Abstract
Recent progress in understanding the molecular mechanisms of bile formation and cholestasis have led to new insights into the pathogenesis of drug induced cholestasis. This review summarizes their variable clinical presentations, examines the, role of transport proteins in hepatic drug clearance and toxicity and addresses the increasing importance of genetic determinants, as well as practical aspects of diagnosis and management.
INTRODUCTION
The liver is the central organ responsible for the selective uptake, metabolism and excretion of drugs, xenobiotics and environmental toxins. This essential function predisposes the liver to drug toxicity and is the primary reason for the failure of pharmaceutical agents during drug development. Hepatic drug toxicity is the most common cause of acute fulminant hepatic failure, accounting for more than 50% of cases (1). More than a thousand drugs and herbal remedies have been reported to cause a variety of different liver disorders. However specific diagnostic markers for drug induced liver injury are lacking, and convincing cause and effect evidence exists for few cases (2). Indeed establishing causality has been a major hindrance in the understanding of drug induced liver injury (3,4).
Cholestatic and mixed cholestatic and hepatocellular injury are two of the most severe manifestations of drug induced liver disease (DILD) (5), and account for close to half of all hepatic drug toxicity in some epidemiologic reports (6). There is increasing evidence that drugs that are excreted by the liver into bile are prime candidates for producing cholestatic liver disease in the susceptible patient (7).
Several forms of cholestatic liver injury can be produced by drugs, and these can present acutely or in the form of chronic liver disease. Drug induced cholestasis may mimic other intrahepatic and extrahepatic cholestatic diseases. Not recognizing a drug as a triggering factor for cholestasis prolongs exposure to the toxic agent, which may lead to worse liver injury and unnecessary diagnostic and therapeutic interventions.
Acute and chronic cholestatic liver injury results from dysfunction of the mechanisms of bile formation. However, drug induced cholestasis can present with asymptomatic disease where the only clinical manifestation is an elevation in alkaline phosphatase. Moreover, the target of injury can vary from a mixed hepatocellular cholestatic injury, to impairment of canalicular bile flow resulting in pure intrahepatic cholestasis, or to an “obstructive” drug induced cholangiopathy where the initial site of injury is located at various levels of the bile duct epithelium (8-10).
The incidence and associated health care costs secondary to drug induced cholestasis are not available, in part because most drugs commonly cause asymptomatic cholestasis associated with mild abnormalities in the serum liver profile. A Danish study of 110 cases of drug induced liver injury (DILI) from 1978 to 1987 reported a 17% prevalence of acute cholestatic injury (11). In the United States the prevalence of drug induced cholestasis was reported to be 20% in the elderly population. However on examination, not all of these cases included patients with cholestasis (12). Approximately 2-5% of hospitalized cases with jaundice are caused by drugs but cholestasis is expressed in only some of these patients (13-16). A Swedish adverse drug reactions advisory committee reviewed 784 reported cases of DILI between 1970 and 2004, almost half of which had either cholestatic or mixed cholestatic hepatic toxicity (6). Nevertheless, a wide variety of commonly used drugs can induce cholestatic liver injury including non-steroidal anti-inflammatory drugs, antihypertensives, antidiabetic, anticonvulsants, lipid-lowering agents, and psychotropic drugs (11-17). Many drugs target the biliary epithelium and result in “drug –induced cholangiopathy” and the “vanishing bile duct syndrome” (VBDS). Terms such as “drug induced bile duct injury” and “disappearing intrahepatic bile ducts” are also used to refer to this type of drug induced injury that can mimic primary biliary cirrhosis or small duct primary sclerosing cholangitis (8). A few rare conditions such as 2-fluoro 2’deoxyuridine can also produce injury to the larger bile ducts; in these cases, injury to the hepatic artery must be excluded as ischemia to the biliary epithelium may result in a similar complication.
CLINICAL FEATURES
Individual drugs that induce drug-induced cholestasis tend to have a characteristic signature, which is composed of a clinical and pathological pattern, but a single drug can exhibit more than one specific signature. Cholestatic reactions tend to be prolonged after the discontinuation of the causative agent, presumably because cholangiocyte repair and regeneration is slower than that of the hepatocyte, and because bile secretory function may be slower to recover than other hepatocyte functions. In some cases, persistence of a self-propagating immune response may play a role in prolonging drug induced cholestasis.
Drug induced cholestasis may present as an acute illness that promptly subsides with the withdrawal of the offending agent. It may present with or without jaundice. However, parenchymal liver injury may elicit non-specific symptoms like nausea, malaise, anorexia and fatigue. Abdominal pain or discomfort may be present in drug induced cholestasis, especially that caused by amoxicillin-clavulanate or erythromycin (18). Symptoms may occur weeks or months after beginning treatment. Chronic drug induced cholestasis can result in development of xanthomas, pruritus and melanoderma (19). Pruritus can be the major reason that patients seek medical care. Chronic drug induced cholestasis often will resolve following withdrawal of the offending agent, but in some cases, if there is significant loss of the interlobular bile ducts (VBDS), drug induced cholestasis can lead to chronic liver disease that may progress to liver failure (20). Rarely drugs can induce cholelithiasis or may mimic large duct sclerosing cholangitis, resulting in extra-hepatic obstruction (21). Occasionally, extrahepatic manifestation of drug toxicity may provide clues to the diagnosis. Amoxicillin-clavulanate can cause acute interstitial nephritis and acute lacrimal gland inflammation along with hepatic injury (22). Similarly, contaminated rapeseed oil poisoning can cause both pulmonary toxicity and drug induced cholestasis concomitantly (23).
Drug induced cholestasis can be categorized into several groups (Table 1 and 2):
Table 1.
Classification of Drug Induced Cholestasis Syndromes
| I. INTRAHEPATIC DRUG INDUCED CHOLESTASIS |
| a) Acute |
| i. Cholestasis without hepatitis (pure, simple, canalicular, or bland cholestasis) |
| ii. Cholestasis with hepatitis (hepatocanalicular hepatitis or mixed cholestatic hepatitis) |
| iii. Cholestasis with bile duct injury (ductular, cholangiolar, or cholangiolytic cholestasis) |
| b) Chronic (Cholangiopathies) |
| i Mild non-specific bile duct injury |
| ii. Vanishing bile duct syndrome (VBDS) |
| iii Primary Sclerosing cholangitis-like |
| ll, EXTRAHEPATIC DRUG INDUCED CHOLESTASIS |
| i. Cholelithiasis |
| ii.Primary Sclerosing cholangitis-like |
TABLE 2.
CLINICAL SYNDROMES OF DRUG INDUCED CHOLESTASIS
| PATHOLOGY | CLINICAL FEATURES | BIOCHEMICAL FEATURES | |
|---|---|---|---|
| INTRAHEPATIC | |||
| ACUTE | |||
| Cholestasis without hepatitis | Dilated canaliculi filled with few bile casts, especially in centrilobular area (acinar zone 3), Minimal or no inflammation or necrosis | Influenza like prodrome (nausea, anorexia, malaise) | Hyperbilirubinemia, < 3 times elevation of AP#, 1-8 times elevation of AST/ALT€ |
| Cholestasis with hepatitis | Early portal inflammation-with or without eosinophils, Hepatocyte necrosis, | Influenza like prodrome, hypersensitivity symptoms, RUQ£ pain (can mimic acute cholangitis or cholecystitis) | Hyperbilirubinemia, > 3 times elevation of AP, 2-10 times elevation of AST/ALT |
| Cholestasis with bile duct injury | Biliary ductules filled with numerous bile casts, scattered steatosis and minimal or no hepatocellular damage | Eosinophilia, renal failure, Stevens-Johnson syndrome, Prolonged Jaundice ( > 6 months), may progress to VBDS | Hyperbilirubinemia, > 3 times elevation of AP, Elevated GGT*, 2-10 times elevation of AST/ALT |
| CHRONIC | |||
| Mils non-specific bile duct injury | Minimal bile duct epithelial disarray phosphatase Occasinal inflammatory cells in or Around biliry epithelia in portal triad | asymptomatic; | Mild elevation in alkaline Or GGT |
| Vanishing bile duct Syndrome (VBDS) | Loss of interlobular or septal bile ducts in ≥ 50 % of portal tracts or complete disappearance, portal tract inflammation, fibrosis, Hepatocellular necrosis, marked ductal destruction | Hepatosplenomegaly, hyperlipidemia, malabsorption, xanthelasmas, xanthomas, leads to cirrhosis | Hyperbilirubinemia, Antimitochondrial antibody absent, > 3 times elevation of AP, 2-10 times elevation of AST/ALT, initial elevation of GGT, Hypercholestrolemia |
| Primry Sclerosing Cholangitis -like | Non specific findings which may resemble PSC, marked ductal destruction, Hepatocellular necrosis | Jaundice develops within 3-6 months of the drug administration, | Hyperbilirubinemia, > 3 times elevation of AP, 2-10 times elevation of AST/ALT Hypercholestrolemia |
| EXTRAHEPATIC | |||
| Cholelithiasis | Biliary colic, gallstone pancreatitis, common bile duct dilatation | Hyperbilirubinemia, Elevated AP | |
| Primary Sclerosing Cholangitis-like | PSC like pathology of extra hepatic ducts | Jaundice develops within 3-6 months of the drug administration, | Hyperbilirubinemia, > 3 times elevation of AP, 2-10 times elevation of |
Alkaline phosphatase
Aspartate aminotransferases/ Alanine aminotransferase
Right upper quadrant
Gamma glutamyl transpeptidase
Acute Drug Induced Cholestasis without Hepatitis
These drug induced cholestatic disorders are rare and cause minimal or no hepatic parenchymal involvement. This form of drug induced cholestasis manifests itself histologically by pure canalicular cholestasis, typically produced by estrogen or anabolic steroids.
Acute Drug Induced Cholestasis with Hepatitis
Cholestasis associated with hepatitis is characterized by portal inflammation and varying degrees of hepatocyte injury and necrosis.
Acute Drug Induced Cholestasis with Bile Duct Injury
These forms of drug induced cholestasis exhibit bile duct injury associated with minimal involvement of parenchymal liver cell injury.
Chronic Drug Induced Cholangiopathies
These drug induced cholestatic disorders vary from asymptomatic patients with isolated elevations in alkaline phosphatase or gammaglutamyl transferase and liver histology showing only mild bile duct disarray or “ductopenia”, to progressive forms of the VBDS (24). While some reports of asymptomatic “idiopathic adulthood ductopenia” fail to identify a causative agent (25) others suggest that these cases may originate from overlooked drug induced bile duct injury (26, 27). The common drugs known to cause the various drug induced cholestasis syndromes are enumerated in Table 3.
TABLE 3.
COMMON DRUGS CAUSING VARIOUS DRUG INDUCED CHOLESTATIC SYNDROMES
| Cholestasis without Hepatitis |
Cholestasis with Hepatitis |
Cholestasis with Bile Duct Injury |
Vanishing Bile Duct Syndrome (Ductopenia) |
Sclerosing Cholangitis like cholestasis |
|---|---|---|---|---|
| Anabolic steroids Estrogens Tamoxifen Azathioprine Cyclosporine Nevirapine Glimepiride Metolazone Infliximab Cetirizine | Isoniazid Halothane Methyldopa Macrolide antibiotics Tricyclic antidepressants Amoxicillin-clavulanate Azathioprine Oxypenicillins NSAID's Chlorpromazine Troglitazone Celecoxib Carbamazepine Repaglinide Terbinafine Cephalexin Fenofibrate Hydrochlorothiazides Ticlopidine Pyritinol Methimazole Metformin Gemcitabine Orlistat Celecoxib Gabapentin Propafenone Acitretin Isoflurane Bupropion Captopril Resperidone Propafenone Chlorambucil Risperidone Glimepiride Proplthiouracil Itraconazole Dextromethorphan Atorvastatin Senna Cascara sagrada Lycopodium serratum | Carmustine Toxins: paraquat, methylenedianiline Flucoxacillin Dextropropxyphene Tenoxicam Gold Therapy Pioglitazone Amoxicillin-clavulanate | Aceprometazine Ajmaline Amineptine Amitriptyline Amoxicillin/clavulanic acid Ampicillin Azathioprine Barbiturates Carbamazepine Carbutamide Chlorothiazide Chlorpromazine Cimetidine Ciprofloxicin Clindamycin Co-trimoxazole Cromolyn sodium Cyamemazine Cyclohexyl propionate Cyproheptadine D-penicillamine Diazepam Erythromycin Estradiol Flucloxacillin Glibenclamide Glycyrrhizin Haloperidol Ibuprofen Imipramine Methyltestosterone Norandrostenolone Phenylbutazone Phenytoin Prochlorperazine Terbinafine Tetracyclines Thiabendazole Tiopronin Trifluoperazine Tolbutamide Trimethoprim-sulfamethoxazole Troleandomycin Xenalamine | Floxuridine Intralesional agents:Hypertonic saline, iodine solution, formaldehyde, absolute alcohol, silver nitrate. |
PATHOPHYSIOLOGY OF DRUG INDUCED CHOLESTASIS
Hepatocytes are highly polarized cells with distinct sinusoidal, lateral, and apical membrane domains. Lipid soluble drugs with molecular weights ~ 500 daltons or greater are selectively removed by the liver across the sinusoidal domain. Although some drugs diffuse across the cellular membrane, most require active or facilitated transporters (Phase 0) (5, 28, 29). Cellular uptake and binding to cytosolic proteins is followed by Phase I & II biotransformation resulting in more water soluble metabolites. Phase I reactions involve the oxidation, hydroxylation and other reactions mediated by the cytochrome P-450 (CYP) system, particularly CYP3A4. The activity of the cytochrome P-450 system varies greatly among individuals and their transcription is highly regulated by xenobiotic sensing nuclear receptors such as the pregnane X receptor (PXR) and the constitutive androstane receptor (CAR). Phase II reactions involve esterification reactions that form conjugates with sulfate, glucuronic acid, amino acids or glutathione molecules. Generally, this results in increased water solubility and decreased pharmacologic activity, which enhances detoxification of the compounds. However, this same process can also lead to the production of toxic intermediates (30). Drug induced cholestasis may occur particularly under conditions of increased drug concentrations, genetic alterations in expression of enzymes or transporters, and/ or reduced hepatic concentrations of anti-oxidants such as glutathione. Drug induced cholestasis can be caused by direct toxic effects of drugs or their metabolites on different cell types of the liver or through an immune mediated process (31).
Role of hepatic transport proteins in drug clearance and toxicity
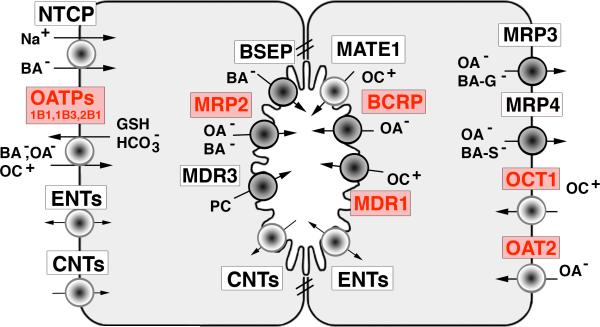
The molecular identification of transport proteins that mediate the sinusoidal uptake and biliary secretion of bile acids and other organic solutes, many of which are drugs, has greatly expanded our understanding of the cellular mechanism for bile formation and its dysregulation in cholestatic conditions including drug induced cholestasis (5,28,29). A list of these membrane transporters, their gene nomenclature and their function in transporting drug substrates is given in Table 4 and illustrated in Figure 1. Drug substrates that are known to induce cholestasis are listed in italics. The rate limiting step in the systemic clearance of lipophilic drugs and their metabolites is their excretion into bile. This process is regulated by ATP-dependent canalicular transporters, including the bile salt export pump (BSEP, ABCB11), the major determinant of bile salt dependent canalicular bile secretion (32), and the multidrug resistance protein-2 (MRP2, ABCC2) that determines bile salt independent bile flow by excretion of glutathione (28). MRP2 also transports drug-conjugates and divalent bile salt conjugates into bile. Other ATP-dependent transporters include the multidrug resistance-1 protein (MDR1, ABCB1), which transports organic cations (often tertiary or quaternary amines), the Breast Cancer Resistance Protein (BCRP, ABCG2) which transports organic anions (including drug conjugates), and the multidrug resistance protein 3 (MDR3, ABCB4), a phospholipid flippase. A number of drug substrates for these transporters are known to produce drug induced cholestasis (see Table 4).
TABLE 4.
Nomenclature, Location and Function of The Major Hepatocyte Membrane Drug Transporters and their known drug substrates (those which can produce cholestasis are in italics).
| NAME | ABBREVIATION | LOCATION | FUNCTION | CLINICALLY RELEVANT POLYMORPHISMS |
|---|---|---|---|---|
| Sodium-taurocholate cotransporter | NTCP, (SLC10A1) | Basolateral membrane | Primary carrier for conjugated bile-salt uptake from portal blood but can transport estrone-3-sulfate and rosuvastatin (115) | |
| Organic-anion-transporting polypeptides | OATPs (SLCO, 1B1,1B3, 2B1) | Basolateral membrane | Broad substrate carriers for organic anions, and other amphipathic organic solutes from portal blood including wide range of drugs as well as sodium-independent uptake of bile salts, | OATP1 *15 variant.(1628T>G), associated with statin induced myopathy (68). |
| Organic cation Transporter 1 | OCT1 (SLC22A1) | Basolateral Membrane | Transports organic cations such as cimetidine, metformin, aclovir and zidovudine | |
| Organic anion transporter 2 | OAT2 (SLC22A7) | Basolateral Membrane | Transports organic anionic drugs like para-aminohippurate, salicylates and prostaglandin E2 | |
| Concentrative and Equilibrative Nucleoside transporters | CNT1 &2 (SLC28A1 &2) ENT1 & 2 (SLC29A1, & 2) | Basolateral Membrane | NTs Transports nucleosides and nucleoside analogues such as adenosine, uridine, didanosine, fialuridine, 5-fluorouridine, ribivirin (117) | |
| Multidrug-resistance-1 P-glycoprotein* | MDR1 (ABCB1) | Canalicular l membrane | ATP-dependent excretion of various organic cations, xenobiotics, and cytotoxins into bile including cholestatic drugs like cyclosporine, verapamil, erythromcin, chlorpromazine,ivermectin and anticancer drugs like daunorubicin and vincristine, cardiac glycosides, HIV protease inhibitors | |
| Multidrug-resistance protein-3* | MDR3 (ABCB4) | Canalicular membrane | Mediates ATP dependent excretion of phosphatidylcholine into bile (a floppase, not a drug transporter); Polymorphisms predispose to drug induced cholestasis. | Herterozygous p.1764L mutation associated with risperidine hepatocellular cholestasis (76); PFIC mutation associated with oral contraceptive induced cholestasis (119). |
| Multidrug-resistance-associated protein (canalicular multispecific organic-anion transporter)* | MRP2 (ABCC2) | Canalicular membrane | Mediates ATP-dependent multispecific organic-anion conjugate efflux into bile (e.g., cholestatic drugs like cephtriaxone, diclofenac, synthetic estrogens; also, pravastitin, methotrexte as well as bilirubin diglucuronide); contributes to bile-salt-independent bile flow by GSH transport | |
| Canalicular bile-salt-export pump* | BSEP (ABCB11) | Canalicular membrane | ATP-dependent bile-salt transport into bile; stimulates bile salt dependent bile flow but also can transport taxol (116) and pravastatin (117) | pV444A SNP more frequently associated with drug cholestasis (76); Heterozygous p.D676Ymutation in patient taking fluvastitin with hepatocellular cholestasis (76) |
| Breast cancer resistance protein* | BCRP (ABCG2) | Canalicular membrane | ATP-dependent excretion into bile of anticancer drugs (Anthracyclins, topotecan), estrogens, rosuvastatin, nitrofurantoin | |
| Multidrug and toxin extrusion transporter | MATE1 (SLC47A1) | Canalicular membrane | Proton gradient mediated efflux of cation drugs like cimetidine, procainamide | |
| Concentrative and Equilibratiave Nucleoside transporters | CNT1 & 2 (SLC28A1 & 2); ENT1 (SLC29A1) | Canalicular membrane | CNTs transport from bile into hepatocyte; ENTs are bidirectional. NTs Transport nucleosides and nucleoside analogues such as adenosine, uridine, didanosine, fialuridine,5-fluorouridine, ribivirin; (118) | |
| Multidrug-resistance-associated protein-3* | MRP3 (ABCC3) | Basolateral membrane of hepatocytes | ATP-dependent efflux of drug glucuronide conjugates like morphine glucuronide. Expression induced in cholestasis | |
| Multidrug resistance associated protein-4* | MRP4 (ABCC4) | Basolateral Membrane of hepatocytes | ATP dependent efflux pump for sulfated drugs and bile acids. Expression up-regulated in cholestasis |
These transporters are members of the ATP-binding cassette family.
Fig 1.
Hepatocyte couplet illustrating location of major transporters that determine bile production and hepatic drug transport. The major drug transporters are indicated in red. ATP dependent transporters are the dark circles. See Table 4 for their definition and function. (Na+ = sodium; BA- = bile acid; OA- = organic anion; OC+ = organic cation; PC= phosphatidylcholine; BA-G = bile acid glucuronides; BA-S = bile acid sulfates);GSH = glutathione;
Insight into specific mechanisms of drug-induced cholestasis in patients has been gained mostly from animal models of cholestasis. These studies carried out in isolated membrane vesicles, hepatocyte cultures and in bile duct cannulated murine models indicate that cholestatic drugs can inhibit bile secretion and bile acid transport at many levels, including uptake and efflux across the sinusoidal membrane, as well as canalicular efflux. For example, rifampicin, cyclosporine A, rifamycin SV, bosentan, troglitazone, erythromycin estolate, and glibenclamide have all been shown to inhibit Bsep in rats in a dose-dependent fashion (33-36). Sulindac also competitively inhibits canalicular bile acid transport (37). Ethinylestradiol-17β- glucuronide is secreted into bile by Mrp2 and then trans-inhibits Bsep from the luminal side of the canalicular membrane (36).
Cyclosporine, an MDR1 substrate, is a prototypical drug that can cause cholestatic liver injury through a number of different mechanisms: i) Competitive inhibition of ATP dependent transporters, (38-40), ii) Iinhibition of intrahepatic vesicle transport and targeting of ATP dependent transporters to the canalicular membrane (41-43), and iii) Iimpairment of bile secretion partly by increasing canalicular membrane fluidity without affecting the expression of canalicular transporters (44). Other studies suggest that cyclosporine reduces the expression of glutathione synthesizing enzymes and the canalicular glutathione efflux system, MRP2, leading to reduced bile salt-independent bile flow. This cholestatic effect is enhanced when the drug is co-administered with sirolimus (rapamycin) (45). It remains uncertain whether these various mechanisms of toxicity also apply to patients who are chronically exposed to drugs like cylcosporine. However long term impairment of hepatobiliary secretory mechanisms and their adverse consequences might be expected.
Other drugs that can be associated with cholestasis, such as the endothelin antagonist bosentan, also inhibit Bsep, an effect that is enhanced by co-administration ofthe oral hypoglycemic agent glibenclamide (33). Troglitazone and troglitazone sulfate, the main troglitazone metabolite eliminated in bile, competitively cis-inhibit Bsep, which could lead to troglitazone-induced intrahepatic cholestasis and liver toxicity (34, 35). Male rats are more susceptible to liver injury than female rats, probably due to higher formation rates of troglitazone sulfate (46). Troglitazone sulfate and troglitazone glucuronide (another important metabolite) are eliminated via MRP2 into bile, suggesting that canalicular elimination via MRP2 may be an important factor in the pathogenesis of troglitazone-induced cholestasis (46). Direct competition of troglitazone metabolites with conjugated bilirubin at the level of MRP2 could result in conjugated hyperbilirubinemia (46). Troglitazone also can produce mitochondrial toxicity and reactive oxygen species so that the pathogenesis may involve more than one mechanism. Fialuridine hepatic toxicity with cholestasis also involves mitochondrial derangement (47).
Although not a drug transporter, MDR3 plays a key role in the biliary secretion of phosphatidylcholine. An aggressive form of progressive familial intrahepatic cholestasis, type III, results from mutations in MDR3. The inability to translocate this phospholipid across the canalicular membrane lipid bilayer results in its absence from bile, and this is thought to result in exposure of the biliary epithelium to the toxic, detergent effects of bile acids that lead to cholangiopathies (48). Impaired expression of MDR3 can lead to development of cholangiolytic cholestasis and the VBDS. Genetic variants in MDR3 and BSEP may predispose individuals to drug induced cholestasis (see section on genetic determinants below and reference 77).
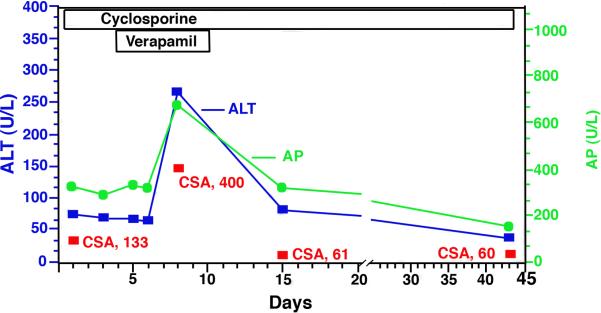
Multiple drugs, particularly quaternary cationic amines are transported into the bile via the MDR1 canalicular transporter. Although mutations in this transporter have not been described in humans, reduced expression or competition for binding sites with other drugs may cause drug accumulation and toxicity. Accumulation of a drug like cyclosporine A can subsequently inhibit Mrp2, resulting in direct toxicity to the cell. Studies in cell monolayers expressing human sodium dependent taurocholate co-transporting polypeptide (NTCP) and BSEP suggest that many well known cholestatic drugs like rifampicin, glibenclamide and cyclosporin A reduce bile acid transport both into the hepatocyte and at the apical canalicular membrane (49). Drug-drug competition for transporter-binding sites may also play a role in drug induced cholestasis in human liver although these effects are not easily recognized and poorly understood mechanistically. See Figure 2 for a possible example of drug - drug competition between two MDR1 substrates resulting in a transient cholestatic effect (50).
Fig 2.
Drug induced cholestasis from interaction of cyclosporine and verapamil in a 46 y WM 5 days post Liver transplant. Note rise in alkaline phosphatase (AP),alanine aminotransferase (ALT) and plasma cyclosposrin levels (CSA) during simultaneous administration of verapamil and cyclosporine, two MDR1 substrates. Drug induced cholestasis resolved quickly after discontinuing the verapamil.
Another potential mechanism for the development of drug induced ductopenia and the VBDS is the biliary excretion of toxic but stable metabolites that injure the bile duct epithelium. α-naphthylisothiocyanate (ANIT) administration to rats results in chronic cholestatic injury characterized by bile duct injury and proliferation. ANIT forms a labile glutathione adduct in hepatocytes, which dissociates after concentrative transport into alkaline bile. Rats with mutated Mrp2 are not able to pump the adduct into bile and thus are protected from ANIT induced cholestatic injury (51).
Flucloxacillin is a beta-lactam semi-synthetic antibiotic commonly used in Europe that causes cholestatic liver injury in ~ 8/100,000 patients. It is an example of a drug that results in liver pathology consistent with the VBDS (52). The mechanism is not known. However, small amounts of the compound form metabolites involving the activity of CYP3A4, which itself may be under genetic control. Whether these metabolites are directly toxic to cholangiocytes after excretion into bile or might also be formed in cholangiocytes is not known. It is generally assumed that an immune mediated response subsequently accounts for the development of the VBDS. Recent studies also support a role for genetic determinants (see below). Isolated reports of VBDS have been described for number of other drugs. (Table 3) (52-61)
Pro-inflammatory cytokines are also powerful down regulators of cytochrome P450 enzymes and biliary transporters (62,63), thereby, lowering the threshold for liver injury and functioning as a critical “second hit” (64). This phenomenon, also known as the “danger hypothesis”, is one of several explanations for the development of idiosyncratic drug toxicity (64). Drug-drug interactions and genetic determinants of drug metabolism enzymes and transporters are other important variables.
GENETIC DETERMINANTS
It is increasingly evident that genetic variants can determine an individual's susceptibility to develop cholestasis, particularly when the drug is a substrate for liver transporters and is excreted into bile (5,29). Very little information exists on the role of hepatic basolateral drug transporters in the development of drug induced cholestasis although it has been speculated that increased expression of the organic anion transporting peptides (OATPs) and other drug uptake transport proteins might enhance the hepatic concentrations of certain drugs, thus, predisposing the subject to cholestatic reactions. Oatp1b2 knockout mice are resistant to the hepatotoxic effects of the mushroom poison, phalloidin, consistent with the role of OATPs in increasing the concentration of substrate drugs/toxins. (65) Although fourteen nonsynonymous SLC1B1 SNPs that encode OATP1B1 have been described by Tirona et al (66) in African Americans and Europeans, 6 reduce rather than enhance the uptake of the OATP1B1 substrates, estrone-3-sulfate and estadiol-17-glucuronide, in in-vitro studies. Genetic variants in OATP1B1 can influence the hepatic uptake of drugs like pravastatin and irinotecan. In one reported case, mutations in this transporter resulted in statin induced myopathy (67) which was explained by a decrease in turnover rate of this transporter (68).
More information is known about the functional and clinical impact of genetic variations in the canalicular transporters and their role in drug induced cholestasis. One study using 110 healthy liver tissues demonstrated considerable variation in the expression of these proteins with 32% expressing low levels of at least one of the canalicular transport proteins, a feature that could predispose individuals to cholestatic drug injury (69). Several common polymorphisms for canalicular ABC transporters have also been identified in healthy individuals by systematic genetic screening of their promoter and coding regions (70-72). Polymorphisms like C1515Y in MRP2, V444A in BSEP, and C3435T in MDR1 were found to be associated with decreased hepatic expression of these proteins (69, 73). These polymorphisms can influence the bioavailability of drugs. For example, the C3435T polymorphism in MDR1 increases oral bioavailability of digoxin, but has no effect on the bioavailability of cyclosporine A (73,74).
However, considerable inter-individual variability exists in the expression of the canalicular membrane ABC transporter proteins with 15-20% of individuals being classified as low or very low expressers of at least one of these proteins in one study (69). Differences in genetic variability of MDR3 and BSEP and haplotype structures in different healthy individuals may predispose different ethnic populations to drug induced cholestasis (75).
Two nonsynonymous SNPs in BSEP have been described for c.1331T>C (p.V444A) in exon 13 and c.2029A>G (p.M677V) in exon 17 with frequencies that are higher than 0.5% in different cohorts (69). In another study, individuals with the p.V444A variant demonstrated lower BSEP expression levels (76). This variant BSEP is now considered a risk factor for drug induced cholestasis since found more frequently in such patients (76), as well as in intrahepatic cholestasis it isof pregnancy (78,79), than in controls (76). In the same Swiss study, full-length sequencing of BSEP and MDR3 also revealed a heterozygous p.D676Y mutation in BSEP in a patient taking fluvastatin and a heterozygous p.I764L mutation in MDR3 in a patient taking risperidone (76). Whether these mutations account for the cholestatic event remains uncertain. A recent study of contraceptive induced cholestasis revealed an association with BSEP 1331T>C polymorphism as a susceptibility factor but not for MRP2 (78).
Other examples of genetically determined drug induced cholestasis involve susceptibility to diclofenac-induced toxicity. Allelic variants in the drug metabolizing enzymes, UGT2B7 and CYP2C8, and canalicular MRP2 presumably lead to an increase in the level of reactive metabolites and higher levels of protein-diclofenac adducts that then produce toxicity (80). Other studies have identified a PXR polymorphism as a risk factor for flucloxacillin-induced liver injury. Flucloxacillin is a PXR agonist. The variant PXR (rs3814055; C-25385T) was found to be more common in patients who developed flucloxacillin drug induced cholestasis and reporter gene experiments demonstrated that the C allele had lower promoter activity than the T allele (81). These findings are a reminder that there is still much to be learned about the role of polymorphisms of nuclear receptors that regulate drug metabolism and transport in patients with drug induced cholestasis (75, (82).
DIAGNOSIS
A detailed history is critical in the diagnosis of drug induced cholestasis. The use of prescribed as well as over the counter medications, herbal drugs and naturopathic substances as well as parenteral nutrition should always be explored (83,84). Temporal relationships between the initiation of the offending agent and development of the symptoms can provide the clue to the diagnosis. The period between drug ingestion and the onset of symptoms may provide a clue as tor the offending agent. This latency period may be short (hours to days), intermediate or delayed (1-8 weeks), or long (1-12 months) depending on the agent . All drugs used by the patient within the last 3 to 6 months should be enumerated. This relationship may not be obvious in patients with chronic liver disease from other causes or when taking multiple medications which may lead to drug-drug interactions . Increase of t serum alkaline phosphatase (AP) activity (usually more than three times the upper limit of normal) is the most common laboratory finding in patients with drug induced cholestasis. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels may be normal or minimally elevated (85).
International criteria for liver toxicity were established by the Council of International Organizations of Medical Sciences (CIOMS) in 1990. This group defined “Cholestatic injury”_ as a patient with AP > 2 × ULN or ALT/AP ≤ 2 while “Mixed Hepatocellular/cholestatic injury” was defined as an ALT/AP ≤ 2-5 in contrast to “Hepatocellular injury” – where the ALT is > 2 × ULN or ALT/AP ≥ 5 (86,857). Diseases which can mimic drug induced cholestasis, such as primary biliary cirrhosis, sepsis (bacterial or viral) should be ruled out .,Evaluations should always include hepatitis and autoimmune serologies and appropriate imaging studies.
On rare occasions patients may develop symptoms on re-exposure to the same medication). However re-challenge with the suspected drug is usually contraindicated particularly if there is active liver injury, because severe or even fatal liver injury can occur.
The role of liver biopsy is controversial. Nevertheless it may be helpful when the diagnosis is not clear or when there are other complicating medical conditions. Occasionally the pathologist will first suggest the possibility of a drug or toxin induced injury. A biopsy may also be useful in predicting prognosis. (see a recent review for a more comprehensive discussion of the role of liver pathology in drug induced liver injury) (88).
MANAGEMENT
Most cases of drug induced cholestasis will resolve with withdrawal of the offending medication and not develop chronic liver disease. A Swedish adverse drug reaction advisory committee report concluded that AST and bilirubin levels are the most important predictors of death or liver transplantation in DILI (6). In another study the persistent use of the offending agent for > 6 months after diagnosis of DILI, predicted the development of chronic liver disease and fibrosis in liver biopsies (89). In addition to watchful waiting after stoppping the suspected agent, it is important to treat pruritus when present Severe pruritus may lead to sleep deprivation and psychological abnormalities especially in elderly patients. The pathophysiological mechanism of pruritus from cholestasisis is still unknown. Suggested mechanisms include high tissue and serum bile salt concentrations, increased opioidergic tone, and alteration of serotonin neurotransmitters. (90-92). A recent study has suggested lysophosphatidic acid as a potential mediator (93).
Mild pruritus can often be managed by nonspecific measures such as emollients and warm baths and/or histamine 1-receptor blockers such as hydroxyzine and diphenhydramine due to their sedative properties. IBile acid resins (cholestyramine or colestipol) are the first line agents in moderate to severe pruritus particularly when associated with excoriations and disturbed sleep (94), Based on the inference that the pruritogens are excreted oin bile,they function to exchange organic anions such as bile acids with chloride anions in the intestine. To be effective, Cholestyramine powder (4 gm packets) or colestipol pills (1 g each), in doses of 4 g, must be taken 20 min before each meal in order to reach the small intestine when food induces bile flow (95) Total doses range from 4-24 g per day. All other medications must be given at least 1-2 hours before or after administration of these binding resins to avoid interference with their absorption. Side-effects like constipation and and hyperchloidemia may occur. Rifampicin (150 – 300 mg twice a day) may also be effective in patients intolerant to binding resins (96,97). Rifampicin is also a ligand for the nuclear receptor PXR and ligand activation of this receptor induces expression of cytochrome P450 isoforms that are capable of detoxification of hydrophobic bile salts (98,99). Side effects of rifampicin include hepatic toxicity. Phenobarbital (1-5 mg/kg/day divided in 3 doses) also induces hepatic microsomal enzymes via activation of CAR (100) and therefore may facilitate detoxification and inactivation of putative peripheral pruritogens (101). However long term administration of microsomal inducers may impair vitamin D metabolism.
Alternatives are limited but include the opiate antagonists, nalaxone and naltrexone (91,102,103). Invasive procedures including plasmapheresis and extracorporeal albumin dialysis using the molecular adsorbent recirculating system (MARS) are reported to relieve severe pruritus (104), but both procedures may require hospitalization and significant input from renal dialysis staff. Severe pruritus even can be an indication for liver transplantation. Ursodeoxycholic acid (UDCA) is currently the only established drug for the treatment of cholestatic liver disease and has cytoprotective, immunomodulatory, anti-apoptotic, and choleretic effects (105). UDCA is also a strong agonist of PXR, an important nuclear receptor that up-regulates CYP3A4 (106).
Experimental studies in rats have demonstrated that UDCA improves cholestasis induced by phalloidin, 17β-estradiol glucuronide and endotoxins (106-109). UDCA increases the expression of canalicular export pumps for bile salts (BSEP) and other organic anions including bilirubin (MRP2) which stimulates bile secretion (110,111). UDCA also stimulates the insertion of these export pumps into the canalicular membrane in a protein kinase C and p38 MAP-kinase dependent fashion (111,112). UDCA stimulates targeting of P- glycoprotein (MDR1) to the canalicular membrane, which could prevent cyclosporine-associated cholestatic effects (112).
Although the mechanism of UDCA's beneficial effect in cholestasis is not clearly understood, it is thought that its primary beneficial effect is decreasing the hydrophobicity of the bile acid pool by replacing toxic (e.g. hydrophobic) with non-toxic (e.g. hydrophilic) bile acids (105). UDCA is best administered at bed time to avoid inhibition of its absorption when administered with cholestyramine or colestipol. However, UDCA is FDA approved only for use in patients with primary biliary cirrhosis and its efficacy in patients with drug induced cholestasis has not been clearly established. For a review of potential future therapeutic approaches for drug induced and other forms of cholestasis see ref 106.
Fat soluble vitamins (A, D and K) should be replaced via the parenteral route in patients with long standing cholestasis. Patients at risk for developing biliary cirrhosis and liver failure, should be promptly referred to liver transplant centers.
In the future, gene-expression profile information, toxicogenomics and proteomics may help to better understand the mechanisms of drug induced cholestasis and provide the technology to better identify individuals at risk (114).
CONCLUSION
Drugs continue to be an important cause of cholestasis and always must be considered in the differential diagnosis of cholestatic syndromes. Prompt recognition and withdrawal of the offending agent is the mainstay of the management. Progress has been made in clarifying potential pathogenetic mechanisms and in establishing the role of genetic susceptibility for the development of drug induced cholestasis.
ACKNOWLEDGEMENTS
The authors thank Carol Soroka, Ph,D. for helpful suggestions and careful review of this manuscript
Abbreviations
- DILD
drug induced liver disease
- DILI
drug induced liver injury
- VBDS
vanishing bile duct syndrome
- CYP
cytochrome P-450
- PXR
pregnance X receptor
- BSEP
bile salt export pump
- MRP
multidrug resistance protein
- MDR1
multidrug resistance-1 protein
- BCRP
Breast Cancer Resistance Protein
- ANIT
α-naphthylisothiocyanate
- OATPs
organic anion transporting peptides
- AST
aspartate aminotransferase
- ALT
alanine aminotransferase
- UDCA
ursodeoxycholic acid
- NTCP
sodium dependent taurocholate co-transporting polypeptide
REFERENCES
- 1.Lee WM. Drug-induced hepatotoxicity. N Engl J Med. 2003;349:474–485. doi: 10.1056/NEJMra021844. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki A, Andrade RJ, Bjornsson E, Lucena MI, Lee WM, Yuen NA, Hunt CM, Freston JW. Drugs associated with hepatotoxicity and their reporting frequency of liver adverse events in VigiBase: unified list based on international collaborative work. Drug Saf. 2010 Jun 1;33(6):503–22. doi: 10.2165/11535340-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Assis DN, Navarro VJ. Human drug hepatotoxicity: a contemporary clinical perspective. Expert Opin Drug Metab Toxicol. 2009 May;5(5):463–73. doi: 10.1517/17425250902927386. [DOI] [PubMed] [Google Scholar]
- 4.Rockey DC, Seeff LB, Rochon J, Freston J, Chalasani N, Bonacini M, Fontana RJ, Hayashi PH, US Drug-Induced Liver Injury Network Causality assessment in drug-induced liver injury using a structured expert opinion process: comparison to the Roussel-Uclaf causality assessment method. HEPATOLOGY. 2010;51:2117–2126. doi: 10.1002/hep.23577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohan A, Boyer JL. Mechanisms of hepatic transport of drugs: implications for cholestatic drug reactions. Semin Liver Dis. 2002;22:123–36. doi: 10.1055/s-2002-30099. [DOI] [PubMed] [Google Scholar]
- 6.Björnsson E, Olsson R. Outcome and prognostic markers in severe drug-induced liver disease. Hepatology. 2005 Aug;42(2):481–9. doi: 10.1002/hep.20800. [DOI] [PubMed] [Google Scholar]
- 7.Lammert C, Bjornsson E, Niklasson A, Chalasani N. Oral medications with significant hepatic metabolism at higher risk for hepatic adverse events. Hepatology. 2010;51:615–620. doi: 10.1002/hep.23317. [DOI] [PubMed] [Google Scholar]
- 8.Geubel AP, Sempoux CL. Drug and toxin-induced bile duct disorders. J Gastroenterol Hepatol. 2000;15:1232–8. [PubMed] [Google Scholar]
- 9.Popper H, Schaffner F. Pathophysiology of cholestasis. Hum Pathol. 1970;1:1–24. doi: 10.1016/s0046-8177(70)80002-8. [DOI] [PubMed] [Google Scholar]
- 10.Zimmerman HJ. Intrahepatic cholestasis. Arch Intern Med. 1979;139:1038–45. [PubMed] [Google Scholar]
- 11.Friis H, Andreasen PB. Drug-induced hepatic injury: an analysis of 1100 cases reported to the Danish Committee on Adverse Drug Reactions between 1978 and 1987. J Intern Med. 1992;232:133–8. doi: 10.1111/j.1365-2796.1992.tb00562.x. [DOI] [PubMed] [Google Scholar]
- 12.Lewis JH. Drug-induced liver disease. Med Clin North Am. 2000;84:1275–311. doi: 10.1016/s0025-7125(05)70287-x. [DOI] [PubMed] [Google Scholar]
- 13.Bjørneboe M, Iversen O, Olsen S. Infective hepatitis and toxic jaundice in a municipal hospital during a five-year period: incidence and prognosis. Acta Med Scand. 1967;182:491–501. doi: 10.1111/j.0954-6820.1967.tb10873.x. [DOI] [PubMed] [Google Scholar]
- 14.Malchow-Møller A, Matzen P, Bjerregaard B, Hilden J, Holst-Christensen J, Staehr Johansen T, et al. Causes and characteristics of 500 consecutive causes of jaundice. Scand J Gastroenterol. 1981;16:1–6. [PubMed] [Google Scholar]
- 15.Whitehead MW, Hainsworth I, Kingham JGC. The causes of obvious jaundice in South West Wales: 2000. Gut. 2001;48:409–413. doi: 10.1136/gut.48.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bjornsson E, Ismael S, Nejdet S, Kilander A. Severe jaundice in Sweden in the new millennium: causes, investigations, treatment and prognosis. Scand J Gastroenterol. 2003;38:86–94. doi: 10.1080/00365520310000492. [DOI] [PubMed] [Google Scholar]
- 17.Chitturi S, George J. Hepatotoxicity of commonly used drugs: nonsteroidal anti-inflammatory drugs, antihypertensives, antidiabetic agents, anticonvulsants, lipid-lowering agents, psychotropic drugs. Semin Liver Dis. 2002;22:169–83. doi: 10.1055/s-2002-30102. [DOI] [PubMed] [Google Scholar]
- 18.Viteri AL, Greene JF, Jr., Dyck WP. Erythromycin ethylsuccinate-induced cholestasis. Gastroenterology. 1979;76:1007–8. [PubMed] [Google Scholar]
- 19.Walker CO, Combes B. Biliary cirrhosis induced by chlorpromazine. Gastroenterology. 1966 Nov;51(5):631–40. [PubMed] [Google Scholar]
- 20.Moradpour D, Altorfer J, Flury R, Greminger P, Meyenberger C, Jost R, Schmid M. Chlorpromazine-induced vanishing bile duct syndrome leading to biliary cirrhosis. Hepatology. 1994 Dec;20(6):1437–41. doi: 10.1002/hep.1840200610. [DOI] [PubMed] [Google Scholar]
- 21.Erlinger S. Drug-induced cholestasis. J Hepatol. 1997;26(Suppl 1):1–4. doi: 10.1016/s0168-8278(97)82326-4. [DOI] [PubMed] [Google Scholar]
- 22.Hautekeete ML, Brenard R, Horsmans Y, et al. Liver injury related to amoxycillin-clavulanic acid: interlobular bile-duct lesions and extrahepatic manifestations. J Hepatol. 1995;22:71–7. doi: 10.1016/0168-8278(95)80262-2. [DOI] [PubMed] [Google Scholar]
- 23.Alonso-Ruiz A, Calabozo M, Perez-Ruiz F, et al. Toxic oil syndrome. A long-term follow-up of a cohort of 332 patients. Medicine (Baltimore) 1993;72:285–95. doi: 10.1097/00005792-199309000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Degott C, Feldmann G, Larrey D, et al. Drug-induced prolonged cholestasis in adults: a histological semiquantitative study demonstrating progressive ductopenia. Hepatology. 1992;15:244–51. doi: 10.1002/hep.1840150212. [DOI] [PubMed] [Google Scholar]
- 25.Moreno A, Carreño V, Cano A, González C. Idiopathic biliary ductopenia in adults without symptoms of liver disease. N Engl J Med. 1997 Mar 20;336(12):835–8. doi: 10.1056/NEJM199703203361204. [DOI] [PubMed] [Google Scholar]
- 26.Geubel AP, Sempoux C, Rahier J. Bile duct disorders. Clin Liver Dis. 2003 May;7(2):295–309. doi: 10.1016/s1089-3261(03)00028-x. [DOI] [PubMed] [Google Scholar]
- 27.Desmet VJ. Destructive intrahepatic bile duct diseases. Recenti Prog Med. 1990 Jun;81(6):392–8. [PubMed] [Google Scholar]
- 28.Trauner M, Meier PJ, Boyer JL. Molecular pathogenesis of cholestasis. N Engl J Med. 1998;339:1217–27. doi: 10.1056/NEJM199810223391707. [DOI] [PubMed] [Google Scholar]
- 29.Pauli-Magnus C, Meier PJ. Hepatobiliary transporters and drug-induced cholestasis. Hepatology. 2006 Oct;44(4):778–87. doi: 10.1002/hep.21359. [DOI] [PubMed] [Google Scholar]
- 30.DeLeve LD, Kaplowitz N. Mechanisms of drug-induced liver disease. Gastroenterol Clin North Am. 1995;24:787–810. [PubMed] [Google Scholar]
- 31.Liu ZX, Kaplowitz N. Immune-mediated drug-induced liver disease. Clin Liver Dis. 2002;6:755–74. doi: 10.1016/s1089-3261(02)00025-9. [DOI] [PubMed] [Google Scholar]
- 32.Stieger B. Role of the bile salt export pump, BSEP, in acquired forms of cholestasis. Drug Metab Rev. 2009 Dec 23; doi: 10.3109/03602530903492004. [DOI] [PubMed] [Google Scholar]
- 33.Fattinger K, Funk C, Pantze M, et al. The endothelin antagonist bosentan inhibits the canalicular bile salt export pump: a potential mechanism for hepatic adverse reactions. Clin Pharmacol Ther. 2001;69:223–31. doi: 10.1067/mcp.2001.114667. [DOI] [PubMed] [Google Scholar]
- 34.Funk C, Pantze M, Jehle L, et al. Troglitazone-induced intrahepatic cholestasis by an interference with the hepatobiliary export of bile acids in male and female rats. Correlation with the gender difference in troglitazone sulfate formation and the inhibition of the canalicular bile salt export pump (Bsep) by troglitazone and troglitazone sulfate. Toxicology. 2001;167:83–98. doi: 10.1016/s0300-483x(01)00460-7. [DOI] [PubMed] [Google Scholar]
- 35.Funk C, Ponelle C, Scheuermann G, et al. Cholestatic potential of troglitazone as a possible factor contributing to troglitazone-induced hepatotoxicity: in vivo and in vitro interaction at the canalicular bile salt export pump (Bsep) in the rat. Mol Pharmacol. 2001;59:627–35. [PubMed] [Google Scholar]
- 36.Stieger B, Fattinger K, Madon J, et al. Drug- and estrogen-induced cholestasis through inhibition of the hepatocellular bile salt export pump (Bsep) of rat liver. Gastroenterology. 2000;118:422–30. doi: 10.1016/s0016-5085(00)70224-1. [DOI] [PubMed] [Google Scholar]
- 37.Bolder U, Trang NV, Hagey LR, et al. Sulindac is excreted into bile by a canalicular bile salt pump and undergoes a cholehepatic circulation in rats. Gastroenterology. 1999;117:962–71. doi: 10.1016/s0016-5085(99)70356-2. [DOI] [PubMed] [Google Scholar]
- 38.Bohme M, Buchler M, Muller M, Keppler D. Differential inhibition by cyclosporins of primary-active ATP-dependent transporters in the hepatocyte canalicular membrane. FEBS Lett. 1993;333:193–6. doi: 10.1016/0014-5793(93)80403-h. [DOI] [PubMed] [Google Scholar]
- 39.Kadmon M, Klunemann C, Bohme M, et al. Inhibition by cyclosporin A of adenosine triphosphate-dependent transport from the hepatocyte into bile. Gastroenterology. 1993;104:1507–14. doi: 10.1016/0016-5085(93)90363-h. [DOI] [PubMed] [Google Scholar]
- 40.Böhme M, Müller M, Leier I, Jedlitschky G, Keppler D. Cholestasis caused by inhibition of the adenosine triphosphate-dependent bile salt transport in rat liver. Gastroenterology. 1994 Jul;107(1):255–65. doi: 10.1016/0016-5085(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 41.Roman ID, Monte MJ, Gonzalez-Buitrago JM, Esteller A, Jimenez R. Inhibition of hepatocytary vesicular transport by cyclosporin A in the rat: relationship with cholestasis and hyperbilirubinemia. Hepatology. 1990;12:83–91. doi: 10.1002/hep.1840120114. [DOI] [PubMed] [Google Scholar]
- 42.Roman ID, Coleman R. Disruption of canalicular function in isolated rat hepatocyte couplets caused by cyclosporin A. Biochem Pharmacol. 1994;48:2181–8. doi: 10.1016/0006-2952(94)90352-2. [DOI] [PubMed] [Google Scholar]
- 43.Roman ID, Fernandez-Moreno MD, Fueyo JA, Roma MG, Coleman R. Cyclosporin A induced internalization of the bile salt export pump in isolated rat hepatocyte couplets. Toxicol Sci. 2003;71:276–81. doi: 10.1093/toxsci/71.2.276. [DOI] [PubMed] [Google Scholar]
- 44.Yasumiba S, Tazuma S, Ochi H, et al. Cyclosporin A reduces canalicular membrane fluidity and regulates transporter function in rats. Biochem J. 2001;354:591–6. doi: 10.1042/0264-6021:3540591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bramow S, Ott P, Thomsen Nielsen F, et al. Cholestasis and regulation of genes related to drug metabolism and biliary transport in rat liver following treatment with cyclosporine A and sirolimus (Rapamycin). Pharmacol Toxicol. 2001;89:133–9. doi: 10.1034/j.1600-0773.2001.d01-147.x. [DOI] [PubMed] [Google Scholar]
- 46.Kostrubsky VE, Vore M, Kindt E, et al. The effect of troglitazone biliary excretion on metabolite distribution and cholestasis in transporter-deficient rats. Drug Metab Dispos. 2001;29:1561–6. [PubMed] [Google Scholar]
- 47.Kleiner DE, Gaffey MJ, Sallie R, Tsokos M, Nichols L, McKenzie R, Straus SE, Hoofnage JH. Histopathologic changes associated with fialuridine hepatotoxicity. Mod Pathol. 1997;10:192–199. [PubMed] [Google Scholar]
- 48.Mauad TH, van Nieuwkerk CM, Dingemans KP, et al. Mice with homozygous disruption of the mdr2 P-glycoprotein gene. A novel animal model for studies of nonsuppurative inflammatory cholangitis and hepatocarcinogenesis. Am J Pathol. 1994;145:1237–45. [PMC free article] [PubMed] [Google Scholar]
- 49.Mita S, Suzuki H, Akita H, Hayashi H, Onuki R, Hofmann AF, Sugiyama Y. Inhibition of bile acid transport across Na+/taurocholate cotransporting polypeptide (SLC10A1) and bile salt export pump (ABCB 11)-coexpressing LLC-PK1 cells by cholestasis-inducing drugs. Drug Metab Dispos. 2006 Sep;34(9):1575–81. doi: 10.1124/dmd.105.008748. [DOI] [PubMed] [Google Scholar]
- 50.Kassianides C, Nussenblatt R, Palestine AG, et al. Liver injury from cyclosporine A. Dig Dis Sci. 1990;35:693–7. doi: 10.1007/BF01540169. [DOI] [PubMed] [Google Scholar]
- 51.Dietrich CG, Ottenhoff R, de Waart DR, et al. Role of MRP2 and GSH in intrahepatic cycling of toxins. Toxicology. 2001;167:73–81. doi: 10.1016/s0300-483x(01)00459-0. [DOI] [PubMed] [Google Scholar]
- 52.Davies MH, Harrison RF, Elias E, Hübscher SG. Antibiotic-associated acute vanishing bile duct syndrome: a pattern associated with severe, prolonged, intrahepatic cholestasis. J Hepatol. 1994 Jan;20(1):112–6. doi: 10.1016/s0168-8278(05)80476-3. [DOI] [PubMed] [Google Scholar]
- 53.Reau NS, Jensen DM. Vanishing bile duct syndrome. Clin Liver Dis. 2008 Feb;12(1):203–17. doi: 10.1016/j.cld.2007.11.007. inaluding ciprofloxacin . a a fluorinated quinolone. [DOI] [PubMed] [Google Scholar]
- 54.Bataille L, Rahier J, Geubel A. Delayed and prolonged cholestatic hepatitis with ductopenia after long-term ciprofloxacin therapy for Crohn's disease. J Hepatol. 2002 Nov;37(5):696–9. doi: 10.1016/s0168-8278(02)00268-4. [DOI] [PubMed] [Google Scholar]
- 55.Okan G, Yaylaci S, Peker O, Kaymakoglu S, Saruc M. Vanishing bile duct and Stevens-Johnson syndrome associated with ciprofloxacin treated with tacrolimus. World J Gastroenterol. 2008 Aug 7;14(29):4697–700. doi: 10.3748/wjg.14.4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Velayudham LS, Farrell GC. Drug-induced cholestasis. Expert Opin Drug Saf. 2003;2(3):287–304. doi: 10.1517/14740338.2.3.287. [DOI] [PubMed] [Google Scholar]
- 57.Choi SH, Yang SH, Song YB, et al. [A case of vanishing bile duct syndrome associated with hypersensitivity to allopurinol]. Korean J Hepatol. 2005;11:80–5. [PubMed] [Google Scholar]
- 58.Capra F, Nicolini N, Morana G, et al. Vanishing bile duct syndrome and inflammatory pseudotumor associated with a case of anabolic steroid abuse. Dig Dis Sci. 2005;50(8):1535–7. doi: 10.1007/s10620-005-2876-2. [DOI] [PubMed] [Google Scholar]
- 59.Srivastava M, Perez-Atayde A, Jonas M. Drug-associated acute-onset vanishing bile duct and Stevens-Johnson syndromes in a child. Gastroenterology. 1998;115:743–6. doi: 10.1016/s0016-5085(98)70154-4. [DOI] [PubMed] [Google Scholar]
- 60.Taghian M, Tran TA, Bresson-Hadni S, et al. Acute vanishing bile duct syndrome after ibuprofen therapy in a child. J Pediatr. 2004;145(2):273–6. doi: 10.1016/j.jpeds.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 61.Hautekeete ML, Horsmans Y, Van Waeyenberge C, et al. HLA association of amoxicillinclavulanate-induced hepatitis. Gastroenterology. 1999;117:1181–6. doi: 10.1016/s0016-5085(99)70404-x. [DOI] [PubMed] [Google Scholar]
- 62.Fardel O, Le Vee M. Regulation of human hepatic drug transporter expression by pro-inflammatory cytokines. Expert Opin.Drug Metab. Toxicol. 2009;5:1469–1481. doi: 10.1517/17425250903304056. [DOI] [PubMed] [Google Scholar]
- 63.Trauner M, Arrese M, Lee H, Boyer JL, Karpen SJ. Endotoxin downregulates rat hepatic ntcp gene expression via decreased activity of critical transcription factors. J Clin Invest. 1998 May 15;101(10):2092–100. doi: 10.1172/JCI1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pirmohamed M, Naisbitt DJ, Gordon F, Park BK. The danger hypothesis--potential role in idiosyncratic drug reactions. Toxicology. 2002 Dec 27;181-182:55–63. doi: 10.1016/s0300-483x(02)00255-x. [DOI] [PubMed] [Google Scholar]
- 65.Lu H, Choudhuri S, Ogura K, Csanaky IL, Lei X, Cheng X, Song PZ, Klaassen CD. Characterization of organic anion transporting polypeptide 1b2-null mice: essential role in hepatic uptake/toxicity of phalloidin and microcystin-LR. Toxicol Sci. 2008 May;103(1):35–45. doi: 10.1093/toxsci/kfn038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tirona RG, Leake BF, Merino G, Kim RB. Polymorphisms in OATP-C: identification of multiple allelic variants associated with altered transport activity among European- and African-Americans. J Biol Chem. 2001;276(38):35669–35675. doi: 10.1074/jbc.M103792200. [DOI] [PubMed] [Google Scholar]
- 67.Morimoto K, Oishi T, Ueda S, Ueda M, Hosokawa M, Chiba K. A novel variant allele of OATP-C (SLCO1B1) found in a Japanese patient with pravastatin-induced myopathy. Drug Metab Pharmacokinet. 2004 Dec;19(6):453–5. doi: 10.2133/dmpk.19.453. [DOI] [PubMed] [Google Scholar]
- 68.Furihata T, Satoh N, Ohishi T, Ugajin M, Kameyama Y, Morimoto K, Matsumoto S, Yamashita K, Kobayashi K, Chiba K. Functional analysis of a mutation in the SLCO1B1 gene (c.1628T>G) identified in a Japanese patient with pravastatin-induced myopathy). Pharmacogenomics J. 2009 Jun;9(3):185–93. doi: 10.1038/tpj.2009.3. [DOI] [PubMed] [Google Scholar]
- 69.Meier Y, Pauli-Magnus C, Zanger UM, Klein K, Schaeffeler E, Nussler AK, Nussler N, Eichelbaum M, Meier PJ, Stieger B. Interindividual variability of canalicular ATP-binding-cassette (ABC)-transporter expression in human liver. Hepatology. 2006 Jul;44(1):62–74. doi: 10.1002/hep.21214. [DOI] [PubMed] [Google Scholar]
- 70.Saito S, Iida A, Sekine A, et al. Three hundred twenty-six genetic variations in genes encoding nine members of ATP-binding cassette, subfamily B (ABCB/MDR/TAP), in the Japanese population. J Hum Genet. 2002;47:38–50. doi: 10.1007/s10038-002-8653-6. [DOI] [PubMed] [Google Scholar]
- 71.Pauli-Magnus C, Meier PJ. Pharmacogenetics of hepatocellular transporters. Pharmacogenetics. 2003;13:189–98. doi: 10.1097/00008571-200304000-00003. [DOI] [PubMed] [Google Scholar]
- 72.Kroetz DL, Pauli-Magnus C, Hodges LM, et al. Sequence diversity and haplotype structure in the human ABCB1 (MDR1, multidrug resistance transporter) gene. Pharmacogenetics. 2003;13:481–94. doi: 10.1097/00008571-200308000-00006. [DOI] [PubMed] [Google Scholar]
- 73.Hoffmeyer S, Burk O, von Richter O, et al. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci U S A. 2000;97:3473–8. doi: 10.1073/pnas.050585397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.von Ahsen N, Richter M, Grupp C, et al. No influence of the MDR-1 C3435T polymorphism or a CYP3A4 promoter polymorphism (CYP3A4-V allele) on dose-adjusted cyclosporin A trough concentrations or rejection incidence in stable renal transplant recipients. Clin Chem. 2001;47:1048–52. [PubMed] [Google Scholar]
- 75.Lang T, Haberl M, Jung D, et al. Genetic variability, haplotype structures, and ethnic diversity of hepatic transporters MDR3 (ABCB4) and bile salt export pump (ABCB11). Drug Metab Dispos. 2006;34:1582–1599. doi: 10.1124/dmd.105.008854. [DOI] [PubMed] [Google Scholar]
- 76.Lang C, Meier Y, Stieger B, et al. Mutations and polymorphisms in the bile salt export pump and the multidrug resistance protein 3 associated with drug induced liver injury. Pharmacogenet Genomics. 2007;17:47–60. doi: 10.1097/01.fpc.0000230418.28091.76. [DOI] [PubMed] [Google Scholar]
- 77.Meier Y, Zodan T, Lang C, et al. Increased susceptibility for Intrahepatic cholestasis of pregnancy and contraceptive-induced cholestasis in carriers of the 1331T>C polymorphism in the bile salt export pump. World J Gastroenterol. 2008;14:38–45. doi: 10.3748/wjg.14.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pauli-Magnus C, Lang T, Meier Y, et al. Sequence analysis of bile salt export pump (ABCB11) and multidrug resistance p-glycoprotein 3 (ABCB4, MDR3) in patients with intrahepatic cholestasis of pregnancy. Pharmacogenetics. 2004;14(2):91–102. doi: 10.1097/00008571-200402000-00003. [DOI] [PubMed] [Google Scholar]
- 79.Dixon PH, van Mil S, Chambers J, et al. Contribution of variant alleles of ABCB11 to susceptibility to intrahepatic cholestasis of pregnancy. Gut. 2009;58:537–544. doi: 10.1136/gut.2008.159541. [DOI] [PubMed] [Google Scholar]
- 80.Daly AK, Aithal GP, Leathart JB, Swainsbury RA, Dang TS, Day CP. Genetic susceptibility to diclofenac-induced hepatotoxicity: contribution of UGT2B7, CYP2C8, and ABCC2 genotypes. Gastroenterology. 2007 Jan;132(1):272–81. doi: 10.1053/j.gastro.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 81.Andrews E, Armstrong M, Tugwood J, Swan D, Glaves P, Pirmohamed M, Aithal GP, Wright MC, Day CP, Daly AK. A role for the pregnane X receptor in flucloxacillin-induced liver injury. Hepatology. 2010 May;51(5):1656–64. doi: 10.1002/hep.23549. [DOI] [PubMed] [Google Scholar]
- 82.Urquhart BL, Tirona RG, Kim RB. Nuclear receptors and the regulation of drug-metabolizing enzymes and drug transporters: implications for interindividual variability in response to drugs. J Clin Pharmacol. 2007 May;47(5):566–78. doi: 10.1177/0091270007299930. [DOI] [PubMed] [Google Scholar]