Abstract
Myeloid-derived suppressor cells (MDSC) are important to the tumour microenvironment as they actively suppress the immune system and promote tumour progression and metastasis. These cells block T-cell activation in the tumour microenvironment, preventing anti-tumour immune activity. The ability of a treatment to alter the suppressive function of these cells and promote an immune response is essential to enhancing overall therapeutic efficacy. Interleukin-12 (IL-12) has the potential not only to promote anti-tumour immune responses but also to block the activity of cells capable of immune suppression. This paper identifies a novel role for IL-12 as a modulator of MDSC activity, with implications for IL-12 as a therapeutic agent. Treatment with IL-12 was found to alter the suppressive function of MDSC by fundamentally altering the cells. Interleukin-12-treated MDSC exhibited up-regulation of surface markers indicative of mature cells as well as decreases in nitric oxide synthase and interferon-γ mRNA both in vitro and in vivo. Treatment with IL-12 was also found to have significant therapeutic benefit by decreasing the percentage of MDSC in the tumour microenvironment and increasing the percentage of active CD8+ T cells. Treatment with IL-12 resulted in an increase in overall survival accompanied by a reduction in metastasis. The findings in this paper identify IL-12 as a modulator of immune suppression with significant potential as a therapeutic agent for metastatic breast cancer.
Keywords: Gr-1/CD11b, IL-12, immune suppressor, macrophage, MDSC
Introduction
Tumour infiltration by immune suppressive cells coupled with T-cell non-responsiveness is critical in tumour-associated immune evasion. The presence of cells capable of blocking immune activation systemically and in the tumour limits the usefulness of therapies meant to promote anti-tumour immunity. Understanding the role of suppressive cells in tumour progression and during immunotherapy is essential for enhancing the effectiveness of therapies that target immune modulation. Of particular interest in recent years, one population of immune modulators capable of suppressing T-cell activation is the myeloid-derived suppressor cells (MDSC).1 The MDSC are bone marrow-derived cells that express both the myeloid lineage differentiation antigen Gr-1 and αm integrin (CD11b) and exhibit the ability to suppress T-cell activation. Gr-1/CD11b double-positive cells represent approximately 20–30% of normal bone marrow cells and 2–4% of nucleated splenocytes, and are nearly absent from the lymph nodes of healthy animals; however, these cells differ from MDSC because they lack the ability to suppress T-cell activity.2–4 The MDSC are a functionally suppressive, heterogeneous population of Gr-1/CD11b double-positive cells composed of polymorphonuclear cells and monocytes at early stages of myeloid differentiation. These cells have been found to increase in the spleens, lymph nodes and peripheral blood of both carcinoma patients and tumour-bearing animals and function to promote tumour progression and block T-cell-mediated immune responses through both antigen-specific and non-specific mechanisms.5–8 In humans, cancer stage and tumour burden have been directly correlated with increases in MDSC populations.9 Findings indicate that tumour-infiltrating MDSC may actually be pleiotropic-inflamed monocytes/macrophages with both activating macrophage (M1) and suppressive macrophage (M2) characteristics.10 The MDSC have a role in the regulation of autoimmune effector cells, but in tumour progression these cells suppress immune system activation and effectively block anti-tumour responses.11
There are several known mechanisms by which MDSC exert their suppressive activity including the production of reactive oxygen species, production of nitric oxide, triggering apoptosis of antigen-activated T cells, depletion of l-arginine via production of arginase, and sequestration of cysteine.12–17 There is also an indirect mechanism through which MDSC suppress immune activation by inducing T regulatory cell development, although this may be specific to only a subset of animal models or tumour types.18 The MDSC are known to cause T-cell dysfunction, suppress T-cell activation and expansion, and induce CD8+ T-cell tolerance.18–22 These cells can also inhibit natural killer (NK) cell activity and NK cell utilization of interleukin-2 (IL-2).23,24 Many of these effects require activation of the MDSC through interferon-γ (IFN-γ), which can be regulated in an autocrine manner. Altering these suppressive activities through depletion or differentiation of MDSC has been shown to result in restored T-cell activation and stimulation of anti-tumour immune responses.25–29
Manipulation of MDSC activity is a promising adjuvant to immunotherapeutic agents. A single-cytokine-based therapy working alone or in combination with an agent that alters MDSC function has the potential to enhance therapeutic efficacy through both the elimination of immune suppression and the activation of an anti-tumour immune response. Altering MDSC suppressive function has been correlated with significant reductions in metastasis even in cases where tumour growth continued.26,30–33 Elucidating the mechanisms of agents with proven therapeutic efficacy is important for designing improved therapies.
Interleukin-12, a 70 000 molecular weight heterodimeric cytokine composed of two disulphide-linked subunits designated p35 and p40, is essential in the interaction between the innate and adaptive arms of immunity and is the major cytokine responsible for the differentiation of T helper cells to promote cell-mediated immunity.34–37 After encountering infectious agents, phagocytic cells, B cells and dendritic cells (DC) produce IL-12 to activate NK cells and T cells and induce their proliferation and production of cytokines, especially IFN-γ, so enhancing the generation and activity of cytotoxic lymphocytes. The IL-12-mediated induction of IFN-γ production occurs predominantly in NK cells, CD4+ T cells and CD8+ T cells. Interleukin-12 has a role in polarizing naive T cells into T helper type 1 (Th1) cells. These Th1 cells are essential in the response against intracellular pathogens through production of IFN-γ and promotion of cell-mediated immunity. In addition to the production of IFN-γ, IL-12 stimulates activated T-cell proliferation and enhances their cytolytic activity. Resting T cells do not proliferate in response to IL-12 stimulation.38,39 This regulation of the adaptive immune response is a major function of IL-12.
It is important to note that the induction of IFN-γ can result in a potent positive feedback loop whereby IL-12 induces IFN-γ, which in turn induces macrophages to generate more IL-12.40 These mechanisms to increase IL-12 production enable IL-12 to enhance the generation and activity of cytotoxic lymphocytes and promote cell-mediated immunity resulting in a potent inflammatory response. These distinctive biological functions of IL-12 are not limited to immune regulation. They have been shown to be effective against tumours in various tumour models and human clinical trials.41–45 In fact, IL-12 remains one of the most potent single cytokine therapies studied to date. Its anti-tumour activity is extensive and includes anti-metastatic and anti-angiogenic properties.46–48 It has been reported that intra-tumoral IL-12 can participate in the induction of apoptosis of tumour-resident regulatory T cells as well as impaired memory CD8+ T cells, permitting an influx of activated tumoricidal CD4+ and CD8+ T cells devoid of a regulatory population.49–52 This implies the ability of IL-12 to simultaneously reverse immune suppression and promote immune activation.
Although IL-12 has been extensively studied in terms of anti-tumour activity and effect on tumour-infiltrating macrophages, the direct effects of IL-12 on MDSC activity are not fully defined. Studies indicate that MDSC decrease IL-12 production from macrophages, indicating a possible role for IL-12 in modulating MDSC activity. As IL-12 can alter the function of tumour-infiltrating macrophages and specifically alter the suppressive activity of M2 macrophages, IL-12 may potentially act on populations of spleen-derived and tumour-derived Gr-1/CD11b double-positive cells.53,54 Whereas changes induced by IL-12 in the functional profile of tumour-infiltrating macrophages have been analysed, the effects of IL-12 on cells within the tumour microenvironment that exhibit a functional MDSC phenotype have not been assessed. Tumour infiltrating and tumour-associated macrophages have been shown to promote recruitment and activation of anti-tumour NK and T cells in response to IL-12-based therapies, indicating that IL-12 can act directly on cells and may affect other cell populations within the tumour. This study demonstrates a unique role for IL-12 as a modulator of MDSC activity with potential therapeutic implications.
Materials and methods
Mice and cell culture
C3H/HeJ and BALB/c mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Murine breast cancer cell lines C3L5 (Dr P. K. Lala, The University of Western Ontario, Canada) and 4T1 (ATCC, Manassas, VA) were maintained in RPMI-1640 and Dulbecco's modified Eagle's medium, respectively. All media were supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. Cells were fed fresh growth media three times per week and maintained at 37° in a 5% CO2 incubator.
Generation of Gr-1/CD11b double-positive cells
C3L5 cells (2·5 × 105) and 4T1 cells (1 × 105) were injected into the fat pads of C3H/HeJ and BALB/c mice, respectively, to induce mammary tumours. Gr-1/CD11b double-positive cells were found to expand in the tumours and spleens. Animals were killed once tumours reached a size of 500 mm3. Tumour specimens were surgically removed and processed by cutting into small pieces, and digested with 5 U/ml collagenase (Roche, Indianapolis, IN) and 5 μg/ml DNase I in RPMI-1640 (10% FBS, 1% penicillin/streptomycin) at 37° for 1·5 hr. The resulting mixture was filtered through 40-μm nylon mesh. The cells were washed with fresh RPMI-1640 and red blood cells were lysed via culture in 1× ACK/red blood cell lysis buffer (0·15 m NH4Cl, 10 mm KHCO3, 0·1 mm Na2-EDTA) at room temperature for 3 min. Cells were washed and maintained in RPMI-1640 (10% FBS, 1% penicillin/streptomycin). Spleens were also surgically removed, mashed, and red blood cells were lysed using the same process as with the digested tumour.
Isolation of Gr-1/CD11b double-positive cells
Whole splenocytes and cells from the digested tumours were stained using standard protocols with fluorochrome-conjugated antibodies, anti-Gr-1 (BD Bioscience, San Diego, CA) and anti-CD11b (BD Bioscience). Briefly, cells were washed with 1× PBS. Antibody was added at a concentration of 2 μg/ml of fluorochrome-conjugated antibody in 1× PBS with 1% BSA or 2 μg/ml mouse IgG (blocking agents; BD Bioscience) for 20 min at 4°. Cells were washed with 1× PBS, resuspended in RPMI-1640 supplemented with 1% FBS and 1% penicillin/streptomycin, and sorted via flow cytometry at the Flow Cytometry Core Facility (IUPUI, Indianapolis, IN).
Analysis of the expression of both subunits of the IL-12 receptor
Sorted Gr-1/CD11b double-positive cells along with whole splenocytes and digested tumour cells were stained with fluorochrome-conjugated anti-IL-12 receptor β1 (IL-12Rβ1) antibody and a combination of anti-IL-12Rβ2 primary antibody with fluorochrome-conjugated secondary antibody (BD Bioscience) in combination with fluorochrome-conjugated anti-Gr-1 and anti-CD11b antibodies. Each antibody was used at a concentration of 2 μg/ml. Cells were blocked with 1% BSA or 2 μg/ml mouse IgG (BD Bioscience). The NK cells were stained with 2 μg/ml fluorochrome-conjugated pan-NK cell marker clone DX5 antibody (eBioscience, San Diego, CA), fluorochrome-conjugated anti-IL-12Rβ1 antibody, and a combination of 2 μg/ml anti-IL-12Rβ2 and 2 μg/ml fluorochrome-conjugated secondary antibody as a positive control. Naive CD4 T cells were stained with 2 μg/ml fluorochrome-conjugated anti-CD4 antibody (eBioscience), 2 μg/ml fluorochrome-conjugated anti-IL-12Rβ1 antibody, and the combination of 2 μg/ml anti-IL-12Rβ2 antibody and 2 μg/ml fluorochrome-conjugated secondary antibody as a negative control. Analysis of the expression of the IL-12R was performed via flow cytometric analysis using a FACScalibur (Becton Dickinson, Mountain View, CA).
5-(and 6-)Carboxyfluorescein diacetate succinimidyl ester labelling of cells
Cells were labelled with carboxyfluorescein succinimidyl ester (CFSE) according to the manufacturer's protocols (Cell Trace CFSE Cell Proliferation kit; Invitrogen, Eugene, OR). Briefly, CFSE was diluted in DMSO at a stock concentration of 5 mm, which was further diluted to a working concentration of 10 μm. Whole splenocytes were suspended at a concentration of 1 × 107 cells/ml in PBS containing 0·1% BSA and combined with the 10 μm working concentration of CFSE. Following a 10-min incubation period at 37°, CFSE labelling was stopped by adding five times volume of ice-cold RPMI-1640 (10% FBS, 1% penicillin/streptomycin) and incubating on ice for 5 min. The CFSE-labelled cells were washed three times with ice-cold fresh RPMI-1640 (10% FBS, 1% penicillin/streptomycin) and resuspended to the desired concentration.
In vitro stimulation of T cells
The CFSE-labelled cells were plated in 96-well plates at a density of 1 × 106 cells/ml in RPMI-1640 (10% FBS, 1% penicillin/streptomycin) and incubated with or without 5 μg/ml soluble anti-CD3 (eBioscience) and anti-CD28 (BD Bioscience) antibodies alone or in combination with sorted Gr-1/CD11b double-positive cells in a 1 : 1 ratio for a total cell density per well of 2 × 105 cells. For analysis of the effect of IL-12, labelled cells and sorted Gr-1/CD11b double-positive cells were incubated with 10 ng/ml recombinant IL-12 separately for 24 hr at 37°, washed, and then co-cultured. Cells were incubated for 4 days at 37°. After this incubation period, cells were harvested and labelled with fluorochrome-conjugated anti-Gr-1 antibody for identification and elimination of the Gr-1+/CD11b+ subset using a FACScalibur (Becton Dickinson). A small sample of cells was also labelled with fluorochrome-conjugated anti-CD4 and anti-CD8 antibodies (eBioscience) to identify the profile of lymphocytes for gating and analysis of lymphocyte activation using a FACScaibur (Becton Dickinson). One hundred thousand total events from triplicate wells were collected and analysed for lymphocyte activation based on loss of CFSE intensity.
Analysis of changes in surface markers
Sorted Gr-1/CD11b double-positive cells from C3H/HeJ and BALB/c naive spleens, tumour-bearing spleens (C3L5 and 4T1, respectively), and digested tumour were incubated alone or in combination with 10 ng/ml recombinant IL-12 for 24 hr. Cells were stained with antibodies at a concentration of 2 μg/ml in 1× PBS containing 1% BSA or 2 μg/ml mouse IgG for 20 min at 4°. Cells were washed with 1× PBS and fixed in 1% paraformaldehyde. Antibodies were all fluorochrome-conjugated and included anti-CD86, anti-CD80, anti-F4/80 and anti-MHClass II antibodies (eBioscience). Analysis for expression of these markers compared with whole splenocyte-positive controls and CD4+ T lymphocyte-negative controls was performed via flow cytometry using the FACScalibur (Becton Dickinson).
Generation of recombinant adenovirus
Adenovirus vectors were generated using a previously described methodology modified to contain the desired genes without prostate specificity.55 Adenovirus containing a luciferase expression cassette (AdLuc) or a recombinant IL-12 expression cassette (AdIL-12) was used as a control for virus effect and to generate IL-12, respectively.
In vivo analysis of IL-12 effects on MDSC
Tumours were generated as described previously and allowed to grow to roughly 350 mm3 in volume. Before treatment, serum was harvested to establish baseline levels of IL-12 in the tumour-bearing animals. Then, 1 × 109 adenovirus particles suspended in 40 μl 1× PBS were injected intramuscularly into the animals. Twenty-four hours after treatment, serum, tumours and spleens were harvested as described previously for further analysis. Serum was used to demonstrate IL-12 production whereas digested tumours and spleens were analysed for changes in the Gr-1/CD11b double-positive cells as described for the in vitro experiments.
In vivo analysis of the anti-tumour effects of AdIL-12
Tumours were also allowed to grow to approximately 65 mm3 as described previously and were treated with intramuscular injections of 1 × 109 adenovirus particles suspended in 40 μl 1× PBS of either AdIL-12 or AdLuc. Tumours were measured twice weekly until they had grown to approximately 500 mm3. Animals were humanely killed and tissues were harvested for further analysis as described previously. Single-cell suspensions of tumours were stained with anti-Gr-1, anti-CD11b, anti-CD8, anti-IFN-γ and anti-CD45 (BD Bioscience) antibodies and analysed via flow cytometry. Lungs were stained in Bouin's fixative (LabChem Inc., Pittsburg, PA) and metastases were counted.
Real-time polymerase chain reaction
Sorted Gr-1/CD11b double-positive cells from naive and tumour-bearing animals were treated for 24 hr with 10 ng/ml IL-12. Cells were lysed and RNA was extracted using the RNAeasy kit (E.Z.N.A. homogenizing columns and E.Z.N.A. RNA Extraction kit; Quanta Biosciences Inc., Gaithersburg, MD). RNA was converted to cDNA via PCR reaction with the qScript cDNA Supermix (Quanta Biosciences Inc.). The PCR protocol was performed on the PTC-100 (MJ Research, Inc., Waltham, MA) and involved three steps: 5 min at 25°, 30 min at 42°, and 5 min at 85°. Primer probes against ArgI, nitric oxide synthase 2 (Nos2) and IFN-γ were purchased from Applied Biosystems (ABI, Carlsbad, CA). The concentration of RNA was obtained via analysis of readings taken at optical density of 260 mn (OD260) versus OD280 readings, and cDNA was diluted in EB buffer (10 mm Tris-HCl, pH 8·5) so that total RNA concentrations were equal for all samples. Using the PCR master mix also obtained from ABI, semi-qualitative real-time PCR was performed on the ABI 7500 Real Time PCR Machine using the following protocol: 10 min at 95° and 40 cycles of 15 seconds at 95° followed by 1 min at 65° (data recorded during this 65° stage).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5.01 for Windows (GraphPad Software, San Diego CA). Statistical significance was defined as a P-value < 0·05 with the actual P-values indicated. Analysis was performed on triplicate experiments.
Results
Gr-1/CD11b double-positive cells from tumour-bearing animals are functionally suppressive of MDSC
Tumour progression promotes MDSC in all mouse models studied to date; however, the type of MDSC induced has been found to vary. C3H/HeJ and BALB/c were selected as models for this study because of differences in the composition of their MDSC populations. C3H/HeJ animals have a population of MDSC that are predominantly monocytes whereas MDSC from BALB/c animals are approximately 2 : 3 polymorphonuclear to monocytes (data not shown). By selecting two models that differ in MDSC composition, we demonstrated that our observations are not strain-specific.
To detect MDSC presence, Gr-1/CD11b double-positive cells were stained with fluorochrome-conjugated antibodies and isolated from the spleens of naive animals as well as the tumours and spleens of tumour-bearing animals and assessed for their suppressive activity via T-cell activation assays. Whole splenocytes were labelled with CFSE and activated with anti-CD3 and anti-CD28 antibodies for 4 days. The CD4+ T-cell activation was determined by CFSE dilution via flow cytometry. C3H/HeJ splenocytes and tumour-derived Gr-1/CD11b double-positive cells are shown in Fig. 1 and BALB/c splenocytes and tumour-derived cells are shown in Fig. 2. Cells cultured in the absence of anti-CD3 and anti-CD28 antibodies served as the negative control and cells cultured with both antibodies in the absence of Gr-1/CD11b double-positive cells served as the positive control (Figs 1 and 2a,b). To demonstrate that the suppressive activity is not a consequence of the experimental design, Gr-1/CD11b double-positive cells from naive animals were co-cultured as well (Figs 1 and 2c).
Figure 1.
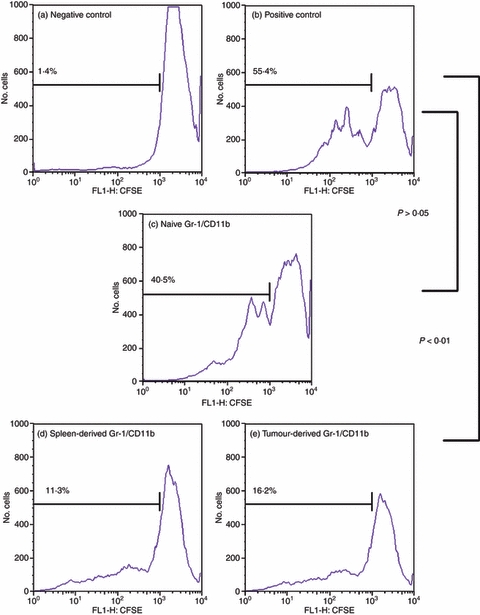
Gr-1/CD11b double-positive cells derived from tumour-bearing C3H/HeJ animals are functional myeloid-derived suppressor cells (MDSC). Whole splenocytes from C3H/HeJ animals were stained with 10 μm CFSE and co-cultured with sorted Gr-1/CD11b double-positive cells at a 1 : 1 ratio for a total of 2 × 105 cells per well. Cells were treated with 5 μg/ml anti-CD3 and anti-CD28 antibodies in 96-well plates for 4 days. The cells were harvested and stained with anti-Gr-1 and anti-CD4 antibodies to isolate only CD4+ T cells for analysis. Cells were gated for CD4+ T cells only and analysed for dilution of CFSE via flow cytometry. A negative control of unstimulated splenocytes (a) and a positive control of stimulated splenocytes only (b) were compared with splenocytes activated in the presence of Gr-1/CD11b double-positive cells from the spleen of naive animals (c) and Gr-1/CD11b double-positive cells from the spleen (d) and tumour (e) of tumour-bearing animals. Percentages indicate per cent activation.
Figure 2.
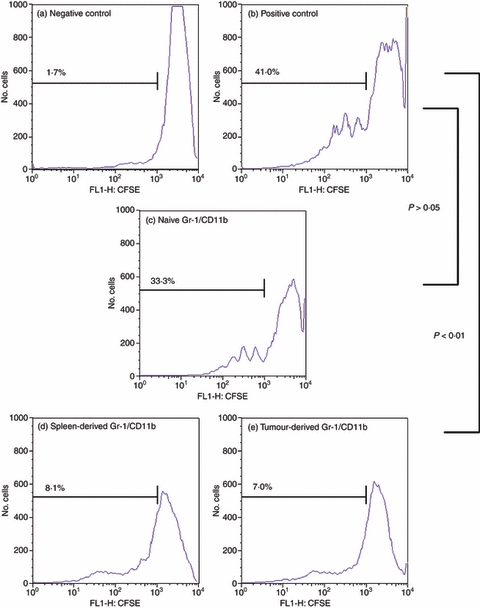
Gr-1/CD11b double-positive cells derived from tumour-bearing BALB/c animals are functional myeloid-derived suppressor cells (MDSC). BALB/c cells were prepared as described previously (Fig. 1). Cells were gated for CD4+ T cells only and analysed for dilution of CFSE via flow cytometry. A negative control of unstimulated splenocytes (a) and a positive control of stimulated splenocytes only (b) were compared with splenocytes activated in the presence of Gr-1/CD11b double-positive cells from the spleen of naive animals (c) and Gr-1/CD11b double-positive cells from the spleen (d) and tumour (e) of tumour-bearing animals. Percentages indicate per cent activation.
Figures 1 and 2 demonstrate that co-culture of T cells with Gr-1/CD11b double-positive cells at a 1 : 1 ratio resulted in the suppression of T-cell activation, dependent on the source of the Gr-1/CD11b cells. Gr-1/CD11b double-positive cells from tumour-bearing animals significantly suppressed T-cell activation regardless of whether they were harvested from the spleen or tumour (P < 0·01) (Figs 1 and 2d,e, respectively). Gr-1/CD11b double-positive cells from naive animals were not capable of significantly suppressing T-cell activation, indicating that Gr-1/CD11b double-positive MDSC are different from Gr-1/CD11b double-positive cells derived from a naive animal (P > 0·05) (Figs 1 and 2c). Functionally suppressive Gr-1/CD11b double-positive cells will be described as MDSC for the remainder of this paper.
MDSC express IL-12Rβ1 and IL-12Rβ2
Treatment of MDSC with factors known to alter their suppressive activity has shown some promise of therapeutic efficacy. Several cytokines have been shown to alter MDSC activity. Interleukin-12 is a cytokine of particular interest in tumour studies because of its efficacy but its role against MDSC remains undetermined.
Interleukin-12 exerts its biological activity through IL-12R, which is composed of two subunits designated β1 and β2 whose genes are located on chromosomes 19p13.1 and 1p31.2, respectively. The subunits are structurally related to the type I cytokine receptor superfamily and are homologous to glycoprotein 130 (gp130), the IL-6 signal transducing chain.56–58 The IL-12Rβ1 subunit associates with the p40 subunit of IL-12 whereas IL-12Rβ2 associates with the p35 subunit and is the signal transducing chain. The IL-12R is expressed on activated T cells, NK cells and DC.37,59–62 It is important to note that resting T cells do not express IL-12R, making this population of cells an ideal negative control for IL-12R expression.
Using NK cells as a positive control and CD4+ resting T cells as a negative control, we tested whether Gr-1/CD11b double-positive cells from naive animals as well as MDSC express either or both of the IL-12Rβ1 and IL-12Rβ2 subunits. Figure 3 demonstrates that naive C3H/HeJ spleen-derived Gr-1/CD11b double-positive cells do not express the IL-12Rβ1 subunit but they do express the IL-12Rβ2 subunit (Fig. 3a,g). BALB/c spleen-derived naive Gr-1/CD11b double-positive cells were found to express both of the receptor subunits (Fig. 3d,j). MDSC from tumour-bearing animals of both strains also express both subunits of the receptor (Fig. 3b,c,e,f,h,i,k,l) regardless of whether the MDSC are derived from the tumours (Fig. 3c,f,i,l) or spleens (Fig. 3b,e,h,k) of tumour-bearing animals. The results of the flow cytometry analysis are summarized in Table 1.
Figure 3.
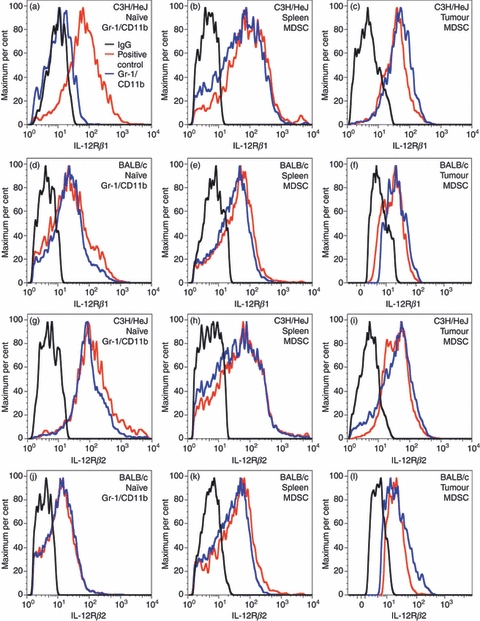
Interleukin-12 receptor β1 (IL-12Rβ1) and IL-12Rβ2 expression on the surface of Gr-1/CD11b double-positive cells. Gr-1/CD11b double-positive cells, natural killer (NK) cells, and naive CD4+ T cells were blocked with 1× PBS containing 1% BSA or 2 μg/ml IgG and stained with 2 μg/ml fluorochrome-conjugated anti-IL-12Rβ1 antibody or 2 μg/ml anti-IL12Rβ2 primary antibody followed by 2 μg/ml phycoerythrin-conjugated secondary antibody and analysed via flow cytometry. IL-12Rβ1 (a–f) and IL-12Rβ2 (g–l) were analysed. Naive Gr-1/CD11b double-positive cells from C3H/HeJ (a, g) and BALB/c (d, j) animals were compared with myeloid-derived suppressor cells from spleen (C3H/HeJ b, h; BALB/c e, k) and tumour (C3H/HeJ c, i; BALB/c f, l). Graphs are representative of three independent stains.
Table 1.
Interleukin-12 receptor β1 (IL-12Rβ1) and IL-12Rβ2 expression on the surface of Gr-1/CD11b double-positive cells
| Cells | IL-12Rβ1 | IL-12Rβ2 |
|---|---|---|
| C3H/HeJ naive spleen Gr-1/CDllb double-positive | − | + |
| C3H/HeJ tumour MDSC | + | + |
| C3H/HeJ tumour-bearing spleen MDSC | + | + |
| BALB/c naive spleen Gr-1/CDllb double-positive | + | + |
| BALB/c tumour MDSC | + | + |
| BALB/c tumour-bearing spleen MDSC | + | + |
MDSC, myeloid-derived suppressor cells.
IL-12 alters the suppressive function of MDSC
After determining that MDSC from tumour-bearing animals are suppressive and express IL-12R components, we sought to define the effect of IL-12 on these cells in terms of MDSC function. Given that the experimental design outlined in Figs 1 and 2 could imply a role for IL-12 to act on either MDSC or T cells or both, we sought to isolate the effect to one cell population. To define which population of cells is involved, cells were pre-treated separately for 24 hr before co-culturing. The MDSC were pre-treated with 10 ng/ml recombinant IL-12 for 24 hr at 37°. Cells were washed with fresh medium and co-cultured with CFSE-stained whole splenocytes and stimulated with anti-CD3 and anti-CD28 antibodies for 4 days (Figs 4 and 5c). Whole splenocytes were also pre-treated with IL-12, followed by anti-CD3 and anti-CD28 antibodies as a control for IL-12 activity (Figs 4 and 5d). Cell activation was determined by CFSE dilution of CD4+ T cells via flow cytometry as with the previous experiments.
Figure 4.
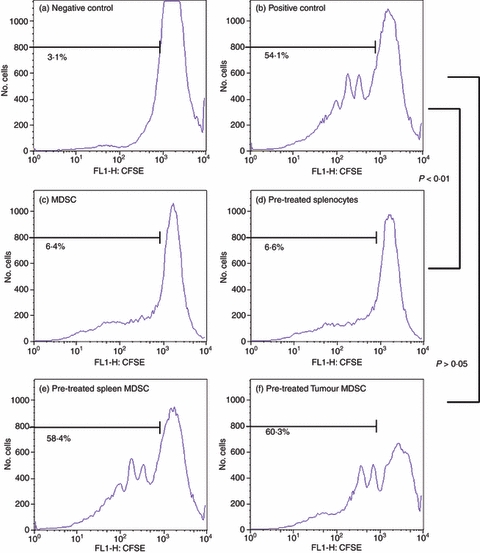
Interleukin-12 (IL-12) alters the suppressive activity of C3H/HeJ myeloid-derived suppressor cells (MDSC). C3H/HeJ naive whole splenocytes were stained with 10 μm CFSE and co-cultured with C3H/HeJ MDSC sorted from digested tumours and spleens of C3L5 tumour-bearing animals. MDSC were pre-treated with or without 10 ng/ml IL-12 for 24 hr. Cells were then co-cultured in 96-well plates at a 1 : 1 ratio of whole splenocytes to MDSC for a total of 2 × 105 cells per well stimulated with anti-CD3 and anti-CD28 antibodies for 4 days. After the 4-day incubation, cells were stained with fluorochrome-conjugated anti-CD4 and anti-Gr-1 antibodies to isolate only the CD4+ T cells for analysis. Using gating and flow cytometric analysis, CD4+ T cells from untreated cells were used as a negative control (a) while a positive control consisted of CD4+ T cells treated with anti-CD-3 and anti-CD-28 antibodies (b). Untreated MDSC co-cultured with CD4+ T cells and stimulated with anti-CD3 and anti-CD28 antibodies (c) were also included as a control for suppression. These controls were compared with whole splenocytes pre-treated with IL-12 followed by co-culture with MDSC (d) IL-12 pre-treated spleen-derived MDSC (e) and IL-12 pre-treated tumour-derived MDSC (f) both co-cultured with CD4+ T cells and stimulated with anti-CD3 and anti-CD28 antibodies. Percentages indicate per cent activation.
Figure 5.
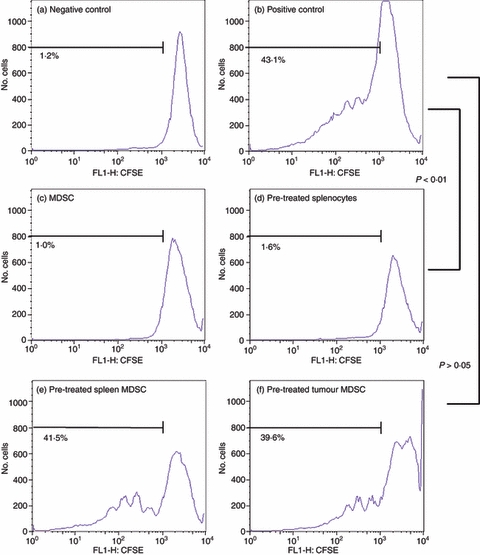
Interleukin-12 (IL-12) alters the suppressive activity of BALB/c myeloid-derived suppressor cells (MDSC). BALB/c cells were obtained and stained as described previously (Fig. 3). After the 4-day incubation, cells were stained with fluorochrome-conjugated anti-CD4 and anti-Gr-1 antibodies to isolate only the CD4+ T cells for analysis. Using gating and flow cytometric analysis, CD4+ T cells from untreated cells were used as a negative control (a) while a positive control consisted of CD4+ T cells treated with anti-CD-3 and anti-CD-28 antibodies (b). Untreated MDSC co-cultured with CD4+ T cells and stimulated with anti-CD3 and anti-CD28 antibodies (c) were also included as a control for suppression. These controls were compared with whole splenocytes pre-treated with IL-12 followed by co-culture with MDSC. (d) IL-12 pre-treated spleen-derived MDSC (e) and IL-12 pre-treated tumour-derived MDSC (f) both co-cultured with CD4+ T cells and stimulated with anti-CD3 and anti-CD28 antibodies. Percentages indicate per cent activation.
As Figs 4 and 5 demonstrate, pre-treatment of whole splenocytes with IL-12 did not alter the ability of MDSC to suppress T-cell activation, indicating that the effects of IL-12 were specific to MDSC (Figs 4 and 5d). The MDSC pre-treated with IL-12 for 24 hr, however, lose the ability to suppress T-cell activation even at the strong 1 : 1 ratio (Figs 4 and 5e,f). The loss of suppressive function is seen in both spleen-derived and tumour-derived MDSC for both C3H/HeJ (Fig. 4e,f) and BALB/c (Fig. 5e,f) mice. This loss of suppressive function when MDSC are pre-treated with IL-12 is an indication that IL-12 can act directly on MDSC in a manner that changes their suppressive function.
IL-12 induces MDSC up-regulation of surface markers in vitro and in vivo
It is clear from the data that treatment of MDSC with IL-12 alters the phenotype and the suppressive function of the cells; however, the mechanism through which IL-12 alters MDSC activity remains to be determined. To assess how MDSC change following IL-12 treatment, stains for changes in key surface markers were performed and analysed. It is known that Gr-1/CD11b double-positive cells can become DC or macrophages. We therefore focused on markers known to be up-regulated on mature DC and macrophages: CD80, CD86, F4/80 and MHCII.63 Changes in the expression of these markers on the surface of MDSC were used to indicate maturation and were compared with Gr-1/CD11b double-positive cells derived from naive animals. The flow cytometric analysis for changes in surface marker expression for naive Gr-1/CD11b double-positive cells and spleen-derived MDSC before and after IL-12 treatment is summarized in Table 2.
Table 2.
Changes in surface marker expression in vitro
| Cell type | CD80 | CD86 | F4/80 | MHCII |
|---|---|---|---|---|
| MFI ≤ 41·53 ± 1·41 (negative control) | MFI ≤ 35·5 ± 1·15 (negative control) | MFI ≤ 16·5 ± 0·95 (negative control) | MFI ≤ 37·87 ± 1·74 (negative control) | |
| MFI = 81·97 ± 3·5 (positive control) | MFI = 79·97 ± 5·99 (positive control) | MFI = 39·97 ± 4·97 (positive control) | MFI = 92·77 ± 3·89 (positive control) | |
| C3H/HeJ | ||||
| Naive Gr-1/CD11b | 82·9 ± 4·76 | 79·2 ± 5·16 | 41·2 ± 2·46 | 93·1 ± 3·43 |
| Naive Gr-1/CD11b + IL-12 | 82·8 ± 3·76 | 74·1 ± 3·10 | 93·1 ± 4·45 | 92·9 ± 4·45 |
| Tumour-bearing spleen MDSC | 40·6 ± 0·91 | 34·2 ± 0·86 | 15·7 ± 0·82 | 38·1 ± 0·96 |
| Tumour-bearing spleen MDSC + IL-12 | 84·5 ± 4·11 | 110 ± 4·73 | 39·0 ± 1·63 | 127 ± 6·51 |
| Tumour MDSC | 41·3 ± 0·49 | 34·6 ± 0·85 | 15·7 ± 0·90 | 38·4 ± 0·73 |
| Tumour MDSC + IL-12 | 41·2 ± 0·57 | 33·7 ± 0·69 | 21·0 ± 0·73 | 65·6 ± 1·97 |
| BALB/c | ||||
| Naive Gr-1/CD11b | 81·5 ± 4·19 | 79·5 ± 5·06 | 40·0 ± 1·84 | 94·1 ± 3·01 |
| Naive Gr-1/CD11b + IL-12 | 78·9 ± 3·32 | 81·2 ± 4·05 | 42·7 ± 1·53 | 93·7 ± 1·68 |
| Tumour-bearing spleen MDSC | 41·8 ± 0·54 | 34·4 ± 0·96 | 15·7 ± 1·00 | 38·1 ± 1·05 |
| Tumour-bearing spleen MDSC + IL-12 | 82·2 ± 4·59 | 74·6 ± 2·63 | 39·4 ± 1·71 | 124 ± 8·77 |
| Tumour MDSC | 41·2 ± 0·60 | 34·9 ± 0·78 | 15·5 ± 0·85 | 37·8 ± 0·70 |
| Tumour MDSC + IL-12 | 40·9 ± 0·58 | 34·4 ± 0·75 | 40·5 ± 1·67 | 65·8 ± 2·87 |
IL-12, interleukin-12; MDSC, myeloid-derived suppressor cells; MFI, mean fluorescence intensity.
Gr-1/CD11b double-positive cells and whole splenocytes from both naive and tumour-bearing animals were incubated for 24 hr with or without 10 ng/ml IL-12. Cells were then stained with 2 μg/ml anti-F4/80, anti-MHCII, anti-CD80 or anti-CD86 antibodies and analysed via flow cytometry. Analysis of both medians and means of histograms were used to quantify the changes in expression of the surface markers studied. MFI values are listed as an average of single-cell suspensions obtained from up to 10 animals stained in three individual studies.
The data show that Gr-1/CD11b double-positive cells from naive animals already express maturation markers for DC and macrophages, adding to the evidence that these cells are fundamentally different populations (Table 2). Spleen-derived and tumour-derived MDSC do not express the DC and macrophage maturation markers. This distinguishes MDSC from other macrophage types such as M1 activating and M2 suppressive tumour-infiltrating macrophages, especially in the tumour microenvironment. Following IL-12 treatment all markers were found to increase on the surface of spleen-derived MDSC, indicating that these cells are capable of up-regulating markers expressed on mature DC and macrophages and that IL-12 causes that up-regulation. Tumour-derived MDSC are distinct from spleen-derived MDSC in terms of IL-12 response. Upon stimulation with IL-12, tumour-derived MDSC in vitro were found to up-regulate only F4/80 and MHCII. As indicated in Table 2, Gr-1/CD11b double-positive cells from naive animals express several markers of mature cells and these markers are consistent with mature cells capable of receiving activation signals.
To determine whether these results could also be observed in vivo, tumours averaging 350 mm3 in size were generated in both C3H/HeJ and BALB/c animals. Intramuscular injections with adenovirus encoding either luciferase or recombinant IL-12 were performed. Twenty-four hours after injection, tumours and spleens were harvested and cells were isolated for further analysis. Serum analysis was used to confirm IL-12 expression and it was determined that only animals treated with adenovirus expressing recombinant IL-12 demonstrated a systemic increase in IL-12 expression, with an average of approximately 9 ng/ml (data not shown). Using identical staining protocols as the in vitro studies outlined in Table 2, treatment with IL-12 was found to be capable of altering the expression of all four markers studied regardless of whether they were spleen-derived or tumour-derived MDSC (Table 3). The ability to see changes in CD80 and CD86 expression for the tumour-derived cells in vivo indicates a small difference between treatments that can be explained by additional in vivo cellular interactions. Interactions of MDSC with other cells capable of responding to IL-12, such as T cells, most probably enhanced the overall effects of the treatment, which implies enhanced efficacy of the treatment under more physiologically relevant conditions.
Table 3.
Changes in surface marker expression in vivo
| Cell type | CD80 | CD86 | F4/80 | MHCII |
|---|---|---|---|---|
| MFI ≤ 41·53 ± 1·41 (negative control) | MFI ≤ 35·5 ± 1·15 (negative control) | MFI ≤ 16·5 ± 0·95 (negative control) | MFI ≤ 37·87 ± 1·74 (negative control) | |
| MFI = 81·97 ± 3·5 (positive control) | MFI = 79·97 ± 5·99 (positive control) | MFI = 39·97 ± 4·97 (positive control) | MFI = 92·77 ± 3·89 (positive control) | |
| C3H/HeJ | ||||
| Tumour-bearing spleen MDSC + AdLuc | 41·0 ± 0·98 | 34·3 ± 1·62 | 16·0 ± 0·88 | 38·3 ± 1·19 |
| Tumour-bearing spleen MDSC + AdlL-12 | 76·8 ± 1·53 | 78·1 ± 3·76 | 38·1 ± 0·82 | 115 ± 2·45 |
| Tumour MDSC + AdLuc | 41·1 ± 0·58 | 34·4 ± 0·79 | 15·8 ± 0·86 | 65·4 ± 0·80 |
| Tumour MDSC + AdlL-12 | 64·8 ± 1·15 | 80·1 ± 4·09 | 41·7 ± 2·23 | 132 ± 11·1 |
| BALB/c | ||||
| Tumour-bearing spleen MDSC + AdLuc | 40·1 ± 0·96 | 33·4 ± 0·76 | 16·1 ± 1·00 | 38·4 ± 0·77 |
| Tumour-bearing spleen MDSC + AdlL-12 | 82·1 ± 4·00 | 76·5 ± 4·12 | 40·1 ± 1·82 | 117 ± 4·29 |
| Tumour MDSC + AdLuc | 40·5 ± 0·98 | 34·1 ± 0·85 | 15·6 ± 0·80 | 67·4 ± 2·94 |
| Tumour MDSC + AdlL-12 | 63·8 ± 0·59 | 53·7 ± 1·68 | 39·9 ± 2·29 | 120 ± 3·24 |
IL-12, interleukin-12; MDSC, myeloid-derived suppressor cells; MFI, mean fluorescence intensity.
C3H/HeJ and BALB/c tumour-bearing animals were treated with intramuscular injections of 1 × 109 adenovirus particles of either AdLuc or AdIL-12. Twenty-four hours after inoculation with virus, spleens and tumours were harvested and digested to obtain single-cell suspensions. Cells were then stained with 2 μg/ml anti-F4/80, anti-MHCII, anti-CD80, or anti-CD86 antibodies and analysed via flow cytometry. Analysis of both medians and means of histograms were used to quantify the changes in expression of the surface markers studied. MFI values are listed as an average of single-cell suspensions obtained from up to 10 animals stained in three individual studies.
IL-12 treatment reduces the expression of ArgI, Nos2 and IFN-γ mRNA
Although differentiation is enough to define the change induced by IL-12, how that change is actually correlated with the loss of suppressive function needs to be determined. It is known that one of the critical mechanisms for MDSC suppressive activity is the production of reactive nitrogen species mediated by the expression of Nos2.13,22 Coinciding with the production of reactive nitrogen species is the depletion of l-arginine mediated by production of ArgI.6,15,64–67 The Nos2 is inducible by IFN-γ expression regardless of whether it is from intracellular production or extracellular sources.68,69 To define whether internal changes in Nos2 and IFN-γ play a role in these cells, RNA was extracted and mRNA levels were analysed using RT-PCR.
Figures 6 and 7 demonstrate the changes in ArgI, Nos2 and IFN-γin vitro and in vivo, respectively. Parts (a), (c) and (e) of both figures demonstrate the changes in expression of ArgI, Nos2 and IFN-γ, respectively for C3H/HeJ-derived cells while parts (b), (d) and (f) of both figures demonstrate the changes in expression for BALB/c-derived cells. The figures demonstrate levels of expression as normalized and compared with naive Gr-1/CD11b double-positive cells and plotted on graphs as a fold change. Expression of ArgI, Nos2 and IFN-γ were increased in MDSC regardless of tissue type. The levels of ArgI, Nos2 and IFN-γ expressed by tumour-derived MDSC were, however, significantly higher than the levels expressed in spleen-derived MDSC. Low levels of IFN-γ expression in the spleen-derived MDSC may account for differences in ability to differentiate in response to IL-12 in vitro and is further evidence that these cells are functionally different from their tumour-derived counterparts. Results for mRNA expression of ArgI, Nos2 and IFN-γ are consistent in vitro (Fig. 6) and in vivo (Fig. 7). It is essential to note that both spleen-derived and tumour-derived cells expressed ArgI and Nos2 at levels exceeding the naive Gr-1/CD11b double-positive controls, supporting the in vitro evidence of a suppressive function for both cell types.
Figure 6.
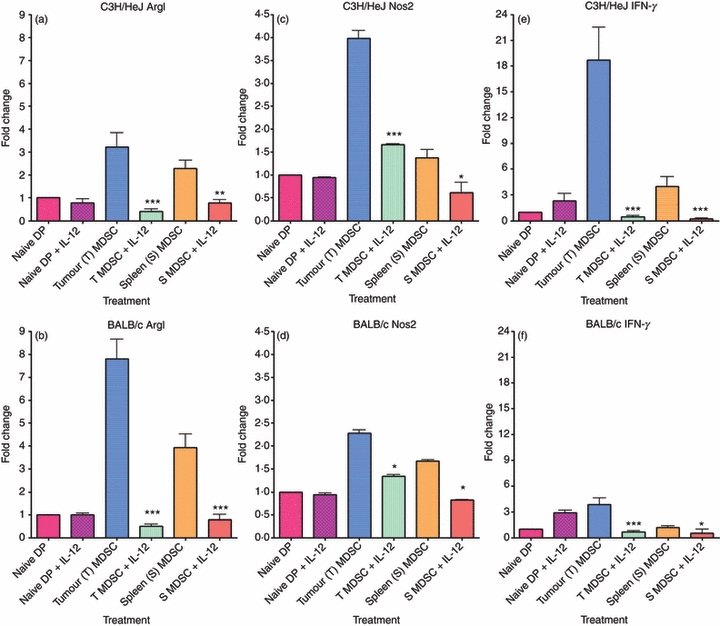
In vitro treatment with interleukin-12 (IL-12) reduces ArgI, Nos2 and interferon-γ (IFN-γ). Gr-1/CD11b double-positive cells were stained using fluorochrome-conjugated antibodies and sorted from the spleens of naive animals as well as the spleens and tumours of tumour-bearing animals. Sorted myeloid-derived suppressor cells (MDSC) were treated for 24 hr with 10 ng/ml recombinant mouse IL-12. RNA was extracted from sorted cell populations, converted to cDNA and analysed for mRNA expression via Real Time PCR. The expression levels for ArgI, Nos2 and IFN-γ were normalized to expression from naive double-positive cells and graphed as a fold change. The expression of ArgI in C3H/HeJ (a) and BALB/c (b), Nos2 in C3H/HeJ (c) and BALB/c (d) and expression of IFN-γ in C3H/HeJ (e) and BALB/c (f) are shown. Statistically significant reductions in mRNA expression were observed following treatment with IL-12 (*P < 0·05; **P < 0·01; ***P < 0·001).
Figure 7.
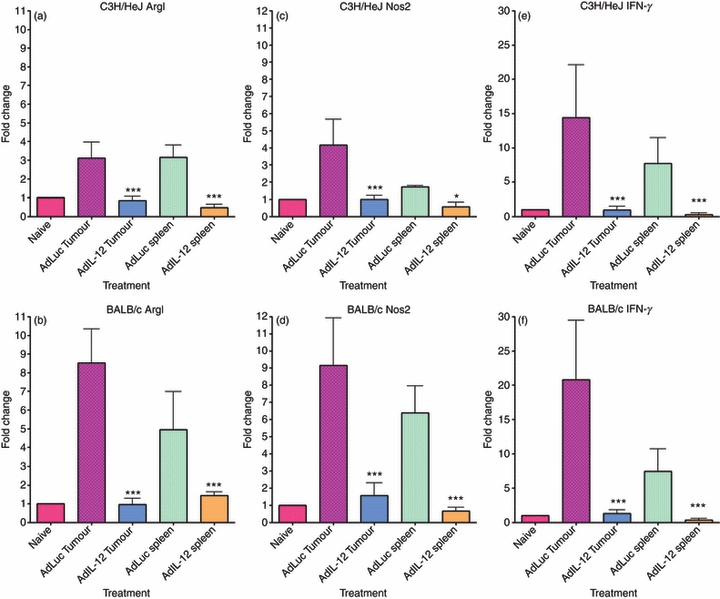
In vivo treatment with interleukin-12 (IL-12) reduces ArgI, Nos2 and interferon-γ (IFN-γ). C3H/HeJ and BALB/c tumour-bearing animals were treated with intramuscular injections of 1 × 109 adenovirus particles of either AdLuc or AdIL-12. Twenty-four hours after inoculation with virus, spleens and tumours were harvested and digested to obtain single-cell suspensions. Gr-1/CD11b double-positive cells were stained using fluorochrome-conjugated antibodies and sorted from the spleens and tumours of AdLuc- or AdIL-12-treated animals. RNA was extracted from sorted cell populations, converted to cDNA and analysed for mRNA expression via Real Time PCR. The expression levels for ArgI, Nos2 and IFN-γ were normalized to expression from naive double-positive cells and plotted on graphs as a fold change. The expression of ArgI in C3H/HeJ (a) and BALB/c (b), Nos2 in C3H/HeJ (c) and BALB/c (d) and expression of IFN-γ in C3H/HeJ (e) and BALB/c (f) are shown. Statistically significant reductions in mRNA expression were observed following treatment with IL-12 (*P < 0·05; ***P < 0·001).
IL-12 treatment induces infiltration of CD8+ T cells and reduces metastasis
Treatment with IL-12 has long been known to have therapeutic benefits including anti-angiogenic and anti-metastatic effects.45,70 In this study, we were interested in whether treatment with IL-12 had an effect on MDSC levels and metastasis. To determine whether MDSC levels were affected in the tumour microenvironment following treatment with adenovirus-mediated IL-12, we studied single-cell suspensions for levels of Gr-1/CD11b double-positive cells. We were also interested in whether infiltration of active CD8+ T cells into the tumour microenvironment occurs after treatment with IL-12 and if the treatment had any significant therapeutic effects on metastasis.
Treatment with IL-12 was found to suppress tumour growth leading to statistically significant increases in survival (Fig. 8). The end-point of the studies was a tumour size of 500 mm3; therefore, decreases in tumour growth were responsible for the increase in overall survival. However, how IL-12 effects on MDSC correlate with this remains to be defined. Figure 9 demonstrates that the percentage of MDSC in the tumours from both C3H/HeJ (a) and BALB/c (b) tumour-bearing animals decreased following treatment with IL-12. The percentage of Gr-1/CD11b double-positive cells is represented as a percentage of CD45+ cells (Fig. 9). Not only were these cells reduced but IL-12 also increased the percentage of IFN-γ-positive CD8+ T cells in the tumour microenvironment for both models as well (Fig. 9c,d). In addition to the effects on the tumour microenvironment, treatment with AdIL-12 was found to significantly reduce metastasis in both the C3H/HeJ (Fig. 10a) and BALB/c (Fig. 10b) models. This reduction in whole numbers of immune suppressors, increase in active CD8+ T cells, increase in overall survival, and decrease in metastasis is an indication that treatment with IL-12 has significant therapeutic benefits related to its MDSC modulating activity.
Figure 8.
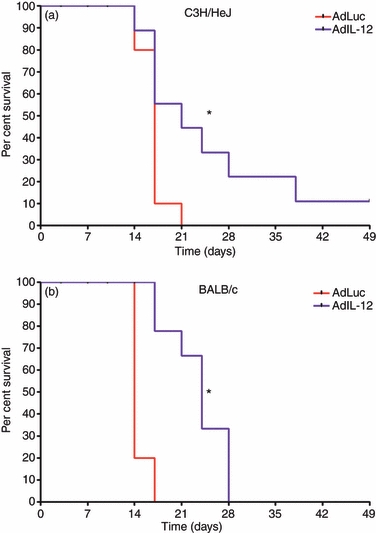
In vivo treatment with interleukin-12 (IL-12) increases overall survival. C3H/HeJ and BALB/c animals were inoculated with 2 × 105 C3L5 and 1 × 105 4T1 cells, respectively. Once tumours reached an average size of 65 mm3, intramuscular injections of 1 × 109 adenovirus particles of AdLuc or AdIL-12 were performed. Tumours were measured twice weekly. C3H/HeJ (a) and BALB/c (b) animals were analysed for overall survival with tumour volumes of 500 mm3 as the end-point (*P < 0·05).
Figure 9.
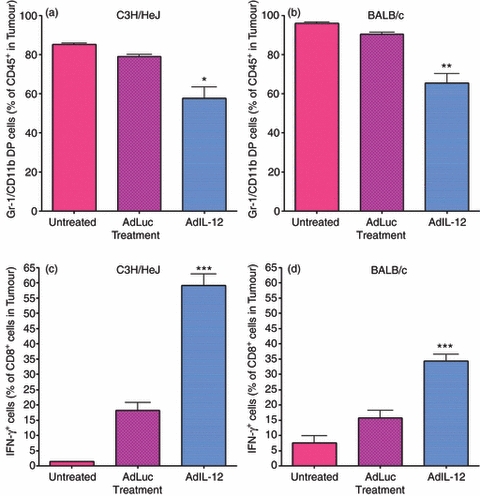
In vivo treatment with interleukin-12 (IL-12) increases active CD8+ T-cell infiltration into the tumour microenvironment. C3H/HeJ and BALB/c animals were inoculated with 2 × 105 C3L5 and 1 × 105 4T1 cells, respectively. Once tumours reached an average size of 65 mm3, intramuscular injections of 1 × 109 adenovirus particles of AdLuc or AdIL-12 were performed. Once tumours reached a volume of 500 mm3, tissues were harvested and single-cell suspensions obtained. Gr-1/CD11b double-positive cells were stained as a set of CD45+ cells using fluorochrome-conjugated anti-Gr-1, anti-CD11b and anti-CD45 antibodies. CD8+ T cells were stained using fluorochrome-conjugated anti-CD8 and anti-interferon-γ (IFN-γ) antibodies and analysed via flow cytometry. The percentage of Gr-1/CD11b double-positive cells in C3H/HeJ (a) and BALB/c (b) tumours as a percentage of CD45+ cells was determined. The percentage of CD8+ T cells that also express IFN-γ in C3H/HeJ (c) and BALB/c (d) tumours are also shown. Statistically significant increases in active IFN-γ+ CD8+ T cells were observed following treatment with AdIL-12 (*P < 0·05; **P < 0·01; ***P < 0·001).
Figure 10.
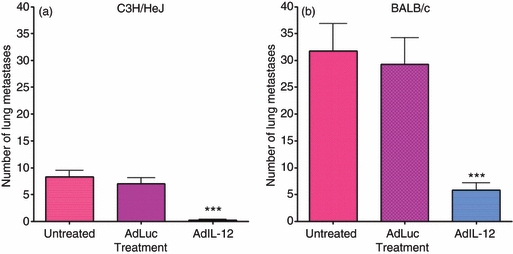
In vivo treatment with interleukin-12 (IL-12) decreases metastasis. C3H/HeJ and BALB/c animals were inoculated with 2 × 105 C3L5 and 1 × 105 4T1 cells, respectively. Once tumours reached an average size of 65 mm3, intramuscular injections of 1 × 109 adenovirus particles of AdLuc or AdIL-12 were performed. Once tumours reached a volume of 500 mm3, lungs were harvested for analysis of metastasis. Lungs were fixed in Bouin's fixative and metastases were counted. Analysis of metastases from C3H/HeJ (a) and BALB/c (b) are also shown. Statistically significant reductions in metastasis were observed following treatment with AdIL-12 (***P < 0·001).
Discussion
The MDSC are a population of cells of great interest to immunologists and cancer researchers. These cells have a wide range of effects in terms of inflammation, immune modulation, autoimmune disease, cancer progression and metastasis. Although MDSC are known as Gr-1/CD11b double-positive cells in mouse models, not all cells expressing both of these markers are MDSC. To be classified as MDSC the cells must be capable of suppressing T-cell activation. These studies confirmed a critical difference between MDSC and cells that are just Gr-1/CD11b double-positive in terms of function, and also identified a new difference: only MDSC up-regulate surface marker expression indicative of maturation in response to IL-12.
The findings in this paper identify a new role of IL-12 in immune modulation. Interleukin-12 was found to alter the suppressive function of MDSC by down-regulating ArgI along with IFN-γ and Nos2 while promoting the up-regulation of markers that indicate cell maturation. Spleen-derived MDSC up-regulate all markers studied, implying a more diverse response to IL-12 treatment, whereas tumour-derived MDSC in vitro appear to be more limited in their capacity to up-regulate surface markers in response to IL-12. This limitation, however, was not apparent from in vivo treatments. This difference is most likely to be the result of interactions with other immune activators affected by both virus treatment and IL-12. The up-regulation of co-stimulatory molecules CD80 and CD86 is important, as it implies the potential for these cells to activate T cells. Whether these cells have in fact been rendered capable of inducing T-cell activation or if they are merely no longer suppressive remains unknown. Although these cells were not found to express F4/80 until after treatment with IL-12, it is still probable that some tumour-derived MDSC are an immature population of macrophages. Further studies are needed to identify whether tumour MDSC exhibit M2 or M1 macrophage characteristics before and after IL-12 treatment, so as to fully define the overall activity of these MDSC. It is interesting that while IL-12 decreases the suppressive function of tumour MDSC in vitro and in vivo, a dramatic increase in maturation marker expression only occurs in vivo, indicating that loss of suppressive function and gain of maturation/differentiation markers are not necessarily coupled.
Several factors such as all-trans retinoic acid and sunitinib are known to act directly on MDSC to alter their suppressive function, leading to reductions in tumour growth.4,25,27–29,71,72 Coupling altered MDSC activity with immune activation may lead to significant improvements in combating metastatic disease and in overall therapeutic efficacy. Interleukin-12 as an immune modulator has the potential to both modulate immune suppression and promote immune activation, giving it significant potential as a therapeutic option. Treatment of tumour-bearing animals with AdIL-12 was found to reduce the percentage of Gr-1/CD11b double-positive cells in the tumour for both mouse strains. This reduction in Gr-1/CD11b double-positive cells could be responsible for the significant increase in IFN-γ+ CD8+ T cells found in the tumours. Treatment with AdIL-12 was also found to result in a significant increase in overall survival and a significant reduction in metastasis. The reduction in metastasis is of particular importance in terms of defining the potential for IL-12 as a cancer therapeutic agent. Overall these findings implicate a significant therapeutic role for treatment with IL-12, though how the reduction in metastasis directly correlates with the effect of IL-12 on MDSC remains undefined. Based on the results outlined in this paper, another role for IL-12 has been defined as well as a potential target for IL-12-based therapies.
Therapies that target immune activation as a means to reject established tumours are limited by the presence of cells capable of suppressing immune activity. The ability of T cells to avoid suppression by MDSC and other suppressive populations is critical for the immune system to promote an anti-tumour immune response. The idea that single cytokine treatments can promote immune responses and also overcome immune suppression is attractive to researchers. Defining the mechanism of IL-12 activity on MDSC is essential to understanding the therapeutic applications of IL-12. It is well established that IL-12 acts to suppress tumour growth in many tumour models. It is clear that IL-12 promotes anti-tumour immune responses but why these vary as dramatically as they do is unknown. This study provides a possible explanation for the discrepancy in IL-12-mediated therapeutic responses. As IL-12 alters the suppressive function of MDSC by inducing phenotypic and functional changes, it is possible that differences in MDSC expression and incomplete reversal of MDSC activity by IL-12 might explain the wide range of tumour responses observed in previous studies. These studies enhance our understanding of IL-12's effects on a suppressive cell population and offer a promising avenue for enhancing the efficacy of combination therapies.
Acknowledgments
We would like to acknowledge Dr Brittney-Shea Herbert and her laboratory technician, Melanie Fox, (Indiana University; Indianapolis, IN) as well as Dr Scott Crist and Jessica Haverkamp (Purdue University; West Layfayette, IN) for assistance with real-time PCR techniques. We would also like to acknowledge Dr Erin Goldblatt (University of California; Irvine, CA) for her assistance with animal research techniques.
Disclosures
The authors declare no financial or commercial conflict of interest.
References
- 1.Gabrilovich DI, Bronte V, Chen SH, Colombo MP, Ochoa A, Ostrand-Rosenberg S, Schreiber H. The terminology issue for myeloid-derived suppressor cells. Cancer Res. 2007;67:425. doi: 10.1158/0008-5472.CAN-06-3037. author reply 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagaraj S, Gabrilovich DI. Tumor escape mechanism governed by myeloid-derived suppressor cells. Cancer Res. 2008;68:2561–3. doi: 10.1158/0008-5472.CAN-07-6229. [DOI] [PubMed] [Google Scholar]
- 3.Kusmartsev S, Gabrilovich DI. Role of immature myeloid cells in mechanisms of immune evasion in cancer. Cancer Immunol Immunother. 2006;55:237–45. doi: 10.1007/s00262-005-0048-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serafini P, Meckel K, Kelso M, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Filipazzi P, Valenti R, Huber V, et al. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte–macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25:2546–53. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 6.Liu CY, Wang YM, Wang CL, et al. Population alterations of L: -arginase- and inducible nitric oxide synthase-expressed CD11b+/CD14−/CD15+/CD33+ myeloid-derived suppressor cells and CD8+ T lymphocytes in patients with advanced-stage non-small cell lung cancer. J Cancer Res Clin Oncol. 2009;136:35–45. doi: 10.1007/s00432-009-0634-0. [DOI] [PubMed] [Google Scholar]
- 7.Mandruzzato S, Solito S, Falisi E, et al. IL4Ralpha+ myeloid-derived suppressor cell expansion in cancer patients. J Immunol. 2009;182:6562–8. doi: 10.4049/jimmunol.0803831. [DOI] [PubMed] [Google Scholar]
- 8.Donkor MK, Lahue E, Hoke TA, et al. Mammary tumor heterogeneity in the expansion of myeloid-derived suppressor cells. Int Immunopharmacol. 2009;9:937–48. doi: 10.1016/j.intimp.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 9.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2008;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Umemura N, Saio M, Suwa T, et al. Tumor-infiltrating myeloid-derived suppressor cells are pleiotropic-inflamed monocytes/macrophages that bear M1- and M2-type characteristics. J Leukoc Biol. 2008;83:1136–44. doi: 10.1189/jlb.0907611. [DOI] [PubMed] [Google Scholar]
- 11.Marhaba R, Vitacolonna M, Hildebrand D, Baniyash M, Freyschmidt-Paul P, Zoller M. The importance of myeloid-derived suppressor cells in the regulation of autoimmune effector cells by a chronic contact eczema. J Immunol. 2007;179:5071–81. doi: 10.4049/jimmunol.179.8.5071. [DOI] [PubMed] [Google Scholar]
- 12.Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol. 2004;172:989–99. doi: 10.4049/jimmunol.172.2.989. [DOI] [PubMed] [Google Scholar]
- 13.Mazzoni A, Bronte V, Visintin A, Spitzer JH, Apolloni E, Serafini P, Zanovello P, Segal DM. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J Immunol. 2002;168:689–95. doi: 10.4049/jimmunol.168.2.689. [DOI] [PubMed] [Google Scholar]
- 14.Apolloni E, Bronte V, Mazzoni A, Serafini P, Cabrelle A, Segal DM, Young HA, Zanovello P. Immortalized myeloid suppressor cells trigger apoptosis in antigen-activated T lymphocytes. J Immunol. 2000;165:6723–30. doi: 10.4049/jimmunol.165.12.6723. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez PC, Ochoa AC. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol Rev. 2008;222:180–91. doi: 10.1111/j.1600-065X.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dolcetti L, Marigo I, Mantelli B, Peranzoni E, Zanovello P, Bronte V. Myeloid-derived suppressor cell role in tumor-related inflammation. Cancer Lett. 2008;267:216–25. doi: 10.1016/j.canlet.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70:68–77. doi: 10.1158/0008-5472.CAN-09-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH. Gr-1+ CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–31. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 19.Young MR, Wright MA, Matthews JP, Malik I, Prechel M. Suppression of T cell proliferation by tumor-induced granulocyte–macrophage progenitor cells producing transforming growth factor-β and nitric oxide. J Immunol. 1996;156:1916–22. [PubMed] [Google Scholar]
- 20.Ochoa AC, Zea AH, Hernandez C, Rodriguez PC. Arginase, prostaglandins, and myeloid-derived suppressor cells in renal cell carcinoma. Clin Cancer Res. 2007;13(2 Pt 2):721s–6s. doi: 10.1158/1078-0432.CCR-06-2197. [DOI] [PubMed] [Google Scholar]
- 21.Sinha P, Clements VK, Miller S, Ostrand-Rosenberg S. Tumor immunity: a balancing act between T cell activation, macrophage activation and tumor-induced immune suppression. Cancer Immunol Immunother. 2005;54:1137–42. doi: 10.1007/s00262-005-0703-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dugast AS, Haudebourg T, Coulon F, et al. Myeloid-derived suppressor cells accumulate in kidney allograft tolerance and specifically suppress effector T cell expansion. J Immunol. 2008;180:7898–906. doi: 10.4049/jimmunol.180.12.7898. [DOI] [PubMed] [Google Scholar]
- 23.Brooks JC, Hoskin DW. The inhibitory effect of cyclophosphamide-induced MAC-1+ natural suppressor cells on IL-2 and IL-4 utilization in MLR. Transplantation. 1994;58:1096–103. [PubMed] [Google Scholar]
- 24.Liu C, Yu S, Kappes J, Wang J, Grizzle WE, Zinn KR, Zhang HG. Expansion of spleen myeloid suppressor cells represses NK cell cytotoxicity in tumor-bearing host. Blood. 2007;109:4336–42. doi: 10.1182/blood-2006-09-046201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan PY, Wang GX, Yin B, Ozao J, Ku T, Divino CM, Chen SH. Reversion of immune tolerance in advanced malignancy: modulation of myeloid-derived suppressor cell development by blockade of stem-cell factor function. Blood. 2008;111:219–28. doi: 10.1182/blood-2007-04-086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sinha P, Clements VK, Ostrand-Rosenberg S. Reduction of myeloid-derived suppressor cells and induction of M1 macrophages facilitate the rejection of established metastatic disease. J Immunol. 2005;174:636–45. doi: 10.4049/jimmunol.174.2.636. [DOI] [PubMed] [Google Scholar]
- 27.Ko JS, Zea AH, Rini BI, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148–57. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 28.Heithoff DM, Enioutina EY, Bareyan D, Daynes RA, Mahan MJ. Conditions that diminish myeloid-derived suppressor cell activities stimulate cross-protective immunity. Infect Immun. 2008;76:5191–9. doi: 10.1128/IAI.00759-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nefedova Y, Fishman M, Sherman S, Wang X, Beg AA, Gabrilovich DI. Mechanism of all-trans retinoic acid effect on tumor-associated myeloid-derived suppressor cells. Cancer Res. 2007;67:11021–8. doi: 10.1158/0008-5472.CAN-07-2593. [DOI] [PubMed] [Google Scholar]
- 30.Young MR, Ihm J, Lozano Y, Wright MA, Prechel MM. Treating tumor-bearing mice with vitamin D3 diminishes tumor-induced myelopoiesis and associated immunosuppression, and reduces tumor metastasis and recurrence. Cancer Immunol Immunother. 1995;41:37–45. doi: 10.1007/BF01788958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Habibi M, Kmieciak M, Graham L, Morales JK, Bear HD, Manjili MH. Radiofrequency thermal ablation of breast tumors combined with intralesional administration of IL-7 and IL-15 augments anti-tumor immune responses and inhibits tumor development and metastasis. Breast Cancer Res Treat. 2009;114:423–31. doi: 10.1007/s10549-008-0024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kusmartsev S, Su Z, Heiser A, et al. Reversal of myeloid cell-mediated immunosuppression in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2008;14:8270–8. doi: 10.1158/1078-0432.CCR-08-0165. [DOI] [PubMed] [Google Scholar]
- 33.Stumpfova M, Ratner D, Desciak EB, Eliezri YD, Owens DM. The immunosuppressive surface ligand CD200 augments the metastatic capacity of squamous cell carcinoma. Cancer Res. 2010;70:2962–72. doi: 10.1158/0008-5472.CAN-09-4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–9. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 35.Schmitt E, Hoehn P, Huels C, Goedert S, Palm N, Rude E, Germann T. T helper type 1 development of naive CD4+ T cells requires the coordinate action of interleukin-12 and interferon-γ and is inhibited by transforming growth factor-β. Eur J Immunol. 1994;24:793–8. doi: 10.1002/eji.1830240403. [DOI] [PubMed] [Google Scholar]
- 36.Romani L, Mencacci A, Tonnetti L, Spaccapelo R, Cenci E, Puccetti P, Wolf SF, Bistoni F. IL-12 is both required and prognostic in vivo for T helper type 1 differentiation in murine candidiasis. J Immunol. 1994;153:5167–75. [PubMed] [Google Scholar]
- 37.Watford WT, Moriguchi M, Morinobu A, O'Shea JJ. The biology of IL-12: coordinating innate and adaptive immune responses. Cytokine Growth Factor Rev. 2003;14:361–8. doi: 10.1016/s1359-6101(03)00043-1. [DOI] [PubMed] [Google Scholar]
- 38.Igarashi O, Yamane H, Imajoh-Ohmi S, Nariuchi H. IL-12 receptor (IL-12R) expression and accumulation of IL-12Rβ1 and IL-12Rβ2 mRNAs in CD4+ T cells by costimulation with B7-2 molecules. J Immunol. 1998;160:1638–46. [PubMed] [Google Scholar]
- 39.Lee SM, Suen Y, Qian J, Knoppel E, Cairo MS. The regulation and biological activity of interleukin 12. Leuk Lymphoma. 1998;29:427–38. doi: 10.3109/10428199809050903. [DOI] [PubMed] [Google Scholar]
- 40.Yoshida A, Koide Y, Uchijima M, Yoshida TO. IFN-γ induces IL-12 mRNA expression by a murine macrophage cell line, J774. Biochem Biophys Res Commun. 1994;198:857–61. doi: 10.1006/bbrc.1994.1122. [DOI] [PubMed] [Google Scholar]
- 41.Leonard JP, Sherman ML, Fisher GL, et al. Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-γ production. Blood. 1997;90:2541–8. [PubMed] [Google Scholar]
- 42.Bramson JL, Hitt M, Addison CL, Muller WJ, Gauldie J, Graham FL. Direct intratumoral injection of an adenovirus expressing interleukin-12 induces regression and long-lasting immunity that is associated with highly localized expression of interleukin-12. Hum Gene Ther. 1996;7:1995–2002. doi: 10.1089/hum.1996.7.16-1995. [DOI] [PubMed] [Google Scholar]
- 43.Lenzi R, Rosenblum M, Verschraegen C, et al. Phase I study of intraperitoneal recombinant human interleukin 12 in patients with Mullerian carcinoma, gastrointestinal primary malignancies, and mesothelioma. Clin Cancer Res. 2002;8:3686–95. [PubMed] [Google Scholar]
- 44.Sung MW, Chen SH, Thung SN, Zhang DY, Huang TG, Mandeli JP, Woo SL. Intratumoral delivery of adenovirus-mediated interleukin-12 gene in mice with metastatic cancer in the liver. Hum Gene Ther. 2002;13:731–43. doi: 10.1089/104303402317322294. [DOI] [PubMed] [Google Scholar]
- 45.Faggioli F, Soldati S, Scanziani E, Cato EM, Adorni F, Vezzoni P, Noonan DM, Sacco MG. Effects of IL-12 gene therapy on spontaneous transgenic and transplanted breast tumors. Breast Cancer Res Treat. 2007;110:223–226. doi: 10.1007/s10549-007-9713-6. [DOI] [PubMed] [Google Scholar]
- 46.Imagawa Y, Satake K, Kato Y, Tahara H, Tsukuda M. Antitumor and antiangiogenic effects of interleukin 12 gene therapy in murine head and neck carcinoma model. Auris Nasus Larynx. 2004;31:239–45. doi: 10.1016/j.anl.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 47.Duda DG, Sunamura M, Lozonschi L, et al. Direct in vitro evidence and in vivo analysis of the antiangiogenesis effects of interleukin 12. Cancer Res. 2000;60:1111–6. [PubMed] [Google Scholar]
- 48.Airoldi I, Di Carlo E, Cocco C, et al. Endogenous IL-12 triggers an antiangiogenic program in melanoma cells. Proc Natl Acad Sci U S A. 2007;104:3996–4001. doi: 10.1073/pnas.0609028104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Broderick L, Yokota SJ, Reineke J, Mathiowitz E, Stewart CC, Barcos M, Kelleher RJ, Jr, Bankert RB. Human CD4+ effector memory T cells persisting in the microenvironment of lung cancer xenografts are activated by local delivery of IL-12 to proliferate, produce IFN-γ, and eradicate tumor cells. J Immunol. 2005;174:898–906. doi: 10.4049/jimmunol.174.2.898. [DOI] [PubMed] [Google Scholar]
- 50.Kilinc MO, Aulakh KS, Nair RE, Jones SA, Alard P, Kosiewicz MM, Egilmez NK. Reversing tumor immune suppression with intratumoral IL-12: activation of tumor-associated T effector/memory cells, induction of T suppressor apoptosis, and infiltration of CD8+ T effectors. J Immunol. 2006;177:6962–73. doi: 10.4049/jimmunol.177.10.6962. [DOI] [PubMed] [Google Scholar]
- 51.Knutson KL, Disis ML. IL-12 enhances the generation of tumour antigen-specific Th1 CD4 T cells during ex vivo expansion. Clin Exp Immunol. 2004;135:322–9. doi: 10.1111/j.1365-2249.2004.02360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Macgregor JN, Li Q, Chang AE, Braun TM, Hughes DP, McDonagh KT. Ex vivo culture with interleukin (IL)-12 improves CD8+ T-cell adoptive immunotherapy for murine leukemia independent of IL-18 or IFN-γ but requires perforin. Cancer Res. 2006;66:4913–21. doi: 10.1158/0008-5472.CAN-05-3507. [DOI] [PubMed] [Google Scholar]
- 53.Bastos KR, Alvarez JM, Marinho CR, Rizzo LV, Lima MR. Macrophages from IL-12p40-deficient mice have a bias toward the M2 activation profile. J Leukoc Biol. 2002;71:271–8. [PubMed] [Google Scholar]
- 54.Watkins SK, Egilmez NK, Suttles J, Stout RD. IL-12 rapidly alters the functional profile of tumor-associated and tumor-infiltrating macrophages in vitro and in vivo. J Immunol. 2007;178:1357–62. doi: 10.4049/jimmunol.178.3.1357. [DOI] [PubMed] [Google Scholar]
- 55.Li X, Raikwar SP, Liu YH, et al. Combination therapy of androgen-independent prostate cancer using a prostate restricted replicative adenovirus and a replication-defective adenovirus encoding human endostatin-angiostatin fusion gene. Mol Cancer Ther. 2006;5:676–84. doi: 10.1158/1535-7163.MCT-05-0339. [DOI] [PubMed] [Google Scholar]
- 56.Chua AO, Wilkinson VL, Presky DH, Gubler U. Cloning and characterization of a mouse IL-12 receptor-β component. J Immunol. 1995;155:4286–94. [PubMed] [Google Scholar]
- 57.Presky DH, Yang H, Minetti LJ, Chua AO, Nabavi N, Wu CY, Gately MK, Gubler U. A functional interleukin 12 receptor complex is composed of two β-type cytokine receptor subunits. Proc Natl Acad Sci U S A. 1996;93:14002–7. doi: 10.1073/pnas.93.24.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chua AO, Chizzonite R, Desai BB, et al. Expression cloning of a human IL-12 receptor component. A new member of the cytokine receptor superfamily with strong homology to gp130. J Immunol. 1994;153:128–36. [PubMed] [Google Scholar]
- 59.Chizzonite R, Truitt T, Desai BB, Nunes P, Podlaski FJ, Stern AS, Gately MK. IL-12 receptor. I. Characterization of the receptor on phytohemagglutinin-activated human lymphoblasts. J Immunol. 1992;148:3117–24. [PubMed] [Google Scholar]
- 60.Wu CY, Warrier RR, Carvajal DM, et al. Biological function and distribution of human interleukin-12 receptor β chain. Eur J Immunol. 1996;26:345–50. doi: 10.1002/eji.1830260212. [DOI] [PubMed] [Google Scholar]
- 61.Grohmann U, Belladonna ML, Bianchi R, Orabona C, Ayroldi E, Fioretti MC, Puccetti P. IL-12 acts directly on DC to promote nuclear localization of NF-kappaB and primes DC for IL-12 production. Immunity. 1998;9:315–23. doi: 10.1016/s1074-7613(00)80614-7. [DOI] [PubMed] [Google Scholar]
- 62.Desai BB, Quinn PM, Wolitzky AG, Mongini PK, Chizzonite R, Gately MK. IL-12 receptor. II. Distribution and regulation of receptor expression. J Immunol. 1992;148:3125–32. [PubMed] [Google Scholar]
- 63.Narita Y, Wakita D, Ohkur T, Chamoto K, Nishimura T. Potential differentiation of tumor bearing mouse CD11b+ Gr-1+ immature myeloid cells into both suppressor macrophages and immunostimulatory dendritic cells. Biomed Res. 2009;30:7–15. doi: 10.2220/biomedres.30.7. [DOI] [PubMed] [Google Scholar]
- 64.Bronte V, Serafini P, De Santo C, et al. IL-4-induced arginase 1 suppresses alloreactive T cells in tumor-bearing mice. J Immunol. 2003;170:270–8. doi: 10.4049/jimmunol.170.1.270. [DOI] [PubMed] [Google Scholar]
- 65.Bronte V, Serafini P, Mazzoni A, Segal DM, Zanovello P. l-Arginine metabolism in myeloid cells controls T-lymphocyte functions. Trends Immunol. 2003;24:302–6. doi: 10.1016/s1471-4906(03)00132-7. [DOI] [PubMed] [Google Scholar]
- 66.Capuano G, Rigamonti N, Grioni M, Freschi M, Bellone M. Modulators of arginine metabolism support cancer immunosurveillance. BMC Immunol. 2009;10:1. doi: 10.1186/1471-2172-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu Y, Yu Y, Yang S, et al. Regulation of arginase I activity and expression by both PD-1 and CTLA-4 on the myeloid-derived suppressor cells. Cancer Immunol Immunother. 2009;58:687–97. doi: 10.1007/s00262-008-0591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Momose I, Terashima M, Nakashima Y, Sakamoto M, Ishino H, Nabika T, Hosokawa Y, Tanigawa Y. Phorbol ester synergistically increases interferon regulatory factor-1 and inducible nitric oxide synthase induction in interferon-γ-treated RAW 264.7 cells. Biochim Biophys Acta. 2000;1498:19–31. doi: 10.1016/s0167-4889(00)00072-0. [DOI] [PubMed] [Google Scholar]
- 69.Martey CA, Vetrano AM, Whittemore MS, Mariano TM, Heck DE, Laskin DL, Heindel ND, Laskin JD. Inhibition of interferon-γ signaling by a mercurio-substituted dihydropsoralen in murine keratinocytes. Biochem Pharmacol. 2005;70:1726–34. doi: 10.1016/j.bcp.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 70.Akhtar N, Padilla ML, Dickerson EB, Steinberg H, Breen M, Auerbach R, Helfand SC. Interleukin-12 inhibits tumor growth in a novel angiogenesis canine hemangiosarcoma xenograft model. Neoplasia. 2004;6:106–16. doi: 10.1593/neo.03334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jiang J, Wang Z, Li Z, Zhang J, Wang C, Xu X, Qin Z. Early exposure of high-dose interleukin-4 to tumor stroma reverses myeloid cell-mediated T-cell suppression. Gene Ther. 2010;17:991–9. doi: 10.1038/gt.2010.54. [DOI] [PubMed] [Google Scholar]
- 72.Ko JS, Rayman P, Ireland J, et al. Direct and differential suppression of myeloid-derived suppressor cell subsets by sunitinib is compartmentally constrained. Cancer Res. 2010;70:3526–36. doi: 10.1158/0008-5472.CAN-09-3278. [DOI] [PMC free article] [PubMed] [Google Scholar]


