Abstract
The reason(s) why individual cytotoxic T lymphocytes (CTL) possess a fast-acting, perforin/granzyme-mediated, as well as a much slower, Fas ligand (FasL) -driven killing mechanism is not clear, nor is the basis for wide variations in killing activity exhibited by individual CTL, ranging from minutes to hours. We show that perforin expression among individual, conjugated CTL varies widely, which can account for the heterogeneity in killing speeds exhibited by individual CTL. Despite a 2-hr lag in FasL-based killing, CTL lytic action is enhanced when the two mechanisms operate in concert. This is explained by finding that the two pathways in fact are jump-started simultaneously with the lag in FasL lytic action reflecting pre-lytic caspase-8 activation and BH3-interacting domain (BID) cleavage. The complementary action of the two lytic pathways, co-expressed at varying levels among individual CTL, facilitates the lytic action of late-stage poor perforin-expressing CTL, ensuring optimal cytocidal action throughout the CTL response.
Keywords: allotransplantation, apoptosis, CD8/cytotoxic T cells, granzymes/perforin
Introduction
Two major cytotoxic T-lymphocyte (CTL) killing mechanisms: one fast-acting, perforin-mediated and one slower, Fas ligand (FasL) -based, account for most CTL activity in short-term in vitro assays.1–3 The fast-acting mechanism is believed to involve secreted perforin, which forms ‘pores’ in the target membrane through which co-secreted granzymes somehow enter cells and activate a cascade of caspases including caspase-3,4 caspase-8 or BH3-interacting domain (BID),5,6 The slower-acting mechanism involves cross-linking of the cell surface death receptor Fas expressed on target cells induced by cell surface FasL expressed on CTL. Cross-linked Fas rapidly induce assembly of an intracellular ‘death-inducing signalling complex’ (DISC),7 recruitment and activation of caspase-8.8 Upon activation, caspase-8 leaves the DISC to cleave BID; translocation of BID to mitochondria releases cytochrome c which activates caspase-9, which in turn activates caspase-3,9 that serves as a common substrate for the two killing mechanisms.
Both mechanisms are believed to be co-expressed and deployed by individual CTL.10–14 Yet a gradual switch in effector CTL from mostly perforin-based to largely FasL-based killers has been reported.3 Related to this is a long-standing open question in CTL biology as to why do CTL co-possess a slow-killing mechanism, which is FasL-based, in addition to the faster, perforin-mediated one, because the latter would make the former redundant. Moreover, symmetric expression and deployment of both mechanisms by individual CTL does not account for the finding of both fast- and slow-acting killers within CTL populations reported previously,15–19 unless perforin (and perhaps FasL) expression in individual CD8 CTL varies widely and the killing strategies employed are complementary, which we aim to investigate.
Materials and methods
Animals and cells
Eight- to ten-week-old C57BL/6 mice and perforin knockout (PKO) mice (both on H-2b background) were supplied by the Animal Breeding Centre of the Weizmann Institute and their use was approved by the Institute's animal use committee. Leukaemia L1210 of DBA/2 (H-2d) was cultured in vitro. LF+ and LF− sub-lines were L1210 variants stably transfected with a Fas over-expressing construct,1 and Fas anti-sense expression vector,20 LF+ and LF−, respectively (cell surface Fas expression on these cells was 85 ± 5% and 25 ± 5%, respectively, Fig. 2). Cells were cultured in RPMI-1640 medium supplemented with heat-inactivated fetal calf serum (10%), glutamine (2 mm), non-essential amino acids (1%), penicillin 100 U/ml, streptomycin 100 μg/ml, β-mercaptoethanol (5 × 10−5 m) and sodium pyruvate (1 mm).
Figure 2.
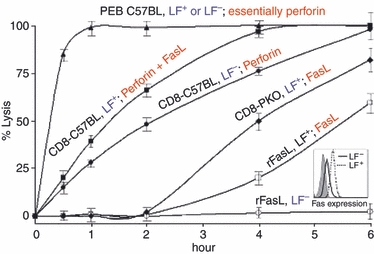
Collaboration between two cytotoxic T lymphocyte (CTL) killing mechanisms: Perforin and Fas ligand (FasL). The CTL were high perforin-expressing C57BL/6 anti-LF peritoneal exudate lymphocyte (PEL) -blasts, CD8+ C57BL/6 anti-LF PEL and CD8+ perforin knockout (PKO) anti-LF PEL, all subjected to a 6-hr cytotoxic assay against LF+ and LF− cells. Strictly Fas/FasL-based killing was induced by soluble recombinant FasL trimmers against LF+ and LF−. Effector cells, black, target cells, blue, and killing mechanisms in red. Mean ± SD of three repeat experiments, each with cells procured from three animals. Cytotoxicity was determined at 0, 1, 2, 4 and 6 hr. Fas expression on LF− and LF+ cells is shown in insert.
In vivo-primed peritoneal exudates CTL (PEL) and PEL-blasts
C57BL/6 and PK0 (H-2b) mice were primed intraperitoneally with allogeneic LF+ tumour cells (25 × 106 per mouse). Peritoneal exudate CD8 CTL (PEL CTL) were extracted and purified as previously described.21 Briefly, 8–10 days after injection, mice were killed, peritoneal cavity cells were collected, purified on nylon wool columns and PEL were sorted by FACS (Fig. 1d). The PEL-blasts were generated by culturing C57BL6 anti-LF+ PEL in RPMI-1640 containing 10 mm HEPES, 10% heat-inactivated newborn calf serum (NCS), 2 mm glutamine, 0·1% combined antibiotics, β-mercaptoethanol (5 × 10−5 m), and supplemented with recombinant interleukin-2 (rIL-2) 500 U/ml, at 37° in a 5% CO2 atmosphere as described before.22
Figure 1.
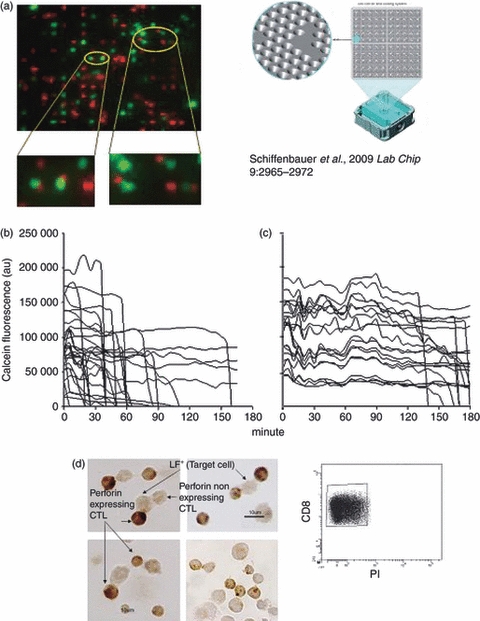
Killing rate and perforin-expression by individual, conjugated cytotoxic T lymphocytes (CTL). (a) CKChip loaded with conjugated as well as non-conjugated cells. Conjugates (encircled yellow) were made with day 8 Cell Tracker Orange-labelled anti-LF+ peritoneal exudate lymphocytes (PEL) and calcein-labelled LF+ cells (red small cells and green fluorescent cells, respectively). Only one-to-one CTL-LF+ pairs (e.g. encircled yellow) were analysed. (b) Killing rates of 35 individual, conjugated target cells induced by perforin+/+ C57BL/6 PEL-CTL and (c) by perforin−/− PKO PEL-CTL were recorded by measuring the decrease in calcein fluorescence intensity over time (fluorescence of non-conjugated LF+ cells imaged in parallel served as internal control). (d) Perforin expression in C57BL/6 anti-LF+ CD8+ PEL (small cells, 7 μm in diameter) conjugated with LF+ cells (larger size cells, 12–15 μm). FACS analysis of sorted CD8+ propidium iodide (PI) -negative population, indicated that only small live cells were employed.
Assessment of cytolytic activity
A 51Cr-release assay was used. Target cells were labelled with 100–200 μCi Na251Cr2O4 (1·5 hr at 37°) and washed three times with cold PBS-NCS (5%). Lytic assays were conducted in U-shaped, 96-well microtitre plates with 105 labelled target cells per well. PEL (pooled from three or four mice per experiment) were added at a 10 : 1 ratio. The plates were centrifuged (200 g, 2 min at room temperature) to promote conjugate formation,23 and incubated at 37° for the times indicated. To terminate the lytic assay, plates were re-centrifuged (700 g, 10 min at 4°) and 100 μl of supernatant from each well was harvested and its radioactivity was determined in a gamma-counter. Cytotoxicity was calculated as follows:
Total release was the amount of radioactivity released by 1 m HCl; spontaneous release was usually below 10%.
Single CTL kinetics measured on a cell array (CKChip)
We employed a novel cell array (CKChip) which enables continuous, fluorescence-based imaging of thousands of individual or conjugated living cells, each held at a given, coded micro-well (‘address’) on the chip24 (Fig. 1). Imaging of cell fluorescence was performed on a Delta Vision RT Olympus 1x71 system (Applied Precision, Issaquah, WA; WoRX software) with a Photometric Cool Snap HQ digital camera equipped with a Nikon DS-Fi1 Digital camera (Nikon Instruments, Melville, NY). The PEL were stained with Cell Tracker Orange (Molecular Probes, Carlsbad, CA), 1–2 μm; target cells were labelled with calcein (Molecular Probes), 1–2 μm. Conjugates were formed as described previously25 by mixing 106 PEL with an equal number of LF+ or LF− target cells suspended in 1 ml PBS–NCS (5%). The suspension was allowed to stand for 10 min at room temperature and then was centrifuged at 200 g for 10 min at this temperature to promote conjugate formation. The cells were then re-suspended vigorously, and loaded on the CKChip. Image analysis was performed using wells software, a dedicated software package designed to automatically quantify the fluorescence emanating from numerous individual cells arranged on the cell chip over time.24
Flow cytometry and cell sorting
Cells were suspended in diluted monoclonal antibody in PBS containing 1% BSA. Goat anti-mouse IgG (Jackson-Immune Research Laboratories Inc, West Grove, PA) was used to prevent non-specific labelling. The following monoclonal antibodies were employed for flow cytometry and cell sorting: allophycocyanin-Cy7-conjugated anti-CD8a/lyt-2 (Biolegend, San Diego, CA), anti-FITC-conjugated anti-mouse CD95/Fas (Pharmingen Inc., San Diego, CA) and propidium iodide (Sigma, Rehovot, Israel) staining was used to exclude dead cells. FACS analysis was performed using a FACScan (Becton Dickinson, San Jose, CA); cell sorting was carried out with an Aria FACS (Becton Dickinson) using cell quest software (Becton Dickinson).
Western blotting
Cells lysates were obtained by incubating 2 × 106 cells in RIPA buffer containing 1% PMSF, a protease inhibitor, at room temperature for 20 min. Twenty micrograms of extracted protein was electrophoresed on a 10% SDS–PAGE gel and then transferred to a nitrocellulose membrane (Schleicher & Schuell Bioscience, Inc., Keene, NH). The resulting protein blot was blocked with goat anti-mouse IgG (Jackson-Immune Research). Caspase-8, BID and truncated BID (tBID) were detected using rat anti-mouse caspase-8 (clone 3B10),26 and anti-BID, which detected BID as well as tBID27,28 (obtained from A. Gross, Weizmann Institute, Rehovot). Blots were developed using a SuperSignal West pico chemiluminescence substrate (Thermo Fisher Scientific Inc, Rockford, IL) and exposed to superRX film (FUJIFILM Global, Tirat Carmel, Israel).
Immunoperoxidase staining of intracellular perforin–immune cytochemistry
Conjugates (105) made of sorted CD8+ CTL and LF+ cells in PBS were placed on poly-l-lysine-treated glass slides and left to adhere for 1 hr at 4°. The slides were then washed in PBS, fixed in 4% paraformaldehyde for 10 min at room temperature and washed in PBS. Cells were permeabilized by 0·2% Triton-X for 10 min at room temperature, washed in Tris–HCl, 50 mm, pH 7·6, and denatured with 0·5% HIO4 for 10 min at room temperature as described elsewhere.29 The slides were then washed in Tris–HCl, quenched with 0·3% H2O2 for 15 min at room temperature, and re-washed with Tris–HCl. Peroxide staining was performed with an ABC Staining System kit (Santa Cruz Biotechnology, Delaware Avenue, Santa Cruz, CA) in conjunction with the P1-8 perforin antibody (obtained from K. Okumura and H. Yagita, Juntendo University, Tokyo). Cover glasses were mounted with Entellan. Perforin (protein) expression was evaluated on a Nikon E800 light microscope.
Results
Variations in killing rates and perforin expression among individual, conjugated CTL
Earlier studies on individual alloreactive CTL–target conjugates have shown that nearly all (> 90%) conjugate-forming cells in the CTL system employed were specific killers.3,15,25 Yet for unknown reason(s) some one-to-one conjugates (i.e. a single CTL bound to one target cell) killed their prey quickly (within 5–20 min), others took up to 2–3 hr. Measuring the actual speed of individual cell lysis in a statistically meaningful number of CTL conjugates, however, has been difficult to pursue using conventional microscopy, requiring micromanipulation of isolated CTL–target cell conjugates.15 To this end, we have exploited a novel cytometric device, the CKChip,24 which allows continuous monitoring of the fate of a considerable number of individual CTL–target conjugates over time, hence providing the life and death history of multiple individual cells. Alloreactive CD8+ CTL were derived from the intra-peritoneal site of tumour allograft rejection (PEL).3,21 C57BL/6 anti-LF+ CTL and PKO anti-LF+ CTL were stained with the permeable red tracker Cell Tracker Orange; target LF+ cells were labelled green with the fluorescent cell viability dye calcein. Mixtures containing conjugated as well as free CTL and LF+ cells (high Fas expressing) were loaded on the CKChip, which was placed on an inverted microscope and monitored at 37° (Fig. 1a). The decay of cell-bound green (calcein) fluorescence in multiple conjugates comprising one CTL bound to a single target cell was recorded by imaging the fluorescence emanating from the CKChip and analysing the data using wells software. Fluorescence of non-conjugated LF+ cells imaged in parallel served as an internal control. The time–course of lytic events (loss of cell-bound fluorescence) in individual conjugated target cells bound each to a single CTL varied from minutes to hours with approximately 70% of conjugated target cells found dead after 90 min (Fig. 1b). Experiments with alloreactive PKO anti-LF+-CTL resulted in slower lysis (Fig. 1c). The pace of lysis measured in individual conjugates clearly differentiated the fast-acting, most likely perforin-mediated, from the slower-acting FasL-based CTL such as those derived from PKO mice (Fig. 1b,c), as well as a range of intermediate rates. Heterogeneity in perforin expression among individual conjugated CTL derived from perforin-expressing (P+/+) C57BL/6 mice could account for the variation in killing speed observed among individual CTL (Fig. 1b). In fact, using perforin immune cytochemistry (ICC), we found that perforin expression among individual conjugated CTL derived from such mice varied widely (Fig. 1d). Eighty to ninety per cent of day 8 conjugated CD8+ CTL expressed perforin,3 and wide variations in perforin content were evident among individual conjugated CTL (Fig. 1d). Some of the conjugated CTL expressed undetectable levels of perforin and were presumably the slower (FasL-based) killers (Fig. 1b), like those derived from PKO mice (Fig. 1c). Killing induced within minutes after conjugation was probably the result of the perforin pathway, as was the case with perforin-rich CTL such as IL-2-driven, PEL-blasts, described elsewhere22 (see Fig. 2). Intermediate rates of killing were most likely the result of CTL expressing reduced amounts of perforin, reflecting the collaborative action of the two CTL killing mechanisms perforin and FasL.
Collaboration between the two CTL killing mechanisms occurs while FasL lytic action is hidden
The activity of the FasL pathway acting alone was determined using CTL procured from alloimmunized PKO mice, as well as of soluble recombinant FasL. Strictly, perforin action was assessed by using low Fas-expressing cognate target cells (LF−). To evaluate the combined action of the two mechanisms, high Fas-expressing target cells (LF+) (Fig. 2, insert) were reacted with CTL from perforin-expressing C57BL/6 mice. We have also employed IL-2 driven PEL-blasts shown to be very high perforin-expressing CTL.3,22 Strictly FasL killing of LF+ cells was detected no sooner than 2 hr after conjugation with CTL, or soluble recombinant FasL. In contrast, perforin-based killing induced by PEL-blasts was immediate, complete within half an hour (Fig. 2) with either LF+ or LF− target cells. Although virtually no killing was induced by FasL CTL in the first 2 hr, a 25% ± 8 enhancement of killing was obvious when the two mechanisms operated in concert, time at which virtually no FasL-mediated lytic activity by PKO-CTL was evident. The effect of soluble recombinant FasL was inferior to that produced by PKO-CTL, possibly because of the enhanced efficacy of FasL action when expressed on the surface membrane of PKO-CTL.9 Combined, these experiments support a concerted action of the two mechanisms at least during the first 2 hr of CTL–target interaction. However, the strict 2-hr delay in FasL-based CTL action (Fig. 2) still constituted a stumbling block for conceiving cooperation between the two mechanisms, which was examined next.
Delayed FasL-based CTL action is jump-started shortly after conjugation
The 2-hr delay in FasL action, observed with either soluble, recombinant FasL or with surface membrane-bound FasL of PKO-CTL (Fig. 2), could be the result of a pre-lytic process(es) induced in the target cell. To this end, pre-formed CTL–target conjugates made of PKO anti-LF CTL and cognate LF+ cells were exposed to sodium EDTA, 5 mm, pH 7·4, shown before to dissociate bound CTL and target cells,30 or to brefeldin A, 10 μg/ml, known to interfere in FasL deployment, by inhibiting its transport from the endoplasmic reticulum to the Golgi apparatus, and then to the surface membrane,31 both added at 0, 45 and 90 min after conjugate formation, times at which overt Fas/FasL-based killing was still undetectable (see Fig. 2). Cytotoxicity determined 5 hr later indicated that a pre-lytic apoptotic process took place in the previously conjugated and subsequently dissociated target cells (Fig. 3a). This result indicated that although killing through the FasL pathway of CTL action lags 2 hr behind perforin-mediated action, both pathways in fact were jump-started shortly after the onset of CTL–target conjugation. Hence, cooperation between the two mechanisms through common downstream signalling steps could account for the observed complementation (see Fig. 2), which was then investigated by searching for FasL–induced pre-lytic caspase-8 and BID activation known to be involved in CTL-induced apoptosis.
Figure 3.
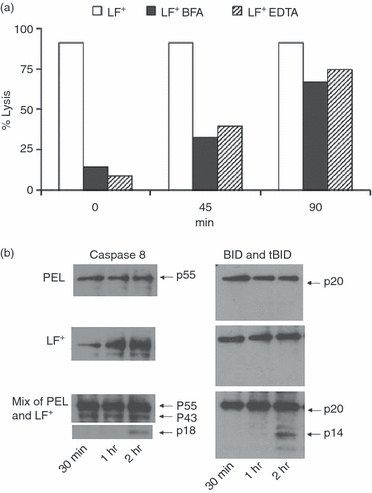
Onset and pre-lytic events during cytotoxic T lymphocyte (CTL) -mediated lysis. Fas ligand (FasL) -based activity of day 9 perforin knockout (PKO) anti-LF peritoneal exudate lymphocyte (PEL) against LF+ cells. (a) Samples containing pre-formed PKO-PEL-LF+ conjugates were treated with 5 mm Na2EDTA or brefeldin A (BFA), added at 0, 45 and 90 min after conjugate formation; lytic activity was determined 5 hr later (one out of three experiments). (b) Kinetics of pro-caspase-8 (p55), active caspase-8 (p43) and (p18), BID (p 20) and tBID (p14) activation was analysed by Western blots.
The downstream signalling events leading to CTL-induced death by either pathway are only partially defined. Once activated, caspases, a family of cysteine proteases coupled to pro-apoptotic signals, are known to cleave and activate downstream effector caspases (including caspase-8 and caspase-3), which in turn execute apoptosis by cleaving cellular proteins. We therefore tested whether FasL-based pre-lytic events could be demonstrated during the 2-hr lag period, before overt killing became obvious. The PKO anti-LF+ CTL were reacted with cognate LF+ cells for 30 min, 1 and 2 hr, before caspase-8 activation and BID cleavage was analysed by Western blots. We found pre-lytic caspase-8 activation, formation of a p43 fragment, after 30 min and ap18 fragment by 2 hr; BID cleavage to tBID (p14) took 1–2 hr (Fig. 3b).
Discussion
In recent years our understanding of how CTL recognize, bind to, communicate with, and subsequently kill their prey has advanced.4 However, an adequate explanation for the specific purpose and rationale for possessing two distinct pathways of CTL action, perforin and FasL, has been and still is the subject of much discussion, as is the question why do CTL equipped with a fast-acting perforin mechanism co-possess the much slower Fas/FasL-based mechanism. Obviously, the two mechanisms can synergize, be additive, or act complementarily, as well as help overcome resistance to either one. That possessing both mechanisms is advantageous is evident from the severe consequences of disruptive mutations (knockout) of the perforin (per−/−) (PKO),2 Fas (lpr) or FasL (gld−/−) genes.32
The kinetics of single target cell killing varies widely and two broadly divided kinds of CTL killers have been observed: ‘fast killers’ and ‘slow killers’. Studies on individual alloreactive CTL conjugates have shown that nearly all (> 90%) conjugate-forming cells were specific killers (Fig. 1d). For an unknown reason some one-to-one conjugates (i.e. a single CTL bound to one target cell) lysed quickly (within 5–20 min), others took up to 2–3 hr to kill. Possessing both killing mechanisms by individual CTL10,11,13,33,34 in itself can explain the finding of fast- and slow-acting killers but not the wide range of intermediate-acting killers detected by the CKChip in CTL populations (Fig. 1b,c). If, however, perforin and FasL were not equally expressed, variable killing rates among individual CTL would be expected as observed (Fig. 1d). The results of the present study suggest that the CTL's ‘kiss of death’ stems from the complementary action of its two lytic mechanisms, FasL and perforin (Fig. 2). The pace of lysis measured in individual conjugates clearly differentiated fast-acting, most likely perforin-mediated, from the slower-acting FasL-based CTL, such as those derived from PKO mice, as previously reported,3,17 as well as a range of intermediate rates in CTL derived from perforin-expressing and FasL-expressing C57BL/6 mice. We have shown that perforin expression among individual, conjugated CTL varied widely at each time-point tested (Fig. 1d). For example, 80–90% of day 8 conjugated CD8+ CTL expressed perforin at different levels, in contrast to stable, constitutive expression of FasL.3 This could account for the wide heterogeneity in killing speeds of individual CTL. Previous results have shown that as the immune response progresses in vivo, responding CD8 CTL gradually switch their killing phenotype, from mostly perforin to largely FasL-based killers.3 The switch has been attributed to the disappearance of antigen and can be reversed in vivo by the constant addition of antigen3 or in vitro by cytokines.22 The CTL killing induced within minutes after conjugation was probably through the perforin pathway, as was the case with perforin-rich CTL (e.g. IL-2-driven, PEL-blasts).3,22 Intermediate rates of killing were most likely to be CTL expressing reduced amounts of perforin, possibly reflecting the collaborative action of the two killing mechanisms perforin and FasL. Despite a 2-hr lag in FasL-based killing, not seen in perforin-based CTL action, CTL lytic action was enhanced (25%) when the two mechanisms operated in concert (Fig. 2). Hence, perforin and FasL death pathways support each other, in particular when perforin expression is diminished upon the decrease in antigen load as shown previously.3
Complementary action of the two CTL mechanisms could play an important role in situations involving CTL resistance, for instance, of tumours, virus-infected or even normal cells. For example, a fraction of fresh human tumours, and some mouse tumours, almost always exhibit partial (and sometimes complete) refractoriness to CTL action,35 possibly because of perforin resistance and hence they require the complementary action of both CTL mechanisms. This situation may be more pronounced with tumour-infiltrating lymphocytes, known to be poor perforin-expressing cells, presumably relying mostly on FasL and hence slow-acting killers.36
We have observed that the two mechanisms in fact were jump-started simultaneously upon conjugate formation. Whereas overt FasL action was delayed by 2 hr, downstream signalling of pre-lytic events related to apoptosis (caspase-8, BID) were initiated shortly after the onset of conjugate formation (Fig. 3a). The comparable delay in the onset of killing induced by either rFasL (determined by chromium release and by DNA degradation; data not shown) or by FasL CTL indicated that a pre-lytic event(s) within the target cells was behind the 2-hr delay, providing a clue to the complementary action of the two mechanisms.
The downstream signalling events leading to CTL-induced cell death by either pathway are only partially defined.4 Once activated, caspases, a family of cysteine proteases coupled to pro-apoptotic signals, are known to cleave and activate downstream caspases (including caspase-8 and caspase-3),9,37 which in turn execute apoptosis by cleaving cellular proteins (Fig. 3b). Granzyme B, a major secreted component of CTL lytic granules, in conjunction with perforin, induce apoptosis by activating caspase-3 or caspase-8,9,38 and by promoting permeability changes of the mitochondrial outer membrane by cleaved BID.39 The BID, in turn, induces cytochrome c release from mitochondria, which then activates caspase-9, resulting in caspase-3 activation, and as a result, cell death.40 FasL signalling, as well as granzyme B action, involves caspase-8 activation and BID cleavage (Fig. 3b).5 Cooperation between the two mechanisms through common downstream signalling observed by measuring the kinetics of caspase-8 and tBid appearance using CTL driven from PKO mice could account for the observed complementation.
The complementary action of the two distinct lytic pathways, FasL and perforin, co-expressed at varying levels among individual CTL, could facilitate the lytic action of even poor perforin-expressing CTL, hence ensuring optimal cytocidal action throughout the CTL response.
Acknowledgments
We thank K. Okumura and H. Yagita, Juntendo University, Tokyo, for providing the perforin antibody, D. Wallach for recombinant FasL trimmers, and A. Gross for the caspase-8 and BID antibodies.
Grant support
These studies were supported by the Israel Science Foundation (ISF) (grant no. 966/05), the Benozio Foundation, the Kirk Foundation, and by the Weizmann Institute – Tel-Aviv Sourasky Medical Centre collaborative grant.
Disclosures
The authors declare that they have no competing financial interests.
References
- 1.Rouvier E, Luciani MF, Golstein P. Fas involvement in Ca2+-independent T cell-mediated cytotoxicity. J Exp Med. 1993;177:195–200. doi: 10.1084/jem.177.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kagi D, Vignaux F, Ledermann B, Burki K, Depraetere V, Nagata S, Hengartner H, Golstein P. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science. 1994;265:528–30. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- 3.Meiraz A, Garber OG, Harari S, Hassin D, Berke G. Switch from perforin-expressing to perforin-deficient CD8+ T cells accounts for two distinct types of effector cytotoxic T lymphocytes in vivo. Immunology. 2009;128:69–82. doi: 10.1111/j.1365-2567.2009.03072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cullen SP, Martin SJ. Mechanisms of granule-dependent killing. Cell Death Differ. 2008;15:251–62. doi: 10.1038/sj.cdd.4402244. [DOI] [PubMed] [Google Scholar]
- 5.Pardo J, Wallich R, Martin P, et al. Granzyme B-induced cell death exerted by ex vivo CTL: discriminating requirements for cell death and some of its signs. Cell Death Differ. 2008;15:567–79. doi: 10.1038/sj.cdd.4402289. [DOI] [PubMed] [Google Scholar]
- 6.Aartman IH, Eijkman MA, Makkes PC. [Treatment of anxious patients in special dental care centers. From local initiatives to organized dentistry] Ned Tijdschr Tandheelkd. 1998;105:365–7. [PubMed] [Google Scholar]
- 7.Kischkel FC, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer PH, Peter ME. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995;14:5579–88. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang TB, Oh GS, Scandella E, Bolinger B, Ludewig B, Kovalenko A, Wallach D. Mutation of a self-processing site in caspase-8 compromises its apoptotic but not its nonapoptotic functions in bacterial artificial chromosome-transgenic mice. J Immunol. 2008;181:2522–32. doi: 10.4049/jimmunol.181.4.2522. [DOI] [PubMed] [Google Scholar]
- 9.Strasser A, Jost PJ, Nagata S. The many roles of FAS receptor signaling in the immune system. Immunity. 2009;30:180–92. doi: 10.1016/j.immuni.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelso A, Costelloe EO, Johnson BJ, Groves P, Buttigieg K, Fitzpatrick DR. The genes for perforin, granzymes A–C and IFN-gamma are differentially expressed in single CD8+ T cells during primary activation. Int Immunol. 2002;14:605–13. doi: 10.1093/intimm/dxf028. [DOI] [PubMed] [Google Scholar]
- 11.Kojima Y, Kawasaki-Koyanagi A, Sueyoshi N, Kanai A, Yagita H, Okumura K. Localization of Fas ligand in cytoplasmic granules of CD8+ cytotoxic T lymphocytes and natural killer cells: participation of Fas ligand in granule exocytosis model of cytotoxicity. Biochem Biophys Res Commun. 2002;296:328–36. doi: 10.1016/s0006-291x(02)00841-0. [DOI] [PubMed] [Google Scholar]
- 12.Stinchcombe JC, Griffiths GM. Secretory mechanisms in cell-mediated cytotoxicity. Annu Rev Cell Dev Biol. 2007;23:495–517. doi: 10.1146/annurev.cellbio.23.090506.123521. [DOI] [PubMed] [Google Scholar]
- 13.He JS, Ostergaard HL. CTLs contain and use intracellular stores of FasL distinct from cytolytic granules. J Immunol. 2007;179:2339–48. doi: 10.4049/jimmunol.179.4.2339. [DOI] [PubMed] [Google Scholar]
- 14.Johnson BJ, Costelloe EO, Fitzpatrick DR, Haanen JB, Schumacher TN, Brown LE, Kelso A. Single-cell perforin and granzyme expression reveals the anatomical localization of effector CD8+ T cells in influenza virus-infected mice. Proc Natl Acad Sci U S A. 2003;100:2657–62. doi: 10.1073/pnas.0538056100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zagury D, Bernard J, Thierness N, Feldman M, Berke G. Isolation and characterization of individual functionally reactive cytotoxic T lymphocytes: conjugation, killing and recycling at the single cell level. Eur J Immunol. 1975;5:818–22. [Google Scholar]
- 16.Sanderson CJ. Morphological aspects of lymphocyte mediated cytotoxicity. Adv Exp Med Biol. 1982;146:3–21. doi: 10.1007/978-1-4684-8959-0_1. [DOI] [PubMed] [Google Scholar]
- 17.Lowin B, Mattman C, Hahne M, Tschopp J. Comparison of Fas(Apo-1/CD95)- and perforin-mediated cytotoxicity in primary T lymphocytes. Int Immunol. 1996;8:57–63. doi: 10.1093/intimm/8.1.57. [DOI] [PubMed] [Google Scholar]
- 18.Breart B, Lemaitre F, Celli S, Bousso P. Two-photon imaging of intratumoral CD8+ T cell cytotoxic activity during adoptive T cell therapy in mice. J Clin Invest. 2008;118:1390–7. doi: 10.1172/JCI34388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Vries JF, von dem Borne PA, van Luxemburg-Heijs SA, Heemskerk MH, Willemze R, Falkenburg JH, Barge RM. Differential activation of the death receptor pathway in human target cells induced by cytotoxic T lymphocytes showing different kinetics of killing. Haematologica. 2007;92:1671–8. doi: 10.3324/haematol.11308. [DOI] [PubMed] [Google Scholar]
- 20.Walsh CM, Glass AA, Chiu V, Clark WR. The role of the Fas lytic pathway in a perforin-less CTL hybridoma. J Immunol. 1994;153:2506–14. [PubMed] [Google Scholar]
- 21.Berke G. Killer Lymphocytes. Berlin: Springer; 2007. [Google Scholar]
- 22.Berke G, Rosen D. Highly lytic in vivo primed cytolytic T lymphocytes devoid of lytic granules and BLT-esterase activity acquire these constituents in the presence of T cell growth factors upon blast transformation in vitro. J Immunol. 1988;141:1429–36. [PubMed] [Google Scholar]
- 23.Berke G, Gabison D. Energy requirements of the binding and lytic steps of T lymphocyte-mediated cytolysis of leukemic cells in vitro. Eur J Immunol. 1975;5:671–5. doi: 10.1002/eji.1830051004. [DOI] [PubMed] [Google Scholar]
- 24.Schiffenbauer YS, Kalma Y, Trubniykov E, Gal-Garber O, Weisz L, Halamish A, Sister M, Berke G. A cell chip for sequential imaging of individual non-adherent live cells reveals transients and oscillations. Lab Chip. 2009;9:2965–72. doi: 10.1039/b904778f. [DOI] [PubMed] [Google Scholar]
- 25.Berke G, Gabison D, Feldman M. The frequency of effector cells in populations containing cytotoxic T lymphocytes. Eur J Immunol. 1975;5:813–8. [Google Scholar]
- 26.O'Reilly LA, Divisekera U, Newton K, et al. Modifications and intracellular trafficking of FADD/MORT1 and caspase-8 after stimulation of T lymphocytes. Cell Death Differ. 2004;11:724–36. doi: 10.1038/sj.cdd.4401408. [DOI] [PubMed] [Google Scholar]
- 27.Wang K, Yin XM, Chao DT, Milliman CL, Korsmeyer SJ. BID: a novel BH3 domain-only death agonist. Genes Dev. 1996;10:2859–69. doi: 10.1101/gad.10.22.2859. [DOI] [PubMed] [Google Scholar]
- 28.Sarig R, Zaltsman Y, Marcellus RC, Flavell R, Mak TW, Gross A. BID-D59A is a potent inducer of apoptosis in primary embryonic fibroblasts. J Biol Chem. 2003;278:10707–15. doi: 10.1074/jbc.M210296200. [DOI] [PubMed] [Google Scholar]
- 29.Kawasaki A, Shinkai Y, Kuwana Y, et al. Perforin, a pore-forming protein detectable by monoclonal antibodies, is a functional marker for killer cells. Int Immunol. 1990;2:677–84. doi: 10.1093/intimm/2.7.677. [DOI] [PubMed] [Google Scholar]
- 30.Stulting RD, Berke G. Nature of lymphocyte–tumor interaction. A general method for cellular immunoabsorption. J Exp Med. 1973;137:932–42. doi: 10.1084/jem.137.4.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li JH, Rosen D, Ronen D, Behrens CK, Krammer PH, Clark WR, Berke G. The regulation of CD95 ligand expression and function in CTL. J Immunol. 1998;161:3943–9. [PubMed] [Google Scholar]
- 32.Voskoboinik I, Smyth MJ, Trapani JA. Perforin-mediated target-cell death and immune homeostasis. Nat Rev Immunol. 2006;6:940–52. doi: 10.1038/nri1983. [DOI] [PubMed] [Google Scholar]
- 33.Bossi G, Griffiths GM. Degranulation plays an essential part in regulating cell surface expression of Fas ligand in T cells and natural killer cells. Nat Med. 1999;5:90–6. doi: 10.1038/4779. [DOI] [PubMed] [Google Scholar]
- 34.Peixoto A, Evaristo C, Munitic I, Monteiro M, Charbit A, Rocha B, Veiga-Fernandes H. CD8 single-cell gene coexpression reveals three different effector types present at distinct phases of the immune response. J Exp Med. 2007;204:1193–205. doi: 10.1084/jem.20062349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21:233–40. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Radoja S, Saio M, Schaer D, Koneru M, Vukmanovic S, Frey AB. CD8+ tumor-infiltrating T cells are deficient in perforin-mediated cytolytic activity due to defective microtubule-organizing center mobilization and lytic granule exocytosis. J Immunol. 2001;167:5042–51. doi: 10.4049/jimmunol.167.9.5042. [DOI] [PubMed] [Google Scholar]
- 37.Afonina IS, Cullen SP, Martin SJ. Cytotoxic and non-cytotoxic roles of the CTL/NK protease granzyme B. Immunol Rev. 2010;235:105–16. doi: 10.1111/j.0105-2896.2010.00908.x. [DOI] [PubMed] [Google Scholar]
- 38.Adrain C, Murphy BM, Martin SJ. Molecular ordering of the caspase activation cascade initiated by the cytotoxic T lymphocyte/natural killer (CTL/NK) protease granzyme B. J Biol Chem. 2005;280:4663–73. doi: 10.1074/jbc.M410915200. [DOI] [PubMed] [Google Scholar]
- 39.Pinkoski MJ, Waterhouse NJ, Heibein JA, et al. Granzyme B-mediated apoptosis proceeds predominantly through a Bcl-2-inhibitable mitochondrial pathway. J Biol Chem. 2001;276:12060–7. doi: 10.1074/jbc.M009038200. [DOI] [PubMed] [Google Scholar]
- 40.Metkar SS, Wang B, Ebbs ML, Kim JH, Lee YJ, Raja SM, Froelich CJ. Granzyme B activates procaspase-3 which signals a mitochondrial amplification loop for maximal apoptosis. J Cell Biol. 2003;160:875–85. doi: 10.1083/jcb.200210158. [DOI] [PMC free article] [PubMed] [Google Scholar]


