Abstract
The mechanisms utilized by Mycobacterium tuberculosis to establish, maintain, or reactivate from latent infection in the host are largely unknown but likely include genes that mediate adaptation to conditions encountered during persistence. Previously, a two-component signal transduction system, mprAB, was found to be required in M. tuberculosis for establishment and maintenance of persistent infection in a tissue- and stage-specific fashion. To begin to characterize the role of this system in M. tuberculosis physiology and virulence, a functional analysis of the mprA and mprB gene products was initiated. Here, evidence is presented demonstrating that sensor kinase MprB and response regulator MprA function as an intact signal-transducing pair in vitro and in vivo. Sensor kinase MprB can be autophosphorylated, can donate phosphate to MprA, and can act as a phospho-MprA phosphatase in vitro. Correspondingly, response regulator MprA can accept phosphate from MprB or from small phosphodonors including acetyl phosphate. Mutagenesis of residues His249 in MprB and Asp48 in MprA abolished the ability of these proteins to be phosphorylated in vitro. Introduction of these alleles into Mycobacterium bovis BCGattenuated virulence in macrophages in vivo. Together, these results support a role for the mprAB two-component system in M. tuberculosis physiology and pathogenesis. Characterization of two-component signal transduction systems will enhance our understanding of processes regulated by M. tuberculosis during acute and/or persistent infection in the host.
Persistent Mycobacterium tuberculosis infections afflict nearly 2 billion people worldwide (31). The combination of M. tuberculosis reactivation in these individuals and subsequent primary infection of immunocompromised hosts unable to control the infection accounts for more than 2.4 million deaths annually due to tuberculosis (34). This disease is the world's leading cause of death due to infection by a single bacterial agent (24). While the majority of individuals latently infected with M. tuberculosis do not undergo reactivation of disease, factors that suppress the host's immune system, including infection with human immunodeficiency virus, steroid therapy, malnutrition, and age, can increase the risk of reactivation tuberculosis to 10% per annum (44). While antitubercular therapeutics are effective in treating individuals with acute primary or reactivated tuberculosis infections, these antibiotics are largely ineffective against M. tuberculosis in the latent stages of infection (40), a poorly understood disease state characterized by little or no bacterial proliferation and the lack of overt disease symptoms. With the continuing increase in human immunodeficiency virus infection in many parts of the world, the emergence of multidrug-resistant isolates of M. tuberculosis (35), and the lack of antitubercular drugs exhibiting activity against organisms during latent infection, new therapeutics targeting persistent bacilli or preventing reactivation are needed.
The ability of M. tuberculosis to establish, maintain, and reactivate from persistent infection is the result of dynamic interactions between host immune components and survival strategies employed by the tubercle bacillus. While information is known about host factors regulating aspects of M. tuberculosis persistence in vivo (6), little is known about the bacterial factors required for growth and survival during persistent stages of infection. Recently, genes encoding secondary metabolism systems, cell surface determinants, and transcriptional factors have been implicated in M. tuberculosis persistence in a mouse model system of latent infection (8, 13, 41). Other genetic determinants, including the two-component signal transduction systems, also play an important role in latency and other stages of the M. tuberculosis life cycle (41). Two-component systems allow organisms to adapt to changing environmental stimuli through phosphotransfer reactions between a membrane-localized histidine kinase sensor and a cytoplasmic response regulator transcription factor. M. tuberculosis encodes 11 putative two-component sensor kinase and response regulator pairs, as well as several unlinked individual components (3). Expression of some of these systems is induced during in vitro growth of M. tuberculosis in macrophages (5, 9, 42, 43), a location where the tubercle bacillus is likely to reside in vivo. Thus, the downstream genes regulated by these systems are likely to be required for aspects of intracellular growth. Others are required for growth of the tubercle bacillus during acute stages of infection in a mouse model of tuberculosis (5, 32). Interestingly, the pathogenicity of M. tuberculosis in immunodeficient SCID mice is enhanced following disruption of some two-component systems, suggesting that these systems may also regulate genes that suppress intracellular growth of M. tuberculosis during the early stages of the infection process (28).
mprAB encodes a putative M. tuberculosis two-component system that is important for virulence during persistent stages of infection (43). Disruption of the mprA response regulator alters the survival characteristics of M. tuberculosis in macrophages in vitro and attenuates the ability of the tubercle bacillus to persist in the lung and spleen during periods of latency in vivo (43). To begin characterizing further the contribution of the mprAB system to M. tuberculosis physiology and pathogenesis, a functional characterization of the mprA and mprB gene products was initiated. Results demonstrating that MprB and MprA function as an intact signal-transducing pair in vitro and in vivo are presented here. Mutations that alter the phosphorylation sites in these proteins abolish their ability to participate in phosphotransfer reactions in vitro and attenuate the survival characteristics of Mycobacterium bovis BCG during in vivo growth in macrophages.
MATERIALS AND METHODS
Media, bacterial growth conditions, and electrotransformation.
Escherichia coli strains were grown in Luria-Bertani (LB) broth or on LB agar. Mycobacteria were grown under standard laboratory conditions in Middlebrook 7H9 broth (Difco) or Middlebrook 7H10 agar medium (Difco) supplemented with 0.5% glycerol, 10% albumin-dextrose-catalase, and 0.05% Tween 80. All strains were incubated at 37°C unless noted otherwise. When required, E. coli media were supplemented with 100 μg of ampicillin/ml, 50 μg of kanamycin sulfate/ml, 20 μg of choramphenicol/ml, or 100 μg of hygromycin B/ml. Mycobacterium media were supplemented with 25 μg of kanamycin sulfate/ml or 100 μg of hygromycin B/ml. Antibiotics were purchased from Sigma. Isopropyl-β-d-thiogalactopyranoside (IPTG; Invitrogen) was added to E. coli cultures at a concentration of 0.01 mM to induce protein overexpression. Preparation and transformation of electrocompetent E. coli or M. bovis BCG were performed as described previously (15).
Bacterial strains and plasmids.
Bacterial strains and plasmids used in this study are listed in Table 1. E. coli strain DH5α was used for all cloning procedures. E. coli BL21(DE3) or BL21(DE3)/pLysS (Novagen) was used for overexpression and purification of recombinant forms of MprA, MprB, or MtrA. M. bovis BCG Pasteur (ATCC 27291) was also used. The mprA::Kmr mutant of BCG was constructed as described previously (43).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Strains | ||
| E. coli DH5α | E. coli cloning strain; Ampr | Laboratory collection |
| E. coli BL21 | F−ompT hsdSB(rB− mB−) gal dcm(DE3) | Novagen |
| E. coli BL21(pLysS) | F−ompT hsdSB(rB− mB−) gal dcm(DE3)(pLysS [Camr]) | Novagen |
| M. bovis BCG Pasteur | Attenuated laboratory strain | ATCC 27291 |
| M. bovis BCG mprA::Kmr | BCG Pasteur isogenic mutant (defective for expression of mprA and mprB) | This study |
| Plasmids | ||
| pTZ215 | E. coli-mycobacterial integrative shuttle plasmid carrying wild-type mprA and mprB | 43 |
| pTZ225 | GST-tagged recombinant MprB expression plasmid | This study |
| pTZ229 | His6-tagged recombinant MprA expression plasmid | This study |
| pTZ310 | His6-tagged recombinant MtrA expression plasmid | This study |
| pTZ313 | His6-tagged recombinant MprA (Asp53-Ala) expression plasmid | This study |
| pTZ324 | His6-tagged recombinant MprA (Asp48-Ala) expression plasmid | This study |
| pTZ332 | GST-tagged recombinant MprB (His249-Gln) expression plasmid | This study |
| pTZ336 | pTZ215 carrying mprA (Asp48-Ala) and mprB | This study |
| pTZ339 | pTZ215 carrying mprA and mprB (His249-Gln) | This study |
Cloning, overexpression, and purification of MprA, MprB, and MtrA.
pGEX4T-1 (Pharmacia) and pET-15b (Novagen) protein overexpression constructs were used to make recombinant forms of MprA, MprB, and MtrA containing N-terminal fusions to glutathione-S-transferase (GST) and His6 tags. Briefly, the coding sequences of the corresponding genes were amplified from M. tuberculosis H37Rv genomic DNA by PCR using the following primers (the indicated restriction endonuclease sites are underlined): mprAstart (5′-GGATCCGTGTCCGTGCGAATTCTTGTC-3′; BamHI site) and mprAstop (5′-GTCGACTCAGGGTGGTGTTTCACGTAG-3′; SalI site), mprBtrunstart (5′-GGATCCATGACCGAAGCGGCCGAGC-3′; BamHI site) and mprBstop (5′-GAATTCCTAGGTTGCGCGCGTGGAC-3′; EcoRI site), and mtrAstart (5′-TGATCAAGGCAAAGGATTTTCCTCGT-3′; BclI site) and mtrAstop (5′-TGATCATCACGGAGGTCCGGCCTTGT-3′; BclI site). Primers mprBtrunstart and mprBstop amplify a truncated form of mprB encoding a protein that lacks the predicted N-terminal periplasmic domain and transmembrane region (amino acids 196 to 504). This truncated form was used to avoid potential toxicity resulting from overexpression of full-length mprB (11). The QuikChange and QuikChange XL site-directed mutagenesis kits (Stratagene) were used to construct mutant alleles of mprA or mprB containing point mutations in putative sites of phosphorylation. The mprA (Asp53-Ala) allele was generated using the following primers (altered nucleotides are underlined): mprAseq8 (5′-TCATGACAGCCAGGACCAACGCGTCG-3′) and mprAseq9 (5′-GGTCCTGGCTGTCATGATGCCGCGGCT-3′). The mprA (Asp48-Ala) allele was generated using the following primers (altered nucleotides are underlined): mprAseq10 (5′-GCGATCGCCCCGCCGCGTTGGTCCTG-3′) and mprAseq11 (5′-CAGGACCAACGCGGCGGGGCGATCGC-3′). The mprB (His249-Gln) allele was generated using the following primers (altered nucleotides are underlined): mprBseq8 (5′-CGACGCCGGACAATTGCGTACCC-3′) and mprBseq9 (5′-GGGTACGGCAATTCTTGTCCGGCGTCG-3′). Constructs were sequenced to confirm the presence of desired mutations and proper orientation. To express recombinant MprA, MprB, and MtrA, BL21(DE3) or BL21(DE3)/pLysS cultures containing overexpression constructs were grown overnight on selective LB agar medium, resuspended into LB containing the appropriate antibiotic, grown to mid-exponential phase (optical density at 600 nm of 0.5), and induced for 3 h in the presence of IPTG. Induced cultures were purified by affinity chromatography over a glutathione-agarose column (for GST tags) or a nickel nitrilotriacetic acid-agarose column (for His6 tags) as recommended by the manufacturer. To prepare cell extracts for chromatography, whole cell pellets were resuspended in lysis buffer (40 mM Tris [pH 7.6], 150 mM NaCl, 10 mM EDTA, 0.1% β-mercaptoethanol, 10 μg of DNase/ml, 10 μg of RNase/ml, 20 μg of leupeptin/ml, 10 μg of aprotinin/ml, and 1 mM phenylmethylsulfonide), passaged through a French press, and centrifuged at 31,000 × g for 30 min. Supernatants containing the soluble fraction were collected and loaded onto the appropriate agarose column and washed, and tagged fusion proteins were eluted with 10 mM reduced glutathione (GST-tagged proteins) or with 250 mM imidazole (His6-tagged proteins). Cellular supernatants and protein eluates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 12.5% polyacrylamide gels and stained with Coomassie brilliant blue.
In vitro autophosphorylation and transphosphorylation reactions.
Protein autophosphorylation reactions with wild-type GST-cMprB or the GST-cMprB (His249-Gln) mutant were carried out for 10 min at room temperature in autophosphorylation buffer (50 mM Tris-HCl [pH 7.6], 50 mM KCl) supplemented with MgCl2, MnCl2, or CaCl2 at a final concentration of 20 mM. Reactions were initiated by the addition of [γ-32P]ATP or [α-32P]ATP (10 mCi/ml, 3,000 Ci/mmol; Perkin-Elmer). For transphosphorylation reactions, wild-type His-MprA, His-MprA (Asp53-Ala), His-MprA (Asp48-Ala), or wild-type His-MtrA was added in 10-fold molar excess to the autophosphorylation reaction mixture containing GST-phospho-cMprB and the mixture was incubated for up to an additional 30 min at 37°C. To stop reactions, samples were incubated with 2× loading dye and heated at 95°C for 5 min. Proteins were subjected to SDS-PAGE on 15% polyacrylamide gels and either stained with Coomassie brilliant blue to confirm equivalent protein loadings or transferred onto an Immobilon-P membrane (Millipore) by electroblotting and exposed to BioMAX MR film (Kodak) overnight. When required, membranes were also subjected to immunoblot analysis using rabbit antiserum generated to M. tuberculosis MprA or cMprB in which N-terminal tags had been cleaved. Donkey anti-rabbit immunoglobulin G conjugated to horseradish peroxidase (Amersham) was used as the secondary antibody. ECL (Pierce) was used to visualize antibodies bound to membrane.
Phosphorylation with acetyl phosphate and resolution by 2D gel electrophoresis.
His-MprA phosphorylation by acetyl phosphate was investigated using two-dimensional (2D) gel electrophoresis. Purified wild-type His-MprA, His-MprA (Asp53-Ala), or His-MprA (Asp48-Ala) was added to phosphorylation buffer supplemented with 20 mM MgCl2 and 10 mM acetyl phosphate, and mixtures were incubated at 37°C for 30 min. To analyze the phospho-MprA phosphatase activity of GST-cMprB, His-MprA phosphorylation reaction mixtures were incubated for an additional 30 min at 37°C in the presence of an equimolar amount of wild-type GST-cMprB or the GST-cMprB (His249-Gln) mutant. Reactions were stopped by the addition of 3 volumes of 100% ice-cold acetone, and mixtures were incubated at −20°C overnight. Precipitated protein was collected by centrifugation, washed with 1 volume of 100% ice-cold acetone, and allowed to air dry. The protein pellet was suspended in 250 μl of rehydration solution (8 M urea, 2% [wt/vol] CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate}, and trace amounts of bromophenol blue) containing 6 mg of dithiothreitol and 0.5% immobilized pH gradient (IPG) buffer (Pharmacia). Isoelectric focusing resolved proteins according to their pIs in the first dimension by using a 7- or 11-cm IPG strip (pH 4.0 to 7.0; Pharmacia) and a Protean isoelectric focusing cell (Bio-Rad) as recommended by the manufacturers. Protein samples were allowed to undergo passive rehydration for 12 h at 20°C before being focused at 500 V for 250 V · h, 1,000 V for 500 V · h, and 8,000 V for 5,000 V · h at 20°C. Proteins were resolved according to molecular mass in the second dimension by SDS-PAGE using 15% polyacrylamide gels. Briefly, focused IPG strips were incubated for 15 min at room temperature in SDS equilibration buffer (50 mM Tris-HCl [pH 8.8], 6 M urea, 30% glycerol, 2% SDS, trace amounts of bromophenol blue) containing 5 mg of dithiothreitol and subsequently for 15 min at room temperature in SDS equilibration buffer containing 125 mg of iodoacetamide. Equilibrated strips were cut to size, loaded onto polyacrylamide gels, and resolved by SDS-PAGE. Proteins were visualized by staining with Coomassie brilliant blue. The apparent pIs of focused proteins were determined by comparison against a control gel containing focused 2D SDS-PAGE protein standards (Bio-Rad).
J774A macrophage infection assays.
Derivatives of M. bovis BCG were used to infect the murine J774Amacrophage cell line (ATCC TIB-67). Bacteria used for infections were grown under static conditions and processed prior to infection as described previously (42). Monolayers were cultured in Dulbecco's modified Eagle's medium (Bio Whittaker) supplemented with 10% fetal bovine serum (Omega Scientific) and 4 mM l-glutamine (Invitrogen) at 37°C in humidified air containing 5% CO2. Twelve-well tissue culture plates (Corning) seeded with 105 macrophages/well were infected with mycobacteria at a multiplicity of infection of 10. Macrophages were allowed to ingest bacteria for 2 h before extracellular bacilli were removed by three washes in phosphate-buffered saline (pH 7.2). At specific times after infection (2 h and 1, 3, and 5 days), macrophage monolayers were lysed with sterile water containing 0.05% Tween 80. Bacterial survival was determined by diluting macrophage lysates in sterile water containing 0.05% Tween, plating them onto 7H10 medium, and incubating them for 3 to 4 weeks at 37°C. The percentages of bacteria surviving at different time points were determined by counting CFU and normalizing to the number of CFU at the initial time point (2 h), which was set at 100%. Equivalent numbers of bacteria (∼105) were recovered from all strains at the initial 2-h time point (data not shown), and no apparent damage to monolayers was observed during the course of infection. Tissue culture media were replenished after 3 days or as required. In vitro growth curve studies performed in parallel did not show significant differences in in vitro growth rates between any of the BCG derivatives examined (data not shown).
Densitometric and statistical analysis.
NIH Image (version 1.62; National Institutes of Health) was used to quantify the relative intensities of MprA or MprB proteins. All statistical analyses (analysis of variance and Fisher's protected least-significant difference) were performed with ANOVA (version 1.11; Abicus Software).
Nucleotide sequence accession number.
The sequence for the mprAB region from M. bovis BCG Pasteur has been deposited in GenBank under accession number AF490842.
RESULTS
Phosphorylation of MprB and MprA.
Sensor kinase MprB and response regulator MprA are predicted to express a functional two-component signal transduction system (3). To determine whether these components work as an intact signal-transducing pair, the mprB and mprA genes from M. tuberculosis H37Rv were cloned into constructs expressing N-terminally GST- or His6-tagged fusion proteins and the proteins were overexpressed in E. coli, purified, and tested for the ability to participate in phosphorylation reactions in vitro. An N-terminally truncated form of MprB lacking the amino-terminal periplasmic domain and membrane-spanning region (amino acids 196 to 504) was used for these reactions, as overexpression of full-length histidine sensor kinase genes is often toxic (11, 12). This truncated protein, called GST- cMprB, was soluble in E. coli and was obtained at ∼90% homogeneity by glutathione Sepharose chromatography (data not shown). The remaining ∼10% of protein that was copurified predominantly comprised three truncated forms of GST-cMprB that retained reactivity with polyclonal antibodies directed against both GST and MprB. In contrast, full-length MprA containing an N-terminal His6 tag was soluble in E. coli and was obtained at greater than 99% homogeneity (data not shown).
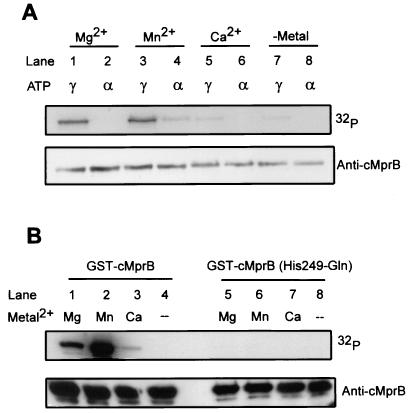
Autophosphorylation of recombinant GST-cMprB in the presence of [γ-32P]ATP was assessed using a standard kinase assay. GST-cMprB was readily phosphorylated when incubated in the presence of divalent cations Mg2+ and Mn2+ (Fig. 1A, lanes 1 and 3) but exhibited little autophosphorylation when incubated in the presence of Ca2+ or in the absence of divalent cation (Fig. 1A, lanes 5 and 7). To determine whether the γ phosphate of ATP was utilized by GST-cMprB in these reactions, MprB kinase reactions were repeated using [α-32P]ATP as the substrate. In these reactions, GST-cMprB phosphorylation was observed at low levels in the presence of Mn2+ (Fig. 1A, lane 4) but was not observed in the presence of Mg2+ or Ca2+ or in the absence of divalent cations (Fig. 1A, lanes 2, 6, and 8). Thus, GST-cMprB possesses kinase activity and requires Mg2+ or Mn2+ as a cofactor for autophosphorylation.
FIG. 1.
In vitro autophosphorylation of GST-cMprB derivatives. (A) Purified GST-cMprB was incubated in the presence of [γ-32P]ATP (lanes 1, 3, 5, and 7) or [α-32P]ATP (lanes 2, 4, 6, and 8) and divalent cations including Mg2+ (lanes 1 and 2), Mn2+ (lanes 3 and 4), and Ca2+ (lanes 5 and 6) or in the absence of metal (lanes 7 and 8). (B) Wild-type GST-cMprB (lanes 1 to 4) or the GST-cMprB (His249-Gln) mutant (lanes 5 to 8) was incubated in the presence of [γ-32P]ATP and Mg2+ (lanes 1 and 5), Mn2+ (lanes 2 and 6), or Ca2+ (lanes 3 and 7) or in the absence of divalent cations (lanes 4 and 8). Phosphorylation of wild-type or mutant GST-cMprB was detected by autoradiography, and polyclonal antibody directed against cMprB was used in Western blotting to confirm similar loading amounts between reactions.
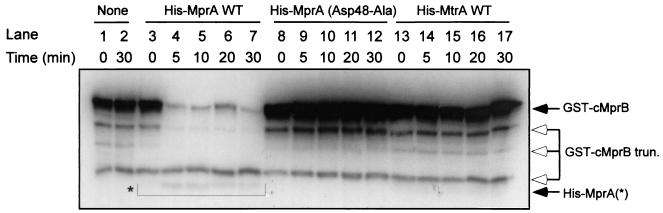
As sensor kinase proteins typically participate in phosphotransfer reactions with their cognate response regulator partners, transfer of radiolabeled phosphate from GST-cMprB to His-MprA was also investigated. Addition of recombinant MprA to phosphorylated GST-cMprB resulted in loss of the radiolabel from GST-cMprB concomitant with a small, but reproducible, incorporation into His-MprA (Fig. 2, lanes 3 to 7). Transfer of the labeled phosphoryl group from GST-cMprB was observed immediately after addition of His-MprA and continued for the first 5 min of incubation, after which no additional transfer was observed. Loss of radiolabel from GST-phospho-cMprB was not simply the result of protein instability, as similar amounts of phosphorylated GST-cMprB protein were observed over the 30-min time course when incubation was in the absence of His-MprA protein (Fig. 2, compare lanes 1 and 2). Incorporation of radiolabeled phosphate into His-MprA in these reactions was also not simply the result of autokinase activity, as phosphorylation was not observed following incubation of His-MprA with [γ-32P]-ATP and divalent cations alone (data not shown). Thus, GST-cMprB is a kinase for response regulator His-MprA in vitro.
FIG. 2.
Transphosphorylation between GST-cMprB and M. tuberculosis response regulators. Wild-type GST-cMprB was autophosporylated with [γ-32P]ATP and then incubated in the absence of other proteins (lanes 1 and 2) or in the presence of wild-type His-MprA (lanes 3 to 7), the His-MprA (Asp48-Ala) mutant (lanes 8 to 12), or wild-type His-MtrA (lanes 13 to 17). Transphosphorylation reactions were allowed to proceed for 0 min (lanes 1, 3, 8, and 13), 5 min (lanes 4, 9, and 14), 10 min (lanes 5, 10, and 15), 20 min (lanes 6, 11, and 16), or 30 min (lanes 2, 7, 12, and 17). Closed arrows indicate the locations of full-length GST-cMprB and response regulator proteins. Open arrows indicate the locations of truncated (trunc.) forms of GST-cMprB. The asterisk indicates the position of phosphorylated His-MprA species. Transfer of radiolabel from GST-cMprB to response regulator proteins was detected by autoradiography. WT, wild type.
Because some two-component signal transduction systems engage in cross talk with other sensor kinase and response regulator pairs (1, 10, 20, 26), transfer of phosphate from GST-cMprB to a noncognate response regulator from M. tuberculosis was also investigated. The MtrA response regulator protein was chosen as a control for these assays, as MtrA has been previously overexpressed, purified, and shown to participate in phosphorylation reactions in vitro (39). Following incubation of His-MtrA with phosphorylated GST-cMprB, loss of 32P from GST-cMprB or concomitant incorporation of radiolabeled phosphate into His-MtrA was not observed over the time course examined (Fig. 2, lanes 13 to 17). The inability to observe phosphorylation in these reactions was not simply due to an inability of His-MtrA to be phosphorylated, as phosphorylation of His-MtrA could be observed following the addition of acetyl phosphate (data not shown). Thus, transfer of phosphate from GST-cMprB to His-MprA in these reactions is specific between this sensor kinase and response regulator pair.
Phosphorylation of MprA by small phosphodonor compounds.
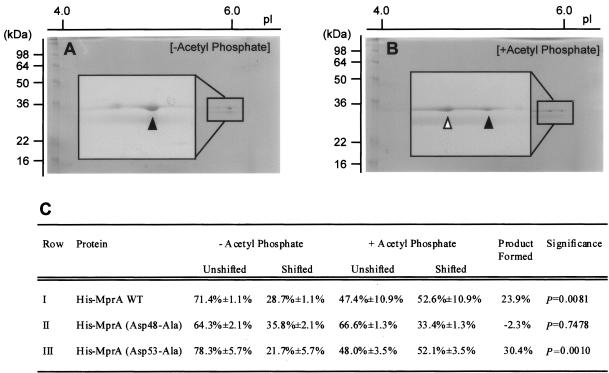
Although transfer of phosphoryl groups to response regulator proteins proceeds from their cognate sensor kinase partners, response regulator proteins can also be phosphorylated, albeit at lower efficiency, with small phosphodonor compounds such as acetyl phosphate (22, 37). To determine whether this was also a characteristic of His-MprA, MprA phosphorylation reactions were performed using acetyl phosphate as a donor substrate. Phosphorylation of His-MprA with acetyl phosphate was evaluated using 2D gel electrophoresis, as addition of a phosphoryl group shifts the pI of a given protein by 0.2 U towards the acidic side (16). In the absence of acetyl phosphate, recombinant MprA focused predominantly as a single protein species with a calculated pI of ∼6.0 (Fig. 3A). This is in good agreement with the predicted pI of 6.1 for His-MprA in the unphosphorylated form. A small fraction of His-MprA also focused at a pI of ∼5.8 in these reactions (Fig. 3A), which is similar to the predicted pI of His-MprA in the phosphorylated form. Because this protein species was observed with all recombinant MprA derivatives tested in the absence of acetyl phosphate, including those incapable of undergoing phosphorylation, this protein species likely represents a conformeric form of unphosphorylated His-MprA that focuses at a slightly altered pI. This phenomenon has also been reported following isoelectric focusing of other purified proteins (18). In contrast, recombinant MprA focused as two predominant protein species following incubation with acetyl phosphate. In these reactions, a His-MprA species that focused at pI 6.0 and another that focused at pI 5.8 were observed (Fig. 3B). To determine whether this apparent shift in the pI of His-MprA following incubation with acetyl phosphate was statistically significant, relative amounts of protein at each pI were determined by densitometry. In the absence of acetyl phosphate, 71.4% ± 1.1% of His-MprA was present in the unshifted form (pI 6.0) and 28.7% ± 1.1% of His-MprA was present at the shifted pI (pI 5.8) (Fig. 3C, row I). In contrast, 47.4% ± 10.9% of His-MprA was present in the unshifted form and 52.6% ± 10.9% of His-MprA was present at the shifted pI following incubation with acetyl phosphate (Fig. 3C, row I). Thus, incubation with acetyl phosphate results in conversion of approximately 23.9% of His-MprA from the unphosphorylated form into the phosphorylated form (P = 0.0081).
FIG. 3.
Phosphorylation of His-MprA with acetyl phosphate and resolution by 2D gel electrophoresis. Wild-type His-MprA was incubated in the absence (A) or presence (B) of acetyl phosphate. His-MprA protein was separated according to charge (pI) and molecular mass by 2D gel electrophoresis. Unphosphorylated (closed arrowheads) and phosphorylated (open arrowhead) forms of His-MprA were quantitated by densitometry, and the amount of product formed was determined (C). The mean and standard error for each category were determined from results of three independent reactions, each run on individual 15% polyacrylamide gels. Significance refers to the comparison between the amount of His-MprA in the shifted form (pI 5.8) following incubation with acetyl phosphate and the amount of His-MprA in the shifted form (pI 5.8) following incubation in the absence of acetyl phosphate. WT, wild type.
Sites of MprB and MprA phosphorylation.
Phosphorylation of sensor kinase and response regulator proteins typically occurs at highly conserved residues. For sensor kinases, phosphorylation occurs at an invariant histidine, while response regulators are generally phosphorylated at a conserved aspartic acid. To define sites of phosphorylation in MprB and MprA, site-directed mutagenesis was performed and purified mutant protein derivatives were tested for the ability to be phosphorylated in vitro. Residues His249 in GST-cMprB and Asp53 in His-MprA were chosen for mutagenesis, as these amino acids are potential sites of phosphorylation based on sequence alignment with other sensor kinases and response regulators from this family (data not shown). In contrast to that of wild-type GST-cMprB, autophosphorylation of the GST-cMprB (His249-Gln) mutant was not observed when the protein was incubated in the presence of [γ-32P]ATP under any condition examined (Fig. 1B). This failure was not the result of protein instability, as both wild-type and mutant cMprB were detected by Western blotting using polyclonal antibody directed against cMprB (Fig. 1B). Thus, His249 is required for phosphorylation in GST-cMprB.
The ability of the His-MprA (Asp53-Ala) mutant to be phosphorylated was also investigated. In assays utilizing both phosphorylated GST-cMprB (data not shown) and acetyl phosphate (Fig. 3C, row III) as substrates, phosphorylation of the His-MprA (Asp53-Ala) mutant was still observed, suggesting that Asp53 is not the primary site of phosphorylation in MprA. As a second aspartic acid residue is present in close proximity at position 48 in MprA, site-directed mutagenesis at this residue was also performed and phosphorylation reactions were repeated. In contrast to wild-type His-MprA and the His-MprA (Asp53-Ala) mutant, His-MprA (Asp48-Ala) was unable to be phosphorylated when incubated in the presence of either GST-phospho-cMprB or acetyl phosphate. For example, GST-cMprB was unable to transfer its 32P-radiolabeled phosphoryl group to the His-MprA (Asp48-Ala) mutant (Fig. 2, lanes 8 to 12), and no observable shift in the pI of mutant His-MprA (Asp48-Ala) following incubation with acetyl phosphate was detected (P = 0.7478) (Fig. 3C, row II). Thus, Asp48 is required for His-MprA to be phosphorylated by GST-cMprB and acetyl phosphate.
MprB possesses phospho-MprA phosphatase activity.
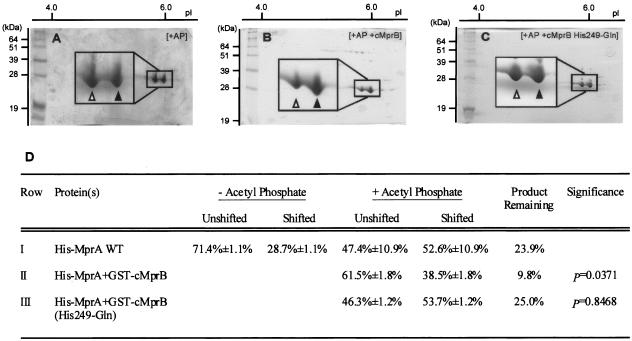
In addition to kinase and phosphotransferase activities, many histidine kinases also facilitate dephosphorylation of their cognate response regulators (36, 37). While His-MprA phosphorylation was detected when His-MprA was incubated in the presence of acetyl phosphate (Fig. 3A), transphosphorylation of His-MprA was not readily observed when this protein was incubated in the presence of GST-phospho-cMprB (Fig. 2, lanes 3 to 7). To determine whether this discrepancy could be attributed to phosphatase activity associated with GST-cMprB, the ability of GST-cMprB to dephosphorylate His-MprA was investigated. As observed previously, incubation of His-MprA with acetyl phosphate resulted in a significant shift of the protein to its phosphorylated form at pI 5.8 (Fig. 4A). The subsequent addition of wild-type GST-cMprB to these reaction mixtures resulted in the transition of His-MprA back to its unphosphorylated form at pI 6.0 (Fig. 4B). For example, while 52.6% ± 10.9% of His-MprA focused at pI 5.8 when the protein was incubated in the absence of cMprB (Fig. 4D, row I), only 38.5% ± 1.8% of His-MprA focused at this pI following addition of cMprB to these reaction mixtures (P = 0.0371) (Fig. 4D, row II). Thus, addition of GST-cMprB induces the dephosphorylation of His-phospho-MprA. As amino acid residues responsible for phosphatase activity in sensor kinase proteins have been shown to overlap those residues involved in the proteins' kinase activity (14), the ability of the GST-cMprB (His249-Gln) mutant to dephosphorylate His-MprA was also tested. In contrast to that of wild-type GST-cMprB, addition of the GST-cMprB (His249-Gln) mutant did not result in dephosphorylation of His-MprA in these reactions (Fig. 4C). Rather, the relative amount of phosphorylated His-MprA remained at levels similar to those observed when His-MprA was incubated in the absence of GST-cMprB (P = 0.8468) (Fig. 4D, row III). Thus, MprB functions as a phospho-MprA phosphatase and this activity requires residue His249.
FIG. 4.
Phospho-MprA dephosphorylation by GST-cMprB. Wild-type His-MprA was incubated in the presence of acetyl phosphate (AP) for 30 min to induce phosphorylation. Reaction mixtures containing His-phospho-MprA were then incubated alone (A), in the presence of wild-type GST-cMprB (B), or in the presence of the GST-cMprB (His249-Gln) mutant (C) for an additional 30 min. Unphosphorylated (closed arrowheads) and phosphorylated (open arrowheads) forms of His-MprA were quantitated by densitometry, and the amount of phosphorylated product remaining was determined (D). The mean and standard error for each category were determined from results of three independent reactions, each run on individual 15% polyacrylamide gels. Significance refers to the comparison between the amount of His-MprA remaining in the shifted form (pI 5.8) following incubation with wild-type GST-cMprB and the amount of His-MprA in the shifted form (pI 5.8) following incubation with the GST-cMprB (His 249-Gln) mutant. WT, wild type.
In vivo analysis of MprB and MprA phosphorylation site mutants.
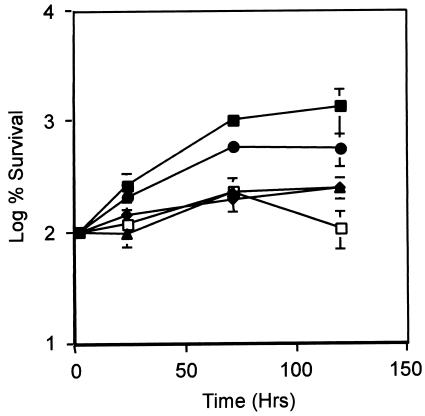
Because our phosphorylation data suggested a role for His249 and Asp48 in the activity of MprB and MprA, respectively, in vitro, the importance of these residues in vivo was examined. Mutant alleles of mprB and mprA containing the His249-Gln and Asp48-Ala substitutions were generated by mutagenic PCR in plasmid pTZ215 (43), an integrative Mycobacterium-E. coli shuttle plasmid containing wild-type mprA and mprB under control of its own promoter. Plasmid constructs were introduced into an mprA::Kmr mutant strain of M. bovis BCG, a derivative defective for expression of both mprA and mprB, and resulting recombinants were evaluated. The physiological consequences of these mutations were examined using a macrophage infection assay, as mprA expression is induced in BCG during growth in macrophages (43) and disruption of mprA alters the growth characteristics of M. tuberculosis H37Rv in macrophages in vitro and in an animal model system of infection in vivo (43). Wild-type BCG-infected J774 macrophages exhibited a >10-fold increase in numbers of CFU after 5 days of growth (Fig. 5). In contrast, numbers of CFU of the BCG mprA::Kmr mutant in J774 macrophages increased by <2.5-fold over the same time period (P = 0.0001) (Fig. 5). Thus, disruption of mprA attenuates M. bovis BCG growth in macrophages. To test whether the observed growth attenuation was a direct result of mprA disruption, macrophages were also infected with a BCG mprA::Kmr mutant derivative expressing wild-type mprA and mprB in single copy in trans (pTZ215). Numbers of CFU of this strain increased by 5.7-fold after 5 days of incubation in macrophages, and this increase partially restored growth to wild-type levels (Fig. 5). The inability to observe full complementation in this strain may be due to expression of wild-type mprA and mprB at an exogenous site in the chromosome, as similar results were also observed during growth of an M. tuberculosis H37Rv mprA::Kmr mutant containing pTZ215 in both J774 and murine bone marrow-derived macrophages in vitro (43). Importantly, attenuation in BCG growth was also observed following infection of J774 macrophages with BCG mprA::Kmr derivatives containing alleles of mprB (wild-type mprA and mprB [His249-Gln] [pTZ339]) or mprA (mprA [Asp48-Ala] and wild-type mprB [pTZ336]) that had been mutated at phosphorylation sites. In these infections, BCG mutants exhibited severe attenuation in growth during the 5-day course of infection and failed to restore growth to wild-type levels [P values of 0.3102 and 0.4840 for BCG mprA::Kmr(pTZ339) and BCG mprA::Kmr(pTZ336), respectively, relative to the BCG mprA::Kmr mutant parent 5 days after infection] (Fig. 5). Thus, site-directed mutations that replace His249 and Asp48 in MprB and MprA, respectively, attenuate growth of M. bovis BCG in macrophages in vitro.
FIG. 5.
Survival of M. bovis BCG derivatives in macrophages. J774 macrophages were infected with various BCG derivatives for 2 h or 1, 3, or 5 days. Numbers of CFU were determined from infected macrophages at each time point and were normalized to the 2-h time point numbers that were set as 100%. Strains examined included wild-type BCG Pasteur (closed squares), the isogenic mprA::Kmr mutant (diamonds), the mprA::Kmr mutant complemented with wild-type mprA and wild-type mprB (pTZ215) in trans (circles), the mprA::Kmr mutant complemented with wild-type mprA and mprB (His249-Gln) (pTZ339) in trans (triangles), and the mprA::Kmr mutant complemented with mprA (Asp48-Ala) and wild-type mprB (pTZ336) in trans (open squares). The means and standard errors of results from three independent infections are shown.
DISCUSSION
Two-component signaling systems regulate various bacterial processes, including respiration, metabolism, development, and drug resistance. In pathogenic bacteria, these systems also regulate expression of virulence determinants, allowing organisms to rapidly adapt to changing environmental conditions encountered within the host. In M. tuberculosis, several two-component systems have been shown to participate in various stages of the bacillus's intracellular life cycle (41). To date, only one M. tuberculosis two-component system has been characterized in detail. This system, devR (dosR) and devS (Rv3132c), is activated by hypoxic conditions and regulates many of the genes induced by M. tuberculosis during growth in environments with low O2 levels (29). In BCG, DevR is required for long-term hypoxic survival (2), and in Mycobacterium smegmatis, DevR acts as a stationary phase regulator required for adaptation to oxygen starvation and resistance to heat stress (27). Thus, the dosR-Rv3132c system regulates gene expression in response to low O2 levels and likely plays a role in the transition of M. tuberculosis in vivo from active growth to nonreplicating persistence (40). In a first step towards understanding the role of another two-component signal transduction system, that of mprA and mprB, in M. tuberculosis pathogenesis, a functional analysis of the gene products encoded by this system has been carried out.
Based on sequence alignment, MprB and MprA are grouped into the E. coli EnvZ-OmpR subfamily of two-component signaling systems (30). MprB contains two major hydrophobic membrane-spanning regions in its N-terminal region and the highly conserved H, N, D or F, and G boxes in the C-terminal region that are typical of sensor kinase proteins from this family. Similarly, MprA possesses the conserved receiver domain motif in its N terminus as well as the winged helix-turn-helix DNA-binding motif in its C terminus found primarily in transcription factors from the OmpR-ROII subclass of response regulator proteins (19). Several lines of evidence support a role for MprA and MprB in phosphorylation-mediated processes characteristic of two-component signaling systems. First, purified recombinant MprB possesses kinase activity and undergoes autophosphorylation in vitro at a conserved histidine residue (His249). Similar to that of many characterized sensor kinase proteins, MprB autophosphorylation is stimulated in the presence of Mg2+. However, phosphorylation also proceeds efficiently in the presence of Mn2+, a characteristic limited to a subset of sensor kinase proteins, including TrcS of M. tuberculosis (11), FrzE and AsgA of Myxococcus xanthus (17, 23), and FixL of Rhizobium meliloti (7). Second, purified recombinant MprA undergoes phosphorylation at a conserved aspartic acid residue (Asp48) in vitro when incubated in the presence of its recombinant cognate sensor kinase partner MprB or acetyl phosphate. Interestingly, identification of residue 48 as the site of MprA phosphorylation is not consistent with predictions specifying the phosphorylated residue based on sequence alignment of MprA with OmpR and other response regulators from the ROII subclass. In these proteins, the invariant aspartic acid residue at position 53, an amino acid that is also conserved in MprA, defines the phosphorylation site (4). The ability of recombinant MprA to be phosphorylated in the absence of its cognate sensor kinase partner adds this protein to the growing list of response regulators that can be phosphorylated by small phosphodonor compounds.
While both recombinant MprB and MprA alone participate in reactions typical of members of the two-component family, it has been difficult to demonstrate that these proteins function as an intact signal-transducing pair to exchange phosphate in vitro. While radiolabeled phosphate is lost from GST-cMprB in transphosphorylation reactions in an MprA-dependent manner, only a minor amount of radiolabel is incorporated into His-MprA (Fig. 2). In many two-component systems of the EnvZ-OmpR family, transfer of radiolabeled phosphate between the sensor kinase and the cognate response regulator is readily observed in vitro (21, 25, 38). However, phosphotransfer reactions between other sensor kinase and response regulator pairs can proceed faster than what can be experimentally observed (33). Several possibilities may explain our inability to readily detect phosphorylation of His-MprA by GST-phospho-cMprB in vitro. (i) Purified His-MprA protein used in these assays may not be active to accept phosphate from GST-cMprB. This possibility is unlikely considering the observation that approximately 25% of His-MprA can be phosphorylated by acetyl phosphate (Fig. 3C), a reaction that is considerably less efficient than phosphotransfer between sensor kinase and response regulator pairs (37). Furthermore, loss of radiolabeled phosphate from GST-cMprB is still observed in these reactions following the addition of recombinant MprA (Fig. 2), suggesting that His-MprA remains functionally able to interact with its sensor kinase partner in vitro. In addition, removal of the His6 tag from recombinant MprA or incubation with recombinant GST-MprA does not result in increased transfer of radiolabeled phosphate from GST-phospho-cMprB to MprA (data not shown). (ii) Recombinant MprA may also possess autophosphatase activity (36, 37). Although His-MprA may contain low-level autophosphatase activity, this possibility is also unlikely considering that a significant amount of His-MprA remains phosphorylated when the protein is incubated in the absence of other proteins (Fig. 3B and 4A). (iii) The inability to readily detect phosphorylated His-MprA following incubation with GST-phospho-cMprB may also be attributed to phosphatase activity associated with GST-cMprB. We believe this model to be correct as incubation of wild-type GST-cMprB but not that of the GST-cMprB (His249-Gln) mutant results in significant conversion of His-MprA from its phosphorylated form into its unphosphorylated form (Fig. 4C). Therefore, although GST-cMprB may be capable of donating phosphate to His-MprA in transphosphorylation reactions in vitro, His-phospho-MprA appears to be short lived due to the phospho-MprA phosphatase activity associated with GST-cMprB. Thus, the MprB phosphatase activity may drive the phosphotransfer equilibrium for this system.
Apart from the importance of His249 and Asp48 in MprB and MprA activity in vitro, these residues are also important for the biological function of this two-component system in vivo. Disruption of mprA, or introduction of mprA and mprB alleles containing mutations in phosphorylation sites, significantly attenuates the ability of M. bovis BCG to grow intracellularly in macrophages. While the exact mechanisms responsible for this attenuation are currently under investigation, the requirement for MprB and MprA to participate in phosphotransfer reactions in vivo underscores the importance of this system for aspects of Mycobacterium pathogenesis. Interestingly, the observation that mutations in mprA and/or mprB attenuate the intracellular growth of BCG in macrophages contradicts the phenotype previously observed during intracellular growth of the M. tuberculosis H37Rv mprA mutant in macrophages (43). In these studies, disruption of mprA enhanced the ability of this strain to grow in J774 macrophages relative to that of wild-type M. tuberculosis H37Rv or a complemented M. tuberculosis mprA::Kmr mutant. While the reasons for this discrepancy remain unclear, differences in mprA expression or regulation may, in part, contribute to the differences in growth characteristics observed between these strains (43). In support of this idea, the sequence of the mprAB region in M. bovis BCG Pasteur (GenBank accession no. AF490842) differs slightly from that found in M. tuberculosis H37Rv (data not shown) and microarray analyses performed with wild-type and mprA::Kmr mutant strains of both M. tuberculosis H37Rv and M. bovis BCG suggest that MprA may regulate different subsets of genes in these strains (V. K. Singh and T. C. Zahrt, unpublished data). Regardless, mutations in the mprAB two-component system alter the survival characteristics of Mycobacterium species both in vitro and in vivo.
The demonstration that MprB and MprA function as an intact two-component signal-transducing pair is the first step towards understanding the role that this system plays in M. tuberculosis physiology and pathogenesis. Continued characterization of two-component signaling systems such as mprAB in M. tuberculosis will continue to provide new insights into the conditions encountered by the tubercle bacillus within the host as well as the genes required for its survival.
Acknowledgments
We thank Dara Frank and members of the Frank and Zahrt laboratories for helpful discussions. We also thank the Clement J. Zablocki VA Medical Center (Milwaukee) for use of their BSL-3 containment laboratory.
We gratefully acknowledge funding from NIH grant AI51669 and a Medical College of Wisconsin Research Affairs award to T.C.Z.
Editor: V. J. DiRita
REFERENCES
- 1.Arthur, M., F. Depardieu, and P. Courvalin. 1999. Regulated interactions between partner and non-partner sensors and response regulators that control glycopeptide resistance gene expression in enterococci. Microbiology 145:1849-1858. [DOI] [PubMed] [Google Scholar]
- 2.Boon, C., and T. Dick. 2002. Mycobacterium bovis BCG response regulator essential for hypoxic dormancy. J. Bacteriol. 184:6760-6767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, A. Krogh, J. McLean, S. Moule, L. Murphy, K. Oliver, J. Osborne, M. A. Quail, M.-A. Rajandream, J. Rogers, S. Rutter, K. Seeger, J. Skelton, R. Squares, S. Squares, J. E. Sulston, K. Taylor, S. Whitehead, and B. G. Barrell. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 4.Delgado, J., S. Forst, S. Harlocker, and M. Inouye. 1993. Identification of a phosphorylation site and functional analysis of conserved aspartic acid residues of OmpR, a transcriptional activator for ompF and ompC in Escherichia coli. Mol. Microbiol. 10:1037-1047. [DOI] [PubMed] [Google Scholar]
- 5.Ewann, F., M. Jackson, K. Pethe, A. Cooper, N. Mielcarek, D. Ensergueix, B. Gicquel, C. Locht, and P. Supply. 2002. Transient requirement of the PrrA-PrrB two-component system for early intracellular multiplication of Mycobacterium tuberculosis. Infect. Immun. 70:2256-2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flynn, J. L., and J. Chan. 2001. Tuberculosis: latency and reactivation. Infect. Immun. 69:4195-4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilles-Gonzalez, M. A., and G. Gonzalez. 1993. Regulation of the kinase activity of heme protein FixL from the two-component system FixL/FixJ of Rhizobium meliloti. J. Biol. Chem. 268:16293-16297. [PubMed] [Google Scholar]
- 8.Glickman, M. S., and W. R. Jacobs, Jr. 2001. Microbial pathogenesis of Mycobacterium tuberculosis: dawn of a discipline. Cell 104:477-485. [DOI] [PubMed] [Google Scholar]
- 9.Graham, J. E., and J. E. Clark-Curtiss. 1999. Identification of Mycobacterium tuberculosis RNAs synthesized in response to phagocytosis by human macrophages by selective capture of transcribed sequences (SCOTS). Proc. Natl. Acad. Sci. USA 96:11554-11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graham, M. R., L. M. Smoot, C. A. Migliaccio, K. Virtaneva, D. E. Sturdevant, S. F. Porcella, M. J. Federle, G. J. Adams, J. R. Scott, and J. M. Musser. 2002. Virulence control in group A Streptococcus by a two-component gene regulatory system: global expression profiling and in vivo infection modeling. Proc. Natl. Acad. Sci. USA 99:13855-13860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haydel, S. E., N. E. Dunlap, and W. H. Benjamin, Jr. 1999. In vitro evidence of two-component system phosphorylation between the Mycobacterium tuberculosis TrcR/TrcS proteins. Microb. Pathog. 26:195-206. [DOI] [PubMed] [Google Scholar]
- 12.Himpens, S., C. Locht, and P. Supply. 2000. Molecular characterization of the mycobacterial SenX3-RegX3 two-component system: evidence for autoregulation. Microbiology 146:3091-3098. [DOI] [PubMed] [Google Scholar]
- 13.Honer zu Bentrup, K., and D. G. Russell. 2001. Mycobacterial persistence: adaptation to a changing environment. Trends Microbiol. 9:597-605. [DOI] [PubMed] [Google Scholar]
- 14.Hsing, W., and T. J. Silhavy. 1997. Function of conserved histidine-243 in phosphatase activity of EnvZ, the sensor for porin osmoregulation in Escherichia coli. J. Bacteriol. 179:3729-3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobs, W. R., Jr., G. V. Kalpana, J. D. Cirillo, L. Pascopella, S. B. Snapper, R. A. Udani, W. Jones, R. G. Barletta, and B. R. Bloom. 1991. Genetic systems for mycobacteria. Methods Enzymol. 204:537-555. [DOI] [PubMed] [Google Scholar]
- 16.Jeon, Y., Y. S. Lee, J. S. Han, J. B. Kim, and D. S. Hwang. 2001. Multimerization of phosphorylated and non-phosphorylated ArcA is necessary for the response regulator function of the Arc two-component signal transduction system. J. Biol. Chem. 276:40873-40879. [DOI] [PubMed] [Google Scholar]
- 17.Li, Y., and L. Plamann. 1996. Purification and in vitro phosphorylation of Myxococcus xanthus AsgA protein. J. Bacteriol. 178:289-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lutter, P., H. E. Meyer, M. Langer, K. Witthohn, W. Dormeyer, A. Sickmann, and M. Bluggel. 2001. Investigation of charge variants of rViscumin by two-dimensional gel electrophoresis and mass spectrometry. Electrophoresis 22:2888-2897. [DOI] [PubMed] [Google Scholar]
- 19.Martinez-Hackert, E., and A. M. Stock. 1997. Structural relationships in the OmpR family of winged-helix transcription factors. J. Mol. Biol. 269:301-312. [DOI] [PubMed] [Google Scholar]
- 20.Matsubara, M., S. I. Kitaoka, S. I. Takeda, and T. Mizuno. 2000. Tuning of the porin expression under anaerobic growth conditions by His-to-Asp cross-phosphorelay through both the EnvZ-osmosensor and ArcB-anaerosensor in Escherichia coli. Genes Cells 5:555-569. [DOI] [PubMed] [Google Scholar]
- 21.Mattison, K., and L. J. Kenney. 2002. Phosphorylation alters the interaction of the response regulator OmpR with its sensor kinase EnvZ. J. Biol. Chem. 277:11143-11148. [DOI] [PubMed] [Google Scholar]
- 22.McCleary, W. R., and J. B. Stock. 1994. Acetyl phosphate and the activation of two-component response regulators. J. Biol. Chem. 269:31567-31572. [PubMed] [Google Scholar]
- 23.McCleary, W. R., and D. R. Zusman. 1990. Purification and characterization of the Myxococcus xanthus FrzE protein shows that it has autophosphorylation activity. J. Bacteriol. 172:6661-6668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murray, C. J., and J. A. Salomon. 1998. Modeling the impact of global tuberculosis control strategies. Proc. Natl. Acad. Sci. USA 95:13881-13886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakano, M. M., and Y. Zhu. 2001. Involvement of ResE phosphatase activity in down-regulation of ResD-controlled genes in Bacillus subtilis during aerobic growth. J. Bacteriol. 183:1938-1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oshima, T., H. Aiba, Y. Masuda, S. Kanaya, M. Sugiura, B. L. Wanner, H. Mori, and T. Mizuno. 2002. Transcriptome analysis of all two-component regulatory system mutants of Escherichia coli K-12. Mol. Microbiol. 46:281-291. [DOI] [PubMed] [Google Scholar]
- 27.O'Toole, R., M. J. Smeulders, M. C. Blokpoel, E. J. Kay, K. Lougheed, and H. D. Williams. 2003. A two-component regulator of universal stress protein expression and adaptation to oxygen starvation in Mycobacterium smegmatis. J. Bacteriol. 185:1543-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parish, T., D. A. Smith, S. Kendall, N. Casali, G. J. Bancroft, and N. G. Stoker. 2003. Deletion of two-component regulatory systems increases the virulence of Mycobacterium tuberculosis. Infect. Immun. 71:1134-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park, H. D., K. M. Guinn, M. I. Harrell, R. Liao, M. I. Voskuil, M. Tompa, G. K. Schoolnik, and D. R. Sherman. 2003. Rv3133c/dosR is a transcription factor that mediates the hypoxic response of Mycobacterium tuberculosis. Mol. Microbiol. 48:833-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parkinson, J. S., and E. C. Kofoid. 1992. Communication modules in bacterial signaling proteins. Annu. Rev. Genet. 26:71-112. [DOI] [PubMed] [Google Scholar]
- 31.Parrish, N. M., J. D. Dick, and W. R. Bishai. 1998. Mechanisms of latency in Mycobacterium tuberculosis. Trends Microbiol. 6:107-112. [DOI] [PubMed] [Google Scholar]
- 32.Perez, E., S. Samper, Y. Bordas, C. Guilhot, B. Gicquel, and C. Martin. 2001. An essential role for phoP in Mycobacterium tuberculosis virulence. Mol. Microbiol. 41:179-187. [DOI] [PubMed] [Google Scholar]
- 33.Porter, S. L., and J. P. Armitage. 2002. Phosphotransfer in Rhodobacter sphaeroides chemotaxis. J. Mol. Biol. 324:35-45. [DOI] [PubMed] [Google Scholar]
- 34.Rook, G. A., and R. Hernandez-Pando. 1996. The pathogenesis of tuberculosis. Annu. Rev. Microbiol. 50:259-284. [DOI] [PubMed] [Google Scholar]
- 35.Schluger, N. W. 2000. The impact of drug resistance on the global tuberculosis epidemic. Int. J. Tuberc. Lung Dis. 4:S71-S75. [PubMed]
- 36.Stock, A. M., V. L. Robinson, and P. N. Goudreau. 2000. Two-component signal transduction. Annu. Rev. Biochem. 69:183-215. [DOI] [PubMed] [Google Scholar]
- 37.Stock, J. B., M. G. Surette, M. Levit, and P. Park. 1995. Two-component signal transduction systems: structure-function relationships and mechanisms of catalysis, p. 25-51. In J. A. Hoch and T. J. Silhavy (ed.), Two-component signal transduction. American Society for Microbiology, Washington, D.C.
- 38.Tran, V. K., R. Oropeza, and L. J. Kenney. 2000. A single amino acid substitution in the C terminus of OmpR alters DNA recognition and phosphorylation. J. Mol. Biol. 299:1257-1270. [DOI] [PubMed] [Google Scholar]
- 39.Via, L. E., R. Curcic, M. H. Mudd, S. Dhandayuthapani, R. J. Ulmer, and V. Deretic. 1996. Elements of signal transduction in Mycobacterium tuberculosis: in vitro phosphorylation and in vivo expression of the response regulator MtrA. J. Bacteriol. 178:3314-3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wayne, L. G., and L. G. Hayes. 1996. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect. Immun. 64:2062-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zahrt, T. C. 2003. Molecular mechanisms regulating persistent Mycobacterium tuberculosis infection. Microbes Infect. 5:159-167. [DOI] [PubMed] [Google Scholar]
- 42.Zahrt, T. C., and V. Deretic. 2000. An essential two-component signal transduction system in Mycobacterium tuberculosis. J. Bacteriol. 182:3832-3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zahrt, T. C., and V. Deretic. 2001. Mycobacterium tuberculosis signal transduction system required for persistent infections. Proc. Natl. Acad. Sci. USA 98:12706-12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zumla, A., P. Mwaba, S. B. Squire, and J. M. Grange. 1999. The tuberculosis pandemic—which way now? J. Infect. 38:74-79. [DOI] [PubMed] [Google Scholar]