Abstract
The immunosuppressive effects of FR 900506 were investigated for one month after canine kidney and liver transplantation. Eight untreated kidney recipients lived for 8 to 19 days, mean 12.1. In contrast, the one month survival of dogs given FR 900506 in daily oral doses of 0.5, 1.0, and 1.5 mg/kg was 2/6, 3/6, and 4/6 respectively. The one month survival of liver recipients was 4/5 compared to one month survival of 1/8 in historical untreated controls. There was no evidence from biochemical tests of hepatotoxicity or nephrotoxicity.
Keywords: immunosuppression, rejection, FR 900506, kidney transplantation, liver transplantation
FR 900506 (FR) is a new immunosuppressive agent produced by Streptomyces tsukubaensis. It inhibits lymphocyte proliferation in in vitro systems several hundred times more effectively than cyclosporine (CyA), (1, 2) and greatly prolongs the survival of heterotopic heart grafts in rats (1, 3). In this study, the effect of FR on canine kidney and liver grafts was determined for the first postoperative month.
MATERIALS AND METHODS
Fasted beagle and mongrel dogs, weighing 13–15kg, were submitted to intraabdominal kidney transplantation and bilateral nephrectomy (4) or to orthotopic liver transplantation during veno-venous bypass (5). Beagle to beagle (B/B) or mongrel to beagle (M/B) strain combinations were used.
FR was supplied as a powder by the Fujisawa Pharmaceutical Company, Inc., Osaka, Japan. The agent was placed into commercial capsules and given orally to the animals each morning, starting on the morning after transplantation. The kidney transplantation groups were as follows:
| Group I: | no treatment | (N=8, 6B/B and 2 M/B) |
| Group II: | 0.5mg/kg/day | (N=6, 6 M/B) |
| Group III: | 1.0mg/kg/day | (N=6, 6 M/B) |
| Group IV: | 1.5mg/kg/day | (N=6, 6 M/B) |
For the 5 liver transplantations, the B/B strain combination was used. The oral FR dose was 1.0mg/kg/day. The antibiotic cephalosporin was given in a one gram dose on the morning of transplantation and intramuscularly (one gram) for 3 days after the operation. Because FR causes vomiting in dogs, 2 mg atropine sulphate were given I.M. twice a day for the first postoperative week, and once a day for the second week. During the third week, their daily atropine dose was reduced to one mg.
Blood samples were drawn every 3 days for determination of blood urea nitrogen (BUN), serum creatinine, serum glutamic oxaloacetic transaminase (SGOT), and total bilirubin. Animals that died were autopsied immediately with one exception. Histopathological studies will be reported elsewhere.
RESULTS
Kidney Transplantation
Of the 27 recipients, one animal died at day 6 from intussusception and was excluded from survival analyses. All of the treated animals had vomiting and emaciation during the first and second postoperative weeks, but this improved gradually thereafter in most of the animals.
Survival
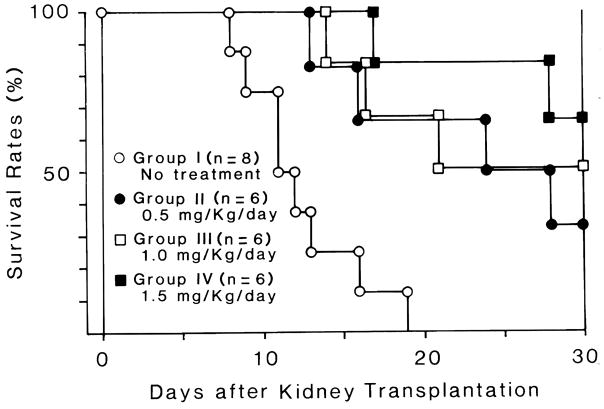
FR had a dose dependent effect on survival (Figure 1). One month survival was 0% (0/8) in Group I, 33% (2/6) in Group II, 50% (3/6) in Group III, and 67% (4/6) in Group IV. All of the deaths in Group I followed severe rejection. Deaths in Group II were caused by rejection (day 13 and 28), intussusception (day 24), and unknown causes (no autopsy, day 16). Deaths in Group III were from rejection (day 16) and lethal emaciation (days 14 and 21). Deaths in Group IV were from unknown cause (day 17) and possible pancreatitis (day 28).
FIGURE 1.
One month survival of Group I (untreated control), Group II (0.5mg/kg/day), Group III (1.0mg/kg/day) and Group IV (1.5mg/kg/day) after kidney transplantation.
Renal and Hepatic Functions
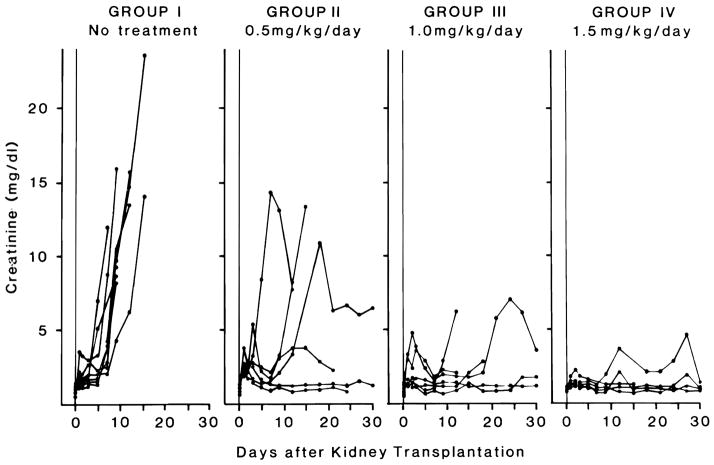
Changes in serum creatinine are shown in Figure 2. FR showed dose-dependent protection of renal function, with the best results in animals treated with 1.5mg/kg/day. There were no abnormalities in SGOT and bilirubin.
FIGURE 2.
Changes in serum creatinine of Group I (untreated control), Group II (0.5mg/kg/day), Group III (1. 0mg/kg/day) and Group IV (1.5mg/kg/day) after kidney transplantation.
Liver Transplantation
Survival
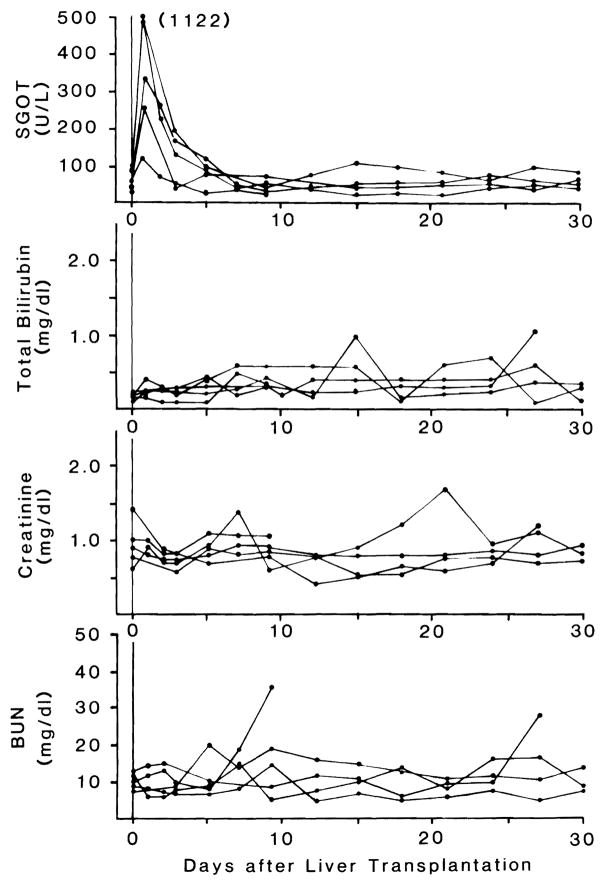
Ten liver transplantation were performed using B/B combinations. Only the 5 animals which survived more than 5 days were used for statistical analysis; the other 5 died perioperatively of technical complications. One of the definitive dogs died of biliary peritonitis 10 days after the operation. The other 4 are still alive after more than 30 days. (Figure 3). There was never an elevation of total bilirubin. The creatinine and BUN were normal except in the dog which died of peritonitis (Figure 3)
FIGURE 3.
Changes in SGOT, total bilirubin, BUN and creatinine of animals after liver transplantaion.
DISCUSSION
This study in dogs has confirmed that FR is a potent immunosuppressive agent when given orally. The low dose of 0.5mg/kg/day had a demonstrable but weak effect. At 1.0mg/kg/day and 1.5mg/kg/day, the results became increasingly predictable.
The side effects of FR were severe with the first doses and included vomiting, and emaciation but these lessened in most of the animals after 2–3 weeks. These toxic manifestations have not been seen in rats (1,3). Intussusception was not commonly observed, possibly because of prophylactic treatment with atropine. Kidney and liver function tests were not adversely effected by FR. Histopathologic studies looking for organ specific toxicity are underway.
After canine liver transplantation, FR gave better results than previously achieved with CyA (6). In those earlier studies, 7 of 8 untreated animals died within 1 months, and the mean survival time was 11.8+9.6 (S.D.) days. When treated with 20mg/kg/day of oral CyA, 3 of 9 animals died within 1 month, 2 as the result of rejection. Two of the remaining 6 CyA treated dogs had persistent elevation of bilirubin at 1 month and they later died of rejection. In contrast 4 of the 5 FR treated liver recipients survived more than 1 month, and none of the 5 had jaundice on late SGOT increases.
Acknowledgments
Supported by Research Grants from the Veterans Administration and Project Grant No. AM-29961 from the National Institutes of Health, Bethesda, Maryland.
References
- 1.OCHIAI T, NAKAJIMA K, NAGATA M, SUZUKI T, ASANO T, UEMATSU T, GOTO T, HORI S, KENMOCHI T, NAGAGOORI T, ISONO K. Effect of a new immunosuppressive agent, FK506, on heterotopic cardiac allotransplantation in the rat. Transplant Proc. 1987;19:1284–1286. [PubMed] [Google Scholar]
- 2.ZEEVI A, DUQUESNOY R, EIRAS G, TODO S, MAKOWKA L, STARZL T. In vitro immunosuppressive effects of FR 900506 on human T lymphocyte alloactivation. Surgical Research Communication. 1987 July; In Press. [PMC free article] [PubMed] [Google Scholar]
- 3.LEE P, MURASE N, TODO S, MAKOWKA L, STARZL TE. The immunosuppressive effects of FR 900506 in rats receiving heterotopic cardiac allografts. Surgical Research Communication. 1987 July; In Press. [PMC free article] [PubMed] [Google Scholar]
- 4.Starzl TE. Experience in renal transplantation. WB Saunders Company; Philadelphia: 1964. [Google Scholar]
- 5.TODO S, KAM I, LYNCH S, STARZL TE. Animal research in liver transplantation with special reference to the dog. Seminar in Liver Disease. 1985;5:309–317. doi: 10.1055/s-2008-1040626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.TODO S, PORTER KA, KAM I, LYNCH S, VENKATARAMANAN R, DEWOLF A, STARZL TE. Canine liver transplantation under Nva2-Cyclosporine versus Cyclosporin. Transplantation. 1986;41:296–300. doi: 10.1097/00007890-198603000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]