Abstract
Autoimmune inner ear disease is characterized by progressive, bilateral although asymmetric, sensorineural hearing loss. Patients with autoimmune inner ear disease had higher frequencies of interferon-γ-producing T cells than did control subjects tested. Human adipose-derived mesenchymal stem cells (hASCs) were recently found to suppress effector T cells and inflammatory responses and therefore have beneficial effects in various autoimmune diseases. The aim of this study was to examine the immunosuppressive activity of hASCs on autoreactive T cells from the experimental autoimmune hearing loss (EAHL) murine model. Female BALB/c mice underwent β-tubulin immunization to develop EAHL; mice with EAHL were given hASCs or PBS intraperitoneally once a week for 6 consecutive weeks. Auditory brainstem responses were examined over time. The T helper type 1 (Th1)/Th17-mediated autoreactive responses were examined by determining the proliferative response and cytokine profile of splenocytes stimulated with β-tubulin. The frequency of regulatory T (Treg) cells and their suppressive capacity on autoreactive T cells were also determined. Systemic infusion of hASCs significantly improved hearing function and protected hair cells in established EAHL. The hASCs decreased the proliferation of antigen-specific Th1/Th17 cells and induced the production of anti-inflammatory cytokine interleukin-10 in splenocytes. They also induced the generation of antigen-specific CD4+ CD25+ Foxp3+ Treg cells with the capacity to suppress autoantigen-specific T-cell responses. The experiment demonstrated that hASCs are one of the important regulators of immune tolerance with the capacity to suppress effector T cells and to induce the generation of antigen-specific Treg cells.
Keywords: autoimmunity, cytokines, stem cells, therapy/immunotherapy
Introduction
Autoimmune inner ear disease (AIED)1,2 is described as progressive, bilateral although asymmetric, sensorineural hearing loss that can be improved by immunosuppressive therapy. It is widely recognized that autoimmune mechanisms are involved in inner ear diseases.2 Tuohy and colleagues3 demonstrated that patients with AIED have higher frequencies of interferon-γ (IFN-γ)-producing T cells and higher serum antibody titres compared with both control subjects with normal hearing and patients with noise- and/or age-related hearing loss. Many autoantigens have been implicated as possible causal antigens in AIED: heat-shock protein 70,4,5 collagen II,6,7 cochlin3,8 and, most recently, β-tubulin.9–13
Yoo et al. demonstrated that 67 (59%) out of 113 patients with Ménière's disease had antibodies to a 55 000 molecular weight protein β-tubulin in guinea-pig inner ear extract.9–13 Moreover, immunohistological studies showed that β-tubulin appears to be the highly expressed protein in inner ear tissues, such as hair cells, supporting cells, spiral ligament of stria vascularis, the neural pathway of the cochlea, as well as the spiral ganglion, indicating that β-tubulin is a fundamental protein in guinea-pig inner ear.9,12 Nevertheless, inner ear immunization with β-tubulin changed its spatial distribution in specific structures12 and caused degeneration of the spiral ganglion,12 thereby affecting the functions of microtubules in the stria vascularis and the spiral ganglion. More recently, Cai et al.13 developed a form of experimental autoimmune hearing loss (EAHL) by immunizing BALB/c mice with recombinant mouse β-tubulin. Mice immunized with β-tubulin developed substantial hearing loss and loss of hair cells in the basal turn of the cochlea. However, peripheral tolerance could be induced by oral administration of low-dose β-tubulin antigen in an animal model of AIED.13 This treatment showed less hearing loss and less inner ear damage; decreased IFN-γ secretion in response to β-tubulin antigen; and demonstrated an effective, antigen-specific method to suppress EAHL.
Mesenchymal stem cells (MSCs) are mesoderm-derived cells that reside in virtually all tissues and function as precursors of non-haematopoietic connective tissues with the capacity to differentiate into mesenchymal and non-mesenchymal cell lineages.14–16 Besides their potential clinical application to repair damaged tissues, bone marrow-derived MSCs (BM-MSCs) have recently been described as potent immunomodulators in various immune disorders, including inhibition of dendritic cell maturation, T-cell proliferation and B-cell function.16–21 However, harvesting BM-MSCs is extremely painful for patients and yields low numbers of cells, but their clinical application requires large numbers of cells for infusion, which in most cases are not available.20–22 When compared with BM-MSCs, human adipose-derived mesenchymal stem cells (hASCs) are equally capable of differentiating into cells and tissues of mesodermal origin.22–26 Abundant numbers of hASCs can be easily derived from lipoaspirate, the waste product of liposuction surgery and rapidly expanded in vitro to generate a clinically effective dosage. Moreover, recent studies have reported that hASCs share some of the immunomodulatory properties that characterize the BM-MSCs.16,22–26 Some researchers have reported that ASCs exert profound immunomodulatory properties and protective effects on acute graft-versus-host disease and experimental arthritis.16,24–26
Our results show that hASC administration has therapeutic effects. Notably, the suppression of EAHL by hASCs was associated with the induction of CD25+ CD4+ Foxp3+ regulatory T (Treg) cells and interleukin-10 (IL-10) that could suppress the in vivo-induced T helper type 1 (Th1) responses in an in vitro co-culture assay.
Materials and methods
Mice and immunization
Female BALB/c mice (Jackson Laboratory, Bar Harbor, ME) were used in this study, and auditory brain responses (ABRs) were measured bilaterally, both pre-treatment and post-treatment, for all the mice to ensure their normal hearing function. Mice were maintained in the animal facility at the University of Tennessee Health Science Center, according to the institutional guidelines for animal care and use. These studies were approved by the Institutional Animal Care and Use Committee of the University of Tennessee. At 6 weeks of age, mice were immunized subcutaneously with 300 μg β-tubulin (recombinant full-length human β-tubulin; Abcam, Cambridge, MA) emulsified with an equal volume of complete Freund's adjuvant (Difco Laboratories, Detroit, MI) containing 2 mg/ml H37Ra Mycobacterium tuberculosis (Difco). The mice were given boosters by subcutaneous injection with β-tubulin emulsified with incomplete Freund's adjuvant (Difco) twice at 1-week intervals, 2 weeks after the initial immunization.
Treatment protocols
The therapeutic treatment was begun after the onset of hearing loss, 2 weeks after immunization. Mice with EAHL received 2 × 106 hASCs (RNL Life Science Inc., Korea) or PBS intraperitoneally, once a week for 6 consecutive weeks.
Hearing tests
During ABR measurements, mice were anaesthetized with avertin (500 mg/kg bodyweight). The far-field auditory brainstem-evoked response was conducted in a sound-attenuating booth and the ABRs were recorded subcutaneously between vertex (active), posterior bulla (reference), and lower back (ground). Click and tone burst stimuli of 8, 16 and 32 kHz were generated and delivered to both ears through a high-frequency transducer. A maximum sound pressure level was stimulated in tone bursts of 100 dB. The evoked potentials were amplified 5000 times and averaged from 600 evoked responses for the first 10-millisecond period following stimulation. Auditory thresholds were determined by increasing the sound intensity of the tone burst for each frequency stimulus and were verified twice. Auditory evoked potential amplitude was calculated from all traces between the maximum intensity of 100 dB and the minimum intensity as hearing threshold was determined.
In vitro cytokine production and lymphocytes proliferation
Single-cell suspensions of spleens were obtained after six hASC infusions, and cells (2 × 105 cells/well) were cultured in 96-well flat-bottomed plates (Costar, Corning, NY) in RPMI-1640 medium supplemented with 5% fetal calf serum (Gibco, Paisley, UK), 50 μm 2-mercaptoethanol, 2 mm l-glutamine and 10 U penicillin/streptomycin (Gibco), and stimulated with 10 μg/ml β-tubulin. Positive control wells contained 2 μg/ml anti-mouse CD3 (BD Biosciences, San Diego, CA), and negative control wells contained only PBS. Supernatants were harvested after 48 hr and stored at −70° for cytokine array. Proliferation assays were determined at 72 hr by measuring bromodeoxyuridine-substituted DNA incorporation (Roche, Madrid, Spain). To examine the suppressive activity of hASCs in vitro, 2 × 105 splenocytes isolated from the EAHL mice were stimulated with 10 μg/ml β-tubulin in the presence of 2 × 104 hASCs. Proliferation and cytokine production were then determined. Some co-cultures of splenocytes with hASCs were treated with anti-IL-10 antibody (10 μg/ml; BD Biosciences).
Milliplex protein array system
The levels of cytokines in culture supernatants were determined by a multiplex cytokine bead array system – MILLIPLEX Mouse Cytokine/Chemokine 22-plex assay (Millipore, St Charles, MO) according to the manufacturer's instructions. The reaction mixture was read using the Bio-Plex protein array reader, and data were analysed with the Bio-Plex Manager software program in the Rheumatic Disease Research Core Center, Veterans Affairs Medical Center (Memphis, TN).
Flow cytometry
To determine the percentage of Treg cells in vivo, flow cytometry was performed on freshly isolated splenocytes usinga Treg cell detection kit (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions. The CD4+ CD25+ Foxp3+ -expressing T cells were identified by staining splenocytes with phycoerythrin-labelled anti-CD4 and allophycocyanin-labelled anti-CD25. For intracellular staining of Foxp3, cells were fixed and permeabilized before incubation with FITC-labelled anti-mouse Foxp3. For all the markers evaluated in this study, appropriate isotype-matched control antibodies were used to determine non-specific staining. Labelled cells were washed with PBS, and a minimum of 10 000 cells was analysed from each sample by flow cytometry with an LSR II (BD Biosciences). The percentage of Treg cells was determined by flowjo software (Tree Star, Ashland, OR).
In vitro suppression assay
Isolation of mouse CD4+, CD4+ CD25+, and CD4+ CD25− T cells was performed by using a mouse Treg cell isolation kit (Miltenyi Biotec) according to the manufacturer's instructions. Briefly, CD4+ T cells were first enriched by negative selection (depleting CD8a, CD11b, CD45R, CD49b and Ter-119-positive cells) with magnetic antibody cell sorting. The CD4+ T cells were incubated with magnetic beads conjugated with an anti-CD25 monoclonal antibody to separate CD4+ CD25+ and CD4+ CD25− T-cell subpopulations. The purity of the resulting T-cell subpopulations was higher than 95% by flow cytometry. To determine the suppressive capacity of hASC-induced Treg cells, proliferation assays were performed in triplicate by culturing CD4+ CD25− cells (responder, 5 × 104 from splenocytes of EAHL mice), CD4+ CD25+ T cells (suppressor, 5 × 104 from splenocytes of β-tubulin-immunized mice treated with either hASCs or PBS) in 96-well plates with irradiated antigen-presenting cells (5 × 104 from splenocytes of normal BALB/c mice) for 72 hr at 37° in complete medium. Cultures were stimulated by β-tubulin (10 μg/ml), and some co-cultures were treated with anti-IL-10 antibody (10 μg/ml). After 72 hr, the proliferation of autoreactive T cells was assayed by measuring bromodeoxyuridine-substituted DNA incorporation.
Statistical analysis
Data were analysed using analysis of variance or Student's t-test to compare differences between the treatment groups.
Results
Ability of hASCs to reverse established EAHL
In the present study, we investigated the potential therapeutic effect of hASCs in an experimental model of murine autoimmune hearing loss. Mice were examined weekly for ABRs for hearing capacity. After three injections (Fig. 1a), the hASC administration group showed that the ABR threshold to click stimulus and wide range of specific frequencies, in comparison with the PBS control group, significantly decreased. After six injections of hASCs (Fig. 1b), ABR click and pure tone thresholds of the hASC administration group showed improved hearing level at all frequencies tested from 8 to 32 kHz. The ABRs detected threshold levels similar to those in naive mice that received no treatment (Fig. 1b), and the hASC administration completely restored hearing in deaf mice, whereas the PBS control group developed EAHL. Therefore, electrophysiology tests demonstrated recovery of hearing to click stimulus and a wide range of specific frequencies after six injections of hASCs.
Figure 1.
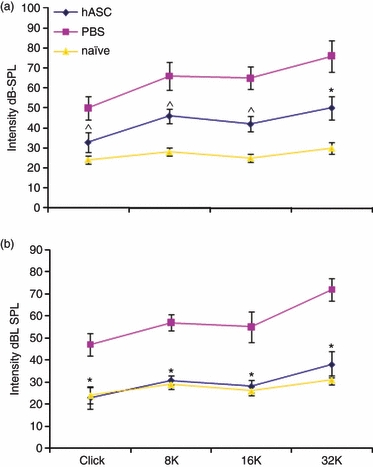
Human adipose-derived mesenchymal stem cells (hASCs) are able to reverse established experimental autoimmune hearing loss (EAHL) in β-tubulin-immunized mice. Female BALB/c mice showing normal auditory behaviour in auditory brainstem responses (ABRs) were immunized with 300 μg β-tubulin emulsified with complete Freund's adjuvant. After 2 weeks, the mice were given boosters by subcutaneous injection with β-tubulin emulsified with incomplete Freund's adjuvant twice at 1-week intervals. Two weeks after immunization, β-tubulin-immunized mice were given 2 × 106 hASCs or PBS intraperitoneally once a week for 6 consecutive weeks. After three injections (a), the hASC administration group had a significantly decreased ABR threshold; after six injections of hASCs (b), the ABR detected similar threshold levels to naive mice at all frequencies tested. dB-SPL, decibels sound pressure level. Values are the mean ± SE of 10 mice per group. *P < 0.001 versus PBS controls; ^P < 0.01 versus PBS controls.
hASC treatment down-regulates the Th1-mediated autoreactive response in EAHL
We investigated the possible immune-modulating effect of hASCs on T-cell priming and differentiation in vivo by examining the recall response to β-tubulin in isolated splenocytes from hASC-treated or PBS-treated mice with EAHL in vitro. To determine the ability of hASC treatment to suppress the ongoing inflammatory process, mice with EAHL were treated with PBS or hASCs once a week for 6 consecutive weeks after β-tubulin immunization, and splenocytes that were isolated 10 days after the last treatment with the hASCs were assessed for proliferative responses to β-tubulin. T cells from hASC-treated mice exhibited a significantly decreased stimulation index compared with that in cells from PBS-treated mice (Fig. 2a). Moreover, T cells from hASC-treated non-immunized mice did not develop a xenogenic response to the hASCs in those non-immunized animals (data not shown). Importantly, splenocytes from the mice that were given hASCs during the ongoing immune process produced significantly lower levels of IL-17 and IFN-γ than did cells from mice given PBS (Fig. 2a). Moreover, hASCs dramatically stimulated the production of IL-10 (Fig. 2a) by β-tubulin-activated T cells, whereas the Th2-type cytokine IL-4 was not significantly affected (data not shown). Hence, our findings indicate that administering hASCs in therapeutic regimens to mice with EAHL was associated with strong immunomodulating effects on the priming of β-tubulin-specific CD4+ T cells, resulting in skewing of activated CD4 T cells toward lower activity of Th1 and Th17 effector cells, but increased activity of the anti-inflammatory cytokine IL-10, suggesting that this treatment may generate IL-10-secreting Treg cells.
Figure 2.
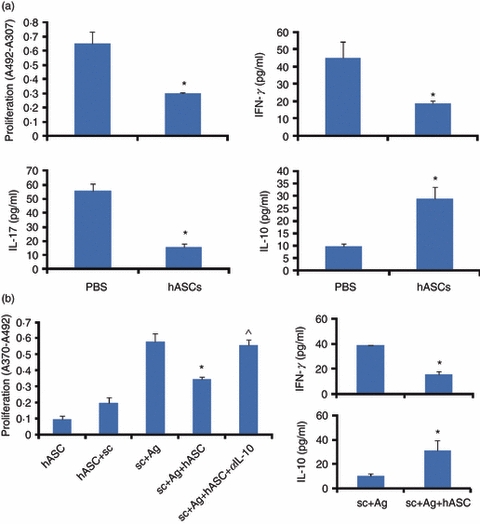
Inhibition of the T helper type 1 (Th1) -mediated response in mice with experimental autoimmune hearing loss (EAHL) by human adipose-derived mesenchymal stem cells (hASC) treatments. β-Tubulin-immunized mice were administered 2 × 106 hASCs or PBS intraperitoneally once a week for 6 consecutive weeks. (a) Proliferative response and cytokine production by splenocytes isolated after six hASC infusions and stimulated in vitro with β-tubulin. Cell proliferation was determined at 72 hr by measuring bromodeoxyuridine-substituted DNA incorporation. Data are shown as the mean incorporation ± SE of triplicate cultures and are representative of three separate experiments with similar results. A total of 2 × 106 splenocytes per well were cultured for 48 hr in the presence of β-tubulin 10 μg/ml. Supernatants were harvested and measured by cytokine array. The data represent the means ± SE (n = 5). *P < 0.001 versus PBS controls. (b) Proliferative response and production of interferon-γ (IFN-γ) and interleukin-10 (IL-10) in splenocytes obtained from EAHL mice. Proliferation (measured after 72 hr) and levels of IFN-γ and IL-10 (measured after 48 hr) were determined in splenocytes (sc) stimulated with or without β-tubulin in the presence or absence of hASCs; in some co-cultures of splenocytes/hASCs treated with anti-IL-10 antibody (αIL-10). Values are the mean ± SE of five mice per group. *P < 0.001 versus β-tubulin-activated splenocytes stimulated with β-tubulin; ^P < 0.005 versus co-cultures of β-tubulin-activated splenocytes/hASCs stimulated with β-tubulin.
To investigate whether hASCs directly deactivated autoreactive Th1 cells, hASCs were co-cultured with splenocytes from mice with EAHL. The hASCs suppressed the proliferation of β-tubulin-activated T cells, and this effect was significantly reversed by anti-IL-10 antibody (Fig. 2b). Moreover, hASCs inhibited the production of IFN-γ and stimulated the production of IL-10 by β-tubulin-activated T cells (Fig. 2b). This suggests that hASCs were able to suppress Th1 responses and to induce Treg cells.
Therapeutic hASC treatment recruits antigen-specific Treg cells in EAHL
Previous studies have indicated that Treg cells can confer significant protection in controlling autoimmunity by suppressing self-reactive T cells.16,27–30 Therefore, defects in Treg cell development, maintenance, or function have been associated with autoimmune diseases. The observed down-regulation of the autoreactive Th1 response and increased levels of regulatory cytokine IL-10 encouraged us to examine the involvement of β-tubulin-specific Treg cells in in vivo immunosuppressive activity of hASCs. Therefore, we compared the proportion and suppressive function of Treg cells between β-tubulin-immunized mice treated with either hASCs or PBS, in view of the critical role of Treg cells in restraining autoaggressive T cells in experimental settings.
Administering hASCs resulted in a significantly higher percentage of CD4+ CD25+ Foxp3+ Treg cells in splenocytes than did PBS in control mice (Fig. 3a) (mean ± SD 7·8% ± 0·6% and 13·5% ± 1·8% in PBS-treated and hASC-treated mice, respectively; P < 0·001). Moreover, we evaluated the suppressive activity of β-tubulin-specific Treg cells generated in the presence of hASCs on the activation of autoreactive T cells isolated from mice with EAHL. CD4+ CD25+ Treg cells from EAHL mice treated with PBS failed to suppress the proliferation of autologous CD4+ CD25− effector T cells (Fig. 3b), whereas CD4+ CD25+ Treg cells isolated from hASC-treated mice could suppress the proliferative response of CD4+ CD25− effectors (Fig. 3b), and this effect was significantly reversed by anti-IL-10 antibody in comparison with hASC-treated mice (Fig. 3b). Hence, administering hASCs might be inducing Treg cells to secrete IL-10, which suppresses the self-reactive T cells.
Figure 3.
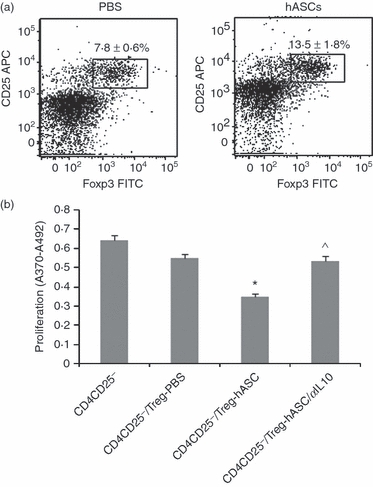
Induction of regulatory T (Treg) cells with suppressive functions in experimental autoimmune hearing loss (EAHL) by human adipose-derived mesenchymal stem cells (hASCs). β-Tubulin-immunized mice were injected with 2 × 106 hASCs or PBS intraperitoneally, once a week for 6 consecutive weeks. (a) Percentage of CD4+ CD25+ Foxp3+ cells. To evaluate the proportion of Treg cells in the experimental groups, spleen cells were stained with antibodies to mouse CD4, CD25 and Foxp3, and the labelled cells in the live lymphocyte gate from each sample were analysed by flow cytometry. (b) The suppressive capacity of hASC-induced Treg cells. CD4+ CD25+ T cells were purified by magnetic antibody cell sorting from spleens of β-tubulin-immunization mice treated with either hASCs or PBS. Responder CD4+ CD25− T cells were isolated from EAHL mice. CD4+ CD25− T cells alone or together with CD4+ CD25+ T cells were cultured with autologous irradiated antigen-presenting cells and β-tubulin (10 μg/ml); some co-cultures treated with anti-interleukin-10 (IL-10) antibody. After 72 hr, the proliferation of autoreactive T cells was assayed by measuring bromodeoxyuridine-substituted DNA incorporation. Values are the means ± SE of five mice per group. *P < 0.001 versus co-cultures of β-tubulin-activated CD4+ CD25− T cells/Treg-PBS cells stimulated with β-tubulin; ^P < 0.005 versus co-cultures of β-tubulin-activated CD4+ CD25− T cells/Treg-hASC cells stimulated with β-tubulin.
hASC administration protects hair cells in established EAHL
Amelioration of EAHL in hASC recipients was confirmed by histological examination of cochlear cross-sections. Cochlear cross-sections from a naive BALB/c mouse (Fig. 4a) revealed a normal density of spiral ganglion cells, as well as three outer hair cell rows with one row of inner hair cells in the basal turn of the cochlea (Fig. 4a). Cross-sections from a PBS-treated mouse (Fig. 4b) revealed a drastic and sizable degeneration in the spiral ganglion cell population of the organ of Corti. Whole-mount preparations of the cochleae showed that significant hair cell loss had occurred in PBS-treated mice (Fig. 4b). It could explain the observed hearing phenotype, because ABR measurements revealed severe deafness in PBS-treated mice. However, in the hASC-treated mice (Fig. 4c), we did not observe abnormal morphological changes. No hair cell loss was found in hASC-treated mice (Fig. 4c); thus, hASC-treated mice had normal hearing compared with naive mice (Fig. 4a).
Figure 4.
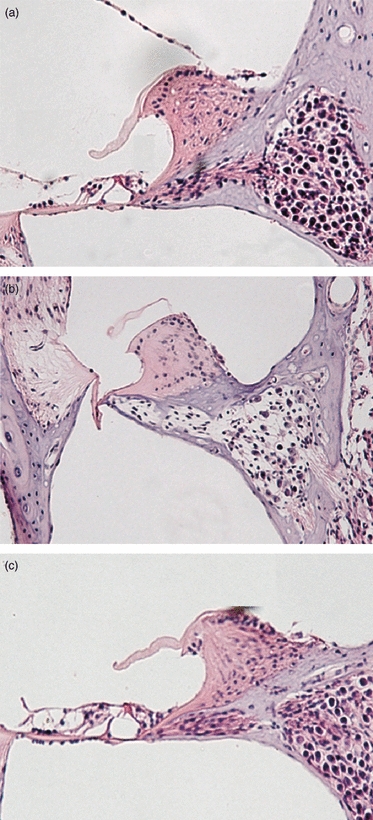
Cochlear morphology. Cochlea whole mounts were prepared, stained with haematoxylin & eosin, and photographed under a light microscope. Representative spiral ganglion and organ of Corti sections are shown at the basal turn of the cochlea. Data shown are representative of mice receiving no treatment (a), PBS (b), or human adipose-derived mesenchymal stem cells (hASC) (c). The figure is representative of three mice per group.
Discussion
There are no specific therapeutic strategies to treat AIED. For this reason, we tested the efficacy of hASCs, a novel cell-based therapeutic strategy, against AIED with autoimmune hearing loss in a murine model. In our study, EAHL mice treated with PBS developed substantial hearing loss, which lasted at least 8 weeks after immunization. Moreover, hair cell loss and degeneration of spiral ganglion cells in the basal turns of the cochlea were also observed in EAHL mice treated with PBS. However, EAHL mice treated with hASCs had significantly improved hearing function. After six infusions, the ABR thresholds in the hASC treatment group and the histological analysis of the cochlear cross-sections were equivalent to naive controls. In addition, hASCs provided a highly effective therapy for EAHL, with the capacity to suppress β-tubulin-reactive T cells by inducing the generation of antigen-specific Treg cells. Therefore, our data showed that the hASC treatment had therapeutic effects.
There are several potential mechanisms for the effect of hASCs on the down-regulation of T-cell responses in vitro and in vivo.16 Our results demonstrated that administering hASCs to mice with established EAHL significantly decreased the proliferation of β-tubulin-specific T cells and the production of the Th1/Th17-type cytokines. The suppression of Th1/Th17 responses might be the result of a direct effect on autoreactive T cells, because autoreactive T cells obtained from mice treated with hASCs were unresponsive in vitro to Th1 restimulation by β-tubulin autoantigens. Accordingly, hASCs directly inhibited the in vitro activation of β-tubulin autoreactive T cells from EAHL mice.
In contrast to the effect on Th1-type cytokines, administering hASCs increased the production of IL-10 in splenocytes. Interleukin-10 is not only a major anti-inflammatory cytokine,16,31,32 it is also an important regulatory factor for Treg cells, which play a key role in the homeostatic regulation of the autoreactive T-cell repertoire and the induction of peripheral tolerance in vivo.16,31,32 The up-regulation of β-tubulin-specific IL-10 production by splenocytes suggests the possibility that hASCs may induce IL-10-producing Treg cells31,33 in EAHL mice. We therefore examined the possibility that this suppression was mediated by the production of Treg cells in vivo. We found a significantly elevated percentage of CD4+ CD25+ Foxp3+ cells from EAHL mice exposed to hASCs compared with the PBS control groups. Also, these hASC-induced Treg cells potently inhibited the proliferative response of autoreactive T cells in vitro, and these effects were significantly abrogated by anti-IL-10 antibodies. Therefore, hASC treatment might induce IL-10-secreting β-tubulin-specific CD4+ CD25+ Foxp3+ Treg cells in mice with EAHL that mediate T-cell tolerance.
In summary, the present study demonstrated that hASCs display a therapeutic potential and suggests that hASCs may provide a novel therapeutic approach for AIED. Mechanistically, our results indicate that the hASCs inhibit the Th1/Th17 cell responses through the generation of IL-10-secreting Treg cells with the capacity to suppress autoreactive T-cell responses, thereby maintaining self-tolerance.
Acknowledgments
We thank RNL-bio (Korea) for providing the funding for this research project.
Glossary
Abbreviations
- ABR
auditory brainstem responses
- AIED
autoimmune inner ear disease
- BM-MSC
bone marrow-derived MSC
- EAHL
experimental autoimmune hearing loss
- hASC
human adipose-derived mesenchymal stem cells
- IFN
interferon
- IL-10
interleukin-10
- MSC
mesenchymal stem cells
- Th1
T helper type 1
- Treg
T regulatory cell
Disclosures
The authors declare no financial conflicts of interest.
References
- 1.McCabe BF. Autoimmune sensorineural hearing loss. Ann Otol Rhinol Laryngol. 1979;88:585–9. doi: 10.1177/000348947908800501. [DOI] [PubMed] [Google Scholar]
- 2.Buniel MC, Geelan-Hansen K, Weber PC, Tuohy VK. Immunosuppressive therapy for autoimmune inner ear disease. Immunotherapy. 2009;1:425–34. doi: 10.2217/imt.09.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baek MJ, Park HM, Johnson JM, et al. Increased frequencies of cochlin-specific T cells in patients with autoimmune sensorineural hearing loss. J Immunol. 2009;6:4203–10. doi: 10.4049/jimmunol.177.6.4203. [DOI] [PubMed] [Google Scholar]
- 4.Billings PB, Keithley EM, Harris JP. Evidence linking the 68 kilodalton antigen identified in progressive sensorineural hearing loss patient sera with heat shock protein 70. Ann Otol Rhinol Laryngol. 1995;104:181–8. doi: 10.1177/000348949510400302. [DOI] [PubMed] [Google Scholar]
- 5.Billings PB, Shin SO, Harris JP. Assessing the role of anti-hsp70 in cochlear impairment. Hear Res. 1998;126:210–3. doi: 10.1016/s0378-5955(98)00172-5. [DOI] [PubMed] [Google Scholar]
- 6.Yoo TJ, Tomoda K, Stuart JM, Cremer TA, Townes AS, Kang AH. Type II collagen-induced autoimmune sensorineural hearing loss and vestibular dysfunction in rats. Ann Otol Rhinol Laryngol. 1983;92:267–71. doi: 10.1177/000348948309200310. [DOI] [PubMed] [Google Scholar]
- 7.Yoo TJ, Yazawa Y, Tomoda K, Floyd R. Type II collagen-induced autoimmune endolymphatic hydrops in guinea pig. Science. 1983;222:65–7. doi: 10.1126/science.6623056. [DOI] [PubMed] [Google Scholar]
- 8.Solares CA, Edling AE, Johnson JM, Baek MJ, Hirose K, Hughes GB, Tuohy VK. Murine autoimmune hearing loss mediated by CD4+ T cells specific for inner ear peptides. J Clin Invest. 2004;113:1210–7. doi: 10.1172/JCI18195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoo TJ, Shea J, Jr, Ge X, et al. Presence of autoantibodies in the sera of Ménière's disease. Ann Otol Rhinol Laryngol. 2001;110:425–9. doi: 10.1177/000348940111000506. [DOI] [PubMed] [Google Scholar]
- 10.Zhou B, Kermany MH, Glickstein J, et al. Murine autoimmune hearing loss mediated by CD4(+) T cells specific for β-tubulin. Clin Immunol. 2011;138:222–30. doi: 10.1016/j.clim.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Yoo TJ, Du X, Kwon SS. Molecular mechanism of autoimmune hearing loss. Acta Otolaryngol Suppl. 2002;548:3–9. doi: 10.1080/00016480260094893. [DOI] [PubMed] [Google Scholar]
- 12.Du X, Yoo TJ, Mora R. Distribution of beta-tubulin in guinea pig inner ear ORL. J Otorhinolaryngol Relat Spec. 2003;65:7–16. doi: 10.1159/000068654. [DOI] [PubMed] [Google Scholar]
- 13.Cai Q, Du X, Zhou B, Cai C, Kermany MH, Yoo T. Induction of tolerance by oral administration of beta-tubulin in an animal model of autoimmune inner ear disease. ORL J Otorhinolaryngol Relat Spec. 2009;71:135–41. doi: 10.1159/000212116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 15.Liu ZJ, Zhuge Y, Velazquez OC. Trafficking and differentiation of mesenchymal stem cells. J Cell Biochem. 2009;106:984–91. doi: 10.1002/jcb.22091. [DOI] [PubMed] [Google Scholar]
- 16.González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum. 2009;60:1006–19. doi: 10.1002/art.24405. [DOI] [PubMed] [Google Scholar]
- 17.Nasef A, Ashammakhi N, Fouillard L. Immunomodulatory effect of mesenchymal stromal cells: possible mechanisms. Regen Med. 2008;3:531–46. doi: 10.2217/17460751.3.4.531. [DOI] [PubMed] [Google Scholar]
- 18.Noël D, Djouad F, Bouffi C, Mrugala D, Jorgensen C. Multipotent mesenchymal stromal cells and immune tolerance. Leuk Lymphoma. 2007;48:1283–9. doi: 10.1080/10428190701361869. [DOI] [PubMed] [Google Scholar]
- 19.Djouad F, Bouffi C, Ghannam S, Noël D, Jorgensen C. Mesenchymal stem cells: innovative therapeutic tools for rheumatic diseases. Nat Rev Rheumatol. 2009;5:392–9. doi: 10.1038/nrrheum.2009.104. [DOI] [PubMed] [Google Scholar]
- 20.Glennie S, Soeiro I, Dyson PJ, Lam W, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821–7. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- 21.Wehner R, Wehrum D, Bornhäuser M, et al. Mesenchymal stem cells efficiently inhibit the proinflammatory properties of 6-sulfo LacNAc dendritic cells. Haematologica. 2009;94:1151–6. doi: 10.3324/haematol.2008.001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouffi C, Djouad F, Mathieu M, Noël D, Jorgensen C. Multipotent mesenchymal stromal cells and rheumatoid arthritis: risk or benefit? Rheumatology (Oxford) 2009;48:1185–9. doi: 10.1093/rheumatology/kep162. [DOI] [PubMed] [Google Scholar]
- 23.Augello A, Tasso R, Negrini SM, Cancedda R, Pennesi G. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum. 2007;56:1175–86. doi: 10.1002/art.22511. [DOI] [PubMed] [Google Scholar]
- 24.Yañez R, Lamana ML, García-Castro J, Colmenero I, Ramírez M, Bueren JA. Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells. 2006;24:2582–91. doi: 10.1634/stemcells.2006-0228. [DOI] [PubMed] [Google Scholar]
- 25.Beyth S, Borovsky Z, Mevorach D, Liebergall M, Gazit Z, Aslan H, Galun E, Rachmilewitz J. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105:2214–9. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- 26.Puissant B, Barreau C, Bourin P, et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005;129:118–29. doi: 10.1111/j.1365-2141.2005.05409.x. [DOI] [PubMed] [Google Scholar]
- 27.Workman CJ, Szymczak-Workman AL, Collison LW, Pillai MR, Vignali DA. The development and function of regulatory T cells. Cell Mol Life Sci. 2009;66:2603–22. doi: 10.1007/s00018-009-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–87. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Morgan ME, Flierman R, van Duivenvoorde LM, et al. Effective treatment of collagen-induced arthritis by adoptive transfer of CD25+ regulatory T cells. Arthritis Rheum. 2005;52:2212–21. doi: 10.1002/art.21195. [DOI] [PubMed] [Google Scholar]
- 30.van Amelsfort JM, van Roon JA, Noordegraaf M, Jacobs KM, Bijlsma JW, Lafeber FP, Taams LS. Proinflammatory mediator-induced reversal of CD4+ CD25+ regulatory T cell-mediated suppression in rheumatoid arthritis. Arthritis Rheum. 2007;56:732–42. doi: 10.1002/art.22414. [DOI] [PubMed] [Google Scholar]
- 31.Battaglia M, Gianfrani C, Gregori S, Roncarolo MG. IL-10-producing T regulatory type 1 cells and oral tolerance. Ann N Y Acad Sci. 2004;1029:142–53. doi: 10.1196/annals.1309.031. [DOI] [PubMed] [Google Scholar]
- 32.Levings MK, Bacchetta R, Schulz U, Roncarolo MG. The role of IL-10 and TGF-beta in the differentiation and effector function of T regulatory cells. Int Arch Allergy Immunol. 2002;129:263–76. doi: 10.1159/000067596. [DOI] [PubMed] [Google Scholar]
- 33.Lloyd CM, Hawrylowicz CM. Regulatory T cells in asthma. Immunity. 2009;31:438–49. doi: 10.1016/j.immuni.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


