Abstract
A major gap in our understanding of the immune response to pathogens and vaccines is how closely the antigen specificity in the memory phase mimics repertoire that is rapidly expanded upon priming. Understanding the diversity of the CD4 T-cell memory compartment after a primary response to pathogens is hampered by the technical challenges of epitope discovery and suitable models to study primary immune responses. Recently, we have used overlapping synthetic peptides to empirically map most of the specificities present in the primary response to live influenza infection. We found that the CD4 T-cell response can be exceptionally diverse, depending on the allele(s) of MHC class II molecules expressed. In the current study, using a mouse model of primary influenza infection and peptide-specific cytokine EliSpots, we have asked how this broad CD4 T-cell immunodominance hierarchy changes as the immune response contracts and memory is established. Our studies revealed that, for the most part, diversity is maintained, and most specificities, including those for relatively minor epitopes, are preserved in the memory CD4 T-cell compartment. A modest, but reproducible shift in specificity toward haemagglutinin-derived epitopes was observed, raising the possibility that protein or peptide persistence might play a role in the evolution of the memory phase of the CD4 T-cell response.
Keywords: CD4 T cells, epitopes, influenza, memory responses, peptides
Introduction
Influenza virus is an immunologically complicated pathogen, particularly in humans, where a long lifespan typically involves multiple encounters with genetically variable influenza viruses and either attenuated or isolated protein vaccines (reviewed in refs 1–7). Worldwide, most humans first encounter influenza virus through natural infection in early childhood. Therefore, the original immunological memory compartment for influenza virus is based on priming through live intranasal infection. After this original contact, individuals encounter influenza virus and influenza virus-derived proteins again periodically, perhaps every 2–3 years, through natural encounters with different subtypes or strains of influenza viruses or through vaccination. It is critical to understand how the influenza-virus-specific CD4 T-cell memory compartment is shaped and remodelled over time, upon periodic encounter with influenza viruses and vaccines, and how these challenges poise us to respond to future encounters with influenza virus. To gain insight into this issue, it is necessary to first understand the diversity and specificity of the primary response and the immunological memory established as the responding lymphocytes contract after the peak of the response.
The factors that are involved in the contraction of the immune response and establishment of immunological memory have been most intensely studied in CD8 T-cell responses (reviewed in refs 8–17). More limited studies have involved CD4 T cells (reviewed in refs 10,18–22) and these have primarily tracked the fate over time of adoptively transferred T-cell receptor (TCR) transgenic CD4 T cells23–26 because of the low frequency of any given CD4 T-cell peptide specificity. It is generally thought that the contraction of T-cell effectors after the peak of the response is the result of programmed cell death, induced either by competition for limiting survival factors and cytokines or through Fas-dependent mechanisms (reviewed in refs 27–29). Once contraction is complete, T-cell memory is primarily maintained by γc cytokines such as interleukin-17 (IL-7) and IL-15 (reviewed in refs 30–32). The role of cognate interactions involving the antigen-specific TCR in selecting or sustaining T-cell memory is controversial and has been the subject of much experimentation. For the most part, a consensus appears to have emerged with CD8 T cells that TCR signalling and MHC class I expression are not needed to sustain functional T-cell memory.33–36 In contrast, there is substantial evidence that TCR-mediated signalling via MHC class II molecules may promote both the persistence and functional capacity of memory CD4 T cells.37–39 The likelihood that TCR-derived signals influence CD4 memory raises the possibility that differences in continued TCR signalling among CD4 T cells during or after transition to memory cells, because of antigen abundance or peptide persistence may alter the final specificity of the memory population compared with that displayed at the peak of the response.
It is not feasible to probe the specificity of the CD4 T-cell repertoire at the memory phase of the primary response in humans both because of the early age of the first contact with influenza virus and because of the limited samples that are available in clinical studies from young children. We have therefore sought to use animal models to study the breadth and specificity of memory CD4 T cells that are elicited after the first response to a live influenza virus infection. In our previously published work, we have evaluated the protein and peptide specificity during the early phases of the primary CD4 T-cell response to live influenza virus infection, using mouse models of primary infection.40–42 These studies have shown that the primary response is diverse. Examination of different strains of mice suggest that the degree of diversity in the elicited CD4 T-cell repertoire depends on the relative promiscuity of the MHC class II molecules expressed, with I-Ab and I-As being examples of the more stringent and selective MHC class II molecule,40 while HLA-DR141–42 and I-Ad and I-Ed (K. Richards, F. Chaves and A. Sant, unpublished data) are more promiscuous. Therefore mice expressing these latter alleles of class II elicit the most diverse CD4 T-cell repertoire. Humans express multiple MHC class II proteins so it is likely that their initial primary CD4 T-cell response is very broad in reactivity.
In the study reported here, we have analysed the CD4 immunodominance hierarchy over time during the primary polyclonal CD4 T-cell response to live influenza infection, using an HLA-DR1 transgenic mouse strain that displays an extensive degree of peptide diversity in the CD4 response at its peak. We asked if and how this pattern of immunodominance changes over time, as the memory compartment becomes established. To our knowledge, there has been no published work that has examined multiple specificities within the polyclonal endogenous CD4 T-cell response to influenza infection over time. In fact, until 2006,40–43 few studies had empirically evaluated the peptide specificity of the CD4 T-cell responses to influenza virus at any stage of the immune response, probably because of the breadth of the response and the paucity and expense of reagents to identify the peptide specificity of CD4 T-cell responses to complex pathogens. In the experiments reported here, we have tracked more than 50 different CD4 T-cell specificities, with peptides derived from four different influenza virus proteins, over time, from the peak to the memory phase of the immune responses. Our studies revealed that the immunodominance hierarchy in the memory CD4 T-cell compartment retains an unexpected degree of diversity, with only a modest perturbation in peptide specificity.
Materials and methods
Cell lines and reagents
DAP-3 fibroblast cells transfected with the genes encoding HLA-DR1, generously provided by E. Long, National Institute of Allergic and Infectious Disease, National Institutes of Health (NIAID, NIH; Bethesda, MD), were used as antigen-presenting cells. They were maintained, as described elsewhere,42 in selective drug that was removed from culture at least 2 days before being used in T-cell stimulation assays.
Mice
The DR1 transgenic mice (B10.M/J-TgN-DR1), obtained from D. Zaller (Merck, Whitehouse Station, NJ) through Taconic Laboratories (Hudson, NY), were maintained in the specific pathogen-free facility at the University of Rochester according to institutional guidelines. Mice were used at 2–4 months of age.
Peptides
Oligomers (18-mers) overlapping by 11 amino acids to encompass the entire sequence of the haemagglutinin (HA) and non-structural protein 1 (NS1) proteins from the H1N1 strain of influenza virus A/New Caledonia/20/99 were obtained from Mimotopes (Clayton, Victoria, Australia) and ProImmune (Oxford, UK), respectively. Seventeen-mer peptides overlapping by 11 amino acids to encompass the entire sequence of the HA and NA proteins from the H1N1 strains of influenza virus A/New Caledonia/20/99, the NS1 sequence from the A/New York/444/2001, and the nucleoprotein (NP) from the H1N1 strain of influenza virus A/New York/348/2003 were used. The following reagents were obtained through the NIH Biodefense and Emerging Infections Research Repository, NIAID, NIH: Peptide Arrays, Influenza Virus A/New Caledonia/20/199 (H1N1) HA protein, NR-2602; neuraminidase (NA) protein, NR-2606; Peptide Array, Influenza Virus A/New York/444/2001 (H1N1) NS1, NR-2612; and Peptide Array, Influenza Virus A/New York/348/2003 highly (98%) conserved between the virus strains A/New Caledonia/20/99, A/New York/348/2003 and A/New York/444/2001. All peptides were reconstituted at 10 mm in PBS, with or without added DMSO, to increase the solubility of hydrophobic peptides, and 1 mm dithiothreitol, for cysteine-containing peptides. Working stocks (100 μm or 1 mm) were prepared in complete Dulbecco's modified Eagle's medium, filter sterilized and stored at −20°, as were the concentrated stocks.
Influenza virus infection of mice
Influenza A/New Caledonia/20/99 virus was produced in eggs as we have previously described.42 HLA-DR1 transgenic mice were infected intranasally at 50 000 EID50 (egg infectious dose), unless otherwise noted, in 30 μl PBS after being anaesthetized by intraperitoneal injection with tribromoethanol (Avertin; 250–300 μl per mouse). Eight, 10, 15, 30 or 60 days post-infection, mice were killed and the spleen and mediastinal lymph nodes were excised and used as sources of CD4 T cells for EliSpot analyses. Lymphocytes were pooled from four to six mice, unless otherwise stated, and depleted of B cells, CD8 cells and macrophages by negative selection using MACS depletion (Miltenyi Biotec, Gladbach, Germany), according to the manufacturer's instructions. Purity was assessed by analytical flow cytometry and indicated that purity of CD4 T cells was between 85 and 92% with no detectable contamination with CD8 T cells.
EliSpot assays
Peptide-specific IL-2 EliSpot assays were performed as previously described.42 Briefly, 96-well filter plates (Millipore, Billerica, MA) were coated with 2 μg/ml purified rat anti-mouse IL-2 (clone JES6-1A12; BD Biosciences, San Jose, CA) in PBS, washed and incubated with medium to block non-specific binding. CD4 T cells (350 000 cells/well) were co-cultured with DAP-3 fibroblasts expressing the HLA-DR1 MHC class II protein (35 000 cells/well) and with the indicated peptide at a final concentration of 10 μm in a total volume of 200 μl for 18–20 hr at 37° in 5% CO2. In most experiments, a previously defined immunodominant peptide (HA-75) was included in the EliSpot assays to control for the degree of CD4 T-cell priming in each experiment. After overnight co-culture, plates were processed to visualize IL-2-producing cells as described elsewhere37,38 and cytokine EliSpots were counted using an Immunospot reader series 2A, using immunospot software version 2 (Shaker Heights, OH).
Results
The primary CD4 T-cell response to live influenza infection in HLA-DR1 transgenic mice displays extensive peptide diversity
In previous studies,41,42 we examined the CD4 T-cell immunodominance hierarchy of the primary CD4 T-cell response to live influenza infection in HLA-DR1 transgenic mice. These studies, evaluating CD4 T-cell specificity at the peak of the response (days 10–15 post-infection in the spleen), revealed the primary response to be extremely diverse, and encompassed more than 70 different peptide specificities. All proteins tested so far, with the exception of the small M2 protein, contain HLA-DR1 restricted peptide epitopes recognized by CD4 T cells. Figure 1 shows representative data examining the immunodominance hierarchy to a subset of these epitopes tested with CD4 T cells isolated from the spleen at days 8–10 post-infection where the responses to 20 peptides derived from HA, six from NS1 and 14 from NP were studied, and peptide-reactive cells were quantified by cytokine EliSpot assays. The data are presented as cytokine-producing cells per million CD4 T cells and data from six independent experiments are shown, with the average values indicated for each peptide. The range in frequency varied from 300–500 per million CD4 T cells, for the most immunodominant peptide epitopes, to approximately 50 cells per million epitope-specific CD4 T cells for the least immunodominant (but consistently positive) epitopes. Included among the most highly immunodominant epitopes were peptides derived from each of the proteins examined (HA, NS1 and NP). The results shown are consistent with our previous studies indicating that the primary response to H1N1 influenza virus infection can lead to priming and expansion of CD4 T cells with highly diverse peptide specificity.
Figure 1.
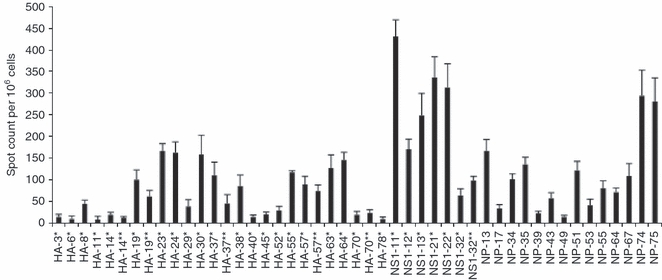
Highly diverse peptide specificity at the peak CD4 T-cell response to live infection in HLA-DR1 transgenic mice. Results from peptide stimulation at the peak of the immune response demonstrate the diversity of the primary CD4 T-cell response to influenza virus, haemagglutinin (HA), non-structural protein 1 (NS1) and nucleoprotein (NP). Mice were infected intranasally with A/New Caledonia/20/99 influenza virus, and the number of interleukin-2 (IL-2) -producing CD4 splenocytes was determined 8–10 days later by 18 hr in vitro stimulation with DAP.3-5.3.1 DR1-positive transfectants cultured with the single 17-mer peptides at a final concentration of 10 μm. The IL-2 EliSpot results were quantified as the averages for duplicate wells with the background value subtracted. The results shown are the average of six independent experiments with the standard error shown. Spleen cells from four to five mice were pooled for each experiment.
The kinetics of the primary response in the draining lymph node peaks early and then contracts over 3–4 weeks
We sought to determine if and how the influenza-virus-specific immunodominance hierarchy becomes remodelled over time as the peak of the response transitions to a memory compartment. Figure 2 shows the kinetics of the influenza-virus-specific CD4 T-cell response over time in the mediastinal lymph node, where priming of influenza-virus-reactive T cells takes place after natural infection. In this study, we performed cytokine EliSpots with CD4 T cells isolated from the lymph nodes from mice infected with live virus, testing days 8–10, 15, 30 and 60–68, using a subset of the individual peptides shown in Fig. 1, or a larger set for later experiments that included epitopes from neuraminidase (NA). To include results from multiple independent experiments, the number of peptide-reactive CD4 T cells detected in the EliSpot assay were summed and then adjusted to reflect the total number of lymph node cells isolated in each experiment. These values, representing the total influenza-virus-specific reactivity within the draining lymph node over time, were then analysed. As can be seen in Fig. 2(a), which shows the results of four independent experiments, the number of influenza-virus-specific CD4 T cells in the draining lymph node decays over time, and at the latest days tested (days 60–70), the frequency of influenza-virus-reactive cells was approximately 10% the level of that detected at days 8–10. Generally, responses peaked at days 8–10 and then decreased by day 30 post-infection in the lymph node. Influenza-virus-specific CD4 T cells in the spleen also peaked at approximately days 10–15 post-infection, lagging a few days behind cell from the draining lymph node. We consider the splenic CD4 T cells as representative of the circulating memory population and have examined these in the most detail. However, in many experiments, lymph node CD4 T cells were examined in parallel with spleen CD4 T cells for specificity with a smaller subset of peptides, because of the modest yield of CD4 T cells recovered from this tissue. An example of the assay of specificity of CD4 T cells from the lymph node over time is shown in Fig. 2(b).
Figure 2.
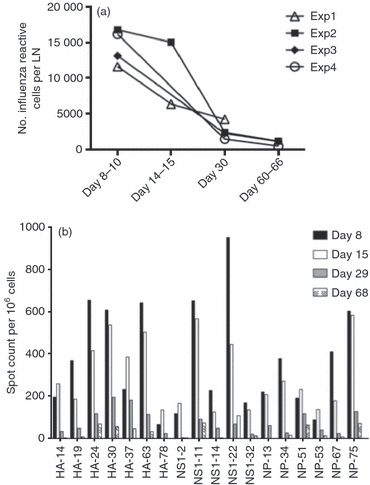
Expansion and contraction of the influenza-specific CD4 T-cell response in the draining lymph node (LN). Interleukin-2 (IL-2) EliSpot assays were used to assess the number of cytokine-producing cells for each influenza-virus-derived peptide. The total number of spots was calculated as the sum of the epitopes tested at each time-point. The data are represented as the total spot count per draining LN for four independent experiments. (a) Total number of influenza-virus-specific CD4 T cells in the draining LN over time in independent experiments. (b) Representative assay showing the specificity of the CD4 T cells from the LN over time. Mice were infected intranasally with A/New Caledonia/20/99 influenza virus, and the number of IL-2-producing CD4 lymphocytes was determined 8, 15, 29 and 68 days post-infection by 18 hr in vitro stimulation with DAP.3-5.3.1 DR1-positive transfectants cultured with the single 17-mer peptides at a final concentration of 10 μm. The IL-2 EliSpot results were quantified as the averages for duplicate wells with the background value subtracted. The results shown are representative of four independent experiments for each peptide. The spleen cells from four to five mice were pooled for each experiment.
The diversity of CD4 T-cell memory response remains high over time and does not display preferential retention of immunodominant specificities
After establishing that it is feasible to identify CD4 T cells of many different peptide specificities, we pursued studies to track the fate of these cells over time. In Fig. 3, we show the results of experiments that have probed the influenza-virus-specific immunodominance hierarchy over time in the spleen, where the number of influenza peptide-reactive CD4 cells has been quantified by cytokine EliSpot assays. In the experiments shown, reactivity to peptide epitopes from four different influenza proteins (HA, NS1, NP and NA) were tested. Data from two independent experiments are presented, with the range in values obtained from the two experiments indicated for each peptide. The results are presented as the number of peptide-reactive cells per million splenic CD4 T cells tested at day 10, day 30 and day 60 post-infection. T cells reactive with different peptides from each protein are readily detectable at each time-point, and the diversity of the memory response remains exceptionally broad, with more than 50 different peptides eliciting CD4 T-cell cytokine production. Overall, the results suggest that as immunological memory is established, there is little evidence of selective retention of the more immunodominant specificities (e.g. HA 35, NS1 13 and NP 74) at the expense of the minor specificities. Even epitopes such as HA 83, NP 35 and NA 1 (arrows), which recruited fewer than 100 T cells per million at day 10 (and so are present at a frequency of < 1 : 10 000) are generally preserved and detectable at day 60 after infection.
Figure 3.
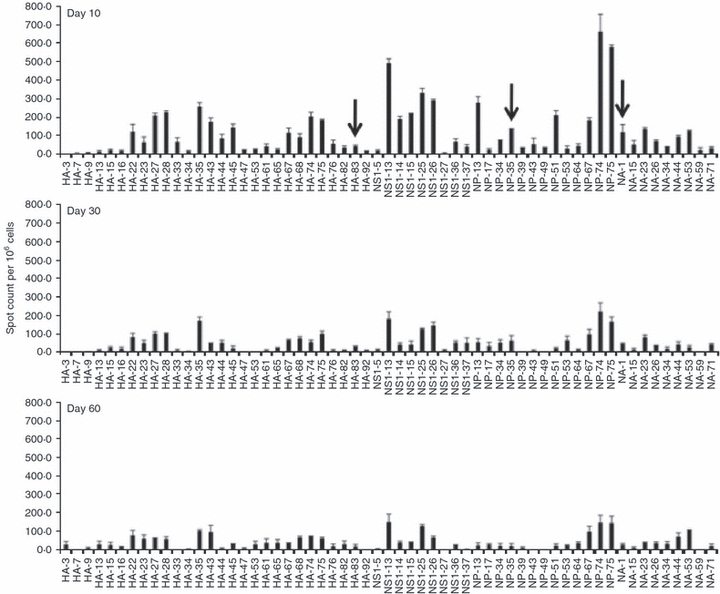
The influenza-virus-specific CD4 T-cell response remains diverse after contraction. Average results of two independent experiments that assessed the influenza-specific immunodominance hierarchy of CD4 T cells in the spleen over time are shown. CD4 T cells isolated from the spleen 10, 30 and 60 days post-infection were screened toward reactivity to the single 17-mer peptide indicated at a final concentration of 10 μm. The sequences of the individual peptides are shown in Table 1. Arrows indicate minor peptide specificities that persist in the memory phase of the response. The range of the response is indicated for each peptide tested.
To evaluate the changes in relative immunodominance of any given peptide over time and to be able to compare the results from many independent experiments, we normalized the data from each experiment. The total number of cytokine-producing cells detected with individual peptides was summed, and this number represents the ‘total’ influenza-virus-reactive CD4 T-cell value for any given experiment. The response to each individual peptide tested in each experiment was then calculated as a fraction of the total influenza-virus-reactive cells. For example, if there were 10 000 total influenza-virus-reactive cells detected when EliSpots from each of the different peptides was summed and peptide #1 recruited 200 cells, then specificity #1 is represented as 200/10 000 or 2% of the response to influenza virus. We calculated this value for each peptide in each experiment and averaged the results of independent assays. In Fig. 4 we show the results of this type of analysis for two groups of experiments, the first set of four experiments (Fig. 4a) assayed at day 8, day 15 and day 30 and the second set of two independent experiments (shown in Fig. 4b) assayed at day 10, days 28–30 and days 60–68. The two sets of experiments differed primarily by the addition of NA epitopes in the second set and some small changes in peptide length and numbering for HA and NS1 peptides (see Table 1, indicating the precise sequence of each peptide tested). Because the total number of epitopes tested in set two was higher than set one, the fractional responses for each peptide in the second set was lower than that in the first set of experiments, even though the absolute number of T cells recruited by any given peptide was similar in the two groups of experiments. It is clear from these multiple, independent experiments that although the total number of influenza-reactive CD4 T cells diminishes over time during the course of an immune response, a given peptide's place in the immunodominance hierarchy does not change dramatically from peak responses to memory responses. T cells specific for peptides that assume immunodominance early remain immunodominant, whereas T cells specific for epitopes that are poorly represented at the peak of the response remain minor in the memory population.
Figure 4.
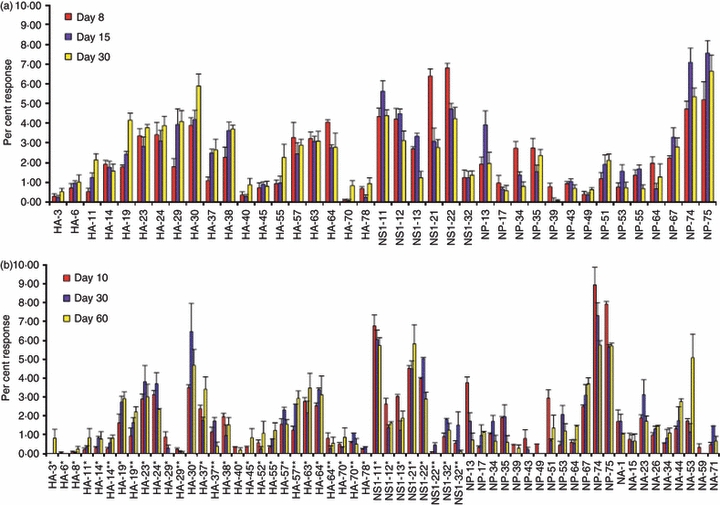
The immunodominance hierarchy displayed at the memory phase of the immune response mimics that seen at the peak of the response. The total number of cytokine-producing CD4 cells isolated from the spleen of previously infected mice cells for all peptides tested were summed to yield the total influenza-virus-reactive CD4 T-cell value for each experiment. This value was then used to calculate the fraction of the response dedicated to each individual peptide over time, represented as the per cent response. (a) Average of four experiments where days 8, 15 and 30 post infection were tested with the error among each of the experiments; (b) the results of two additional experiments testing responses at days 10, 30 and 60 post-infection that included peptides from neuraminidase (NA), with the range in the experiments shown.
Table 1.
Specific sequences for the peptides tested in these experiments
| Peptide | Amino acid sequence | Related amino acid sequence | |
|---|---|---|---|
| HAp3* | TATYADTICIGYHANNS | TYADTICIGYHANNSTDT | HAp3 |
| HA-6* | LEKNVTVTHSVNLLEDS | VLEKNVTVTHSVNLLEDS | HAp6 |
| HA-8* | NLLEDSHNGKLCLLKGI | LEDSHNGKLCLLKGIAPL | HAp8 |
| HA-11* | CSVAGWILGNPECELLI | NCSVAGWILGNPECELLI | HApll |
| HA-14* | CELLISKESWSYIVETP | SWSYIVETPNPENGTCYP | HApl4 |
| HA-14** | KESWSYIVETPNPENGT | ||
| HA-19* | SSFERFEIFPKESSWPN | SFERFEIFPKESSWPNHT | HApl9 |
| HA-19** | EIFPKESSWPNHTVTGV | ||
| HA-23* | GKSSFYRNLLWLTGKNG | NGKSSFYRNLLWLTGKNG | HAp23 |
| HA-24* | RNLLWLTGKNGLYPNLS | RNLLWLTGKNGLYPNLSK | HAp24 |
| HA-29* | LWGVHHPPNIGNQRALY | HPPNIGDQRALYHTENAY | HAp29 |
| HA-29** | PPNIGNQRALYHTENAY | ||
| HA-30* | NQRALYHTENAYVSWS | QRALYHTENAYVSWSSH | HAp30 |
| HA-37* | LEPGDTIIFEANGNLIA | GDTIIFEANGNLIAPWYA | HAp37 |
| HA-37** | IIFEANGNLIAPWYAFA | ||
| HA-38* | GNLIAPWYAFALSRGFG | ANGNLIAPWYAFALSRGF | HAp38 |
| HA-40* | SRGFGSGIITSNAPMDE | SRGFGSGIITSNAPMDEC | HAp40 |
| HA-45* | NVHPVTIGECPKYVRSA | QNVHPVTIGECPKYVRSA | HAp45 |
| HA-52* | TGMVDGWYGYHHQNEQG | TGMVDGWYGYHHQNEQGS | HA-52 |
| HA-55* | DQKSTQNAINGITNKVN | ADQKSTQNAINGITNKVN | HAp55 |
| HA-57* | TNKVNSVIEKMNTQFTA | NKVNSVIEKMNTQFTAVG | HAp57 |
| HA-57** | VIEKMNTQFTAVGKEFN | ||
| HA-63* | IWTYNAELLVLLENERT | WTYNAELLVLLENERTLD | HAp63 |
| HA-64* | ELLVLLENERTLDFHDS | LVLLENERTLDFHDSNVK | HAp64 |
| HA-64** | ENERTLDFHDSNVKNLY | ||
| HA-70* | CFEFYHKCNNECMESVK | YHKCNNECMESVKNGTYD | HAp70 |
| HA-70** | KCNNECMESVKNGTYDY | ||
| HA-78* | SLVLLVSLGAISFWMCS | LVLLVSLGAISFWMCSNG | HAp78 |
| NS1-11* | ESDEAFKMTMASALASR | EESDEAFKMTMASALASR | NSlpll |
| NS1-12* | KMTMASALASRYLTDMT | KMTMASALASRYLTDMTI | NSlpl2 |
| NS1-13* | ALASRYLTDMTIEEMSR | LASRYLTDMTIEEMSRDW | NSlpl3 |
| NS1-21* | LTLLRAFTEEGAIVGEI | LENLTLLRAFTEEGAIVG | NSlp21 |
| NS1-22* | FTEEGAIVGEISPLPSL | RAFTEEGAIVGEISPLPS | NSlp22 |
| NS1-22** | IVGEISPLPSLPGHTNE | ||
| NS1-32* | GGPPFTPTQKRKMAGTI | PFTPTQKRKMAGTIRSEV | NSlp32 |
| NS1-32** | PTQKRKMAGTIRSEV | ||
| NPpl3 | ERRNKYLEEHPSAGKDP | ||
| NPpl7 | YKRVDGKWVRELVLYDK | ||
| JPp34 | RGINDRNFWRGENGRKT | ||
| JPp35 | NFWRGENGRKTRIAYER | ||
| JPp39 | KFQTAAQKAMMDQVRES | ||
| JPp43 | EIEDLTFLARSALILRG | ||
| JPp49 | GYDFEKEGYSLVGVDPF | ||
| JPp53 | VYSLIRPNENPAHKSQL | ||
| NPp64 | TLELRSRYWAIRTRSGG | ||
| NPp67 | TNQQRASAGQISTQPTF | ||
| NPp74 | SDMRAEIIKMMESARPE | ||
| NPp75 | IIKMMESARPEEVSFQG | ||
| NApl | MNPNQKIITIGSISIAI | ||
| NApl5 | TLAGNSSLCSISGWAIY | ||
| NAp23 | RTFFLTQGALLNDKHSN | ||
| NAp26 | GTVKDRSPYRALMSCPL | ||
| NAp34 | ISGPDNGAVAVLKYNGI | ||
| NAp44 | IFKIEKGKVTKSIELNA | ||
| NAp53 | NLDYQIGYICSGVFGDN | ||
| NAp59 | GVKGFSYKYGNGVWIGR | ||
| NAp71 | DCIRPCFWVELVRGLPR |
An * indicates an alternative, but related, peptide that was tested in later experiments that differ because of differences in length of 17-mer versus 18-mer peptide sets. The double asterisks is a second sequence that overlaps with the sequence of the single * and is partially contained within the related sequence.
A shift in specificities toward HA
As discussed above, for the most part, each of the CD4 T-cell peptide specificity responses was maintained in the memory population. However, there was one pattern of change in reactivity that was consistently observed in independent experiments. Generally, CD4 T cells specific for HA became somewhat enriched over time, from the early peak of the response (at days 8–10) to later time-points (day 30 or day 60). This increased representation of CD4 T cells toward HA occurs somewhat at the ‘expense’ of CD4 T cells specific for NS1, which become less represented over time. The changes are modest, but were observed with multiple peptides in these two proteins. The pattern is best visualized when the number of CD4 T cells reactive with each peptide within a given protein are summed and represented as a fraction of the total number of influenza-virus-reactive CD4 T cells assayed. This calculation is represented as a set of pie graphs (Fig. 5) that show the distribution of reactivities over time, for each of the six independent experiments we have performed, where we omit the values for NA-reactive cells to make comparison among the six experiments easier to visualize. Experiments one to four were performed at days 8, 15 and 30 post infection and included peptide epitopes from HA, NS1 and NP, whereas experiments five and six evaluated immunodominance at day 10, day 30 and day 60 post infection and included NA. These data demonstrate that the ‘slice’ of the pie representing all HA specificities in the primary response grows over time, while the ‘slice’ of the NS1 pie contracts as memory is established.
Figure 5.
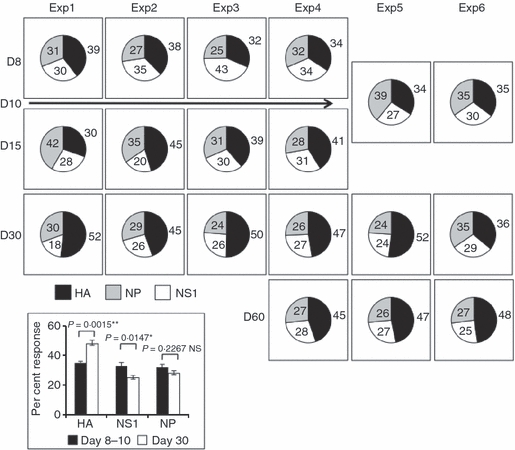
The influenza virus protein-specific CD4 T-cell response shows a shift in reactivity toward haemagglutinin (HA) -derived specificities after the peak of the response. Values from individual peptides tested in the experiments shown in Fig. 4(a,b) were grouped according to the protein from which the peptide was derived and then summed. The fractional response of the CD4 T cells specific for each protein over the time–course of the immune response were then represented as shown, with the per cent of the response specific for each protein at each time-point indicated within or beside each ‘slice’ of the pie shown. The inset shows the average (n = 6) total per cent CD4 T-cell response at the peak (days 8–10, black bars) and memory (day 30, white bars) phases of the immune response for each protein. The standard errors are indicated by error bars for each protein. Statistical analysis was completed using a Student's t-test with a 95% confidence interval and demonstrates that the shift toward HA is statistically significant, as is the loss in non-structural protein-1 (NS1). There is no statistically significant change in nucleoprotein (NP).
The inset to Fig. 5 shows that the changes observed in the relative reactivity of CD4 T cells toward HA-derived and NS1-derived peptides over time are statistically significant, with NP-reactive cells maintaining a steady representation in the total immune response.
Discussion
In this study, we have evaluated if and how the memory CD4 T-cell repertoire changes over time with regard to specificity. In the HLA-DR1 transgenic model that we have analysed, the primary response is exceptionally diverse and includes as many as 70 different peptide specificities, drawn from many different influenza proteins, including HA, NA, NP and NS1. We sought to evaluate if the immunodominance hierarchy narrows or shifts after contraction of the response to the long-term memory compartment. At the initiation of these studies, we could envision at least three possible patterns. First, we thought it possible that all CD4 T-cell specificities would be preserved in the memory compartment, with each peptide specificity diminishing to some constant fractional representation of that present at the peak of the response. Second, as the response contracts, the memory compartment could become populated primarily by the most immunodominant specificities, perhaps preferentially retained by the greater strength or duration of the TCR that initially primed the CD4 T cells19,23,24,44–48 or by limitations in the ‘niche’ allowed for T-cell memory in vivo,11,20,24,49–54 hence selectively retaining only the highest frequency specificities. In this case, the immunodominant hierarchy could become narrowed by exclusion of less abundant specificities. Finally, the immunodominance hierarchy might shift over time, with gains and losses in specificity that track with particular influenza proteins or with subsets of peptides, but independently of whether the specificity was highly or poorly represented early in the response. Such shifts might occur if there were asymmetries in the half-lives of the different influenza proteins, hence allowing for more prolonged presentation during priming,55,56 or differences in the persistence of MHC class II–peptide complexes after pathogen clearance. Our laboratory has shown that immunogenic MHC class II–peptide complexes can have dissociation half-times that vary between 5 and 250 hr.57–62 If MHC class II–peptide complexes displayed by antigen-presenting cells influence the representation or homeostasis of memory CD4 T cells over time, then changes in the repertoire might take place, with enrichment of CD4 T cells with specificity for the most persistent complexes.
The studies reported here revealed that almost all specificities that were originally detected at the peak of the primary response were preserved in the memory compartment. In preliminary experiments with strains of mice with more limited diversity, such as those of the H-2b and H-2s MHC haplotypes (40) where most epitopes recruit large numbers of CD4 T cells, we have found that each of the specificities detected at the peak of the response persist in the memory compartment, agreeing with our results in the HLA-DR1 strain presented here. Our major conclusion, therefore, is that the initial CD4 memory that is established from the first encounter with live influenza virus largely reflects the broad diversity that is initiated during the primary immune response. It is interesting to consider how the memory repertoire is re-shaped over time, with periodic encounter with viruses and vaccines that share some, but not all, protein sequences in their viral proteins. One might expect that the fraction of pre-existing memory that is specific for sequences shared among the different strains of virus would become boosted and respond more rapidly than new specificities, and that the final new memory composition might become disproportionately occupied by the shared and boosted peptide specificities. Presumably in humans who encounter influenza virus or vaccines ever few years, this remodelling takes place continually. However, despite intuitive predictions, to our knowledge, this issue has not been addressed experimentally in an unbiased and comprehensive way, by tracking the rise and fall of multiple specificities during the primary, secondary and subsequent immune responses to viral challenge and this issue is the subject of current investigations in our laboratory. We have addressed this question at a single time-point in normal human adults that have presumably been exposed to multiple strains of viruses and their derived vaccines over their lifetimes and we found, somewhat surprisingly, that the memory CD4 compartment is not disproportionately occupied by CD4 epitopes derived from the genetically conserved internal viral proteins. It is possible that memory CD4 T-cell reactivation does not preclude expansion of new specificities.
One modest change in specificity did occur between the peak of the immune response and the memory phase. Collectively, CD4 T cells specific for the HA protein were better represented in the memory phase than they were at the peak of the response. Whereas most epitopes maintained their place in the immunodominance hierarchy, T cells specific for some HA epitopes (e.g. HA 11, HA 19, HA 30) increased their representation at later time-points. The converse was seen for NS1 epitopes (NS1 12, NS1 13) as well as some NP-derived epitopes (NP 13, NP 34). The scatter among the responses to different epitopes among independent experiments can be quite large, probably as the result of stochastic variation in T-cell precursor frequencies in individual mice for different epitopes. Therefore, the overall increased representation of HA-specific T cells over time was most readily observed when the responses of all of the HA-specific CD4 T cells were summed and displayed as a fraction of the total influenza-virus-specific responses, as depicted in Fig. 5. We have not yet been able to determine why HA-specific cells become enriched over time, but several mechanisms are possible. The HA protein itself might persist for longer in the draining lymph node compared with other proteins and so remain available for MHC class II-restricted presentation. A recent study by Jelley-Gibbs et al.55 suggested that one HA-derived epitope recognized by a TCR transgenic mouse persisted for > 5 weeks, long after viral RNA was detectable. An extended half-life of HA protein compared with cytosolic proteins such as NP and NS1 might relate to its membrane localization. Alternatively, peptides derived from the HA protein might be better presented by subsets of antigen-presenting cells that have been implicated in promoting memory, such as B cells.63,64 Finally, some HA-derived peptides might have extended persistence on the presenting class II molecule, which might promote continued expansion or selective retention of these peptide-specific CD4 T cells, although it is difficult to imagine that off-rates of peptides with class II will track with a particular protein rather than the peptide sequence. In a separate set of studies, we have measured the dissociation half-times of two immunodominant HA epitopes (HA 63 and HA 23) and find that these peptides have relatively stable interactions with the HLA-DR1 molecule (t½ of 360 and 85 hr, respectively, data not shown) but the CD4 T cells specific for the more stable MHC class II–peptide complex (HA 63) is not preferentially enriched in the memory compartment. In future experiments we plan to determine whether other strains of mice show preference for HA-derived epitopes in the memory CD4 T-cell compartment and will explore if there are any functional consequences associated with the shift in specificities over time.
The main conclusion of the studies presented here is that after a primary immune response to live influenza virus infection, the CD4 memory compartment retains a remarkable degree of peptide-specific diversity, with even the minor specificities displayed at the peak of the response preserved. Therefore, if protein specificity does determine the efficiency of CD4 T-cell help to B cells, all protein-specific responses are likely to be available long after the initial contact with influenza virus. One major question raised by these studies is how the broad peptide specificity in the memory compartment shifts upon re-challenge with viruses that share some but not all of the epitopes present in the original priming virus. One would expect that upon challenge, the memory cells reactive toward the conserved epitopes, even those that represent minor specificities, would become rapidly boosted and expand, perhaps at some expense of the novel specificities present in the new challenge virus or vaccine. How CD4 T-cell memory is re-established after multiple challenges is clearly an important area to understand, as there must be considerable constraints on the absolute size of the niche11,24,51–54 that is allocated to the CD4 T-cell memory compartment.
Acknowledgments
The authors would like to thank Dr Jim Miller and Scott Leddon (URMC) for editorial suggestions during preparation of this manuscript and members of the D.H. Smith Center for Vaccine Biology and Immunology for helpful discussions. This work was supported with funding from NIAID, NIH (HHSN266200700008C and R01 AI51542 to A.J.S.).
Disclosures
The authors have no conflict of interest.
References
- 1.Boni MF. Vaccination and antigenic drift in influenza. Vaccine. 2008;26(Suppl. 3):C8–14. doi: 10.1016/j.vaccine.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Treanor JD. Influenza – the goal of control. N Engl J Med. 2007;357:1439–41. doi: 10.1056/NEJMe078140. [DOI] [PubMed] [Google Scholar]
- 3.Johansson BE, Brett IC. Changing perspective on immunization against influenza. Vaccine. 2007;25:3062–5. doi: 10.1016/j.vaccine.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 4.Cox NJ, Subbarao K. Global epidemiology of influenza: past and present. Annu Rev Med. 2000;51:407–21. doi: 10.1146/annurev.med.51.1.407. [DOI] [PubMed] [Google Scholar]
- 5.Luke CJ, Subbarao K. Vaccines for pandemic influenza. Emerg Infect Dis. 2006;12:66–72. doi: 10.3201/eid1201.051147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Treanor J. Influenza vaccine – outmaneuvering antigenic shift and drift. N Engl J Med. 2004;350:218–20. doi: 10.1056/NEJMp038238. [DOI] [PubMed] [Google Scholar]
- 7.Ellebedy AH, Webby RJ. Influenza vaccines. Vaccine. 2009;27(Suppl. 4):D65–8. doi: 10.1016/j.vaccine.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schluns KS, Lefrancois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003;3:269–79. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- 9.Prlic M, Lefrancois L, Jameson SC. Multiple choices: regulation of memory CD8 T cell generation and homeostasis by interleukin (IL)-7 and IL-15. J Exp Med. 2002;195:F49–52. doi: 10.1084/jem.20020767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sallusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity. 2010;33:451–63. doi: 10.1016/j.immuni.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–62. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J Virol. 2004;78:5535–45. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masopust D, Ahmed R. Reflections on CD8 T-cell activation and memory. Immunol Res. 2004;3:151–60. doi: 10.1385/IR:29:1-3:151. [DOI] [PubMed] [Google Scholar]
- 14.Kim HR, Hwang KA, Park SH, Kang I. IL-7 and IL-15: biology and roles in T-cell immunity in health and disease. Crit Rev Immunol. 2008;28:325–39. doi: 10.1615/critrevimmunol.v28.i4.40. [DOI] [PubMed] [Google Scholar]
- 15.Gourley TS, Wherry EJ, Masopust D, Ahmed R. Generation and maintenance of immunological memory. Semin Immunol. 2004;16:323–33. doi: 10.1016/j.smim.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 16.Rodrigues L, Bonorino C. Role of IL-15 and IL-21 in viral immunity: applications for vaccines and therapies. Expert Rev Vaccines. 2009;8:167–77. doi: 10.1586/14760584.8.2.167. [DOI] [PubMed] [Google Scholar]
- 17.Jameson SC, Masopust D. Diversity in T cell memory: an embarrassment of riches. Immunity. 2009;31:859–71. doi: 10.1016/j.immuni.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stockinger B, Kassiotis G, Bourgeois C. CD4 T-cell memory. Semin Immunol. 2004;16:295–303. doi: 10.1016/j.smim.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Sallusto F, Lanzavecchia A. Heterogeneity of CD4+ memory T cells: functional modules for tailored immunity. Eur J Immunol. 2009;39:2076–82. doi: 10.1002/eji.200939722. [DOI] [PubMed] [Google Scholar]
- 20.McKinstry KK, Strutt TM, Swain SL. The potential of CD4 T-cell memory. Immunology. 2010;130:1–9. doi: 10.1111/j.1365-2567.2010.03259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lees JR, Farber DL. Generation, persistence and plasticity of CD4 T-cell memories. Immunology. 2010;130:463–70. doi: 10.1111/j.1365-2567.2010.03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swain SL, Agrewala JN, Brown DM, et al. CD4+ T-cell memory: generation and multi-faceted roles for CD4+ T cells in protective immunity to influenza. Immunol Rev. 2006;211:8–22. doi: 10.1111/j.0105-2896.2006.00388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blair DA, Lefrancois L. Increased competition for antigen during priming negatively impacts the generation of memory CD4 T cells. Proc Natl Acad Sci USA. 2007;104:15045–50. doi: 10.1073/pnas.0703767104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams MA, Ravkov EV, Bevan MJ. Rapid culling of the CD4+ T cell repertoire in the transition from effector to memory. Immunity. 2008;28:533–45. doi: 10.1016/j.immuni.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu H, Huston G, Duso D, Lepak N, Roman E, Swain SL. CD4+ T cell effectors can become memory cells with high efficiency and without further division. Nat Immunol. 2001;2:705–10. doi: 10.1038/90643. [DOI] [PubMed] [Google Scholar]
- 26.Teijaro JR, Verhoeven D, Page CA, Turner D, Farber DL. Memory CD4 T cells direct protective responses to influenza virus in the lungs through helper-independent mechanisms. J Virol. 2010;84:9217–26. doi: 10.1128/JVI.01069-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouillet P, O'Reilly LA. CD95, BIM and T cell homeostasis. Nat Rev Immunol. 2009;9:514–9. doi: 10.1038/nri2570. [DOI] [PubMed] [Google Scholar]
- 28.Weant AE, Michalek RD, Khan IU, Holbrook BC, Willingham MC, Grayson JM. Apoptosis regulators Bim and Fas function concurrently to control autoimmunity and CD8+ T cell contraction. Immunity. 2008;28:218–30. doi: 10.1016/j.immuni.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 29.Green DR. Fas Bim boom! Immunity. 2008;28:141–3. doi: 10.1016/j.immuni.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 30.D'Cruz LM, Rubinstein MP, Goldrath AW. Surviving the crash: transitioning from effector to memory CD8+ T cell. Semin Immunol. 2009;21:92–8. doi: 10.1016/j.smim.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Osborne LC, Abraham N. Regulation of memory T cells by gammac cytokines. Cytokine. 2010;50:105–13. doi: 10.1016/j.cyto.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 32.Sandau MM, Kohlmeier JE, Woodland DL, Jameson SC. IL-15 regulates both quantitative and qualitative features of the memory CD8 T cell pool. J Immunol. 2010;184:35–44. doi: 10.4049/jimmunol.0803355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leignadier J, Hardy MP, Cloutier M, Rooney J, Labrecque N. Memory T-lymphocyte survival does not require T-cell receptor expression. Proc Natl Acad Sci USA. 2008;105:20440–5. doi: 10.1073/pnas.0806289106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choo DK, Murali-Krishna K, Anita R, Ahmed R. Homeostatic turnover of virus-specific memory CD8 T cells occurs stochastically and is independent of CD4 T cell help. J Immunol. 2010;185:3436–44. doi: 10.4049/jimmunol.1001421. [DOI] [PubMed] [Google Scholar]
- 35.Lau LL, Jamieson BD, Somasundaram T, Ahmed R. Cytotoxic T-cell memory without antigen. Nature. 1994;369:648–52. doi: 10.1038/369648a0. [DOI] [PubMed] [Google Scholar]
- 36.Murali-Krishna K, Lau LL, Sambhara S, Lemonnier F, Altman J, Ahmed R. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science. 1999;286:1377–81. doi: 10.1126/science.286.5443.1377. [DOI] [PubMed] [Google Scholar]
- 37.Bushar ND, Corbo E, Schmidt M, Maltzman JS, Farber DL. Ablation of SLP-76 signaling after T cell priming generates memory CD4 T cells impaired in steady-state and cytokine-driven homeostasis. Proc Natl Acad Sci USA. 2010;107:827–31. doi: 10.1073/pnas.0908126107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kassiotis G, Garcia S, Simpson E, Stockinger B. Impairment of immunological memory in the absence of MHC despite survival of memory T cells. Nat Immunol. 2002;3:244–50. doi: 10.1038/ni766. [DOI] [PubMed] [Google Scholar]
- 39.Seddon B, Tomlinson P, Zamoyska R. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat Immunol. 2003;4:680–6. doi: 10.1038/ni946. [DOI] [PubMed] [Google Scholar]
- 40.Nayak J, Richards KA, Chaves FA, Sant AJ. Analysis of the specificity of CD4 T cells during the primary immune response to influenza virus reveals dramatic MHC-linked asymmetries in reactivity to individual viral proteins. Viral Immunol. 2010;23:169–80. doi: 10.1089/vim.2009.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richards KA, Chaves FA, Sant AJ. Infection of HLA-DR1 transgenic mice with a human isolate of influenza A virus (H1N1) primes a diverse CD4 T-cell repertoire that includes CD4 T cells with heterosubtypic cross-reactivity to avian (H5N1) influenza virus. J Virol. 2009;83:6566–77. doi: 10.1128/JVI.00302-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richards KA, Chaves FA, Krafcik FR, Topham DJ, Lazarski CA, Sant AJ. Direct ex vivo analyses of HLA-DR1 transgenic mice reveal an exceptionally broad pattern of immunodominance in the primary HLA-DR1-restricted CD4 T-cell response to influenza virus hemagglutinin. J Virol. 2007;81:7608–19. doi: 10.1128/JVI.02834-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crowe SR, Miller SC, Brown DM, et al. Uneven distribution of MHC class II epitopes within the influenza virus. Vaccine. 2006;24:457–67. doi: 10.1016/j.vaccine.2005.07.096. [DOI] [PubMed] [Google Scholar]
- 44.Lanzavecchia A, Sallusto F. Antigen decoding by T lymphocytes: from synapses to fate determination. Nat Immunol. 2001;2:487–92. doi: 10.1038/88678. [DOI] [PubMed] [Google Scholar]
- 45.De Boer RJ, Perelson AS. T cell repertoires and competitive exclusion. J Theor Biol. 1994;169:375–90. doi: 10.1006/jtbi.1994.1160. [DOI] [PubMed] [Google Scholar]
- 46.Fishman MA, Perelson AS. Lymphocyte memory and affinity selection. J Theor Biol. 1995;173:241–62. doi: 10.1016/s0022-5193(95)80003-4. [DOI] [PubMed] [Google Scholar]
- 47.Kassiotis G, Zamoyska R, Stockinger B. Involvement of avidity for major histocompatibility complex in homeostasis of naive and memory T cells. J Exp Med. 2003;197:1007–16. doi: 10.1084/jem.20021812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gett AV, Sallusto F, Lanzavecchia A, Geginat J. T cell fitness determined by signal strength. Nat Immunol. 2003;4:355–60. doi: 10.1038/ni908. [DOI] [PubMed] [Google Scholar]
- 49.Stockinger B, Bourgeois C, Kassiotis G. CD4+ memory T cells: functional differentiation and homeostasis. Immunol Rev. 2006;211:39–48. doi: 10.1111/j.0105-2896.2006.00381.x. [DOI] [PubMed] [Google Scholar]
- 50.Stockinger B, Barthlott T, Kassiotis G. The concept of space and competition in immune regulation. Immunology. 2004;111:241–7. doi: 10.1111/j.1365-2567.2004.01831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Freitas AA, Agenes F, Coutinho GC. Cellular competition modulates survival and selection of CD8+ T cells. Eur J Immunol. 1996;26:2640–9. doi: 10.1002/eji.1830261115. [DOI] [PubMed] [Google Scholar]
- 52.Utzny C, Burroughs NJ. Long-term stability of diverse immunological memory. J Theor Biol. 2001;211:393–402. doi: 10.1006/jtbi.2001.2352. [DOI] [PubMed] [Google Scholar]
- 53.Di Rosa F, Santoni A. Memory T-cell competition for bone marrow seeding. Immunology. 2003;108:296–304. doi: 10.1046/j.1365-2567.2003.01593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mahajan VS, Leskov IB, Chen JZ. Homeostasis of T cell diversity. Cell Mol Immunol. 2005;2:1–10. [PubMed] [Google Scholar]
- 55.Jelley-Gibbs DM, Brown DM, Dibble JP, Haynes L, Eaton SM, Swain SL. Unexpected prolonged presentation of influenza antigens promotes CD4 T cell memory generation. J Exp Med. 2005;202:697–706. doi: 10.1084/jem.20050227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim TS, Hufford MM, Sun J, Fu YX, Braciale TJ. Antigen persistence and the control of local T cell memory by migrant respiratory dendritic cells after acute virus infection. J Exp Med. 2010;207:1161–72. doi: 10.1084/jem.20092017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weaver JM, Chaves FA, Sant AJ. Abortive activation of CD4 T cell responses during competitive priming in vivo. Proc Natl Acad Sci USA. 2009;106:8647–52. doi: 10.1073/pnas.0811584106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weaver JM, Lazarski CA, Richards KA, Chaves FA, Jenks SA, Menges PR, Sant AJ. Immunodominance of CD4 T cells to foreign antigens is peptide intrinsic and independent of molecular context: implications for vaccine design. J Immunol. 2008;181:3039–48. doi: 10.4049/jimmunol.181.5.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lazarski CA, Chaves FA, Sant AJ. The impact of DM on MHC class II-restricted antigen presentation can be altered by manipulation of MHC-peptide kinetic stability. J Exp Med. 2006;203:1319–28. doi: 10.1084/jem.20060058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lazarski CL, Chaves F, Jenks S, Wu S, Richards K, Weaver JM, Sant A. The kinetic stability of MHC class II:peptide complexes is a key parameter that dictates immunodominance. Immunity. 2005;23:29–40. doi: 10.1016/j.immuni.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 61.Sant AJ, Chaves FA, Jenks SA, Richards KA, Menges P, Weaver JM, Lazarski CA. The relationship between immunodominance, DM editing, and the kinetic stability of MHC class II:peptide complexes. Immunol Rev. 2005;207:261–78. doi: 10.1111/j.0105-2896.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- 62.Sant AJ, Chaves FA, Krafcik FR, Lazarski CA, Menges P, Richards K, Weaver JM. Immunodominance in CD4 T-cell responses: implications for immune responses to influenza virus and for vaccine design. Expert Rev Vaccines. 2007;6:357–68. doi: 10.1586/14760584.6.3.357. [DOI] [PubMed] [Google Scholar]
- 63.Linton PJ, Harbertson J, Bradley LM. A critical role for B cells in the development of memory CD4 cells. J Immunol. 2000;165:5558–65. doi: 10.4049/jimmunol.165.10.5558. [DOI] [PubMed] [Google Scholar]
- 64.MacLeod M, Kwakkenbos MJ, Crawford A, Brown S, Stockinger B, Schepers K, Schumacher T, Gray D. CD4 memory T cells survive and proliferate but fail to differentiate in the absence of CD40. J Exp Med. 2006;203:897–906. doi: 10.1084/jem.20050711. [DOI] [PMC free article] [PubMed] [Google Scholar]


