Abstract
In this study, we focused on three leukocyte-rich guinea pig cell populations, bronchoalveolar lavage (BAL) cells, resident peritoneal cells (PC), and splenocytes (SPC). BAL cells, SPC, and PC were stimulated either with live attenuated Mycobacterium tuberculosis H37Ra or with live or heat-killed virulent M. tuberculosis H37Rv (multiplicity of infection of 1:100). Each cell population was determined to proliferate in response to heat-killed virulent H37Rv, whereas no measurable proliferative response could be detected upon stimulation with live mycobacteria. Additionally, this proliferative capacity (in SPC and PC populations) was significantly enhanced upon prior vaccination with Mycobacterium bovis BCG. Accordingly, in a parallel set of experiments we found a strong positive correlation between production of antigen-specific bioactive tumor necrosis factor alpha (TNF-α) and prior vaccination with BCG. A nonspecific stimulus, lipopolysaccharide, failed to induce this effect on BAL cells, SPC, and PC. These results showed that production of bioactive TNF-α from mycobacterium-stimulated guinea pig cell cultures positively correlates with the vaccination status of the host and with the virulence of the mycobacterial strain.
Tuberculosis (TB) is a pandemic disease that represents a major public health, social, and economic problem throughout the world. Mycobacterium tuberculosis, the causative agent of TB, is estimated to have infected nearly one-third of the world's population, annually causing approximately 8 million cases and claiming the lives of nearly 2 million people. Although combination drug therapies are available for this deadly disease, the only realistic hope of eradicating this ancient killer is through the development of a standard, universally efficacious form(s) of vaccination. Although the current TB vaccine, Mycobacterium bovis bacille Calmette-Guérin (BCG), has yielded widely divergent results in several human field trials, considerable protection can be afforded by BCG in the guinea pig model of TB (30). Accordingly, the mechanisms of vaccine-induced protection in the guinea pig model can teach us important lessons about TB immunology and vaccinology that can fuel our search for a superior vaccine.
Effective vaccination against an intracellular pathogen such as M. tuberculosis requires the induction of cell-mediated immunity, which is characterized by the bidirectional interactions between T lymphocytes and cells of the monocyte/macrophage lineage. The specificity of this interaction is governed by the T-cell antigen receptor, which recognizes small peptide fragments in association with cell surface glycoproteins encoded by genes of the major histocompatibility complex (39).
The subsequent response is the stimulation of T lymphocytes to proliferate and produce interleukin-2 (IL-2) (2). In addition, this initial response to M. tuberculosis involves the production of a variety of other immunomodulatory cytokines, chemokines, and other immune cell products from resident alveolar macrophages (36). These host cell factors operate in a complex network of cell-cell communication that no doubt is crucial to the control of the infection but may also contribute to chronic infection and associated immunopathology (35). One such host cell factor, tumor necrosis factor alpha (TNF-α), is a proinflammatory cytokine produced by a wide array of immune cells, including macrophages, T lymphocytes, and polymorphonuclear cells (1). This cytokine is believed to play multiple roles in both the immune and pathological responses to TB infection. For example, in the murine system, TNF-α was discovered to synergize with gamma interferon to activate infected macrophages and enhance antimicrobicidal activities (12). TNF-α has also been shown to be intimately involved in the protective architecture of the granulomatous response in the mouse model (5, 13, 18). In addition to this cytokine's essential protective effects in the generation of immunity to TB, TNF-α may also play a role in the wasting and tissue necrosis which characterize active TB infection (8, 32). These paradoxical findings with respect to TNF-α biology may be explained by an in vivo dose dependency (6); however, additional studies in other models of TB are needed to further characterize the role of this multifunctional cytokine in immune defenses and/or pathogenesis of TB. Biologically active recombinant guinea pig TNF-α (secreted form, ∼17 kDa) has been cloned and expressed in Escherichia coli (37). Intratracheal infusion of recombinant guinea pig TNF-α effectedairway leukocyte recruitment by increasing the numbers of monocyte/macrophage, neutrophil, and eosinophil populations recoverable by bronchoalveolar lavage (BAL) (37).
Previous work in this laboratory demonstrated that BCG vaccination in guinea pigs enhances both chemokine (RANTES and IL-8) and cytokine (IL-1-β and IFN-γ) mRNA responses from various cell cultures (i.e., alveolar macrophages, adherence-purified peritoneal exudate cells, and whole splenocytes [SPC]) (15, 16, 19). This vaccination effect was observed in response to different multiplicities of infection (MOIs) with various attenuated and virulent mycobacterial strains. In this study, we focused on three distinct leukocyte-rich cell populations: SPC, resident peritoneal cells (PC), and BAL cells. Using these guinea pig cell populations, we determined whether BCG vaccination influenced the ability of these distinct cell populations to proliferate and produce TNF-α in response to both mycobacterial and nonmycobacterial stimuli. In a general sense, our results indicate that BCG vaccination enhanced the capacity of these cell populations to undergo proliferation and enhanced TNF-α production in response to various agonists, including lipopolysaccharide (LPS), concanavalin A (ConA), and whole mycobacteria. Furthermore, a positive correlation between TNF-α production and mycobacterial strain virulence was observed.
MATERIALS AND METHODS
Animals.
Specific-pathogen-free outbred Hartley strain guinea pigs (Charles River Breeding Laboratories, Inc., Wilmington, Mass.) were individually housed in polycarbonate cages on stainless-steel grid floors and provided commercial chow (Ralston Purina, St. Louis, Mo.) in stainless-steel feeders and tap water ad libitum. The guinea pigs were maintained in a temperature- and humidity-controlled environment and exposed to a 12-h light-dark cycle. Each animal was randomly assigned to a vaccination treatment group. All procedures were reviewed and approved by the Texas A&M University Laboratory Animal Care Committee.
BCG vaccination.
M. bovis BCG (Danish 1331; Statens Seruminstitut, Copenhagen, Denmark) was reconstituted in 0.9% physiological saline, and 0.1 ml (103 CFU) was administered to the experimental animals subcutaneously into the left inguinal region. The guinea pigs were rested for 6 to 8 weeks postvaccination before being used in these studies.
Bacteria.
M. tuberculosis H37Ra (ATCC 25177; American Type Culture Collection [ATCC], Manassas, Va.) and M. tuberculosis H37Rv (ATCC 27294) were cultured in Middlebrook 7H9 medium with albumin-dextrose-catalase enrichment, and frozen stocks were prepared according to a published procedure (14). Heat-killed M. tuberculosis H37Rv (hkRv) was prepared by heating at 80°C for 3 h. Before use, both live and heat-killed mycobacterial preparations were sonicated briefly (60 s) with a cell disruptor (Heat Systems-Ultrasonics, Inc.) to disperse bacterial clumps. Viability was determined by plating appropriate dilutions onto Middlebrook 7H10 agar (Difco, Detroit, Mich.).
Isolation and preparation of BAL cells.
Euthanasia was carried out by intramuscular injection of 100 mg of sodium pentobarbital (Sleepaway; Fort Dodge Laboratories Inc.)/kg of body weight. The abdominal and thoracic cavities of each guinea pig were opened aseptically, and the trachea was separated from the surrounding tissue. A longitudinal 1/4-in. incision was made along the ventral surface of the trachea. After an 18-gauge cannula was inserted into the opening, BAL was performed by instilling ice-cold 12 mM lidocaine in phosphate-buffered saline (PBS) (pH 7.4) with 2% heat-inactivated fetal bovine serum (FBS) (Atlanta Biologicals, Norcross, Ga.) into the lungs via the trachea by affixing a 20-ml syringe to the cannula. Five 10-ml washes were performed by injection of the wash solution into the lungs and subsequent withdrawal of the fluid after 1 min to allow for detachment of adherent cells. BAL fluid was collected in sterile 50-ml conical tubes. Lavage cells were washed twice in RPMI 1640 (Irvine Scientific, Santa Ana, Calif.) with 1% FBS (wash medium) by centrifugation at 320 × g for 10 min at 4°C. After the second wash, the cell pellet was resuspended in RPMI 1640 medium supplemented with 2 mM l-glutamine, 100 U of penicillin per ml, and 10% FBS (RPMI complete medium). Viable BAL cells were enumerated by trypan blue exclusion (Gibco Life Technologies, Grand Island, N.Y.), and the cell concentration was adjusted to 2 × 106 cells/ml just prior to stimulation.
Isolation and preparation of resident PC.
After recovery of the BAL cells, the peritoneal cavity was washed twice with ice-cold PBS (30 ml/wash) containing 10 U of heparin per ml and 2% FBS. Peritoneal fluid was pooled into a 50-ml conical tube and centrifuged at 320 × g for 10 min (4°C). The cell pellets were resuspended in wash medium and washed twice (as for BAL cells). After the viable PC were enumerated (by trypan blue exclusion), the cell concentration was adjusted to 106 cells/ml immediately prior to stimulation.
Preparation of SPC.
The spleen was removed aseptically from each guinea pig and placed in 15 ml of wash medium at necropsy. The spleens were then gently homogenized in wash medium with glass homogenizers, and after the second wash, the cell pellet was then resuspended in complete medium and viable SPC were enumerated by trypan blue exclusion. The number of cells was then adjusted to 107 cells/ml. Total cell recovery and calculated viabilities (based on trypan blue exclusion method) are summarized in Table 1.
TABLE 1.
Cell viability and total cell counts for BAL cells, PL, and SPC
| Vaccination status | Guinea pig cell subpopulation | Mean total cell recovery (SEM)a | Mean % viability (range)a |
|---|---|---|---|
| Unvaccinated | BAL cells | 5.13 × 106 (1.80 × 106) | 81.50 (73-86) |
| PC | 2.19 × 107 (0.375 × 107) | 96.00 (90-99) | |
| SPC | 2.21 × 108 (0.404 × 108) | 64.00 (57-71) | |
| BCG vaccinated | BAL cells | 8.48 × 106 (2.13 × 106) | 80.75 (69-88) |
| PC | 2.68 × 107 (0.398 × 107) | 97.75 (91-99) | |
| SPC | 2.70 × 108 (0.298 × 108) | 62.75 (52-73) |
For four guinea pigs per treatment group.
Cell stimulation.
Each cell population (at the specified concentration) was individually cultured in 24-, 48-, or 96-well tissue culture plates and either incubated in medium alone or infected in vitro with live attenuated M. tuberculosis H37Ra or live virulent M. tuberculosis H37Rv (MOI of 1:100). Parallel cultures were also set up with hkRv at the same MOI or with LPS at 100 ng/ml (E. coli serotype 0111:B4; Sigma Chemical Co., St. Louis, Mo.). Supernatants were then obtained from each of the culture wells at 24, 48, and 72 h; centrifuged at 12,000 × g; and stored at −20°C until analyzed by the L929 bioassay.
Lymphoproliferation assay.
BAL cells, SPC, and PC were seeded into 96-well flat-bottomed tissue culture plates at the identical concentrations described above in RPMI complete medium. Triplicate cultures were stimulated with ConA (Sigma) at a final concentration of 10 μg/ml or with LPS, live H37Ra or H37Rv, or hkRv at the identical concentration described above. The cultures were incubated for 4 days at 37°C in a 5% CO2 atmosphere, labeled with 1.0 μCi of tritiated thymidine per well for the final 6 h of the incubation, and harvested onto glass wool fiber filters by using a cell harvester. The results were expressed as a stimulation index (SI), which was defined as the counts per minute of tritiated thymidine taken up by stimulated cells divided by the counts per minute of unstimulated cells from the same source.
Differential cell counts.
Each of the three cell populations (BAL cells, PC, and SPC) was characterized by Diff-Quik staining (Dade Behring Inc., Newark, Del.) to determine the relative immune cell composition by cell morphology. Briefly, 106 cells from each prepared cell population were diluted into 1 ml of complete medium. The cells were spun for 5 min at 140 × g in a cytospin centrifuge, air dried for 1 to 2 min, and fixed and stained separately with Diff-Quik. After being rinsed with tap water, the slides were air dried, cover slips were applied, and cells were quantified with a light microscope.
Bioassay of TNF.
The culture supernatants were assessed for TNF activity by measuring their cytotoxicity on L929 cells as described by Espevik and Nissen-Meyer (11) with some modifications. L929 cells were suspended in RPMI 1640 without phenol red supplemented with 2 μM l-glutamine (Gibco Life Technologies), 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 5% FBS at 4 × 105 cells/ml. Aliquots (100 μl) of L929 cell suspension (ATCC) were seeded into 96-well flat-bottomed plates and incubated overnight in a CO2 incubator at 37°C. On the following day, 50 μl of serially diluted culture supernatants and 50 μl of an 8-μg/ml actinomycin D solution (final concentration, 2 μg/ml) were added to each well and incubated for an additional 20 h. A tetrazolium reagent, 2-(4-idophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2-H-tetrazolium salt (WST-1; Dojindo, Kumamoto, Japan) and 1-methoxymethyl phenazium methylsulfate (Dojindo) were dissolved at 6 and 0.4 mM, respectively, in PBS. These were mixed at a ratio of 1:1, and 20 μl was added to each well. The cells were incubated for 2 h in a CO2 incubator at 37°C to allow the color to develop, and 25 μl of 1 N H2SO4 was added to stop further development. Both the optical density at 450 nm (OD450) and the OD630 in each well were measured with a microplate reader for the test and reference wavelengths. The net change (net OD450 − OD630) for each well was calculated by the following equation: net OD450 − OD630 = [(OD450 − OD630 of test well) − (OD450 − OD630 of TNF-α-treated control)]. A standard curve was generated with recombinant human TNF-α (R&D Systems Inc., Minneapolis, Minn.). All of the experimental data were expressed as the 50% cytotoxicity value based on the standard curve. To verify the specificity of TNF in the culture supernatants, some of the supernatant samples were incubated with rabbit anti-guinea pig TNF-α polyclonal antiserum or normal rabbit serum at room temperature for 30 min prior to addition to the L929 cells.
Cell viability.
Culture conditions representing each cell population and stimulus were duplicated in a series of 96-well plates in ∼150-μl volumes. At 24, 48, and 72 h, 25 μl of a 5-mg/ml solution of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide was added to triplicate wells. The plates were then incubated at 37°C for 4 h in a 5% CO2 atmosphere. Insoluble formazan crystals were solubilized with lysis buffer (20% sodium dodecyl sulfate and 50% 2,2-dimethylformamide in distilled water [pH 4.7]), and the absorbance of the converted dye was measured at a wavelength of 570 nm with a microplate reader. The results obtained from this assay revealed that the cell viability did not vary with vaccination status or in vitro stimulus and was not significantly different from the cell viability of negative controls at each of the indicated time points (data not shown).
Statistical analysis.
Differences between groups were compared by Student's one-tailed t test, assuming equal variances. P values of <0.05 were considered significant.
RESULTS
Cellular composition of distinct guinea pig leukocyte populations.
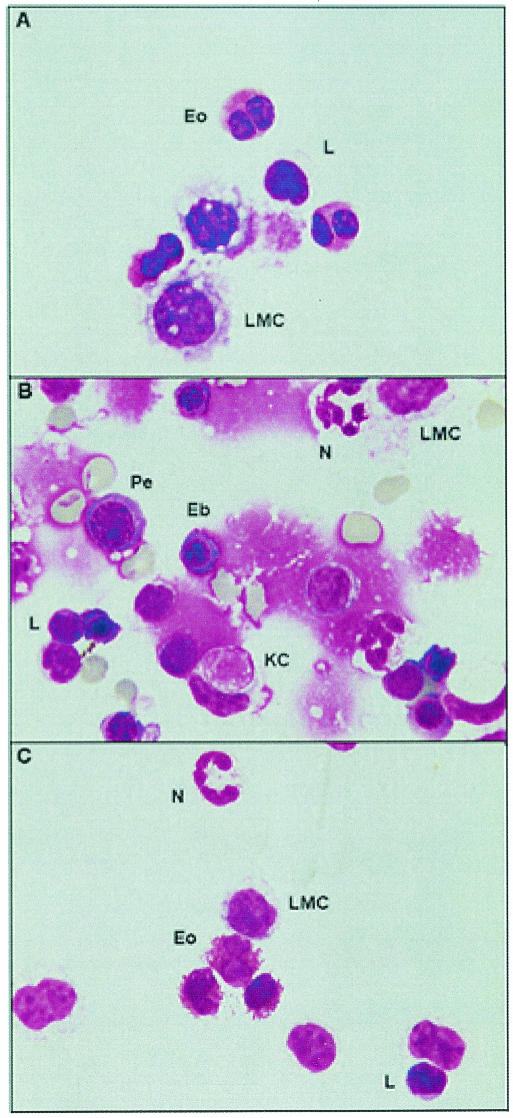
Table 2 shows guinea pig leukocytes from each distinct subpopulation as identified by cell morphology and counted with a light microscope under oil immersion (magnification, ×100). For the BAL cells and resident PC, the large mononuclear cells (LMC) were differentiated from the lymphocytes on the basis of size, nuclear morphology, and total cytoplasmic area. This contrast can be seen in Fig. 1A and C, where the large mononuclear cells show a greater cytoplasmic area than and typically have an irregularly shaped cell membrane in comparison to smaller lymphocytes. In addition, guinea pig neutrophils (sometimes referred to as pseudoeosinophils due to their eosinophilic granules present in the cytoplasm [24]) can be distinguished from eosinophils by the characteristic hypersegmented nucleus of the neutrophil and punctate eosinophilic granules. In contrast, the guinea pig eosinophil contains a round-to-bilobed nucleus and large globular, densely packed, eosinophilic granules that fill the entire cytoplasm.
TABLE 2.
Cell differential counts for guinea pig BAL cells, PC, and SPC
| Vaccination status | Cell subtypea | Mean % ± SEM in the following guinea pig cell subpopulationb:
|
||
|---|---|---|---|---|
| BAL cells | PC | SPC | ||
| Unvaccinated | LMC | 57.8 ± 3.3 | 61.9 ± 9.1 | 11.3 ± 2.1 |
| Lymphocytes | 2.60 ± 0.7 | 3.10 ± 0.9 | 54.3 ± 1.7 | |
| Eosinophils | 39.0 ± 4.4 | 33.4 ± 7.7 | 3.70 ± 0.5 | |
| Neutrophils | 0.60 ± 0.1 | 1.60 ± 0.7 | 17.9 ± 2.5 | |
| Kurloff cells | 0.00 ± 0.0 | 0.00 ± 0.0 | 12.8 ± 3.1 | |
| BCG vaccinated | LMC | 49.5 ± 3.1 | 60.3 ± 1.1 | 9.80 ± 1.6 |
| Lymphocytes | 3.60 ± 1.3 | 4.00 ± 1.5 | 52.7 ± 5.5 | |
| Eosinophils | 45.5 ± 2.0 | 34.6 ± 0.9 | 4.10 ± 0.6 | |
| Neutrophils | 1.40 ± 0.5 | 1.10 ± 0.4 | 18.6 ± 4.9 | |
| Kurloff cells | 0.00 ± 0.0 | 0.00 ± 0.0 | 14.8 ± 1.2 | |
Cellular differentials were determined by morphology.
Cytospins were performed on BAL cells, PC, and SPC as described in Materials and Methods. One slide was prepared for each cell population per guinea pig per treatment group (n = 4); 250 cells per slide were enumerated, and erythrocyte contamination for BAL cell and PC preparations was consistently <5% for this study (lysed cells were not counted).
FIG. 1.
Morphology of the cell subtypes identified in BAL cells (A), SPC (B), and PC (C). A total of 7 × 104 cells from each subpopulation were mounted onto silanated slides by means of a cytospin. After brief air drying, the slides were fixed and stained with Dif-Quik, and a coverslip was applied with mounting medium. Representative photographs depict individual cell types as determined by morphology. Eo, eosinophil; L, lymphocyte; N, neutrophil; KC, Kurloff cell; Eb, erythroblast; Pe, proerythrocyte (Pe). Magnifications, ×100 (A and C) and ×40 (B).
The criteria used to distinguish between cell subtypes from the spleen were the same as for BAL cells and PC; however, additional cell subtypes, greater artifacts from the isolation process, and poor viability (Table 1) complicated the analysis. Briefly, while the red blood cells, as well as hematopoetic cells (i.e., erythroblasts, and proerythrocytes) (Fig. 1B), were not included as part of the analysis, a unique mononuclear cell of the guinea pig, the Kurloff cell, which has been reported to possess natural killer cell activity (9), was included as part of the differential counts and could be distinguished by its characteristic intracytoplasmic inclusion body, which resembles a phagocytized erythrocyte.
The cellular differential counts are summarized in Table 2. Both BAL cells and PC yielded very similar cellular constituents with modest differences in the ratio of specific cell subsets between vaccination groups, but no significant differences in the absolute number of cell subtypes were found. These differential counts revealed that the resident LMC and eosinophils made up approximately 95% of the lavaged leukocytes, with neutrophils and lymphocytes comprising the remaining 5%. Among the cells isolated from the spleen, the lymphocyte counts increased dramatically from the lavage counts, as did the numbers of neutrophils. In contrast, few LMC and eosinophils were associated with this tissue, while Kurloff cells comprised approximately 14% of the enumerated splenocytes. In addition, as with the BAL cells and PC, little effect of vaccination was observed.
Antigen-specific proliferation in BAL cells, SPC, and PC.
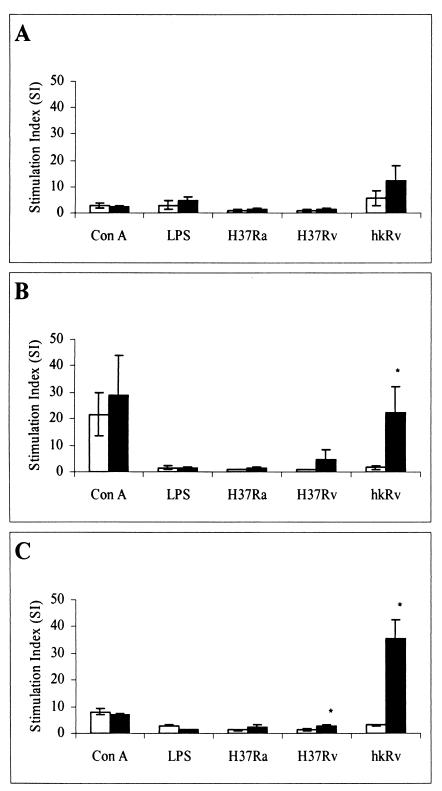
The capacity of the guinea pig leukocyte-rich BAL cell, SPC, and PC populations to proliferate in response to both mitogenic and mycobacterium-specific stimuli was evaluated. As shown in Fig. 2, mitogenic responses were high among the various cell populations, particularly in splenocytes. While ConA-induced proliferation was the most dramatic (SI of 3 to 29), LPS also induced measurable proliferation, but only in the in the BAL cell population (SI of 3 to 5). If an SI of ≥3 is used, the responses to these defined stimuli were independent of the vaccination status. Background counts were uniformly low and were not different between treatments.
FIG. 2.
Proliferative responses of leukocyte-rich guinea pig cell populations. BAL cells (A), SPC (B), and PC (C) from both unvaccinated (open bars) and BCG-vaccinated (closed bars) guinea pigs were prepared as described in the text and incubated for 4 days in the presence of mitogenic stimuli (ConA or LPS) or mycobacterium-specific stimuli, including a live attenuated (H37Ra) or virulent (H37Rv) strain of M. tuberculosis at an infectivity ratio of 1:100 or hkRv. The SI is defined as the ratio of counts per minute of tritiated thymidine taken up by stimulated cells to counts per minute of unstimulated cells from the same source. Results are given as the means ± standard errors of the means for four animals per group. Differences between the vaccination groups were compared by Student's t test, assuming equal variances. P values of <0.05 (*) were considered significant.
In addition, antigen-specific responses in each distinct cell population were assessed by pulsing the cell cultures with either live attenuated M. tuberculosis (H37Ra) or virulent M. tuberculosis (H37Rv). In either case, stimulation with live mycobacteria (with the exception of H37Rv-stimulated SPC [SI = 4.5]) failed to induce proliferation above that for unstimulated controls. In contrast, heat treatment of H37Rv clearly resulted in the exposure of H37Rv antigens that greatly enhanced lymphocyte proliferation in comparison to stimulation with live mycobacteria among each of the cell populations. Furthermore, enhanced proliferative responses were observed from both SPC (P < 0.032) and PC (P < 0.002) derived from BCG-vaccinated guinea pigs.
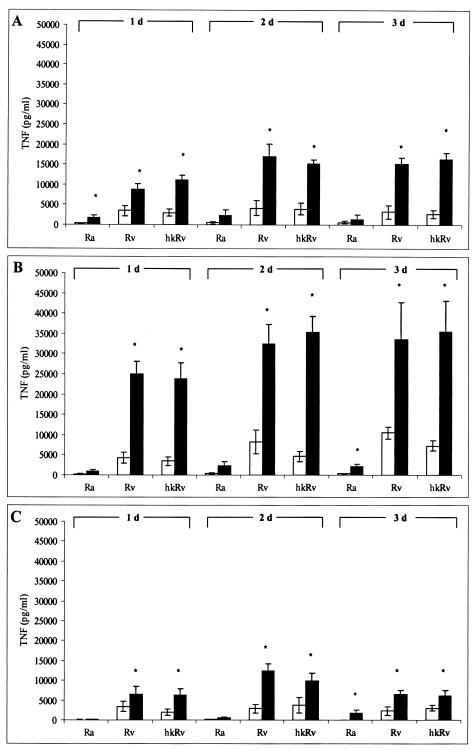
Effect of BCG vaccination on bioactive TNF-α protein production from differentially stimulated guinea pig cell populations.
BAL cells, SPC, and PC were obtained from BCG-vaccinated and unvaccinated guinea pigs and stimulated with live M. tuberculosis H37Ra or H37Rv or hkRv for 1, 2, and 3 days. Supernatants were collected and tested by L929 bioassay for levels of bioactive TNF protein. As revealed in Fig. 3, very clear differences were seen between BCG-vaccinated and nonvaccinated guinea pigs in response to mycobacterial stimuli. These differences were highly significant among each cell population studied. SPC-induced TNF protein was the most dramatic with respect to absolute TNF production, which remained relatively constant throughout the time interval (1 to 3 days) studied. Interestingly, virulent M. tuberculosis H37Rv-infected SPC consistently produced significantly more bioactive TNF than did the attenuated H37Ra strain. In addition, BAL cells and PC demonstrated trends analogous to those for the SPC with respect to vaccination status and the positive correlation observed between TNF production and mycobacterial strain virulence. In addition, vaccination status did not affect LPS-induced TNF-α production from any of the cell populations (data not shown). Thus, the responses of the cell populations from BCG-vaccinated guinea pigs examined in this study can be described as an antigen-specific phenomenon. Polyclonal antisera raised against our recombinant guinea pig TNF-α completely eliminated the bioactivity of selected samples, thus verifying that the supernatants contained bioactive TNF-α specifically (data not shown).
FIG. 3.
Total bioactive TNF-α production from BAL cells, SPC, and PC and effect of BCG vaccination. BAL cells (A), SPC (B), and PC (C) from both vaccinated (closed bars) and unvaccinated (open bars) guinea pigs were cultured with either live or heat-killed mycobacteria for 1, 2, and 3 days. At the indicated time points, supernatants were collected and tested for levels of bioactive TNF by the L929 bioassay. Results are means ± standard errors of the means for three animals per treatment group. Differences between vaccination treatment groups were compared by Student's t test, assuming equal variances. P values of <0.05 (*) were considered significant.
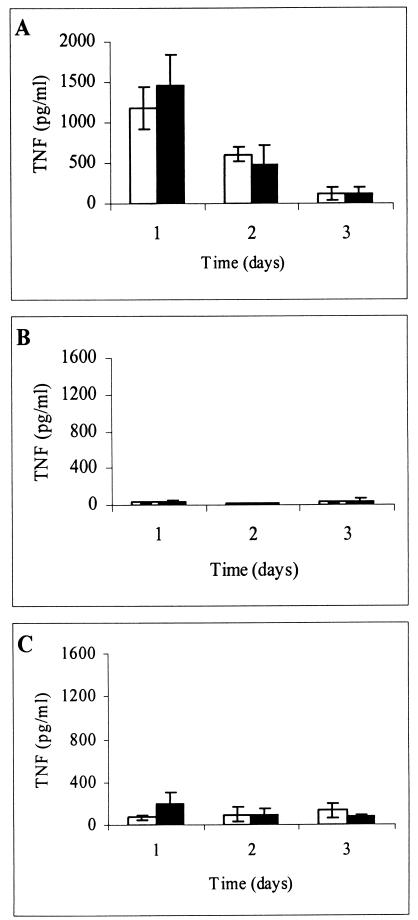
Spontaneous production of TNF by BAL cells, SPC, and PC.
It is conceivable that upon plating each cell population, cellular adherence to the culture wells could have induced background activation in the negative controls which may have interfered with the analysis. To test this hypothesis, each cell population was plated into 48-well plates in RPMI complete medium alone. Supernatants were obtained at 24, 48, and 72 h after plating and subsequently tested by the L929 bioassay for total TNF production. As Fig. 4 indicates, the levels of spontaneous TNF production were most dramatic in BAL cells but could also be observed in PC. These levels of TNF release from unstimulated BAL cells and PC were approximately 81-and 3-fold higher at 24 h, respectively, than SPC spontaneous TNF production. The heightened spontaneous production of TNF-α from the BAL cell population is most likely due to this compartment being the most exposed to LPS in environment. Alternatively, LPS contamination could have occurred during the tracheal manipulations in our harvest procedure. However, no statistically significant differences were seen between the treatment groups with respect to spontaneous TNF production in any of the cell populations.
FIG. 4.
Spontaneous bioactive TNF production from BAL cells, SPC, and PC. BAL cells (A), SPC (B), and PC (C) from both vaccinated (closed bars) and unvaccinated (open bars) guinea pigs were cultured in medium alone for the indicated time intervals. At the indicated time points, supernatants were collected and tested for levels of total bioactive TNF. The limit of detection for this assay was routinely 40 pg/ml; consequently, 20 pg/ml was used for determining the averages when results fell below the threshold of detection. Results are means ± standard errors of the means for four animals per treatment group.
DISCUSSION
An essential focus in our laboratory includes the characterization of immunological indicators of vaccine-induced protective immunity in experimental TB. One of the strategies employed to study these immunological indicators (“correlates of protection”) has been to compare the responses of immunologically naive (unvaccinated) guinea pigs to those of animals in which the degree of TB resistance (to low-dose aerosol challenge with M. tuberculosis) has been elevated by prior vaccination with BCG (21). In these studies, the underlying research hypothesis is that the ability of resident guinea pig leukocyte-rich populations to proliferate and produce the proinflammatory cytokine TNF-α correlates with antigen-specific, protective immune responses.
Nearly all expressions of antigen-specific lymphocyte activation, including in vivo or in vitro proliferation, delayed hypersensitivity, and cytokine production, require antigen presentation by an antigen-presenting cell (APC), such as the macrophage. For this reason, BAL cells, SPC, and PC were examined as a heterogeneous population of leukocytes containing both lymphocytes and APCs (Table 2). Blastogenic responses to nonliving as well as viable preparations of mycobacteria have been a reliable standard for predicting the relative success of host resistance to mycobacterial infection (25). Our lab has previously demonstrated that SPC, bronchotracheal lymph node cells, and peripheral blood lymphocytes obtained from aerogenically challenged guinea pigs proliferate in response to protein antigens of M. tuberculosis (purified protein derivative); furthermore, this blastogenic response correlates with the vaccination status of the host (3, 4). That is, prior vaccination with BCG sensitizes the guinea pigs to mount brisk in vitro lymphocyte proliferation in comparison to their unvaccinated counterparts, especially at the critical early stages (1 to 3 weeks). Likewise, in human TB patients, evidence demonstrates a reciprocal relationship between lymphocyte proliferation and disease severity (31). The results obtained from SPC and PC proliferation (Fig. 2) in response to hkRv agree with these previous studies, as there were clear differences between the vaccination treatment groups. Infection with live mycobacteria (H37Ra or H37Rv) routinely did not induce cellular proliferation greater than an SI of 2. These comparatively low indices induced by viable mycobacteria are most likely related to the low bacterium/cell ratio (MOI) employed in these studies (0.01) rather than to the inability of whole mycobacteria to effectproliferation from these cell populations. Previous studies have shown that both heat-killed and viable mycobacteria can induce dramatic cellular proliferation from human tuberculin-positive peripheral blood mononuclear cells; however, most of those studies were carried out with bacterium/cell ratios of ≥1:1 (27, 33), which are lethal to guinea pig cells over short-term culture (2 to 3 days) (data not shown) and therefore, were not attempted in this study. In addition, it can be assumed that alveolar macrophages most likely comprise an overwhelming majority of the LMC of the BAL cells. Given the propensity of this cell type for the suppression of T-cell proliferation (22, 38), it was not surprising that BAL cell proliferation showed a depressed SI when stimulated with ConA and hkRv. Although the T-cell mitogen ConA induced measurable proliferation (SI of ≥3) from SPC and PC, LPS (which is mitogenic for both murine B (26) and human T (34) lymphocytes failed to induce measurable proliferation except in the case of BAL cells derived from BCG-vaccinated guinea pigs (SI = 4.8). It is unclear to us why LPS did not induce a greater proliferative index, but this may be attributed to the comparatively low dose of LPS used in this study, the low frequency (<1:1,000) of lymphocytes responding to this agonist, or quite possibly the absence of the appropriate APCs (34).
In this paper, we demonstrate for the first time that various leukocyte populations harvested from BCG-vaccinated guinea pigs and stimulated in vitro with both live and heat-killed mycobacteria produce significantly higher levels of bioactive TNF-α than identical leukocyte-rich populations obtained from unvaccinated guinea pigs (Fig. 3). We speculate that mycobacterium-driven TNF-α production occurs both as a result of direct stimulation of APCs and through subsequent activation of memory T cells in BCG-vaccinated guinea pigs via T-cell receptor engagement of cognate antigen. We further hypothesize that this direct correlation of vaccination status with the ability to produce bioactive TNF-α is related to the capacity of sensitized T cells specifically present in cell cultures derived from BCG-vaccinated guinea pigs to engage APCs. In driving this process, APCs display mycobacterial peptides in the context of either the major histocompatibility complex class I and II or antigen-presenting molecules of the CD1 family (23). Although these results may, in fact, be explained by BCG-induced alterations in intrinsic accessory cell functions, no differences could be detected between the vaccination groups (TNF expression in response to LPS), which argues that this is most likely not the case.
Controversy exists over whether attenuated or virulent mycobacteria induce higher levels of TNF-α. Some studies have demonstrated that attenuated strains of mycobacteria induce higher TNF-α production in human macrophages (7, 17), while other reports show that the opposite may be true (29, 32). In either case, TNF-α induction in primary macrophages appears to be highly dependent on the experimental protocol and the origin of cells and bacterial strains under investigation. Our findings that TNF-α production positively correlates with the virulence of the mycobacterial strain employed demonstrates that, in guinea pigs, virulent mycobacteria may not effect a broad immunological “silence” implicated from previous studies in our laboratory (15, 16, 19). Instead, it is conceivable that M. tuberculosis H37Rv elevates host phagocyte TNF-α levels to facilitate its own survival by interfering with the production of IL-12 (20) and possibly increasing its availability to iron (10).
In conclusion, these studies strongly indicate that BCG vaccination enhances both proliferative and TNF-α responses in guinea pig leukocyte populations. These immunological measurements could potentially lead to the development and standardization of these techniques as a method of quantitatively predicting the level of resistance induced by a novel vaccine. It is unclear at this point in our studies whether the production of TNF-α serves to promote TB pathogenesis or to protect the host. Accordingly, studies are currently under way to determine the involvement of TNF-α production in M. tuberculosis growth in guinea pig macrophages, as well as the contribution of TNF-α to host defense and/or disease pathogenesis in aerosol-infected guinea pigs.
Acknowledgments
This work was supported by National Institutes of Health grant RO1 AI 15495 to D.N.M.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Aggarwal, B. B., and K. Natarajan. 1996. Tumor necrosis factors: developments during the last decade. Eur. Cytokine Netw. 2:93-124. [PubMed] [Google Scholar]
- 2.Allen, P. M., and E. R. Unanue. 1984. Differential requirements for antigen processing by macrophages for lysozyme-specific T cell hybridomas. J. Immunol. 132:1077-1079. [PubMed] [Google Scholar]
- 3.Bartow, R. A., and D. N. McMurray. 1997. Cellular and humoral responses to mycobacterial stress proteins in experimental pulmonary tuberculosis. Tub. Lung Dis. 78:185-193. [DOI] [PubMed] [Google Scholar]
- 4.Bartow, R. A., and D. N. McMurray. 1989. Vaccination with Mycobacterium bovis BCG affects the distribution of Fc receptor-bearing T lymphocytes in experimental pulmonary tuberculosis. Infect. Immun. 57:1374-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bean, A. G., D. R. Roach, H. Briscoe, M. P. France, H. Korner, J. D. Sedgwick, and W. J. Britton. 1999. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J. Immunol. 162:3504-3511. [PubMed] [Google Scholar]
- 6.Bekker, L. G., A. Moreira, A. Bergtold, S. Freeman, B. Ryffel, and G. Kaplan. 2000. Immunopathologic effects of tumor necrosis factor alpha in murine mycobacterial infection are dose dependent. Infect. Immun. 68:6954-6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beltan, E., L. Horgen, and N. Rastogi. 2000. Secretion of cytokines by human macrophages upon infection by pathogenic and non-pathogenic mycobacteria. Microb. Pathog. 28:313-320. [DOI] [PubMed] [Google Scholar]
- 8.Beutler, B., and A. Cerami. 1988. Tumor necrosis, cachexia, shock, and inflammation: a common mediator. Annu. Rev. Biochem. 57:505-518. [DOI] [PubMed] [Google Scholar]
- 9.Debout, C., A. M. Griveau, and J. Izard. 1991. The Kurloff cell in estrogenized guinea pigs as a CT7+ 8BE6− CT6− MR-1− CT10− IgM− lymphocyte with natural killer activity. Nat. Immun. Cell Growth Regul. 10:327-335. [PubMed] [Google Scholar]
- 10.Engele, M., E. Stossel, K. Castiglione, N. Schwerdtner, M. Wagner, P. Bolcskei, M. Rollinghoff, and S. Stenger. 2002. Induction of TNF in human alveolar macrophages as a potential evasion mechanism of virulent Mycobacterium tuberculosis. J. Immunol. 168:1328-1334. [DOI] [PubMed] [Google Scholar]
- 11.Espevik, T., and J. Nissen-Meyer. 1986. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J. Immunol. Methods 95:99-103. [DOI] [PubMed] [Google Scholar]
- 12.Flesch, I. E. A., and S. H. E. Kaufmann. 1990. Activation of tuberculostatic macrophage functions by gamma interferon, interleukin-4, and tumor necrosis factor. Infect. Immun. 58:2675-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flynn, J. L., M. M. Goldstein, J. Chan, K. J. Triebold, K. Pfeffer, C. J. Lowenstein, R. Schreiber, T. W. Mak, and B. R. Bloom. 1995. Tumor necrosis factor-α is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 2:561-572. [DOI] [PubMed] [Google Scholar]
- 14.Grover, A. A., H. K. Kim, E. H. Wiegeshaus, and D. W. Smith. 1967. Host-parasite relationships in experimental airborne tuberculosis. II. Reproducible infection by means of an inoculum preserved at −70°C. J. Bacteriol. 94:832-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeevan, A., T. Yoshimura, K. E. Lee, and D. N. McMurray. 2003. Differential expression of gamma interferon mRNA induced by attenuated and virulent Mycobacterium tuberculosis in guinea pig cells after Mycobacterium bovis BCG vaccination. Infect. Immun. 71:354-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeevan, A., T. Yoshimura, G. Foster, and D. N. McMurray. 2002. Effect of Mycobacterium bovis BCG vaccination on interleukin-1β and RANTES mRNA expression in guinea pig cells exposed to attenuated and virulent mycobacteria. Infect. Immun. 70:1245-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keane, J., M. K. Balcewicz-Sablinska, H. G. Remold, G. L. Chupp, B. B. Meek, M. J. Fenton, and H. Kornfeld. 1997. Infection by Mycobacterium tuberculosis promotes human alveolar macrophage apoptosis. Infect. Immun. 65:298-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kindler, V., A. Sappino, G. E. Grau, P. Piguet, and P. Vassalli. 1989. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell 56:731-740. [DOI] [PubMed] [Google Scholar]
- 19.Lyons, M. J., T. Yoshimura, and D. N. McMurray. 2002. Differential expression of interleukin (IL)-8 (CXCL8) and monocyte chemoattractant protein (MCP-1) in guinea pig alveolar macrophages infected with Mycobacterium tuberculosis. Infect. Immun. 70:5471-5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma, X. 2001. TNF-α and IL-12: a balancing act in macrophage functioning. Microb. Infect. 3:121-129. [DOI] [PubMed] [Google Scholar]
- 21.McMurray, D. N. 1994. Guinea pig model of pulmonary tuberculosis, p. 135-147. In B. R. Bloom (ed.), Tuberculosis: pathogenesis, protection, and control. American Society for Microbiology, Washington, D.C.
- 22.Metzger, Z., J. T. Hoffeld, and J. J. Oppenheim. 1980. Macrophage-mediated suppression. I. Evidence for participation of both hydrogen peroxide and prostaglandins in suppression of murine lymphocyte proliferation. J. Immunol. 124:983-988. [PubMed] [Google Scholar]
- 23.Moody, D. B., and G. S. Besra. 2001. Glycolipid targets of CD1-mediated T-cell responses. Immunology 104:243-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore, D. M. 2000. Hematology of the guinea pig, p. 1107-1115. In C. Feldman, M. Zinkl, and J. Jain (ed.), Schalm's veterinary hematology. Lippincott, Williams, and Wilkins, Baltimore, Md.
- 25.Orme, I. M., P. Anderson, and W. H. Boom. 1993. T cell response to Mycobacterium tuberculosis. J. Infect. Dis. 167:1481-1497. [DOI] [PubMed] [Google Scholar]
- 26.Peavy, D. L., W. H. Adler, and R. T. Smith. 1970. The mitogenic effects of endotoxin and staphylococcal enterotoxin B on mouse spleen cells and human peripheral lymphocytes. J. Immunol. 105:1453-1458. [PubMed] [Google Scholar]
- 27.Rojas, R. E., K. N. Balaji, A. Subramanian, and W. H. Bloom. 1999. Regulation of human CD4+ αβ T-cell-receptor-positive (TCR+) and γδ TCR+ T-cell responses to Mycobacterium tuberculosis by interleukin-10 and transforming growth factor β. Infect. Immun. 67:6461-6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rook, G. A., J. Taverne, C. Leveton, and J. Steele. 1987. The role of γ-interferon vitamin D3 metabolites and tumor necrosis factor in the pathogenesis of tuberculosis. Immunology 62:229-235. [PMC free article] [PubMed] [Google Scholar]
- 29.Silver, R. F., Q. Li, and J. J. Ellner. 1998. Expression of virulence of Mycobacterium tuberculosis within human monocytes: virulence correlated with intracellular growth and induction of tumor necrosis factor α but not with evasion of lymphocyte-dependent monocyte effector functions. Infect. Immun. 66:1190-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith, D. W., D. N. McMurray, E. H. Wiegeshaus, A. A. Grover, and G. E. Harding. 1970. Host-parasite relationships in experimental airborne tuberculosis. IV. Early events in the course of infection in vaccinated and nonvaccinated guinea pigs. Am. Rev. Respir. Dis. 102:937-949. [DOI] [PubMed] [Google Scholar]
- 31.Toossi, Z., M. E. Kleinherz, and J. J. Ellner. 1986. Defective IL-2 production and responsiveness in human tuberculosis. J. Exp. Med. 163:1162-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tracey, K. J., and A. Cerami. 1992. Tumor necrosis factor and regulation of metabolism in infection: role of systemic versus tissue levels. Exp. Biol. Med. 200:233-239. [DOI] [PubMed] [Google Scholar]
- 33.Tsukaguchi, K., K. N. Balaji, and W. H. Boom. 1995. CD4+ αβ T-cell and γδ T-cell responses to Mycobacterium tuberculosis. J. Immunol. 154:1786-1796. [PubMed] [Google Scholar]
- 34.Ulmer, A. J., H. D. Flad, T. Rietschel, and T. Mattern. 2000. Induction of proliferation and cytokine production in human T lymphocytes by lipopolysaccharide (LPS). Toxicology 152:37-45. [DOI] [PubMed] [Google Scholar]
- 35.Vanham, G., Z. Toossi, C. S. Hirsch, R. S. Wallis, S. K. Schwander, E. A. Rich, and J. J. Ellner. 1997. Examining a paradox in the pathogenesis of human pulmonary tuberculosis: immune activation and suppression/anergy. Tub. Lung Dis. 78:145-149. [DOI] [PubMed] [Google Scholar]
- 36.Wallis, R. S., and J. J. Ellner. 1994. Cytokines and tuberculosis. J. Leukoc. Biol. 55:676-681. [DOI] [PubMed] [Google Scholar]
- 37.White, A. M., T. Yoshimura, A. W. Smith, J. Westwick, and M. L. Watson. 1997. Airway inflammation induced by recombinant guinea pig tumor necrosis factor-α. Am. J. Physiol. 273:L524-L530. [DOI] [PubMed] [Google Scholar]
- 38.Zhang, X., and D. N. McMurray. 1998. Suppression of lymphoproliferation by alveolar macrophages in the guinea pig. Tub. Lung Dis. 79:119-126. [DOI] [PubMed] [Google Scholar]
- 39.Ziegler, K., and E. R. Unanue. 1981. Identification of macrophage antigen-processing event required for I-region-restricted antigen presentation to T lymphocytes. J. Immunol. 127:1869-1875. [PubMed] [Google Scholar]