgG1 and IgG3, followed by IgG4 and IgG2.62 Furthermore, FcγRI is highly competent in phagocytosis,63 and important in the uptake of bactaeremia-causing bacteria such as Staphylococcus aureus by human neutrophils.64 However, FcγRIIA-transfected non-phagocytic cells are able to phagocytose Escherichia coli efficiently65 and we found that IgG2-mediated bacterial uptake has a greater dependency on FcγRIIA over FcγRI. Hence, both FcγRI and FcγRIIA are important in mediating phagocytosis in THP-1 cells, and the relative importance of each FcγR differs, depending on the antibody subclass in question. Although FcγRIII has also been shown to mediate bacterial phagocytosis,63 we observed little effect of FcγRIII on bacterial internalization by the THP-1 cells. This is probably because of the very low expression levels of FcγRIII on these cells and our data therefore by no means discard the possibility that this receptor may play a role in the uptake of Salmonella by other cell types.44
There have been a number of studies on the feasibility of the Omp proteins as candidate targets for vaccine development for protection against S. enterica infections as they are highly immunogenic and can mediate protection in immunized mice.37–39 In line with the above, our in vitro findings illustrate that antibody targeting OmpA is efficient in mediating phagocytosis, and suggest that antibodies targeting OmpA might mediate protection. OmpA could be a promising target for vaccine development.
Acknowledgments
This work was supported by grants from the Wellcome Trust and from the Medical Research Council, UK.
References
- 1.Bhutta ZA, Threlfall J. Addressing the global disease burden of typhoid fever. JAMA. 2009;302:898–9. doi: 10.1001/jama.2009.1259. [DOI] [PubMed] [Google Scholar]
- 2.Gilks CF, Brindle RJ, Otieno LS, et al. Life-threatening bacteraemia in HIV-1 seropositive adults admitted to hospital in Nairobi, Kenya. Lancet. 1990;336:545–9. doi: 10.1016/0140-6736(90)92096-z. [DOI] [PubMed] [Google Scholar]
- 3.Graham SM, Molyneux EM, Walsh AL, Cheesbrough JS, Molyneux ME, Hart CA. Nontyphoidal Salmonella infections of children in tropical Africa. Pediatr Infect Dis J. 2000;19:1189–96. doi: 10.1097/00006454-200012000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Graham SM, Hart CA, Molyneux EM, Walsh AL, Molyneux ME. Malaria and Salmonella infections: cause or coincidence? Trans R Soc Trop Med Hyg. 2000;94:227. doi: 10.1016/s0035-9203(00)90286-4. [DOI] [PubMed] [Google Scholar]
- 5.Bronzan RN, Taylor TE, Mwenechanya J, et al. Bacteremia in Malawian children with severe malaria: prevalence, etiology, HIV coinfection, and outcome. J Infect Dis. 2007;195:895–904. doi: 10.1086/511437. [DOI] [PubMed] [Google Scholar]
- 6.Graham SM, Walsh AL, Molyneux EM, Phiri AJ, Molyneux ME. Clinical presentation of non-typhoidal Salmonella bacteraemia in Malawian children. Trans R Soc Trop Med Hyg. 2000;94:310–4. doi: 10.1016/s0035-9203(00)90337-7. [DOI] [PubMed] [Google Scholar]
- 7.Hohmann EL. Nontyphoidal salmonellosis. Clin Infect Dis. 2001;32:263–9. doi: 10.1086/318457. [DOI] [PubMed] [Google Scholar]
- 8.Mirza SH, Beeching NJ, Hart CA. Multi-drug resistant typhoid: a global problem. J Med Microbiol. 1996;44:317–9. doi: 10.1099/00222615-44-5-317. [DOI] [PubMed] [Google Scholar]
- 9.Holmberg SD, Solomon SL, Blake PA. Health and economic impacts of antimicrobial resistance. Rev Infect Dis. 1987;9:1065–78. doi: 10.1093/clinids/9.6.1065. [DOI] [PubMed] [Google Scholar]
- 10.Coovadia YM, Gathiram V, Bhamjee A, Garratt RM, Mlisana K, Pillay N, Madlalose T, Short M. An outbreak of multiresistant Salmonella typhi in South Africa. Q J Med. 1992;82:91–100. [PubMed] [Google Scholar]
- 11.Lee CA, Falkow S. Isolation of hyperinvasive mutants of Salmonella. Methods Enzymol. 1994;236:531–45. doi: 10.1016/0076-6879(94)36041-3. [DOI] [PubMed] [Google Scholar]
- 12.Richter-Dahlfors A, Buchan AM, Finlay BB. Murine Salmonellosis studies by confocal microscopy: Salmonella typhimurium resides intracellularly inside macrophages and exerts a cytotoxic effects on phagocytes in vivo. J Exp Med. 1997;186:569–80. doi: 10.1084/jem.186.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheppard M, Webb C, Heath F, Mallows V, Emilianus R, Maskell D, Mastroeni P. Dynamics of bacterial growth and distribution within the liver during Salmonella infection. Cell Microbiol. 2003;5:593–600. doi: 10.1046/j.1462-5822.2003.00296.x. [DOI] [PubMed] [Google Scholar]
- 14.Fields PI, Swarson RV, Haidaris CG, Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci U S A. 1986;83:5189–93. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunlap NE, Benjamin WH, Jr, Berry AK, Eldridge JH, Briles DE. A ‘safe-site’ for Salmonella typhimurium is within splenic polymorphonuclear cells. Microb Pathog. 1992;13:181–90. doi: 10.1016/0882-4010(92)90019-k. [DOI] [PubMed] [Google Scholar]
- 16.Mastroeni P, Skepper JN, Hormaeche CE. Effect of anti-tumor necrosis factor alpha antibodies on histopathology of primary Salmonella infections. Infect Immun. 1995;63:3674–82. doi: 10.1128/iai.63.9.3674-3682.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groisman EA, Parra-Lopez C, Salcedo M, Lipps CJ, Heffron F. Resistance to host antimicrobial peptides is necessary for Salmonella virulence. Proc Natl Acad Sci U S A. 1992;89:11939–43. doi: 10.1073/pnas.89.24.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Groote MA, Testerman T, Xu Y, Stauffer G, Fang FC. Homocysteine antagonism of nitric oxide-related cytostasis in Salmonella typhimurium. Science. 1996;272:414–7. doi: 10.1126/science.272.5260.414. [DOI] [PubMed] [Google Scholar]
- 19.Shiloh MU, Ruan J, Nathan C. Evaluation of bacterial survival and phagocyte function with a fluorescence-based microplate assay. Infect Immun. 1997;65:3193–8. doi: 10.1128/iai.65.8.3193-3198.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shiloh MU, MacMicking JD, Nicholson S, et al. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity. 1999;10:29–38. doi: 10.1016/s1074-7613(00)80004-7. [DOI] [PubMed] [Google Scholar]
- 21.Vazquez-Torrez A, Jones-Carson J, Mastroeni P, Ischiropoulos H, Fang FC. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. I. Effect on microbial killing by activated peritoneal macrophages in vitro. J Exp Med. 2000;192:227–36. doi: 10.1084/jem.192.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mastroeni P, Vazquez-Torrez A, Fang FC, Xu Y, Khan S, Hormaeche CE, Dougan G. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. II. Effects of microbial proliferation and host survival in vivo. J Exp Med. 2000;192:237–47. doi: 10.1084/jem.192.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vazquez-Torres A, Xu Y, Holden D, Lucia S, Dinauer M, Mastroeni P, Fang FC. Salmonella pathogenicity Island 2 (SPI-2)-dependent evasion of the phagocyte NADPH oxidase. Science. 2000;287:1655–8. doi: 10.1126/science.287.5458.1655. [DOI] [PubMed] [Google Scholar]
- 24.Mastroeni P, Villarreal-Ramos B, Hormaeche CE. Adoptive transfer of immunity to oral challenge with virulent salmonellae in innately susceptible BALB/c mice requires both immune serum and T cells. Infect Immun. 1993;61:3981–4. doi: 10.1128/iai.61.9.3981-3984.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grant AJ, Restif O, McKinley TJ, Sheppard M, Maskell DJ, Mastroeni P. Modelling within-host spatiotemporal dynamics of invasive bacterial disease. PLoS Biol. 2008;6:e74. doi: 10.1371/journal.pbio.0060074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mastroeni P, Ugrinovic S, Chandra A, MacLennan C, Doffinger R, Kumararatne D. Resistance and susceptibility to Salmonella infections: lessons from mice and patients with immunodeficiencies. Rev Med Microbiol. 2003;14:53–62. [Google Scholar]
- 27.MacLennan CA, Gondwe EN, Msefula CL, et al. The neglected role of antibody in protection against bacteremia caused by nontyphoidal strains of Salmonella in African children. J Clin Invest. 2008;118:1553–62. doi: 10.1172/JCI33998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collins FM. Vaccines and cell-mediated immunity. Bacteriol Rev. 1974;38:371–402. doi: 10.1128/br.38.4.371-402.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gondwe EN, Molyneux ME, Goodall M, Graham SM, Mastroeni P, Drayson MT, MacLennan CA. Importance of antibody and complement for oxidative burst and killing of invasive nontyphoidal Salmonella by blood cells in Africans. Proc Natl Acad Sci U S A. 2010;107:3070–5. doi: 10.1073/pnas.0910497107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaheen HI, Girgis NI, Rodier GR, Kamal KA. Evaluation of the response of human humoral antibodies to Salmonella typhi lipopolysaccharide in an area of endemic typhoid fever. Clin Infect Dis. 1995;21:1012–3. doi: 10.1093/clinids/21.4.1012. [DOI] [PubMed] [Google Scholar]
- 31.Fomsgaard A, Galanos C. Protective properties of a human IgG preparation rich in antibodies to a wide spectrum of lipopolysaccharides. APMIS. 1989;97:1114–20. doi: 10.1111/j.1699-0463.1989.tb00525.x. [DOI] [PubMed] [Google Scholar]
- 32.Harrison JA, Villarreal-Ramos B, Mastroeni P, Demarco de Hormaeche R, Hormaeche CE. Correlates of protection induced by live Aro−Salmonella typhimurium vaccines in the murine typhoid model. Immunology. 1997;90:618–25. doi: 10.1046/j.1365-2567.1997.00158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levine MM, Ferreccio C, Black RE, Tacket CO, Germanier R. Progress in vaccines against typhoid fever. Rev Infect Dis. 1989;11(Suppl 3):S552–67. doi: 10.1093/clinids/11.supplement_3.s552. [DOI] [PubMed] [Google Scholar]
- 34.Uppington H, Menager N, Boross P, Wood J, Sheppard M, Verbeek S, Mastroeni P. Effect of immune serum and role of individual Fcgamma receptors on the intracellular distribution and survival of Salmonella enterica serovar Typhimurium in murine macrophages. Immunology. 2006;119:147–58. doi: 10.1111/j.1365-2567.2006.02416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Menager N, Foster G, Ugrinovic S, Uppington H, Verbeek S, Mastroeni P. Fcgamma receptors are crucial for the expression of acquired resistance to virulent Salmonella enterica serovar Typhimurium in vivo but are not required for the induction of humoral or T-cell-mediated immunity. Immunology. 2007;120:424–32. doi: 10.1111/j.1365-2567.2006.02527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crowley MT, Costello PS, Fitzer-Attas CJ, Turner M, Meng F, Lowell C, Tybulewicz VL, DeFranco AL. A critical role for Syk in signal transduction and phagocytosis mediated by Fcgamma receptors on macrophages. J Exp Med. 1997;186:1027–39. doi: 10.1084/jem.186.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gil-Cruz C, Bobat S, Marshall JL, et al. The porin OmpD from nontyphoidal Salmonella is a key target for a protective B1b cell antibody response. Proc Natl Acad Sci U S A. 2009;106:9803–8. doi: 10.1073/pnas.0812431106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamid N, Jain SK. Characterization of an outer membrane protein of Salmonella enterica serovar typhimurium that confers protection against typhoid. Clin Vaccine Immunol. 2008;15:1461–71. doi: 10.1128/CVI.00093-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Udhayakumar V, Muthukkaruppan VR. Protective immunity induced by outer membrane proteins of Salmonella typhimurium in mice. Infect Immun. 1987;55:816–21. doi: 10.1128/iai.55.3.816-821.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoiseth SK, Stocker BA. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–9. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 41.Hautefort I, Proenca MJ, Hinton JC. Single-copy green fluorescent protein gene fusions allow accurate measurement of Salmonella gene expression in vitro and during infection of mammalian cells. Appl Environ Microbiol. 2003;69:7480–91. doi: 10.1128/AEM.69.12.7480-7491.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riechmann L, Clark MR, Waldmann H, Winter G. Reshaping human antibodies for therapy. Nature. 1988;332:323–7. doi: 10.1038/332323a0. [DOI] [PubMed] [Google Scholar]
- 43.Redpath S, Michaelsen TE, Sandlie I, Clark MR. The influence of the hinge region length in binding of human IgG to human Fcγ receptors. Hum Immunol. 1998;59:720–7. doi: 10.1016/s0198-8859(98)00075-5. [DOI] [PubMed] [Google Scholar]
- 44.Armour KL, Clark MR, Hadley AG, Williamson LM. Recombinant human IgG molecules lacking Fcgamma receptor I binding and monocyte triggering activities. Eur J Immunol. 1999;29:13–24. doi: 10.1002/(SICI)1521-4141(199908)29:08<2613::AID-IMMU2613>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 45.Shen L, Graziano RF, Fanger MW. The functional properties of FcγRI, II and III on myeloid cells: a comparative study of killing of erythrocytes and tumour cells mediated through the different Fc receptors. Mol Immunol. 1989;26:959–69. doi: 10.1016/0161-5890(89)90114-4. [DOI] [PubMed] [Google Scholar]
- 46.Dougherty GJ, Selvendran Y, Murdoch S, Palmer DG, Hogg N. The human mononuclear phagocyte high-affinity Fc receptor, FcRI, defined by a monoclonal antibody, 10.1. Eur J Immunol. 1987;17:1453–9. doi: 10.1002/eji.1830171011. [DOI] [PubMed] [Google Scholar]
- 47.Bredius RG, Fijen CA, De Haas M, Kuijper EJ, Weening RS, Van de Winkel JG, Out TA. Role of neutrophil Fc gamma RIIa (CD32) and Fc gamma RIIIb (CD16) polymorphic forms in phagocytosis of human IgG1- and IgG3-opsonised bacteria and erythrocytes. Immunology. 1994;83:624–30. [PMC free article] [PubMed] [Google Scholar]
- 48.Edberg JC, Kimberly RP. Cell type-specific glycoforms of Fc gamma RIIIa (CD16): differential ligand binding. J Immunol. 1997;159:3849–57. [PubMed] [Google Scholar]
- 49.Rozsnyay Z, Sármay G, Walker M, Maslanka K, Valasek Z, Jefferis R, Gergely J. Distinctive role of IgG1 and IgG3 isotypes in Fc gamma R-mediated functions. Immunology. 1989;66:491–8. [PMC free article] [PubMed] [Google Scholar]
- 50.Aase A, Michaelsen TE. Opsonophagocytic activity induced by chimeric antibodies of the four human IgG subclasses with or without help from complement. Scand J Immunol. 1994;39:581–9. doi: 10.1111/j.1365-3083.1994.tb03416.x. [DOI] [PubMed] [Google Scholar]
- 51.Roux KH, Strelets L, Michaelsen TE. Flexibility of human IgG subclasses. J Immunol. 1997;159:3372–82. [PubMed] [Google Scholar]
- 52.Burton DR, Woof JM. Human antibody effector function. Adv Immunol. 1992;51:1–84. doi: 10.1016/s0065-2776(08)60486-1. [DOI] [PubMed] [Google Scholar]
- 53.Taylor RR, Allen SJ, Greenwood BM, Riley EM. IgG3 antibodies to Plasmodium falciparum merozoite surface protein 2 (MSP2): increasing prevalence with age and association with clinical immunity to malaria. Am J Trop Med Hyg. 1998;58:406–13. doi: 10.4269/ajtmh.1998.58.406. [DOI] [PubMed] [Google Scholar]
- 54.Cavacini LA, Emes CL, Power J, Desharnais FD, Duval M, Montefiori D, Posner MR. Influence of heavy chain constant regions on antigen binding and HIV-1 neutralization by a human monoclonal antibody. J Immunol. 1995;155:3638–44. [PubMed] [Google Scholar]
- 55.Carter PB, Collins FM. The route of enteric infection in normal mice. J Exp Med. 1974;139:1189–203. doi: 10.1084/jem.139.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wain J, Diep TS, Ho VA, Walsh AM, Nguyen TT, Parry CM, White NJ. Quantitation of bacteria in blood of typhoid fever patients and relationship between counts and clinical features, transmissibility, and antibiotic resistance. J Clin Microbiol. 1998;36:1683–7. doi: 10.1128/jcm.36.6.1683-1687.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robbins JD, Robbins JB. Reexamination of the protective role of the capsular polysaccharide (Vi antigen) of Salmonella typhi. J Infect Dis. 1984;150:436–49. doi: 10.1093/infdis/150.3.436. [DOI] [PubMed] [Google Scholar]
- 58.Landy M. Studies on Vi antigen. VI. Immunization of human beings with purified Vi antigen. Am J Hyg. 1954;60:52–62. doi: 10.1093/oxfordjournals.aje.a119703. [DOI] [PubMed] [Google Scholar]
- 59.Keitel WA, Bond NL, Zahradnik JM, Cramton TA, Robbins JB. Clinical and serological responses following primary and booster immunization with Salmonella typhi Vi capsular polysaccharide vaccines. Vaccine. 1994;12:195–9. doi: 10.1016/0264-410x(94)90194-5. [DOI] [PubMed] [Google Scholar]
- 60.Brugier JC, Barra A, Schulz D, Preud'homme JL. Subclasses of human vaccinal antibodies to the Vi capsular polysaccharide of Salmonella typhi. Int J Clin Lab Res. 1993;23:38–41. doi: 10.1007/BF02592279. [DOI] [PubMed] [Google Scholar]
- 61.Aase A, Naess LM, Sandin RH, et al. Comparison of functional immune responses in humans after intranasal and intramuscular immunisations with outer membrane vesicle vaccines against group B meningococcal disease. Vaccine. 2003;21:2042–51. doi: 10.1016/s0264-410x(02)00774-0. [DOI] [PubMed] [Google Scholar]
- 62.Van de Winkel JG, Anderson CL. Biology of human immunoglobulin G Fc receptors. J Leukoc Biol. 1991;49:511–24. doi: 10.1002/jlb.49.5.511. [DOI] [PubMed] [Google Scholar]
- 63.Anderson CL, Shen L, Eichen DM, Wewers MD, Gill JK. Phagocytosis mediated by three distinct Fc gamma receptor classes on human leukocytes. J Exp Med. 1990;171:1333–45. doi: 10.1084/jem.171.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schiff DE, Rae J, Martin TR, Davis BH, Curnutte JT. Increased phagocyte FcgammaRI expression and improved Fc gamma-receptor-mediated phagocytosis after in vivo recombinant human interferon-gamma treatment of normal human subjects. Blood. 1997;90:3187–94. [PubMed] [Google Scholar]
- 65.Downey GP, Botelho RJ, Butler JR, Moltyaner Y, Chien P, Schreiber AD, Grinstein S. Phagosomal maturation, acidification, and inhibition of bacterial growth in nonphagocytic cells transfected with FcgammaRIIA receptors. J Biol Chem. 1999;274:28436–44. doi: 10.1074/jbc.274.40.28436. [DOI] [PubMed] [Google Scholar]


